Mesomorphic, Computational Investigations and Dyeing Applications of Laterally Substituted Dyes
Abstract
:1. Introduction
2. Results and Discussion
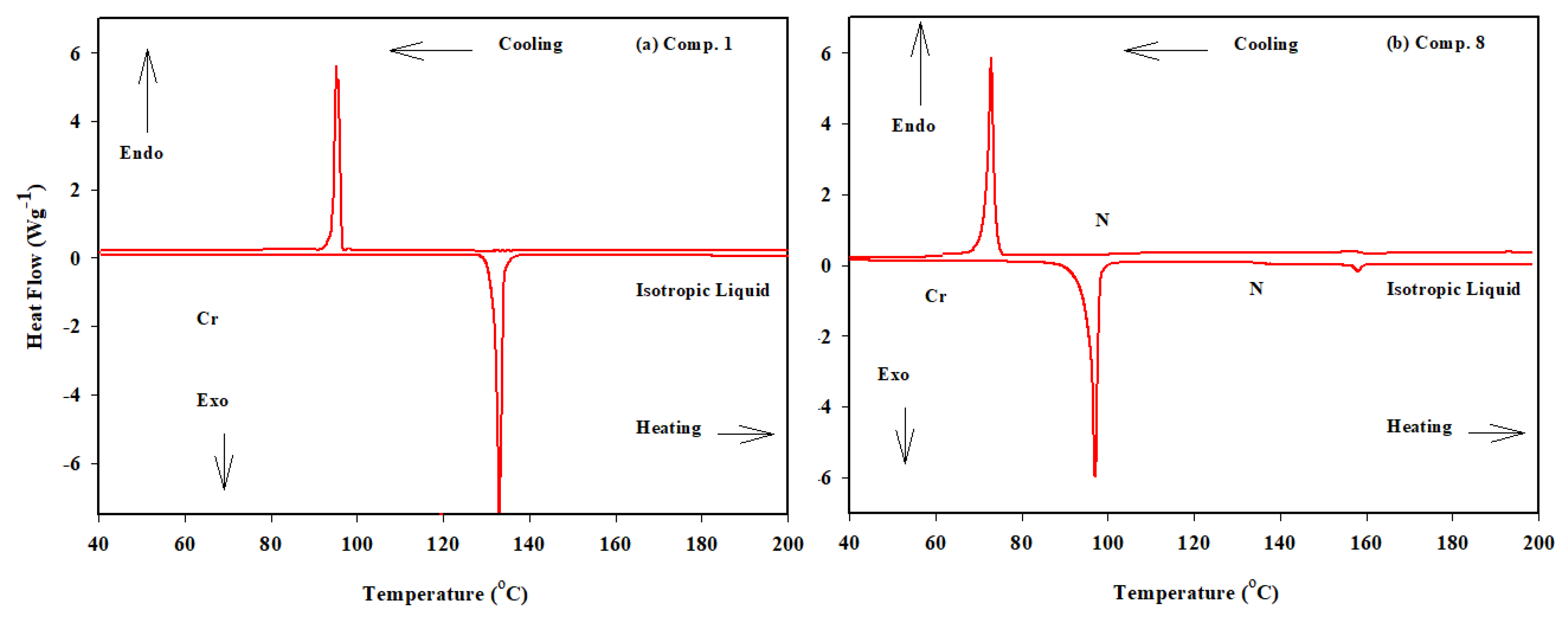
2.1. Thermal and Mesomorphic Properties
2.2. Electronic Absorption Spectra and Substituent Effect
2.3. Dyeing Process and Fastness Properties
2.4. Dye Exhaustion, Reflectance, and Color Strength
2.5. Theoretical Study and the Molecular Descriptors
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Aitken, D.; Burkinshaw, S.; Griffiths, J.; Towns, A. Textile applications of thennochromic systems. Rev. Prog. Coloration Relat. Top. 1996, 26, 1–8. [Google Scholar] [CrossRef]
- Tao, X. Smart Fibres, Fabrics and Clothing; The Textile Institute: Cambridge, UK, 2001. [Google Scholar]
- Kulčar, R.; Friškovec, M.; Hauptman, N.; Vesel, A.; Gunde, M.K. Colorimetric properties of reversible thermochromic printing inks. Dyes Pigments 2010, 86, 271–277. [Google Scholar] [CrossRef]
- Seeboth, A.; Klukowska, A.; Ruhmann, R.; Lötzsch, D. Thermochromic polymer materials. Chin. J. Polym. Sci. 2007, 25, 123–135. [Google Scholar] [CrossRef]
- Rizk, H.; Ibrahim, S.; El-Borai, M. Synthesis, fastness properties, color assessment and antimicrobial activity of some azo reactive dyes having pyrazole moiety. Dyes Pigments 2015, 112, 86–92. [Google Scholar] [CrossRef]
- Satam, M.A.; Raut, R.K.; Sekar, N. Fluorescent azo disperse dyes from 3-(1, 3-benzothiazol-2-yl) naphthalen-2-ol and comparison with 2-naphthol analogs. Dyes Pigments 2013, 96, 92–103. [Google Scholar] [CrossRef]
- Shukla, S.; Mathur, M.R. Low-temperature ultrasonic dyeing of silk. J. Soc. Dye. Colour. 1995, 111, 342–345. [Google Scholar] [CrossRef]
- Fang, S.; Feng, G.; Guo, Y.; Chen, W.; Qian, H. Synthesis and application of urethane-containing azo disperse dyes on polyamide fabrics. Dyes Pigments 2020, 176, 108225. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.W. A study of novel bisazo reactive red dyes with good wet fastness. Coloration Technol. 2009, 125, 216–221. [Google Scholar] [CrossRef]
- Soliman, H.; Yahia, I. Synthesis and technical analysis of 6-butyl-3-[(4-chlorophenyl) diazenyl]-4-hydroxy-2H-pyrano [3, 2-c] quinoline-2, 5 (6H)-dione as a new organic semiconductor: Structural, optical and electronic properties. Dyes Pigments 2020, 176, 108199. [Google Scholar] [CrossRef]
- Omar, A.Z.; Mahmoud, M.N.; El-Sadany, S.K.; Hamed, E.A.; El-atawy, M.A. A combined experimental and DFT investigation of mono azo thiobarbituric acid based chalcone disperse dyes. Dyes Pigments 2021, 185, 108887. [Google Scholar] [CrossRef]
- Alaasar, M.; Schmidt, J.-C.; Darweesh, A.F.; Tschierske, C. Azobenzene-based supramolecular liquid crystals: The role of core fluorination. J. Mol. Liq. 2020, 310, 113252. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alhaddad, O. Mesomorphic and geometrical orientation study of the relative position of fluorine atom in some thermotropic liquid crystal systems. Liq. Cryst. 2020, 47, 404–413. [Google Scholar] [CrossRef]
- Bremer, M.; Kirsch, P.; Klasen-Memmer, M.; Tarumi, K. The TV in your pocket: Development of liquid-crystal materials for the new millennium. Angew. Chem. Int. Ed. 2013, 52, 8880–8896. [Google Scholar] [CrossRef]
- Pauluth, D.; Tarumi, K. Advanced liquid crystals for television. J. Mater. Chem. 2004, 14, 1219–1227. [Google Scholar] [CrossRef]
- Hird, M. Fluorinated liquid crystals–properties and applications. Chem. Soc. Rev. 2007, 36, 2070–2095. [Google Scholar] [CrossRef]
- Sun, G.; Chen, B.; Tang, H.; Shi, G.; Xu, S. Synthesis and physical properties of laterally fluorinated liquid crystals containing 1, 3, 2-dioxaborinane and cyclohexyl units. Liq. Cryst. 2004, 31, 1151–1158. [Google Scholar] [CrossRef]
- Jessy, P.; Radha, S.; Patel, N. Morphological, optical and dielectric behavior of chiral nematic liquid crystal mixture: Study on effect of different amount of chirality. J. Mol. Liq. 2018, 255, 215–223. [Google Scholar] [CrossRef]
- Mishra, R.; Hazarika, J.; Hazarika, A.; Gogoi, B.; Dubey, R.; Bhattacharjee, D.; Singh, K.N.; Alapati, P.R. Dielectric properties of a strongly polar nematic liquid crystal compound doped with gold nanoparticles. Liq. Cryst. 2018, 45, 1661–1671. [Google Scholar] [CrossRef]
- Zaki, A. Optical measurements of phase transitions in difluorophenylazophenyl benzoate thermotropic liquid crystal with specific orientated fluorine atoms. Phase Transit. 2019, 92, 135–148. [Google Scholar] [CrossRef]
- Zaki, A.A.; Ahmed, H.; Hagar, M. Impact of fluorine orientation on the optical properties of difluorophenylazophenyl benzoates liquid crystal. Mater. Chem. Phys. 2018, 216, 316–324. [Google Scholar] [CrossRef]
- Alhaddad, O.A.; Khushaim, M.S.; Gomha, S.M.; Ahmed, H.A.; Naoum, M.M. Mesophase behavior of four ring ester/azomethine/ester liquid crystals in pure and mixed states. Liquid Cryst. 2022, 49, 1395–1402. [Google Scholar] [CrossRef]
- Alrefaee, S.H.; Ahmed, H.A.; Khan, M.T.; Al-Ola, K.A.; Al-Refai, H.; El-Atawy, M.A. New Self-Organizing Optical Materials and Induced Polymorphic Phases of Their Mixtures Targeted for Energy Investigations. Polymers 2022, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.A.; El-Atawy, M.A. Synthesis, mesomorphic and geometrical approaches of new non-symmetrical system based on central naphthalene moiety. Liq. Cryst. 2021, 48, 1940–1952. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S.; Alamro, F.S.; Popoola, S.A.; Gomha, S.M.; Bedowr, N.S.; Al-Juhani, S.S.; Ahmed, H.A. Novel Imidazole Liquid Crystals; Experimental and Computational Approaches. Molecules 2022, 27, 4607. [Google Scholar] [CrossRef] [PubMed]
- Alamro, F.S.; Gomha, S.M.; Shaban, M.; Altowyan, A.S.; Abolibda, T.Z.; Ahmed, H.A. Optical investigations and photoactive solar energy applications of new synthesized Schiff base liquid crystal derivatives. Sci. Rep. 2021, 11, 15046. [Google Scholar] [CrossRef]
- Alshabanah, L.A.; Al-Mutabagani, L.A.; Gomha, S.M.; Ahmed, H.A.; Popoola, S.A.; Shaban, M. Novel sulphonic acid liquid crystal derivatives: Experimental, computational and optoelectrical characterizations. RSC Adv. 2021, 11, 27937–27949. [Google Scholar] [CrossRef]
- Selivanova, G.; Tretyakov, E.; Amosov, E.; Bagryanskaya, I.Y.; Vasiliev, V.; Vasilyev, E.; Tikhova, V.; Karpova, E.; Basova, T.; Stass, D. X-ray induced phase transitions in 4-((4-(dibutylamino) phenyl) diazenyl)-biphenyl-2, 3′, 4′-tricarbonitrile. J. Mol. Struct. 2016, 1107, 242–248. [Google Scholar] [CrossRef]
- Shelkovnikov, V.; Selivanova, G.; Lyubas, G.; Korotaev, S.; Shundrina, I.; Tretyakov, E.; Zueva, E.; Plekhanov, A.; Mikerin, S.; Simanchuk, A. Second-order nonlinear optical properties of composite material of an azo-chromophore with a tricyanodiphenyl acceptor in a poly (styrene-co-methyl methacrylate) matrix. Opt. Mater. 2017, 69, 67–72. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.A.; Alshabanah, L.A.; Ahmed, H.A.; El-Atawy, M.A. Synthesis, optical and DFT characterizations of laterally fluorinated phenyl cinnamate liquid crystal non-symmetric system. Symmetry 2021, 13, 1145. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Alhaddad, O.A.; Ahmed, H.A. Experimental and geometrical structure characterizations of new synthesized laterally fluorinated nematogenic system. Liq. Cryst. 2021, 48, 2106–2116. [Google Scholar] [CrossRef]
- Al-Zahrani, S.A.; Ahmed, H.A.; El-Atawy, M.A.; Abu Al-Ola, K.A.; Omar, A.Z. Synthetic, mesomorphic, and DFT investigations of new nematogenic polar naphthyl benzoate ester derivatives. Materials 2021, 14, 2587. [Google Scholar] [CrossRef]
- Alamro, F.S.; Tolan, D.A.; El-Nahas, A.M.; Ahmed, H.A.; El-Atawy, M.A.; Al-Kadhi, N.S.; Aziz, S.G.; Shibl, M.F. Wide Nematogenic Azomethine/Ester Liquid Crystals Based on New Biphenyl Derivatives: Mesomorphic and Computational Studies. Molecules 2022, 27, 4150. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Aboelnaga, A. Synthesis and mesomorphic study of new phenylthiophene liquid crystals. Liq. Cryst. 2022, 49, 804–811. [Google Scholar] [CrossRef]
- Koopmans, T. Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Kaya, S.; Kariper, S.E.; Ungördü, A.; Kaya, C. Effect of some electron donor and electron acceptor groups on stability of complexes according to the principle of HSAB. J. New Results Sci. 2014, 3, 1. [Google Scholar]
- Alexander, D.; Moccari, A. Evaluation of corrosion inhibitors for component cooling water systems. Corrosion 1993, 49. [Google Scholar] [CrossRef]
- Sastri, V.; Perumareddi, J. Molecular orbital theoretical studies of some organic corrosion inhibitors. Corrosion 1997, 53. [Google Scholar] [CrossRef]
- Omar, A.Z.; Mosa, T.M.; El-Sadany, S.K.; Hamed, E.A.; El-Atawy, M. Novel piperazine based compounds as potential inhibitors for SARS-CoV-2 Protease Enzyme: Synthesis and molecular docking study. J. Mol. Struct. 2021, 1245, 131020. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L. Quantum density oscillations in an inhomogeneous electron gas. Phys. Rev. 1965, 137, A1697. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Govindarasu, K.; Kavitha, E. Vibrational spectra, molecular structure, NBO, UV, NMR, first order hyperpolarizability, analysis of 4-Methoxy-4′-Nitrobiphenyl by density functional theory. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.Z.; Hamdy, E.; Hamed, E.A.; Hafez, E.; Abdelkhalek, A. The curative activity of some arylidene dihydropyrimidine hydrazone against Tobacco mosaic virus infestation. J. Saudi Chem. Soc. 2022, 26, 101504. [Google Scholar] [CrossRef]
- Martinez, S. Inhibitory mechanism of mimosa tannin using molecular modeling and substitutional adsorption isotherms. Mater. Chem. Phys. 2003, 77, 97–102. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Subramanian, V.; Roy, D.R.; Chattaraj, P. Electrophilicity index as a possible descriptor of biological activity. Bioorg. Med. Chem. 2004, 12, 5533–5543. [Google Scholar] [CrossRef] [PubMed]





| Comp. | TCr-I | ΔHCr-I | TCr-N | ΔHCr-N | TN-I | ΔHN-I | ΔTN |
|---|---|---|---|---|---|---|---|
| 1 | 138.0 | 57.9 | - | - | - | - | - |
| 2 | 120.8 | 60.1 | - | - | - | - | - |
| 3 | 111.0 | 55.7 | - | - | - | - | - |
| 4 | 116.0 | 49.4 | - | - | - | - | - |
| 5 | - | - | 96.3 | 39.38 | 177.3 | 1.23 | 81.0 |
| 6 | - | - | 103.1 | 42.30 | 137.1 | 1.36 | 34.0 |
| 7 | - | - | 68.9 | 44.1 | 140.2 | 1.91 | 71.3 |
| 8 | - | - | 96.4 | 30.82 | 156.7 | 2.89 | 60.3 |
| 9 | - | - | 83.1 | 39.83 | 152.7 | 1.90 | 69.6 |
| 10 | - | - | 84.3 | 48.58 | 121.9 | 2.35 | 37.6 |
| Dye no. | Observed Color | Experimental (nm) | Calculated (nm) |
|---|---|---|---|
| 1 | yellow | 430, 283 | 374 |
| 2 | orange | 408 | 307 |
| 3 | orange | 394 | 307 |
| 4 | orange | 389 | 341 |
| 5 | yellow | 390, 258 | 307 |
| 6 | yellow | 260 | 339 |
| 7 | yellow | 379 | 307 |
| 8 | orange | 383, 262 | 340 |
| 9 | orange | 403 | 340 |
| 10 | orange | 382, 262 | 339 |
| Dye no. | Dyed PE Fabrics | Wash * | Perspiration ** | Scorch *** | Light **** | ||
|---|---|---|---|---|---|---|---|
| Acidic | Alkaline | Cotton | Poly Ester | ||||
| 1 |  | 5 | 5 | 5 | 5 | 5 | 5–6 |
| 2 |  | 5 | 4–5 | 5 | 4-5 | 5 | 5–6 |
| 3 |  | 4–5 | 4–5 | 5 | 5 | 5 | 3–4 |
| 4 |  | 5 | 5 | 5 | 5 | 5 | 3–4 |
| 6 |  | 4–5 | 3–4 | 5 | 4–5 | 3–4 | 5–6 |
| 8 |  | 4–5 | 5 | 5 | 4–5 | 5 | 5–6 |
| Dye No | Dye Exhaustion | Reflectance (%) | K/S |
|---|---|---|---|
| 1 | 82% | 11.42 | 3.43 |
| 2 | 65% | 13.80 | 2.69 |
| 3 | 54% | 18.78 | 1.77 |
| 4 | 69% | 13.54 | 2.75 |
| 6 | 46% | 20.88 | 1.49 |
| 8 | 71% | 13.59 | 2.74 |
| Parameter | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| EHOMO (eV) | −0.4649 | −0.2876 | −0.2875 | −0.2643 | −0.2928 | −0.2726 | −0.2912 | −0.2705 | −0.2704 | −0.2707 |
| ELUMO (eV) | 0.0277 | 0.0497 | 0.0498 | 0.0481 | 0.0426 | 0.0400 | −0.0447 | 0.0419 | 0.0420 | 0.0419 |
| ∆E (eV) | 0.4926 | 0.3373 | 0.3373 | 0.3124 | 0.3354 | 0.3126 | 0.2465 | 0.3124 | 0.3124 | 0.3126 |
| IP (eV) | 0.4649 | 0.2876 | 0.2875 | 0.2643 | 0.2928 | 0.2726 | 0.2912 | 0.2705 | 0.2704 | 0.2707 |
| EA (eV) | −0.0277 | −0.0497 | −0.0498 | −0.0481 | −0.0426 | −0.0400 | 0.0447 | −0.0419 | −0.0420 | −0.0419 |
| χ (eV) | 0.2186 | 0.1189 | 0.1189 | 0.1081 | 0.1251 | 0.1163 | 0.1679 | 0.1143 | 0.1142 | 0.1144 |
| µ (eV) | −0.2186 | −0.1189 | −0.1189 | −0.1081 | −0.1251 | −0.1163 | −0.1679 | −0.1143 | −0.1142 | −0.1144 |
| η (eV) | 0.2463 | 0.1687 | 0.1687 | 0.1562 | 0.1677 | 0.1563 | 0.1233 | 0.1562 | 0.1562 | 0.1563 |
| S (eV−1) | 4.0601 | 5.9294 | 5.9293 | 6.4016 | 5.9634 | 6.3980 | 8.1133 | 6.4016 | 6.4025 | 6.3977 |
| ω (eV) | 0.0970 | 0.0419 | 0.0419 | 0.0374 | 0.0467 | 0.0432 | 0.1144 | 0.0418 | 0.0418 | 0.0419 |
| µ (D) | 3.2995 | 6.4157 | 6.4206 | 3.3629 | 4.3809 | 2.5306 | 2.7504 | 2.6160 | 2.6316 | 2.7973 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, H.A.; El-Atawy, M.A.; Alamro, F.S.; Al-Kadhi, N.S.; Alhaddad, O.A.; Omar, A.Z. Mesomorphic, Computational Investigations and Dyeing Applications of Laterally Substituted Dyes. Molecules 2022, 27, 8980. https://doi.org/10.3390/molecules27248980
Ahmed HA, El-Atawy MA, Alamro FS, Al-Kadhi NS, Alhaddad OA, Omar AZ. Mesomorphic, Computational Investigations and Dyeing Applications of Laterally Substituted Dyes. Molecules. 2022; 27(24):8980. https://doi.org/10.3390/molecules27248980
Chicago/Turabian StyleAhmed, Hoda A., Mohamed A. El-Atawy, Fowzia S. Alamro, Nada S. Al-Kadhi, Omaima A. Alhaddad, and Alaa Z. Omar. 2022. "Mesomorphic, Computational Investigations and Dyeing Applications of Laterally Substituted Dyes" Molecules 27, no. 24: 8980. https://doi.org/10.3390/molecules27248980
APA StyleAhmed, H. A., El-Atawy, M. A., Alamro, F. S., Al-Kadhi, N. S., Alhaddad, O. A., & Omar, A. Z. (2022). Mesomorphic, Computational Investigations and Dyeing Applications of Laterally Substituted Dyes. Molecules, 27(24), 8980. https://doi.org/10.3390/molecules27248980








