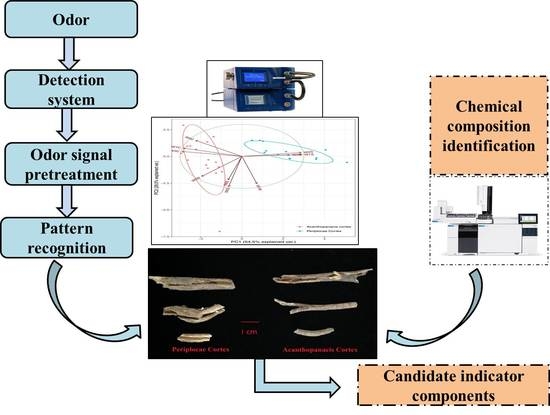
Comparative Analysis of Acanthopanacis Cortex and Periplocae Cortex Using an Electronic Nose and Gas Chromatography–Mass Spectrometry Coupled with Multivariate Statistical Analysis
Abstract
:1. Introduction
2. Results
2.1. Differentiation of Acanthopanacis Cortex and Periplocae Cortex by E-Nose
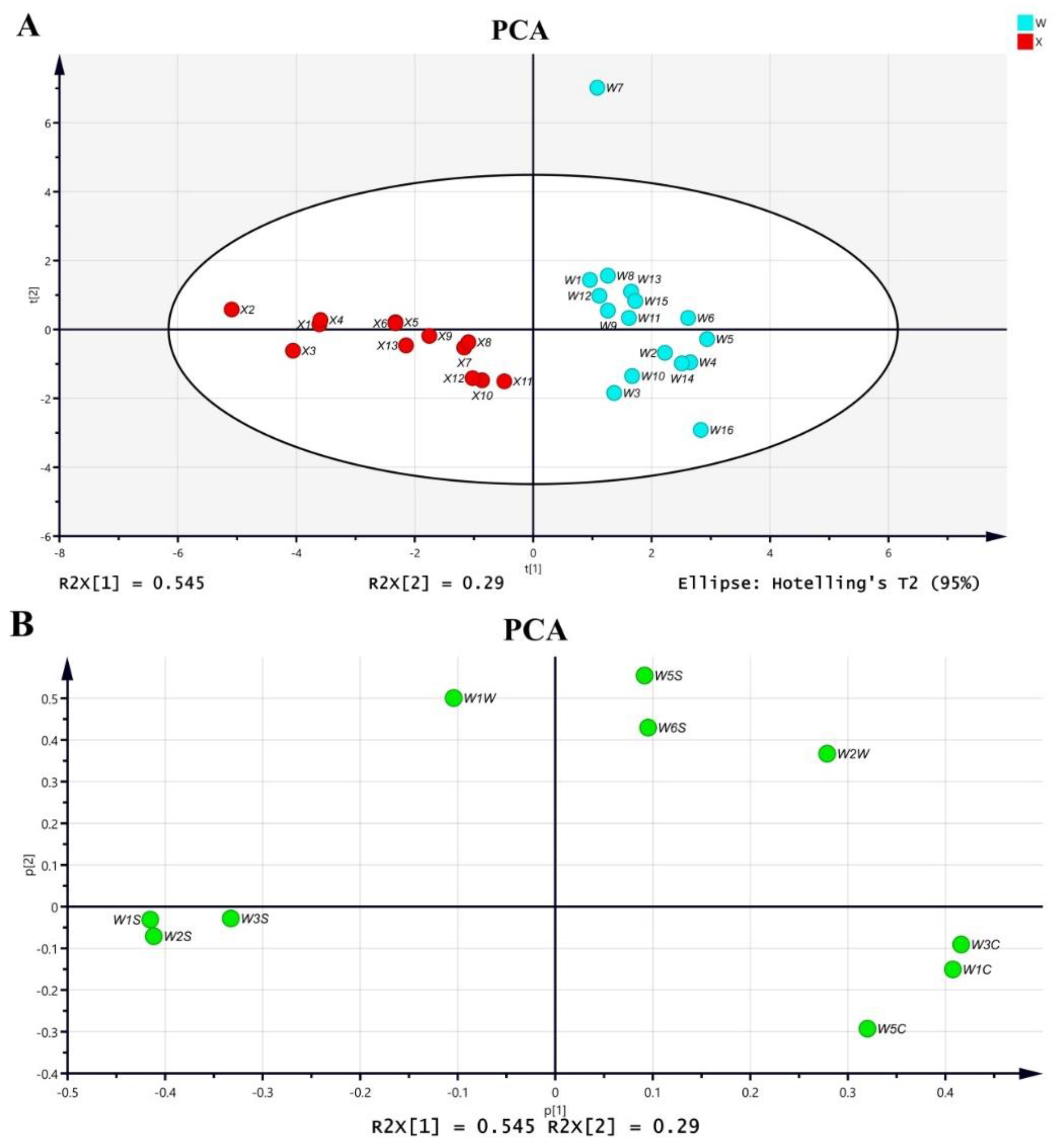
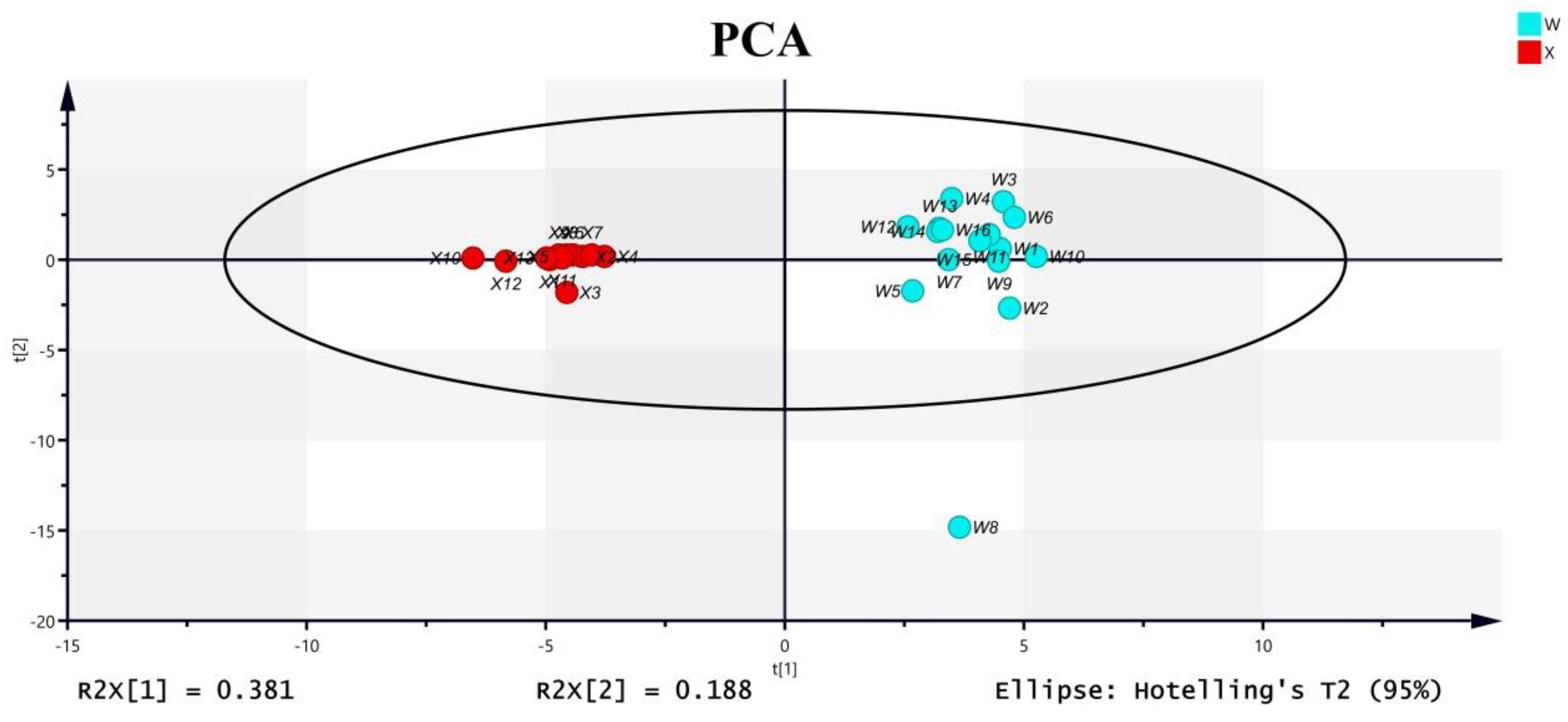
2.1.1. PCA
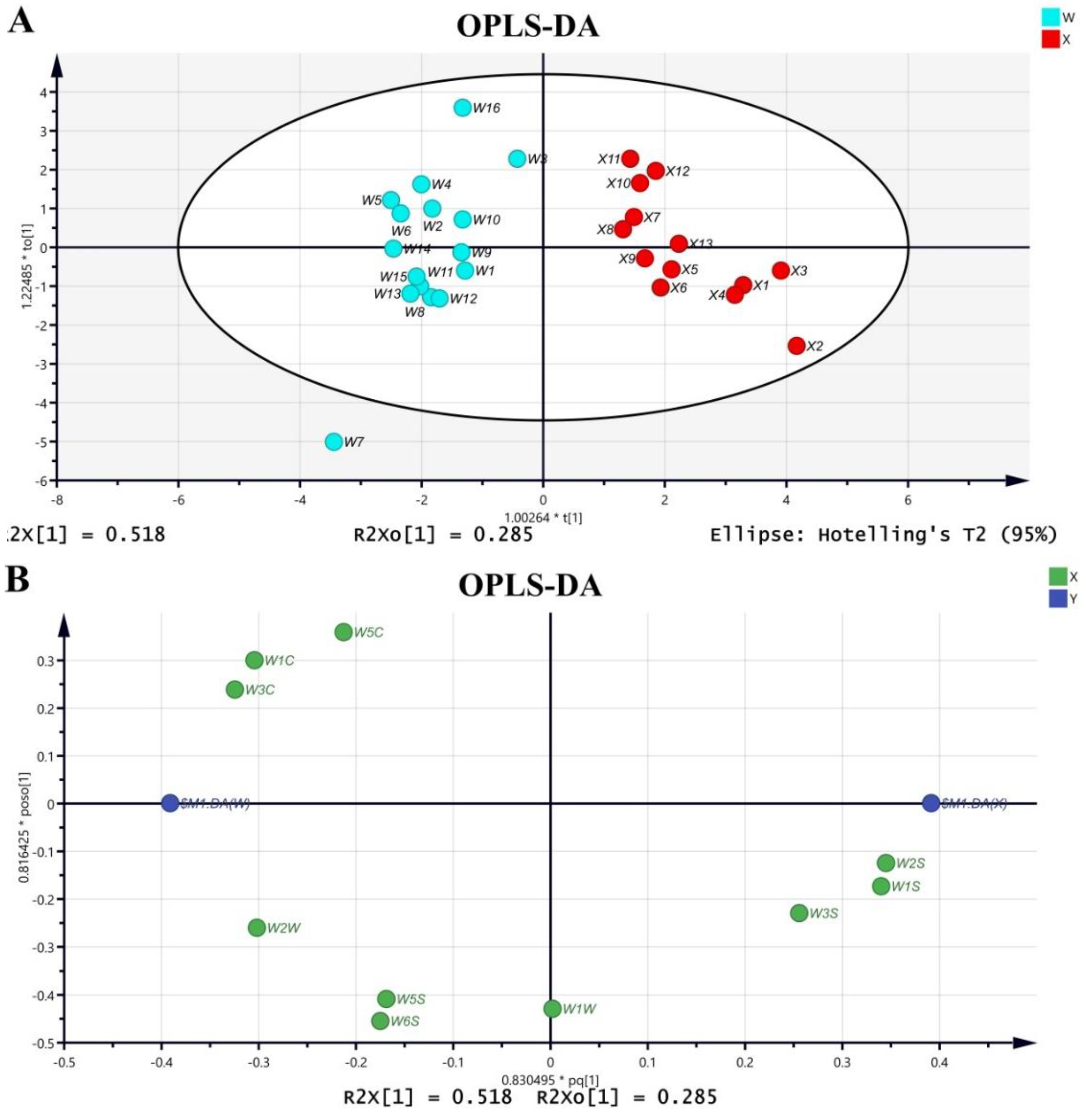
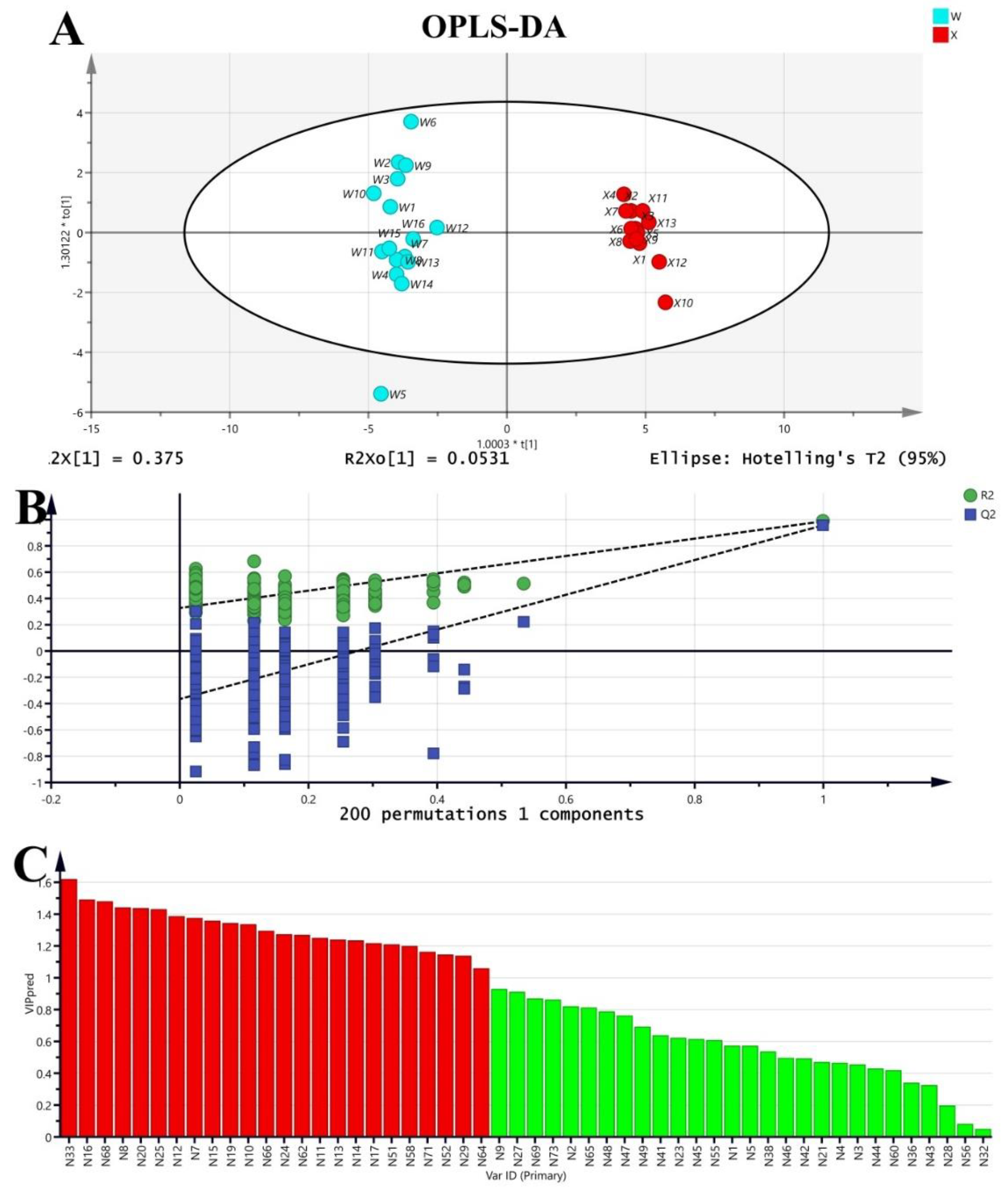
2.1.2. OPLS-DA
2.1.3. Relationship between Sample Odor and E-Nose Sensor
2.2. Composition and Relative Contents of Volatile Aroma Compounds in Acanthopanacis Cortex and Periplocae Cortex
2.3. Metabonomics Difference Analysis of Volatile Aroma Compounds in Acanthopanacis Cortex and Periplocae Cortex
2.3.1. Chemometric Analysis
2.3.2. Comparative Analysis of Potential Chemical Markers
3. Discussion
3.1. Acanthopanacis Cortex and Periplocae Cortex Should Be Correctly Identifed and Used
3.2. E-Nose Effectively Identifies Acanthopanacis Cortex and Periplocae Cortex
3.3. GC-MS Combined with Multivariate Statistical Analysis Effectively Classifies and Identifies Acanthopanacis Cortex and Periplocae Cortex
3.4. The Standards for Periplocae Cortex Need to Be Further Improved
3.5. E-Nose Technology Is Expected to Become a Technical Tool for Quality Supervision and Improvement of CHMS
4. Materials and Methods
4.1. Plant Materials
4.2. Reagents
4.3. E-Nose Analysis
4.4. Extract of Volatile Oil and GC-MS Analysis
4.4.1. Extraction of Volatile Oil
4.4.2. Instrumentation and GC-MS Conditions
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ahn, S.; Singh, P.; Jang, M.; Kim, Y.J.; Castro-Aceituno, V.; Simu, S.Y.; Kim, Y.J.; Yang, D.C. Gold nanoflowers synthesized using Acanthopanacis cortex extract inhibit inflammatory mediators in LPS-induced RAW264.7 macrophages via NF-κB and AP-1 pathways. Colloids Surf. B Biointerfaces 2018, 162, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.E.; Zeki, K.; Sugiura, T.; Yoshida, Y.; Yamashita, U. Chinese medicinal herb, Acanthopanax gracilistylus, extract induces cell cycle arrest of human tumor cells in vitro. Jpn. J. Cancer Res. 2000, 91, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dong, J.; Liu, M.; Li, Y.; Pan, J.; Liu, H.; Wang, W.; Bai, D.; Xiang, L.; Xiao, G.G.; et al. Therapeutic Effects of Cortex acanthopanacis Aqueous Extract on Bone Metabolism of Ovariectomized Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 492627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.X.; Li, N.; Zhang, Z.P.; Liu, H.B.; Zhou, R.R.; Zhong, B.Y.; Zou, M.X.; Dai, X.H.; Xiao, M.F.; Liu, X.Q.; et al. Protective effect of Acanthopanax gracilistylus-extracted Acankoreanogenin A on mice with fulminant hepatitis. Int. Immunopharmacol. 2011, 11, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, X.; Song, J.; Pang, X.; Chen, S. Internal transcribed spacer 2 barcode: A good tool for identifying Acanthopanacis cortex. Front. Plant Sci. 2015, 6, 840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.T.; Zhang, F.X.; Chen, W.W.; Chen, M.H.; Tang, X.Y.; Ye, M.N.; Yao, Z.H.; Yao, X.S.; Dai, Y. Characterization of chemical components of Periplocae Cortex and their metabolites in rats using ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Biomed. Chromatogr. 2020, 34, e4807. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Deng, F.; Xing, H.; Wen, H.; Shi, X.; Martey, O.N.; Koomson, E.; He, X. P-glycoprotein- and organic anion-transporting polypeptide-mediated transport of periplocin may lead to drug-herb/drug-drug interactions. Drug Des. Dev. Ther. 2014, 8, 475–483. [Google Scholar]
- Peris, M.; Escuder-Gilabert, L. Electronic noses and tongues to assess food authenticity and adulteration. Trends Food Sci. Technol. 2016, 58, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, J.; Horrillo, M.C. Advances in artificial olfaction: Sensors and applications. Talanta 2014, 124, 95–105. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, J.; Wang, Y.; Cheng, S. A novel framework for analyzing MOS E-nose data based on voting theory: Application to evaluate the internal quality of Chinese pecans. Sens. Actuators B Chem. 2017, 242, 511–521. [Google Scholar] [CrossRef]
- Gebicki, J. Application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. Trends Anal. Chem. 2016, 77, 1–13. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.; Liu, L.; Zhang, R. A novel method for qualitative analysis of edible oil oxidation using an electronic nose. Food Chem. 2016, 202, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.; Bandyopadhyay, R.; Bhattacharyya, N.; Pandey, R.A.; Jana, A. Application of electronic nose for industrial odors and gaseous emissions measurement and monitoring—An overview. Talanta 2015, 144, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Luo, D.; GholamHosseini, H.; Li, Z.; He, J. Identification of Chinese Herbal Medicines with Electronic Nose Technology: Applications and Challenges. Sensors 2017, 17, 1073–1094. [Google Scholar] [CrossRef] [Green Version]
- Cui, S.; Wu, J.; Wang, J.; Wang, X. Discrimination of American ginseng and Asian ginseng using electronic nose and gas chromatography-mass spectrometry coupled with chemometrics. J. Ginseng Res. 2017, 41, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Ye, T.; Jin, C.; Zhou, J.; Li, X.; Wang, H.; Deng, P.; Yang, Y.; Wu, Y.; Xiao, X. Can odors of TCM be captured by electronic nose? The novel quality control method for musk by electronic nose coupled with chemometrics. J. Pharm. Biomed. Anal. 2011, 55, 1239–1244. [Google Scholar] [CrossRef]
- Luo, D.; Chen, H.; Yu, H.; Sun, Y. A Novel Approach for Classification of Chinese Herbal Medicines Using Diffusion Maps. Intern. J. Pattern Recognit. Artif. Intell. 2015, 29, 1550003. [Google Scholar] [CrossRef]
- Zheng, S.; Ren, W.; Huang, L. Geoherbalism evaluation of Radix Angelica sinensis based on electronic nose. J. Pharm. Biomed. Anal. 2015, 105, 101–106. [Google Scholar] [CrossRef]
- Long, Q.; Li, Z.; Han, B.; Gholam Hosseini, H.; Zhou, H.; Wang, S.; Luo, D. Discrimination of Two Cultivars of Alpinia Officinarum Hance Using an Electronic Nose and Gas Chromatography-Mass Spectrometry Coupled with Chemometrics. Sensors 2019, 19, 572. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Serra, D.; Suraci, F.; Di Sanzo, R.; Fuda, S.; Postorino, S. The potential of e-nose aroma profiling for identifying the geographical origin of licorice (Glycyrrhiza glabra L.) roots. Food Chem. 2014, 165, 467–474. [Google Scholar] [CrossRef]
- Gao, M.; Jia, X.; Huang, X.; Wang, W.; Yao, G.; Chang, Y.; Ouyang, H.; Li, T.; He, J. Correlation between Quality and Geographical Origins of Cortex Periplocae, Based on the Qualitative and Quantitative Determination of Chemical Markers Combined with Chemical Pattern Recognition. Molecules 2019, 24, 3621. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.T.; Zhang, F.X.; Fan, C.L.; Ye, M.N.; Chen, W.W.; Yao, Z.H.; Yao, X.S.; Dai, Y. Discovery of potential Q-marker of traditional Chinese medicine based on plant metabolomics and network pharmacology: Periplocae Cortex as an example. Phytomedicine 2021, 85, 153535. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yu, P.H.; Zhao, X.; Zhong, N.; Zheng, H.F. HS-SPME-GC-MS Analysis of Volatile Components of Congou Black Tea Processed from Baojing Huangjincha 1 from Different Harvesting Seasons. Food Sci. 2020, 41, 188–196. [Google Scholar]
- Li, Y.B. Application of GC-MS and LC-MS for Screening and Identification of Different Markers in Agarwood. Ph.D. Dissertation, Guangzhou University of Chinese Medicine, Guangzhou, China, 2017. [Google Scholar]
- Zhang, L.; Zeng, Z.; Zhao, C.; Kong, H.; Lu, X.; Xu, G. A comparative study of volatile components in green, oolong and black teas by using comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry and multivariate data analysis. J. Chromatogr. A 2013, 1313, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Z.; Zhang, L.X.; Sun, Y.Q. Semipreparative Separation and Determination of Eleutheroside E in Acanthopanax giraldii Harms by High-Performance Liquid Chromatography. J. Chromatogr. Sci. 2005, 43, 249–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Deng, Y.; Huang, D.; Wen, J.; Liu, Z.; Li, F. LC-MS/MS determination and pharmacokinetic study of four lignan components in rat plasma after oral administration of Acanthopanax sessiliflorus extract. J. Ethnopharmacol. 2012, 141, 957–963. [Google Scholar] [CrossRef]
- Yang, Y.J.; Gao, Q.Q.; E, X.H. Simultaneous determination of periplocoside and 4-methoxysalicylaldehyde in Periplocae Cortex from different habitats by HPLC. Tianjin Pharm. 2019, 31, 4–7. [Google Scholar]
- Guo, H.; Mao, H.; Pan, G.; Zhang, H.; Fan, G.; Li, W.; Zhou, K.; Zhu, Y.; Yanagihara, N.; Gao, X. Antagonism of Cortex Periplocae extract-induced catecholamines secretion by Panax notoginseng saponins in cultured bovine adrenal medullary cells by drug combinations. J. Ethnopharmacol. 2013, 147, 447–455. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, S. Identification of cortex herbs using the DNA barcode nrITS2. J. Nat. Med. 2013, 67, 296–302. [Google Scholar] [CrossRef]
- Huang, X.; Liang, Z.; Chen, H.; Zhao, Z.; Li, P. Identification of Chinese herbal medicines by fluorescence microscopy: Fluorescent characteristics of medicinal bark. J. Microsc. 2014, 256, 6–22. [Google Scholar] [CrossRef]
- Commission, C.P. The Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020; Volume I. [Google Scholar]
- Ren, X.L.; Liu, H.; QI, A.D.; Wang, Y. Improving of simultaneous determination of periplocin and 4-methoxy salicylic aldehyde in cortex periplocae with HPLC method. Tianjin J. Tradit. Chin. Med. 2007, 24, 252–254. [Google Scholar]
- Tong, L.; Tan, X.J.; Ling, J.R.; Chen, X.H.; Bi, K.S. RP-HPLC determination of isovanillin, periplocin and 4-methoxy salicylaldehyde in root bark of Cortex Periplocae. Chin. J. Pharm. Anal. 2009, 29, 961–962. [Google Scholar]
- Li, D.; Lei, T.; Zhang, S.; Shao, X.; Xie, C. A novel headspace integrated E-nose and its application in discrimination of Chinese medical herbs. Sens. Actuators B Chem. 2015, 221, 556–563. [Google Scholar] [CrossRef]
- Bhandari, M.P.; Carmona, E.N.; Galstyan, V.; Sberveglieri, V. Quality Evaluation of Parmigiano Reggiano Cheese by a Novel Nanowire Device S3 and Evaluation of the VOCs Profile. Procedia Eng. 2016, 168, 460–464. [Google Scholar] [CrossRef]
- Sberveglieri, V.; Bhandari, M.P.; Nunez Carmona, E.; Betto, G.; Sberveglieri, G. A Novel MOS Nanowire Gas Sensor Device (S3) and GC-MS-Based Approach for the Characterization of Grated Parmigiano Reggiano Cheese. Biosensors 2016, 6, 60. [Google Scholar] [CrossRef]
- Peng, L.; Zou, H.Q.; Bauer, R.; Liu, Y.; Tao, O.; Yan, S.R.; Han, Y.; Li, J.H.; Ren, Z.Y.; Yan, Y.H. Identification of chinese herbal medicines from zingiberaceae family using feature extraction and cascade classifier based on response signals from e-nose. Evid. Based Complement. Altern. Med. 2014, 7, 963035. [Google Scholar]
- Bhandari, M.P.; Carmona, E.N.; Abbatangelo, M.; Sberveglieri, V.; Duina, G.; Malla, R.; Comini, E.; Sberveglieri, G. Discrimination of Quality and Geographical Origin of Extra Virgin Olive Oil by S3 Device with Metal Oxides Gas Sensors. Proceedings 2018, 2, 1061. [Google Scholar]
- Gómez, A.H.; Wang, J.; Hu, G.; Pereira, A.G. Monitoring storage shelf life of tomato using electronic nose technique. J. Food Eng. 2008, 85, 625–631. [Google Scholar] [CrossRef]
- Feng, T.; Zhuang, H.; Ye, R.; Jin, Z.; Xu, X.; Xie, Z. Analysis of volatile compounds of Mesona Blumes gum/rice extrudates via GC–MS and electronic nose. Sens. Actuators B Chem. 2011, 160, 964–973. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Zhang, Y.B.; Zhu, K.K.; Luo, C.; Zhang, J.X.; Cheng, C.R.; Feng, R.H.; Yang, W.Z.; Zeng, F.; Wang, Y.; et al. Anti-inflammatory diterpenoids from the root bark of Acanthopanax gracilistylus. J. Nat. Prod. 2014, 77, 2342–2351. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhou, K.; He, J.; Cao, J.; An, M.; Chang, Y.X. A Review on Phytochemistry and Pharmacology of Cortex Periplocae. Molecules 2016, 21, 1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, N. Studies on the Active Ingredients from the Leaves of Acanthopanax gracilistylus W.W. Smith and other Acanthopanax Miq. plants. Master’s Thesis, Central South University, Changsha, China, 2008. [Google Scholar]
- Yang, J.B.; Cai, W.; Li, M.H.; Li, N.X.; Ma, S.C.; Cheng, X.L.; Wei, F. Progress in Chemical and Pharmacological Research of Acanthopanax gracilistylus. Mod. Chin. Med. 2020, 22, 652–662. [Google Scholar]
- Boulechfar, S.; Zellagui, A.; Asan-Ozusaglam, M.; Bensouici, C.; Erenler, R.; Yildiz, İ.; Tacer, S.; Boural, H.; Demirtas, I. Chemical composition, antioxidant, and antimicrobial activities of two essential oils from Algerian propolis. Z. Nat. C J. Biosci. 2022, 77, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Liu, H.; Dong, J.P. Studies on chemical constituents of essential of the Acanthopanax giraldii Harms var. hispidus Hoo. Chin. Pharm. J. 1994, 29, 83–86. [Google Scholar]
- An, S.Y.; Qian, S.H.; Jiang, J.Q.; Wang, K.C. Chemical constituents in leaves of Acanthopanax gracilistylus. Chin. Tradit. Herb. Drugs 2009, 40, 1528–1534. [Google Scholar]
- Wang, L.; Yin, Z.Q.; Zhang, L.H.; Ye, W.C.; Zhang, X.Q. Chemical constituents from root barks of Periploca sepium. Zhongguo Zhong Yao Za Zhi 2007, 32, 1300–1302. [Google Scholar]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Trygg, J.; Holmes, E.; Lundstedt, T. Chemometrics in Metabonomics. J. Proteome Res. 2007, 6, 469–479. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Holmes, E.; Khan, F.; Kochhar, S.; Scanlan, P.; Shanahan, F.; Wilson, I.D.; Wang, Y. Rapid and Noninvasive Metabonomic Characterization of Inflammatory Bowel Disease. J. Proteome Res. 2007, 6, 546–551. [Google Scholar] [CrossRef]

| No. | Retention Time/Min | Constituents | Formula | Compatibility | Relative Contents (% Average) | Structure Type | |||
|---|---|---|---|---|---|---|---|---|---|
| W | X | W | X | W | X | ||||
| 1 | 6.195 | - | 1-methyl-4-prop-1-en-2-ylcyclohexa-1,3-diene | C10H14 | 92.64 | - | 0.72 ± 0.34 | - | Monoterpenoids |
| 2 | 6.731 | - | 1-methyl-2-propan-2-ylbenzene | C10H14 | 93.19 | - | 1.94 ± 0.6 | - | Monoterpenoids |
| 3 | 6.872 | - | (4R)-1-methyl-4-prop-1-en-2-ylcyclohexene | C10H16 | 86.85 | - | 0.25 ± 0.16 | - | Monoterpenoids |
| 4 | 7.81 | - | 1-methyl-4-propan-2-ylcyclohexa-1,4-diene | C10H16 | 92.43 | - | 0.2 ± 0.12 | - | Monoterpenoids |
| 5 | 8.417 | - | 1-methoxy-2-propylbenzene | C10H14O | 82.38 | - | 0.17 ± 0.08 | - | Aromatic ethers |
| 6 | 8.933 | - | 1-Ethenyl-3,5-dimethylbenzene | C10H12 | 80.21 | - | 0.19 ± 0.19 | - | Monoterpenoids |
| 7 | 10.519 | - | 1-(2,2,3-trimethyl-1-cyclopent-3-enyl)ethanone | C10H16O | 96.46 | - | 6.65 ± 0.72 | - | Monoterpenoids |
| 8 | 11.171 | - | (1S,5S)-7,7-dimethyl-4-methylidenebicyclo [3.1.1]heptan-3-ol | C10H16O | 95.71 | - | 10.98 ± 0.97 | - | Monoterpenoids |
| 9 | 11.421 | - | (1S,2R,5S)-4,6,6-trimethylbicyclo[3.1.1]hept-3-en-2-ol | C10H16O | 92.19 | - | 3.82 ± 0.94 | - | Monoterpenoids |
| 10 | 11.63 | - | 2-(4-methylidene-1-cyclohex-2-enyl)propan-2-ol | C10H16O | 85.82 | - | 4.46 ± 0.53 | - | Alkenes |
| 11 | 12.39 | - | 7,7-dimethyl-4-methylidenebicyclo[3.1.1]heptan-3-one | C10H14O | 93.79 | - | 1.76 ± 0.25 | - | Monoterpenoids |
| 12 | 12.58 | - | 2-(4-methyl-1-cyclohexa-2,4-dienyl)propan-2-ol | C10H16O | 94.68 | - | 12.13 ± 1.27 | - | Alkanes |
| 13 | 13.118 | - | (1R)-4-methyl-1-propan-2-ylcyclohex-3-en-1-ol | C10H18O | 90.29 | - | 1.04 ± 0.15 | - | Monoterpenoids |
| 14 | 13.448 | - | 2-(4-methylphenyl)propan-2-ol | C10H14O | 87.44 | - | 1.61 ± 0.25 | - | Alcohols |
| 15 | 13.731 | - | 2-[(1R)-4-methyl-1-cyclohex-3-enyl]propan-2-ol | C10H18O | 93.2 | - | 1.27 ± 0.14 | - | Monoterpenoids |
| 16 | 13.942 | - | (1R,5S)-6,6-dimethyl-bicyclo[3.3.1]hept-2-en-2-carbaldehyde | C10H14O | 95.17 | - | 9.84 ± 0.72 | - | Monoterpenoids |
| 17 | 14.044 | 9.127 | Dodecane | C12H26 | 84.64 | 97.3 | 0.12 ± 0.12 | 1.54 ± 0.25 | Alkanes |
| 18 | 14.194 | - | 1,7,7-trimethylbicyclo[2.2.1]heptan-6-ol | C10H18O | 85.15 | - | 0.04 ± 0.04 | - | Monoterpenoids |
| 19 | 14.446 | - | (1S,5S)-2,7,7-trimethylbicyclo[3.1.1]hept-2-en-4-one | C10H14O | 97.45 | - | 11.6 ± 1.37 | - | Monoterpenoids |
| 20 | 14.746 | - | (1R,5S)-2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-ol | C10H16O | 96.49 | - | 3.86 ± 0.35 | - | Monoterpenoids |
| 21 | 15.126 | - | 2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-ol | C10H16O | 89.83 | - | 0.08 ± 0.04 | - | Monoterpenoids |
| 22 | 15.267 | - | 2-methoxy-4-methyl-1-propan-2-ylbenzene | C11H16O | 88.87 | - | 0.02 ± 0.02 | - | Ether |
| 23 | 15.434 | - | 4-propan-2-ylbenzaldehyde | C10H12O | 84.86 | - | 0.13 ± 0.05 | - | Aldehyde |
| 24 | 15.536 | - | (5S)-2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one | C10H14O | 94.2 | - | 0.66 ± 0.09 | - | Monoterpenoids |
| 25 | 15.795 | - | 2,7,7-trimethylbicyclo[3.1.1]hept-2-en-4-one | C10H14O | 85.38 | - | 1 ± 0.09 | - | Monoterpenoids |
| 26 | 15.989 | - | [(1S,2R,5R)-6,6-dimethylbicyclo[3.1.1]hept-2-yl]methanol | C10H18O | 86.84 | - | 0.06 ± 0.04 | - | Alcohols |
| 27 | 16.693 | - | 1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol acetate | C12H20O2 | 86.53 | - | 0.27 ± 0.07 | - | Esters |
| 28 | 16.849 | 10.793 | (2E,4Z)-deca-2,4-dienal | C10H16O | 83.19 | 92.02 | 0.08 ± 0.05 | 0.12 ± 0.06 | Aldehyde |
| 29 | 16.979 | - | [(4S)-4-prop-1-en-2-yl-1-cyclohexenyl]methanol | C10H16O | 81.6 | - | 0.62 ± 0.11 | - | Monoterpenoids |
| 30 | 17.315 | - | 1-(2-hydroxy-5-methylphenyl)ethanone | C9H10O2 | 83.2 | - | 0.02 ± 0.02 | - | Ketones |
| 31 | 17.359 | - | 4-(2,2,6-trimethyl-1-bicyclo[4.1.0]heptanyl)butan-2-one | C14H24O | 82.36 | - | 0.03 ± 0.03 | - | Ketones |
| 32 | 17.382 | 11.195 | (2E,4E)-deca-2,4-dienal | C10H16O | 92.36 | - | 0.16 ± 0.1 | 0.18 ± 0.09 | Aldehyde |
| 33 | 17.631 | 11.481 | 2-Hydroxy-4-methoxybenzaldehyde | C8H8O3 | 97.42 | - | 2.62 ± 0.74 | 91.74 ± 1.15 | Aldehyde |
| 34 | 18.125 | - | (3R,3aS,7S,8aS)-3,6,8,8-Tetramethyl-2,3,4,7,8,8a-hexahydro-1H-3a,7-methanoazulene | C15H24 | 80.4 | - | 0.07 ± 0.07 | - | Sesquiterpenes |
| 35 | 18.241 | - | 2-Methoxy-4-prop-2-enylphenol | C10H12O2 | 94.36 | - | 0.32 ± 0.32 | - | Phenols |
| 36 | 18.661 | 12.302 | Tricyclo[4.4.0.02,7]dec-3-ene,1,3-dimethyl-8-(1-methylethyl)-, stereoisomer | C15H24 | 83.88 | - | 0.43 ± 0.16 | 0.18 ± 0.18 | Sesquiterpenes |
| 37 | 18.752 | - | (4aR,8aS)-7-Isopropylidene-4a-methyl-1-methylene-decahydro-naphthalene | C15H24 | 84.81 | - | 0.14 ± 0.1 | - | Sesquiterpenes |
| 38 | 18.771 | - | [1S-(1α,3aβ,4α,8aβ,9S*)]-decahydro-4,8,8-trimethyl-1,4-methanoazulene-9-methyl acetate | C17H28O2 | 80.84 | - | 0.17 ± 0.08 | - | Esters |
| 39 | 18.873 | - | (1R,3aS,5aS,8aR)-1,3a,4,5a-Tetramethyl-1,2,3,3a,5a,6,7,8-octahydrocyclopenta[c]pentalene | C15H24 | 81.46 | - | 0.02 ± 0.02 | - | Sesquiterpenes |
| 40 | 18.934 | - | 1-ethenyl-1-methyl-2,4-di(prop-1-en-2-yl)cyclohexane | C15H24 | 81.93 | - | 0.02 ± 0.02 | - | Sesquiterpenes |
| 41 | 19.453 | 13. 051 | (1R,4E,9S)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene | C15H24 | 84 | - | 0.63 ± 0.18 | 0.15 ± 0.15 | Sesquiterpenes |
| 42 | 19.653 | - | (1S,2E,10R)-3,7,11,11-Tetramethylbicyclo[8.1.0]undeca-2,6-diene | C15H24 | 84.06 | - | 0.18 ± 0.11 | - | Sesquiterpenes |
| 43 | 19.784 | 13.326 | 1-(2-Hydroxy-4-methoxyphenyl)ethanone | C9H10O3 | 95.59 | - | 0.5 ± 0.39 | 0.05 ± 0.05 | Phenols |
| 44 | 20.011 | - | 1,3a-Ethano-3aH-indene, 1,2,3,6,7,7a-hexahydro-2,2,4,7a-tetramethyl-, [1R-(1α,3aα,7aα)]- | C15H24 | 91.19 | - | 0.49 ± 0.32 | - | Sesquiterpenes |
| 45 | 20.353 | - | 1,4-dimethyl-7-propan-2-ylidene-2,3,4,5,6,8-hexahydro-1H-azulene | C15H24 | 88.63 | - | 0.27 ± 0.12 | - | Sesquiterpenes |
| 46 | 20.538 | - | 1,4-dimethyl-7-prop-1-en-2-yl-1,2,3,3a,4,5,6,7-octahydroazulene | C15H24 | 87.79 | - | 0.77 ± 0.45 | - | Sesquiterpenes |
| 47 | 20.719 | - | (1S,4aS,8aR)-4,7-dimethyl-1-(propan-2-yl)-1,2,4a,5,6,8a-hexahydronaphthalene | C15H24 | 84.66 | - | 0.48 ± 0.17 | - | Sesquiterpenes |
| 48 | 21.04 | 14.593 | (1S,8aR)-4,7-Dimethyl-1-(propan-2-yl)-1,2,3,5,6,8a-hexahydronaphthalene | C15H24 | 83.12 | - | 1.57 ± 0.42 | 0.19 ± 0.19 | Sesquiterpenes |
| 49 | 21.26 | - | (1R,4aR,4bS,7R,10aR)-1,4a,7-Trimethyl-7-vinyl-1,2,3,4,4a,4b,5,6,7,9,10,10a-dodecahydrophenanthrene-1-carbaldehyde | C20H30O | 89.3 | - | 4.95 ± 1.79 | - | Sandaracopimaral |
| 50 | 21.41 | - | ent-Kaur-16-en-19-al | C20H30O | 89.14 | - | 1.2 ± 1.16 | - | Diterpene |
| 51 | 21.914 | - | (1aR,4aR,7S,7aR,7bR)-1,1,7-Trimethyl-4-methylenedecahydro-1H-cyclopropa[e]azulen-7-ol | C15H24O | 93.04 | - | 1.31 ± 0.2 | - | Sesquiterpenes |
| 52 | 22.03 | - | (1R,4R,6R,10S)-4,12,12-Trimethyl-9-methylene-5-oxatricyclo[8.2.0.0]dodecane | C15H24O | 82.04 | - | 1.28 ± 0.25 | - | Sesquiterpenes |
| 53 | 22.742 | - | (1aR,7S,7aS,7bR)-1,1,4,7-Tetramethyl-1a,2,3,5,6,7,7a,7b-octahydro-1H-cyclopropa[e]azulen-7-ol | C15H24O | 86.65 | - | 0.14 ± 0.1 | - | Sesquiterpenes |
| 54 | 22.955 | - | 2-[(3S,5R,8S)-3,8-dimethyl-1,2,3,4,5,6,7,8-octahydroazulen-5-yl]propan-2-ol | C15H26O | 86.34 | - | 0.19 ± 0.19 | - | Sesquiterpenes |
| 55 | 23.179 | - | 2-[(2R,4aR,8aS)-4a-methyl-8-methylidene-1,2,3,4,5,6,7,8a-octahydronaphthalen-2-yl]propan-2-ol | C15H26O | 91.92 | - | 0.91 ± 0.44 | - | Sesquiterpenes |
| 56 | 23.315 | 16.639 | (4aS,8aR)-3,8a-Dimethyl-5-methylene-4,4a,5,6,7,8,8a,9-octahydronaphtho[2,3-b]furan | C15H20O | 86.49 | - | 0.29 ± 0.22 | 0.41 ± 0.14 | Sesquiterpenes |
| 57 | 24.173 | - | (Z)-octadec-9-en-1-ol | C18H36O | 84.2 | - | 0.1 ± 0.08 | - | Alcohols |
| 58 | 25.548 | - | 5-(5,5,8a-trimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl)-3-methylpent-1-en-3-ol | C20H34O | 87.84 | - | 4.9 ± 0.78 | - | Diterpene |
| 59 | 26.726 | - | (3R)-5-[(1S,4aR,5S,8aR)-5-(Hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydro-1-naphthalenyl]-3-methyl-1-penten-3-ol | C20H34O2 | 81.12 | - | 0.26 ± 0.26 | - | Diterpene |
| 60 | 29.794 | - | 2-ethenyl-2,4b-dimethyl-8-methylidene-3,4,4a,5,6,7,8a,9-octahydro-1H-phenanthrene | C19H28 | 82.09 | - | 0.33 ± 0.23 | - | Alkanes |
| 61 | - | 9.182 | (E)-2-ethylhex-2-enal | C8H14O | - | 83.58 | - | 0.04 ± 0.04 | Aldehyde |
| 62 | - | 9.701 | 3,4,5-trimethyloxolan-2-one | C7H12O2 | - | 87.35 | - | 0.5 ± 0.1 | Ketones |
| 63 | - | 10.197 | 4-Hydroxy-3-methylbenzaldehyde | C8H8O2 | - | 84.32 | - | 0.01 ± 0.01 | Aldehyde |
| 64 | - | 12.579 | 3-Hydroxy-4-methoxybenzaldehyde | C8H8O3 | - | 86.95 | - | 0.13 ± 0.04 | Alkanes |
| 65 | - | 13.187 | 1-ethenyl-1-methyl-4-propan-2-ylidene-2-prop-1-en-2-ylcyclohexane | C15H24 | - | 85.61 | - | 0.05 ± 0.02 | Sesquiterpenes |
| 66 | - | 13.474 | 5-formyl-2-methoxyphenyl acetate | C10H10O4 | - | 91.55 | - | 2.1 ± 0.37 | Monoterpenoids |
| 67 | - | 13.589 | (1E,4E,8E)-2,6,6,9-tetramethylcycloundeca-1,4,8-triene | C15H24 | - | 87.39 | - | 0.05 ± 0.05 | Sesquiterpenes |
| 68 | - | 13.688 | methyl 2-hydroxy-4-methoxybenzoate | C9H10O4 | - | 97.04 | - | 0.53 ± 0.06 | Esters |
| 69 | - | 14.096 | (3R,4aS,8aR)-8a-methyl-5-methylidene-3-prop-1-en-2-yl-1,2,3,4,4a,6,7,8-octahydronaphthalene | C15H24 | - | 85.68 | - | 0.12 ± 0.04 | Sesquiterpenes |
| 70 | - | 14.193 | (3S,3aR,3bR,4S,7R,7aR)-4-Isopropyl-3,7-dimethyloctahydro-1H-cyclopenta[1,3]cyclopropa[1,2]benzen-3-ol | C15H26O | - | 92.71 | - | 0.06 ± 0.06 | Sesquiterpenes |
| 71 | - | 14.282 | 2,4-ditert-butylphenol | C14H22O | - | 93.44 | - | 0.58 ± 0.13 | Phenols |
| 72 | - | 14.507 | (3R,3aR,3bR,4S,7R,7aR)-4-Isopropyl-3,7-dimethyloctahydro-1H-cyclopenta[1,3]cyclopropa[1,2]benzen-3-ol | C15H26O | - | 92.58 | - | 0.27 ± 0.27 | Sesquiterpenes |
| 73 | - | 14.834 | 1,2,3,5,6,7,8,8a-octahydro-1,8a-dimethyl-7-(1-methylethenyl)-,[1R-(1a,7b,8aa)]-Naphthalene | C15H24 | - | 85.36 | - | 0.13 ± 0.05 | Sesquiterpenes |
| 74 | - | 14.801 | (4aR,8aR)-4a,8-dimethyl-2-(1-methylethylidene)-1,2,3,4,4a,5,6,8a-octahydronaphthalene | C15H24 | - | 81.81 | - | 0.03 ± 0.02 | Sesquiterpenes |
| 75 | - | 16.354 | (1S,4S,4aR,8aR)-1,6-dimethyl-4-propan-2-yl-3,4,4a,7,8,8a-hexahydro-2H-naphthalen-1-ol | C15H26O | - | 93.83 | - | 0.25 ± 0.25 | Sesquiterpenes |
| 76 | - | 17.521 | 7-methoxychromen-2-one | C10H8O3 | - | 86.21 | - | 0.01 ± 0.01 | Phenylpropanoids |
| 77 | - | 18.672 | hexadecanal | C16H32O | - | 92.5 | - | 0.06 ± 0.06 | Aldehyde |
| 78 | - | 18.932 | (9Z,12Z)-octadeca-9,12-dienoic acid | C18H32O2 | - | 81.53 | - | 0.35 ± 0.35 | Organic acids |
| 79 | - | 19.113 | (E)-octadec-9-enoic acid | C18H34O2 | - | 80.34 | - | 0.03 ± 0.03 | Organic acids |
| 80 | - | 19.615 | O1-cyclohexyl O2-(2-methylpropyl) benzene-1,2-dicarboxylate | C18H24O4 | - | 83.82 | - | 0.01 ± 0.01 | Esters |
| 81 | - | 20.321 | (3S,3aS,6S,7S,7aS)-7-Isopropenyl-3,6-dimethyl-6-vinyl-hexahydro-benzofuran-2-one | C15H22O2 | - | 84.77 | - | 0.08 ± 0.08 | Esters |
| 82 | - | 21.551 | O2-butyl O1-cyclohexyl benzene-1,2-dicarboxylate | C18H24O4 | - | 87.88 | - | 0.05 ± 0.05 | Esters |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Wu, J.; Wang, K.; Liang, T.; Liu, Q.; Yan, J.; Yang, Y.; Qiao, K.; Ma, S.; Wang, D. Comparative Analysis of Acanthopanacis Cortex and Periplocae Cortex Using an Electronic Nose and Gas Chromatography–Mass Spectrometry Coupled with Multivariate Statistical Analysis. Molecules 2022, 27, 8964. https://doi.org/10.3390/molecules27248964
Sun L, Wu J, Wang K, Liang T, Liu Q, Yan J, Yang Y, Qiao K, Ma S, Wang D. Comparative Analysis of Acanthopanacis Cortex and Periplocae Cortex Using an Electronic Nose and Gas Chromatography–Mass Spectrometry Coupled with Multivariate Statistical Analysis. Molecules. 2022; 27(24):8964. https://doi.org/10.3390/molecules27248964
Chicago/Turabian StyleSun, Li, Jing Wu, Kang Wang, Tiantian Liang, Quanhui Liu, Junfeng Yan, Ying Yang, Ke Qiao, Sui Ma, and Di Wang. 2022. "Comparative Analysis of Acanthopanacis Cortex and Periplocae Cortex Using an Electronic Nose and Gas Chromatography–Mass Spectrometry Coupled with Multivariate Statistical Analysis" Molecules 27, no. 24: 8964. https://doi.org/10.3390/molecules27248964
APA StyleSun, L., Wu, J., Wang, K., Liang, T., Liu, Q., Yan, J., Yang, Y., Qiao, K., Ma, S., & Wang, D. (2022). Comparative Analysis of Acanthopanacis Cortex and Periplocae Cortex Using an Electronic Nose and Gas Chromatography–Mass Spectrometry Coupled with Multivariate Statistical Analysis. Molecules, 27(24), 8964. https://doi.org/10.3390/molecules27248964