Effect of Esculetin on Tert-Butyl Hydroperoxide-Induced Oxidative Injury in Retinal Pigment Epithelial Cells In Vitro
Abstract
1. Introduction
2. Results
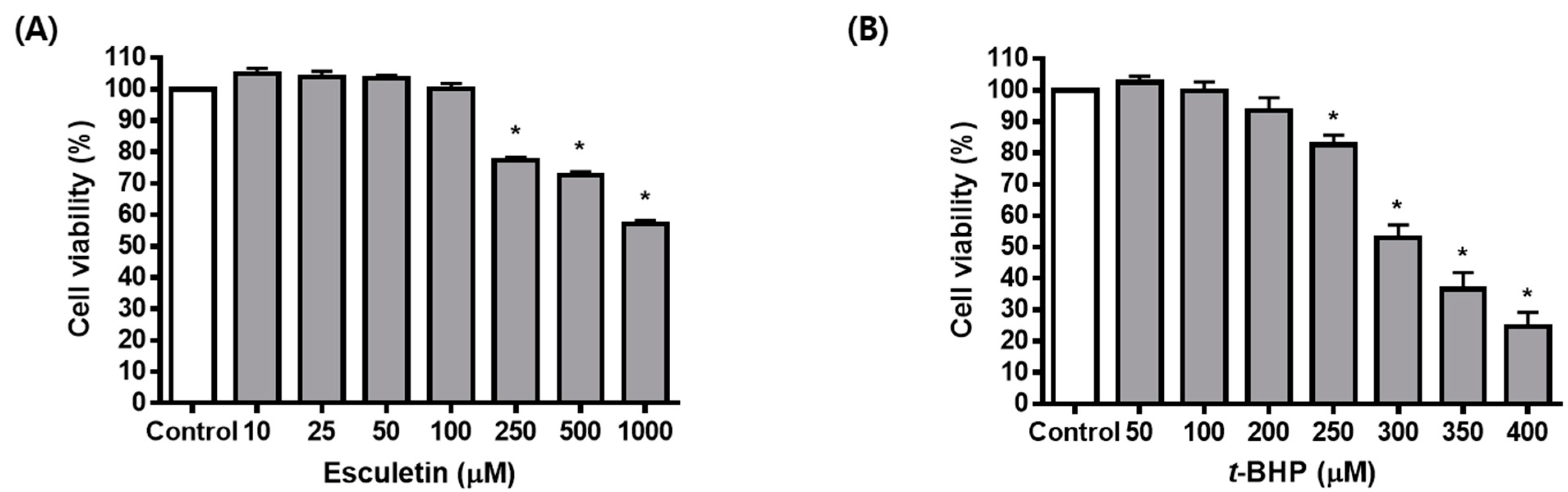
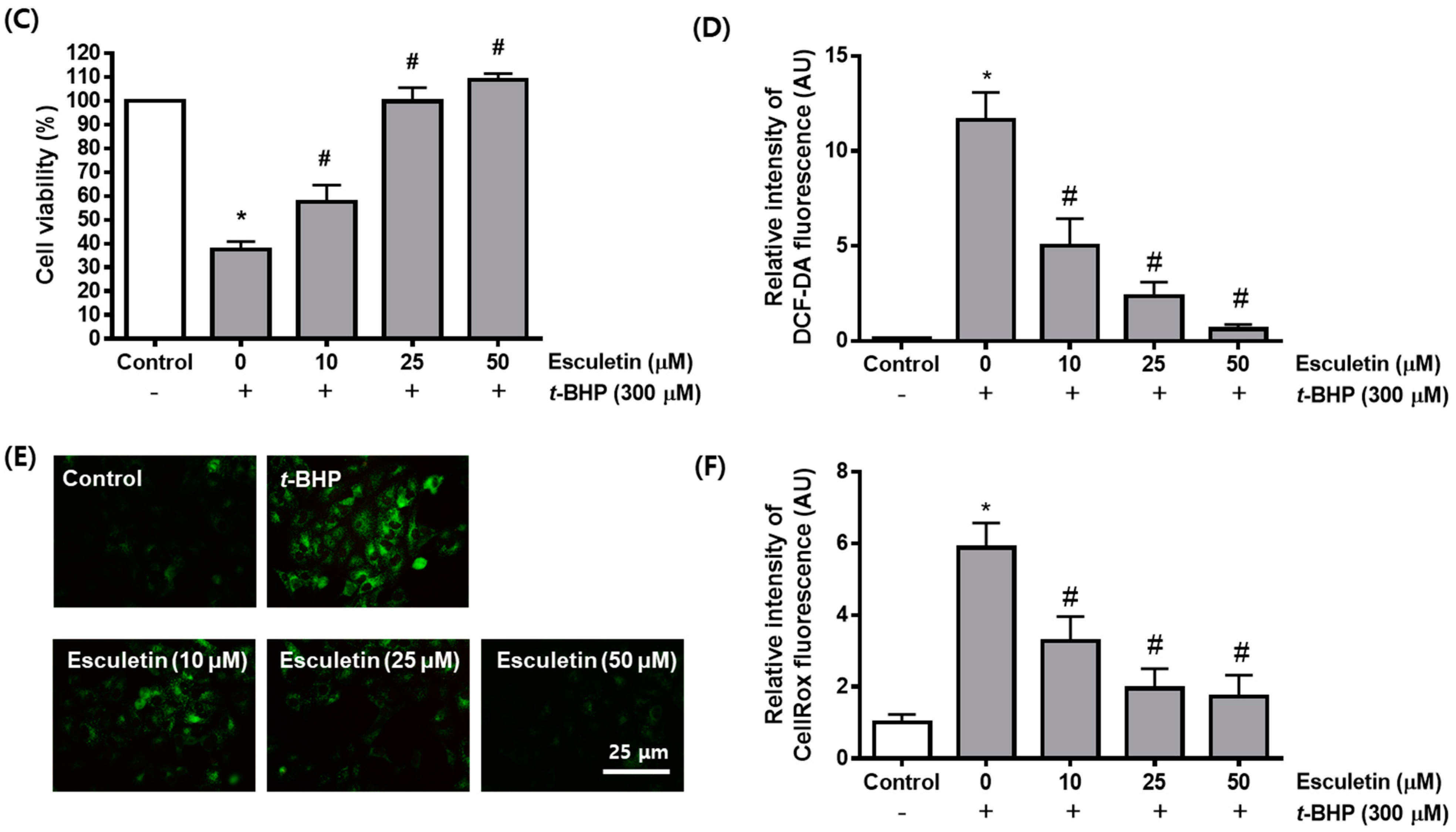
2.1. Esculetin Inhibits t-BHP-Induced Oxidative Injury of ARPE-19 Cells
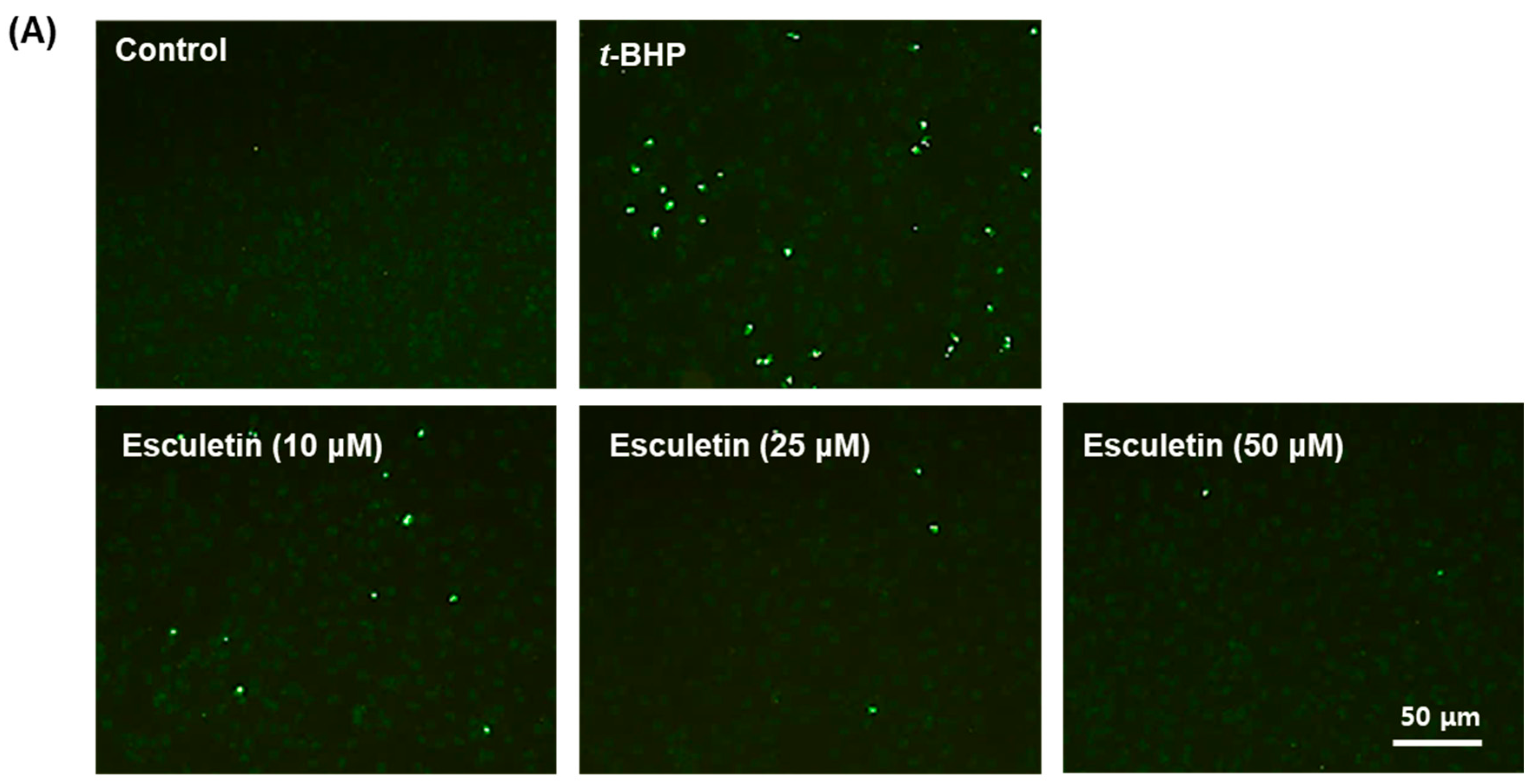
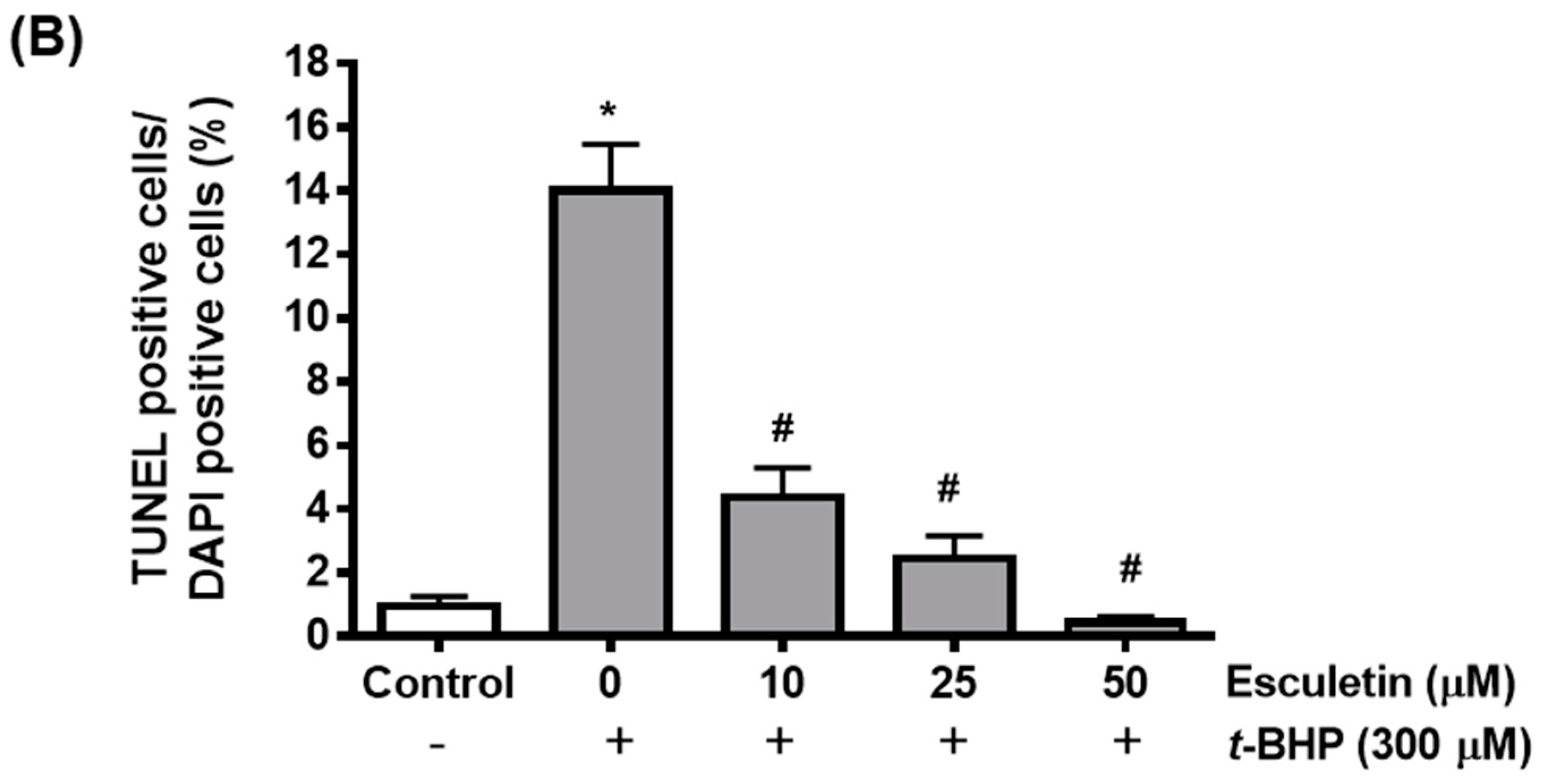
2.2. Esculetin Reduces t-BHP-Induced Apoptosis of ARPE-19 Cells
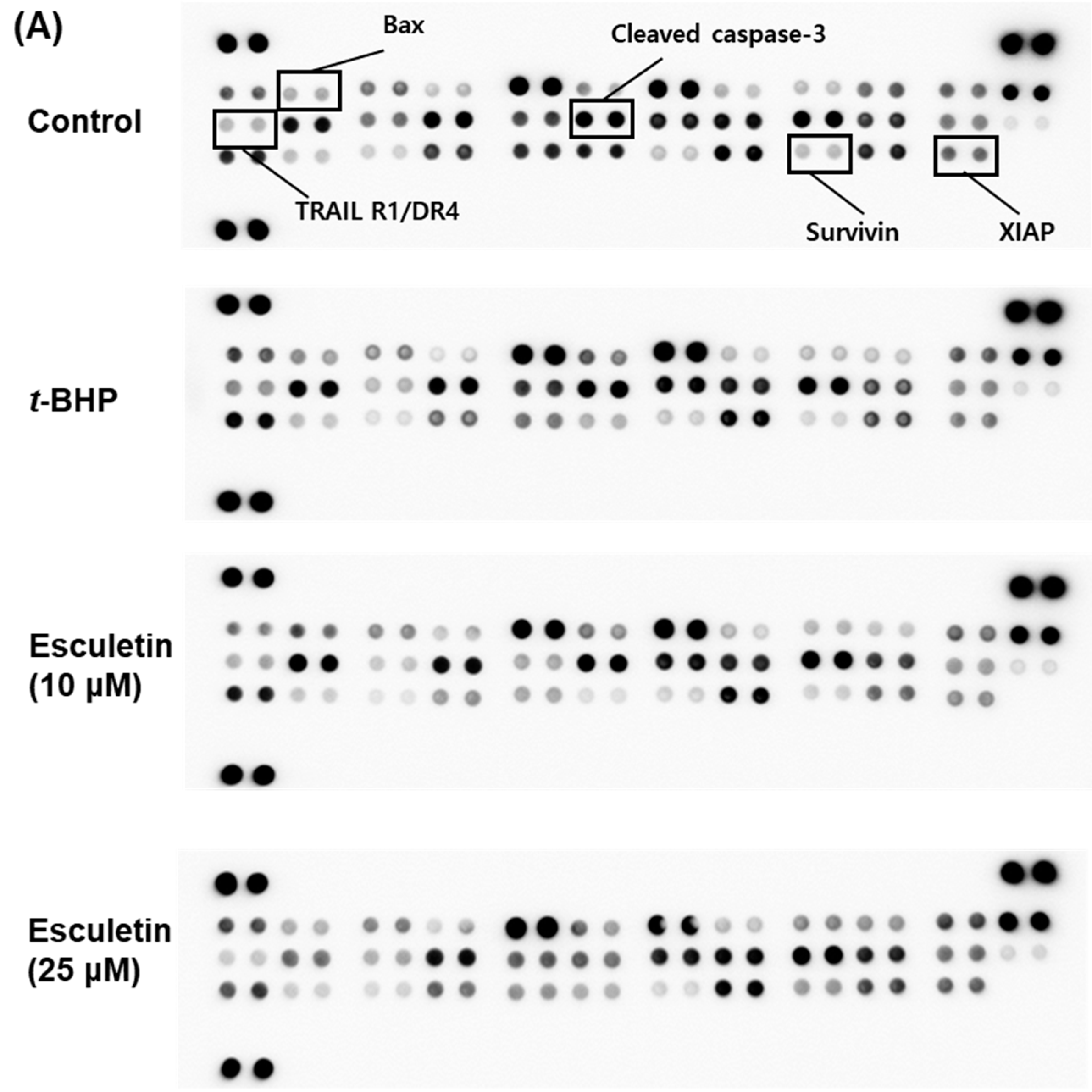
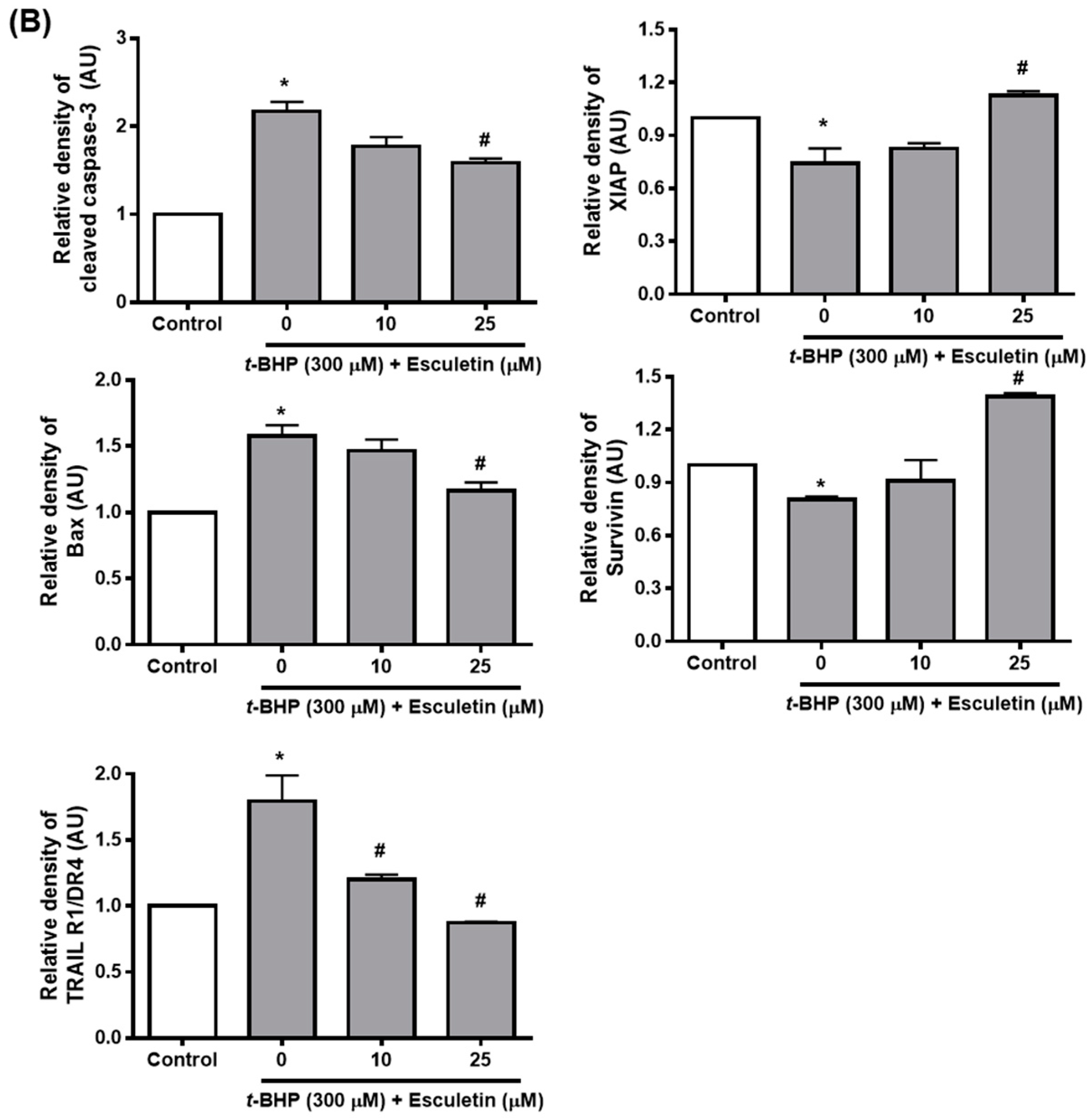
2.3. Esculetin Regulates Apoptosis-Related Signaling Pathways in ARPE-19 Cells
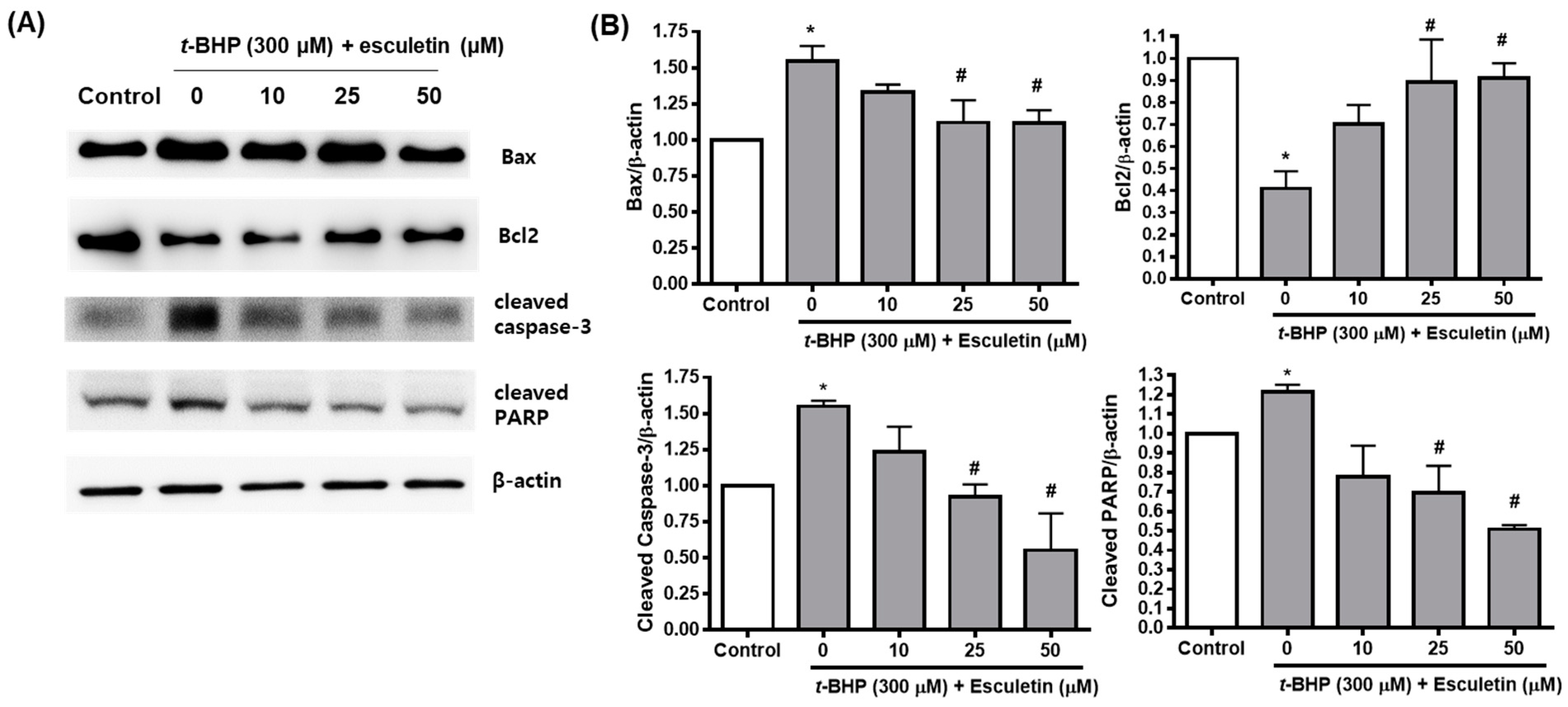
2.4. Esculetin Regulates the Expression of Apoptosis-Related Proteins in ARPE-19 Cells
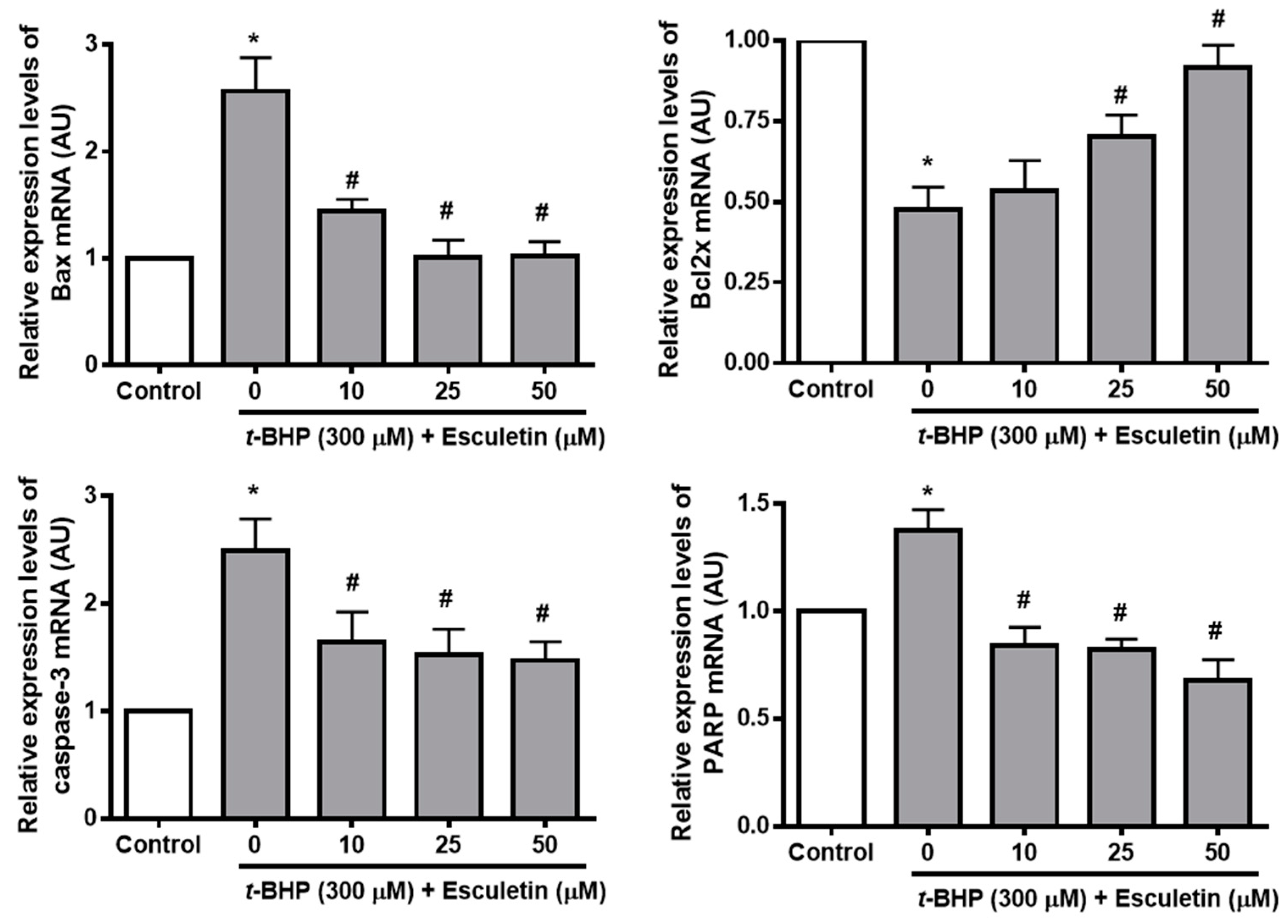
2.5. Esculetin Regulates the Expression of Apoptosis-Related mRNA in ARPE-19 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Oxidative Injury of ARPE-19 Cells
4.3. Measurement of Reactive Oxygen Species (ROS) Generation
4.4. TUNEL Staining
4.5. Apoptosis Antibody Array
4.6. Western Blot Analysis
4.7. Real-Time PCR
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Klein, R.; Klein, B.E. The prevalence of age-related eye diseases and visual impairment in aging: Current estimates. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF5–ORSF13. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M.A.; Casaroli-Marano, R.P.; Rosenfeld, P.J. Age-related macular degeneration: Clinical findings, histopathology and imaging techniques. Cell-Based Ther. Retin. Degener. Dis. 2014, 53, 1–32. [Google Scholar]
- Ishikawa, M.; Jin, D.; Sawada, Y.; Abe, S.; Yoshitomi, T. Future therapies of wet age-related macular degeneration. J. Ophthalmol. 2015, 2015, 138070. [Google Scholar] [CrossRef] [PubMed]
- Eyetech Study, G. Anti-vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age-related macular degeneration: Phase II study results. Ophthalmology 2003, 110, 979–986. [Google Scholar]
- Campa, C.; Harding, S.P. Anti-VEGF compounds in the treatment of neovascular age related macular degeneration. Curr. Drug Targets 2011, 12, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Augustin, A.; Hunt, S.; Breipohl, W.; Böker, T.; Spitznas, M. Influence of oxygen free radicals and free radical scavengers on the growth behaviour and oxidative tissue damage of bovine retinal pigment epithelium cells in vitro. Graefe’s Arch. Clin. Exp. Ophthalmol. 1996, 234, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Newsome, D.; Dobard, E.; Liles, M.; Oliver, P. Human retinal pigment epithelium contains two distinct species of superoxide dismutase. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2508–2513. [Google Scholar]
- Steinberg, R.H.; Miller, S.S. The relationship of the retinal pigment epithelium. In The Retinal Pigment Epithelium; Zin, K.M., Marmor, M.F., Eds.; Harvard University Press: Cambridge, MA, USA, 1979; pp. 205–225. [Google Scholar]
- Basinger, S.F.; Hoffman, R.T. Biochemistry of the pigment epithelium. In Biochemistry of the Eye; Anderson, R.E., Ed.; American Academy of Ophthalmology Manuals Program: San Francisco, CA, USA, 1983; pp. 256–264. [Google Scholar]
- Harper, F.H.; Liversidge, J.; Thomson, A.W.; Forrester, J. Interphotoreceptor retinoid binding protein induced experimental autoimmune uveitis: An immunophenotypic analysis using alkaline phosphatase anti-alkaline phosphatase staining, dual immunofluorescence and confocal microscopy. Curr. Eye Res. 1992, 11, 129–134. [Google Scholar] [CrossRef]
- Charteris, D.G.; Hiscott, P.; Grierson, I.; Lightman, S.L. Proliferative vitreoretinopathy: Lymphocytes in epiretinal membranes. Ophthalmology 1992, 99, 1364–1367. [Google Scholar] [CrossRef]
- Lopez, P.F.; Grossniklaus, H.E.; Lambert, H.M.; Aaberg, T.M.; Capone, A., Jr.; Sternberg, P., Jr.; L’Hernault, N. Pathologic features of surgically excised subretinal neovascular membranes in age-related macular degeneration. Am. J. Ophthalmol. 1991, 112, 647–656. [Google Scholar] [CrossRef]
- Dorey, C.K.; Wu, G.; Ebenstein, D.; Garsd, A.; Weiter, J. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1691–1699. [Google Scholar]
- Hageman, G.S.; Luthert, P.J.; Chong, N.V.; Johnson, L.V.; Anderson, D.H.; Mullins, R.F. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001, 20, 705–732. [Google Scholar] [CrossRef] [PubMed]
- Rhone, M.; Basu, A. Phytochemicals and age-related eye diseases. Nutr. Rev. 2008, 66, 465–472. [Google Scholar] [CrossRef]
- Hogg, R.; Chakravarthy, U. AMD and micronutrient antioxidants. Curr. Eye Res. 2004, 29, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research, G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar]
- Chang, W.-S.; Lin, C.-C.; Chuang, S.-C.; Chiang, H.-C. Superoxide anion scavenging effect of coumarins. Am. J. Chin. Med. 1996, 24, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Egan, D.; O’kennedy, R.; Moran, E.; Cox, D.; Prosser, E.; Thornes, R.D. The pharmacology, metabolism, analysis, and applications of coumarin and coumarin-related compounds. Drug Metab. Rev. 1990, 22, 503–529. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Yoshimi, N.; Yamada, Y.; Shimizu, M.; Kawabata, K.; Ozawa, Y.; Hara, A.; Mori, H. Inhibitory effects of nabumetone, a cyclooxygenase-2 inhibitor, and esculetin, a lipoxygenase inhibitor, on N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats. Jpn. J. Cancer Res. 1998, 89, 496–501. [Google Scholar] [CrossRef]
- Okada, Y.; Miyauchi, N.; Suzuki, K.; Kobayashi, T.; Tsutsui, C.; Mayuzumi, K.; Nishibe, S.; Okuyama, T. Search for naturally occurring substances to prevent the complications of diabetes. II. Inhibitory effect of coumarin and flavonoid derivatives on bovine lens aldose reductase and rabbit platelet aggregation. Chem. Pharm. Bull. 1995, 43, 1385–1387. [Google Scholar] [CrossRef]
- Kwon, O.S.; Choi, J.S.; Islam, M.N.; Kim, Y.S.; Kim, H.P. Inhibition of 5-lipoxygenase and skin inflammation by the aerial parts of Artemisia capillaris and its constituents. Arch. Pharmacal Res. 2011, 34, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; An, Y.; He, X.; Zhang, D.; He, W. Esculetin protects human corneal epithelial cells from oxidative stress through Nrf-2 signaling pathway. Exp. Eye Res. 2021, 202, 108360. [Google Scholar] [CrossRef] [PubMed]
- Ozal, S.A.; Turkekul, K.; Gurlu, V.; Guclu, H.; Erdogan, S. Esculetin Protects Human Retinal Pigment Epithelial Cells from Lipopolysaccharide-induced Inflammation and Cell Death. Curr. Eye Res. 2018, 43, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Dayhaw-Barker, P. The role of the retinal pigment epithelium: Topographical variation and ageing changes. Eye 2001, 15, 384–389. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, Z.; Wang, X.; Li, R.; Hou, W.; Bi, W.; Zhang, X. Inhibition of autophagy induces IL-1β release from ARPE-19 cells via ROS mediated NLRP3 inflammasome activation under high glucose stress. Biochem. Biophys. Res. Commun. 2015, 463, 1071–1076. [Google Scholar] [CrossRef]
- Vainio, I.; Khamidakh, A.A.; Paci, M.; Skottman, H.; Juuti-Uusitalo, K.; Hyttinen, J.; Nymark, S. Computational model of Ca2+ wave propagation in human retinal pigment epithelial ARPE-19 cells. PLoS ONE 2015, 10, e0128434. [Google Scholar] [CrossRef]
- Wang, P.; Xing, Y.; Chen, C.; Chen, Z.; Qian, Z. Advanced glycation end-product (AGE) induces apoptosis in human retinal ARPE-19 cells via promoting mitochondrial dysfunction and activating the Fas-FasL signaling. Biosci. Biotechnol. Biochem. 2016, 80, 250–256. [Google Scholar] [CrossRef]
- Winkler, B.S.; Boulton, M.E.; Gottsch, J.D.; Sternberg, P. Oxidative damage and age-related macular degeneration. Mol. Vis. 1999, 5, 32. [Google Scholar]
- Anderson, B., Jr. Ocular effects of changes in oxygen and carbon dioxide tension. Trans. Am. Ophthalmol. Soc. 1968, 66, 423–474. [Google Scholar] [PubMed]
- Brennan, L.A.; Kantorow, M. Mitochondrial function and redox control in the aging eye: Role of MsrA and other repair systems in cataract and macular degenerations. Exp. Eye Res. 2009, 88, 195–203. [Google Scholar] [CrossRef]
- Rattner, A.; Nathans, J. Macular degeneration: Recent advances and therapeutic opportunities. Nat. Rev. Neurosci. 2006, 7, 860–872. [Google Scholar] [CrossRef]
- Querques, G.; Rosenfeld, P.J.; Cavallero, E.; Borrelli, E.; Corvi, F.; Querques, L.; Bandello, F.M.; Zarbin, M.A. Treatment of dry age-related macular degeneration. Ophthalmic Res. 2014, 52, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Yiğit, M.; Güneş, A.; Uğuz, C.; Yalçın, T.Ö.; Tök, L.; Öz, A.; Nazıroğlu, M. Effects of astaxanthin on antioxidant parameters in ARPE-19 cells on oxidative stress model. Int. J. Ophthalmol. 2019, 12, 930. [Google Scholar] [PubMed]
- Oh, S.; Kim, Y.J.; Lee, E.K.; Park, S.W.; Yu, H.G. Antioxidative effects of ascorbic acid and astaxanthin on arpe-19 cells in an oxidative stress model. Antioxidants 2020, 9, 833. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.T.; Kam, W.; Cunningham, D.; Harrington, M.; Hammel, K.; Meyerle, C.B.; Cukras, C.; Chew, E.Y.; Sadda, S.R.; Ferris, F.L. Treatment of geographic atrophy by the topical administration of OT-551: Results of a phase II clinical trial. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6131–6139. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.; Różanowska, M.; Sarna, T. Zeaxanthin in combination with ascorbic acid or α-tocopherol protects ARPE-19 cells against photosensitized peroxidation of lipids. Free Radic. Biol. Med. 2004, 36, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Hanneken, A.; Lin, F.F.; Johnson, J.; Maher, P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3164–3177. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hackett, S.F.; Mincey, A.; Lai, H.; Campochiaro, P.A. Effects of different types of oxidative stress in RPE cells. J. Cell. Physiol. 2006, 206, 119–125. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef]
- Johnson, A.R.; Munoz, A.; Gottlieb, J.L.; Jarrard, D.F. High dose zinc increases hospital admissions due to genitourinary complications. J. Urol. 2007, 177, 639–643. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). Jama 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Lonn, E.; Bosch, J.; Yusuf, S.; Sheridan, P.; Pogue, J.; Arnold, J.M.; Ross, C.; Arnold, A.; Sleight, P.; Probstfield, J.; et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial. Jama 2005, 293, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.A.; Kanuga, N.; Romero, I.A.; Greenwood, J.; Luthert, P.J.; Cheetham, M.E. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 675–684. [Google Scholar] [CrossRef]
- Liu, H.; Miller, E.; van de Water, B.; Stevens, J.L. Endoplasmic reticulum stress proteins block oxidant-induced Ca2+ increases and cell death. J. Biol. Chem. 1998, 273, 12858–12862. [Google Scholar] [CrossRef]
- Henschke, P.N.; Elliott, S.J. Oxidized glutathione decreases luminal Ca2+ content of the endothelial cell ins (1, 4, 5) P 3-sensitive Ca2+ store. Biochem. J. 1995, 312, 485–489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakaida, I.; Thomas, A.; Farber, J. Increases in cytosolic calcium ion concentration can be dissociated from the killing of cultured hepatocytes by tert-butyl hydroperoxide. J. Biol. Chem. 1991, 266, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Castilho, R.F.; Kowaltowski, A.J.; Meinicke, A.; Bechara, E.J.; Vercesi, A.E. Permeabilization of the inner mitochondrial membrane by Ca2+ ions is stimulated by t-butyl hydroperoxide and mediated by reactive oxygen species generated by mitochondria. Free Radic. Biol. Med. 1995, 18, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, A.; Saylor, A.; Tesfai, S.; Herman, B.; Lemasters, J. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem. J. 1995, 307, 99–106. [Google Scholar] [CrossRef]
- Shertzer, H.G.; Bannenberg, G.L.; Zhu, H.; Liu, R.-M.; Moldeus, P. The role of thiols in mitochondrial susceptibility to iron and tert-butyl hydroperoxide-mediated toxicity in cultured mouse hepatocytes. Chem. Res. Toxicol. 1994, 7, 358–366. [Google Scholar] [CrossRef]
- Liang, F.-Q.; Godley, B.F. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: A possible mechanism for RPE aging and age-related macular degeneration. Exp. Eye Res. 2003, 76, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Sil, P.C. Tertiary butyl hydroperoxide induced oxidative damage in mice erythrocytes: Protection by taurine. Pathophysiology 2012, 19, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Feng, H.; Sun, W.; Liu, K.; Lu, J.J.; Chen, X. Tert-butyl hydroperoxide (t-BHP) induced apoptosis and necroptosis in endothelial cells: Roles of NOX4 and mitochondrion. Redox Biol. 2017, 11, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Kučera, O.; Endlicher, R.; Roušar, T.; Lotková, H.; Garnol, T.; Drahota, Z.; Cervinková, Z. The effect of tert-butyl hydroperoxide-induced oxidative stress on lean and steatotic rat hepatocytes in vitro. Oxid. Med. Cell. Longev. 2014, 2014, 752506. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Min, W.; Bradley, J.R. Mechanisms of endothelial dysfunction, injury, and death. Annu. Rev. Pathol. 2009, 4, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-C.; Liu, T.-J.; Lai, H.-C. Pathobiological Mechanisms of Endothelial Dysfunction Induced by tert-Butyl Hydroperoxide via Apoptosis, Necrosis and Senescence in a Rat Model. Int. J. Med. Sci. 2020, 17, 368–382. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.I.; Jang, M.; Namkoong, S.; Park, R.; Ju, H.; Choi, I.; Oh, W.K.; Park, J. Conessine Interferes with Oxidative Stress-Induced C2C12 Myoblast Cell Death through Inhibition of Autophagic Flux. PLoS ONE 2016, 11, e0157096. [Google Scholar] [CrossRef]
- Sriset, Y.; Chatuphonprasert, W.; Jarukamjorn, K. Optimized models of xenobiotic-induced oxidative stress in HepG2 cells. Trop. J. Pharm. Res. 2019, 18, 1001–1007. [Google Scholar] [CrossRef]
- Tien, Y.C.; Liao, J.C.; Chiu, C.S.; Huang, T.H.; Huang, C.Y.; Chang, W.T.; Peng, W.H. Esculetin ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. Int. J. Mol. Sci. 2011, 12, 4053–4067. [Google Scholar] [CrossRef]
- Turkekul, K.; Colpan, R.D.; Baykul, T.; Ozdemir, M.D.; Erdogan, S. Esculetin Inhibits the Survival of Human Prostate Cancer Cells by Inducing Apoptosis and Arresting the Cell Cycle. J. Cancer Prev. 2018, 23, 10–17. [Google Scholar] [CrossRef]
- Park, S.S.; Park, S.K.; Lim, J.H.; Choi, Y.H.; Kim, W.J.; Moon, S.K. Esculetin inhibits cell proliferation through the Ras/ERK1/2 pathway in human colon cancer cells. Oncol. Rep. 2011, 25, 223–230. [Google Scholar]
- Lin, W.-L.; Wang, C.-J.; Tsai, Y.-Y.; Liu, C.-L.; Hwang, J.-M.; Tseng, T.-H. Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver. Arch. Toxicol. 2000, 74, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, K.A.; Zhang, R.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Chae, S.W.; Kang, S.S.; Lee, K.H.; Kang, H.K.; et al. Protective effect of esculetin against oxidative stress-induced cell damage via scavenging reactive oxygen species. Acta Pharm. Sin. 2008, 29, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, C.; Ma, Q.; Chen, S. Esculetin inhibits oxidative stress and apoptosis in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Biochem. Biophys. Res. Commun. 2018, 501, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pei, A.; Chen, J.; Yu, H.; Sun, M.L.; Liu, C.F.; Xu, X. A natural coumarin derivative esculetin offers neuroprotection on cerebral ischemia/reperfusion injury in mice. J. Neurochem. 2012, 121, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, W.K.; Park, S.-B.; Yu, H.Y.; Kim, Y.H.; Kim, J. Effect of Esculetin on Tert-Butyl Hydroperoxide-Induced Oxidative Injury in Retinal Pigment Epithelial Cells In Vitro. Molecules 2022, 27, 8970. https://doi.org/10.3390/molecules27248970
Jung WK, Park S-B, Yu HY, Kim YH, Kim J. Effect of Esculetin on Tert-Butyl Hydroperoxide-Induced Oxidative Injury in Retinal Pigment Epithelial Cells In Vitro. Molecules. 2022; 27(24):8970. https://doi.org/10.3390/molecules27248970
Chicago/Turabian StyleJung, Woo Kwon, Su-Bin Park, Hwa Young Yu, Yong Hwan Kim, and Junghyun Kim. 2022. "Effect of Esculetin on Tert-Butyl Hydroperoxide-Induced Oxidative Injury in Retinal Pigment Epithelial Cells In Vitro" Molecules 27, no. 24: 8970. https://doi.org/10.3390/molecules27248970
APA StyleJung, W. K., Park, S.-B., Yu, H. Y., Kim, Y. H., & Kim, J. (2022). Effect of Esculetin on Tert-Butyl Hydroperoxide-Induced Oxidative Injury in Retinal Pigment Epithelial Cells In Vitro. Molecules, 27(24), 8970. https://doi.org/10.3390/molecules27248970







