Ursolic Acid Analogs as Potential Therapeutics for Cancer
Abstract
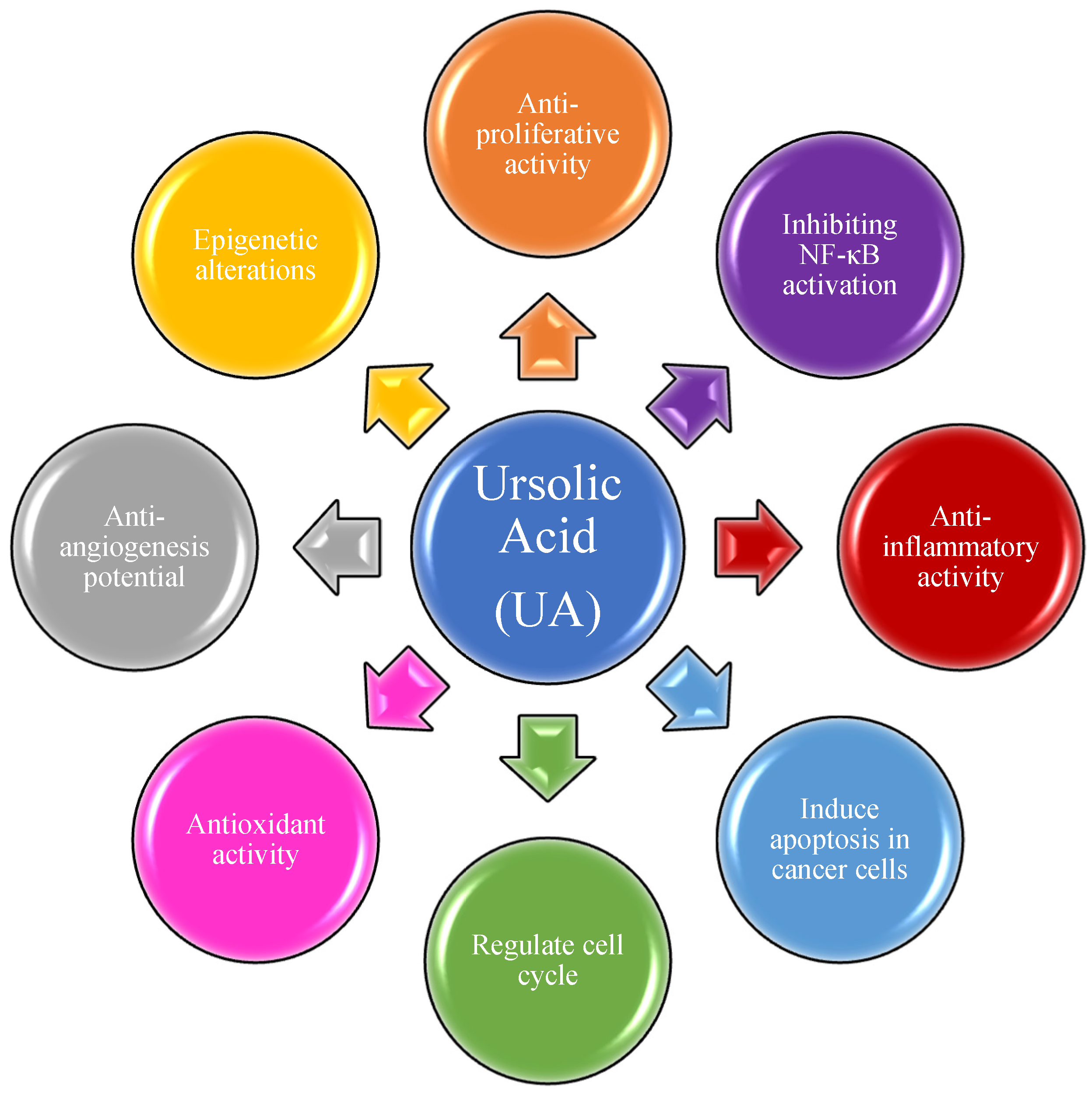
:1. Introduction
2. Triterpenes as Bioactive NPs
3. Materials and Methods
4. Synthetic Analogs of UA
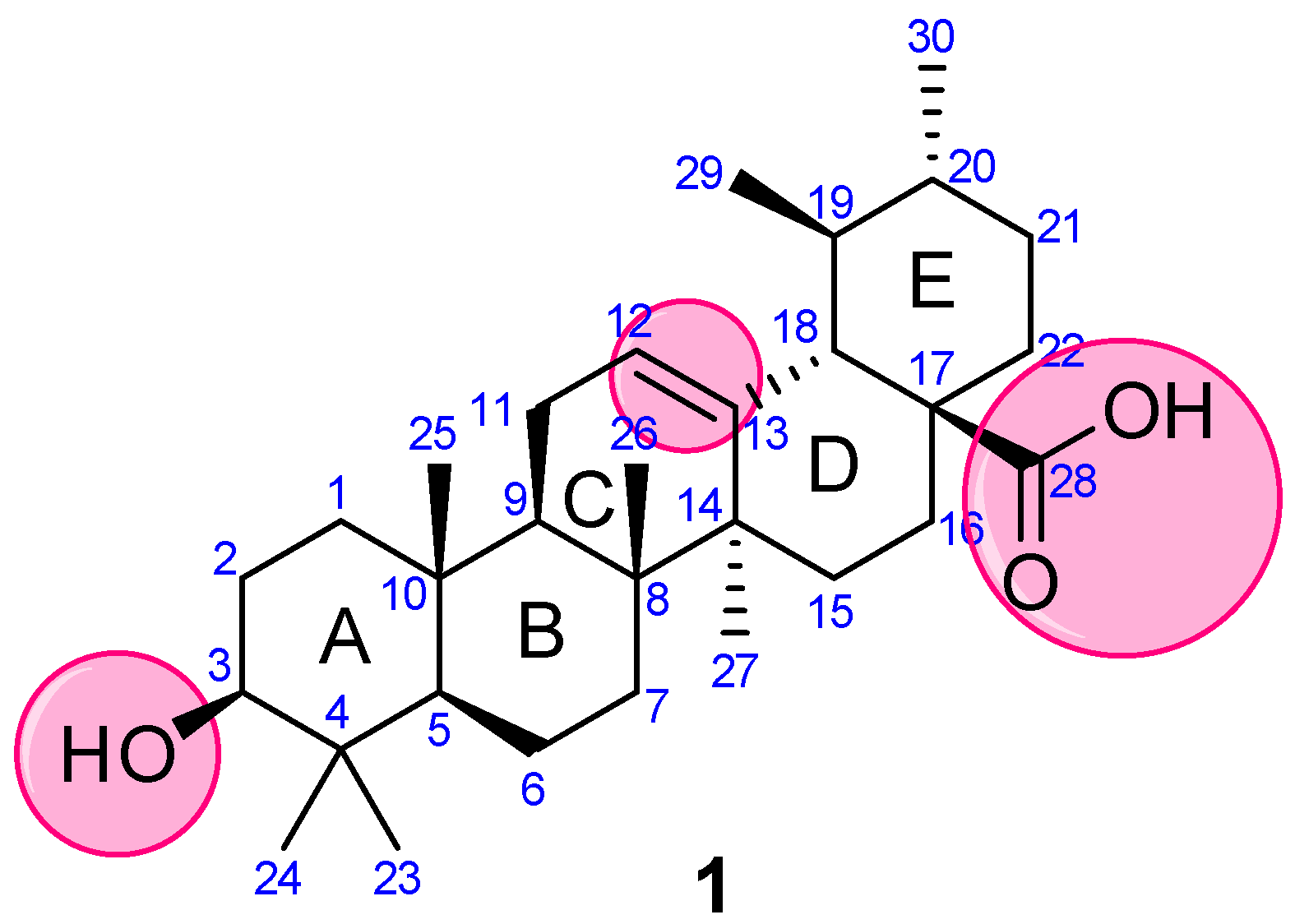
4.1. Modification of UA at C3
4.2. Modification of UA at C28
4.3. Modification of UA at Both C3 and C28
4.4. Modification of UA at Other Positions
5. Conclusions and Future
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Yabroff, K.R.; Mariotto, A.M.; Tangka, F.; Zhao, J.; Islami, F.; Sung, H.; Sherman, R.L.; Henley, S.J.; Jemal, A.; Ward, E.M. Annual Report to the Nation on the Status of Cancer, Part II: Patient Economic Burden Associated with Cancer Care. J. Natl. Cancer Inst. 2021, 113, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar]
- Pejin, B.; Jovanovi, K.K.; Mojovi, M.; Savi, A.G. New and Highly Potent Antitumor Natural Products from Marine-Derived Fungi: Covering the Period from 2003 to 2012. Curr. Top. Med. Chem. 2013, 13, 2745–2766. [Google Scholar] [CrossRef]
- Sisodiya, P.S. Plant Derived Anticancer Agents: A Review. Int. J. Res. Dev. Pharm. Life Sci. 2013, 2, 293–308. [Google Scholar]
- Pejin, B.; Glumac, M.A. brief review of potent anti-CNS tumourics from marine sponges: Covering the period from 1994 to 2014. Nat. Prod. Res. 2018, 32, 375–384. [Google Scholar] [CrossRef]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine Sponge Natural Products with Anticancer Potential: An Updated Review. Mar. Drugs 2017, 15, 310. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, E.; Sankari, L.S.; Malathi, L.; Krupaa, J.R. Naturally occurring products in cancer therapy. J. Pharm. Bioallied Sci. 2015, 7, S181–S183. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. 2011, 16, 980–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksit, H.; Gozcu, S.; Altay, A. Isolation and cytotoxic activities of undescribed iridoid and xanthone glycosides from Centaurium erythraea Rafn. (Gentianaceae). Phytochemistry 2023, 205, 113484. [Google Scholar] [CrossRef]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, D.Y.; Lee, S.R.; Heo, J.-W.; No, M.-H.; Rhee, B.D.; Ko, K.S.; Kwak, H.-B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Hortas, L.; Perez-Larran, P.; Gonzalez-Munoz, M.J.; Falque, E.; Dominguez, H. Recent developments on the extraction and application of ursolic acid. A review. Food Res. Int. 2018, 103, 130–149. [Google Scholar] [CrossRef]
- Li, C.; Chen, W.; Zheng, L.; Zhang, B.; Yang, X.; Zhang, Q.; Wang, N.; Wang, Y.; Yang, J.; Sha, J. Ameliorative effect of ursolic acid on ochratoxin A-induced renal cytotoxicity mediated by Lonp1/Aco2/Hsp75. Toxicon 2019, 168, 141–146. [Google Scholar] [CrossRef]
- Ozdemir, Z.; Wimmer, Z. Selected plant triterpenoids and their amide derivatives in cancer treatment: A review. Phytochemistry 2022, 203, 113340. [Google Scholar] [CrossRef] [PubMed]
- Kornel, A.; Nadile, M.; Tsiani, E. Evidence of the Beneficial Effects of Ursolic Acid against Lung Cancer. Molecules 2022, 27, 7466. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, Q.; Liu, C.; Tang, Y.; Sun, C.; Zhuang, J. Nanoformulations of ursolic acid: A modern natural anticancer molecule. Front. Pharmacol. 2021, 12, 706121. [Google Scholar] [CrossRef] [PubMed]
- Sycz, Z.; Tichaczek-Goska, D.; Wojnicz, D. Anti-Planktonic and Anti-Biofilm Properties of Pentacyclic Triterpenes-Asiatic Acid and Ursolic Acid as Promising Antibacterial Future Pharmaceuticals. Biomolecules 2022, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid. Based Complement. Altern. Med. 2015, 2015, 620472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Negm, W.A.; Alexiou, A.; Batiha, G.E.S. Ursolic acid and SARS-CoV-2 infection: A new horizon and perspective. Inflammopharmacology 2022, 30, 1493–1501. [Google Scholar] [CrossRef]
- Bachar, S.C.; Mazumder, K.; Bachar, R.; Aktar, A.; Al Mahtab, M. A review of medicinal plants with antiviral activity available in Bangladesh and mechanistic insight into their bioactive metabolites on SARS-CoV-2, HIV and HBV. Front. Pharmacol. 2021, 12, 732891. [Google Scholar] [CrossRef]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and oleanolic acids: Plant metabolites with neuroprotective potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef]
- Li, W.; Sun, K.; Hu, F.; Chen, L.; Zhang, X.; Wang, F.; Yan, B. Protective effects of natural compounds against oxidative stress in ischemic diseases and cancers via activating the Nrf2 signaling pathway: A mini review. J. Biochem. Mol. Toxicol. 2021, 35, e22658. [Google Scholar] [CrossRef]
- Habtemariam, S. Antioxidant and anti-inflammatory mechanisms of neuroprotection by ursolic acid: Addressing brain injury, cerebral ischemia, cognition deficit, anxiety, and depression. Oxid. Med. Cell. Longev. 2019, 2019, 8512048. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Punia, S.; Sharma, A.K. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent Pat. Inflamm. Allergy Drug Discov. 2016, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Luan, M.; Wang, H.; Wang, J.; Zhang, X.; Zhao, F.; Liu, Z.; Meng, Q. Advances in anti-inflammatory activity, mechanism and therapeutic application of ursolic acid. Mini-Revi. Med. Chem. 2022, 22, 423–437. [Google Scholar]
- Achiwa, Y.; Hasegawa, K.; Udagawa, Y.; Achiwa, Y.; Hasegawa, K.; Udagawa, Y. Effect of ursolic acid on MAPK in cyclin D1 signaling and RING-type E3 ligase (SCF E3s) in two endometrial cancer cell lines. Nutr. Cancer 2013, 65, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Yadav, V.R.; Sung, B.; Gupta, S.C.; Tyagi, A.K. Ursolic acid inhibits the growth of human pancreatic cancer and enhances the antitumor potential of gemcitabine in an orthotopic mouse model through suppression of the inflammatory microenvironment. Oncotarget 2016, 7, 13182. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lin, C.Y.; Tsai, C.W.; Yin, M.C. Inhibition of cell proliferation, invasion and migration by ursolic acid in human lung cancer cell lines. Toxicol. Vitr. 2011, 25, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ryu, H.G.; Lee, J.; Shin, J.; Harikishore, A.; Jung, H.Y.; Kim, Y.S.; Lyu, H.N.; Oh, E.; Baek, N.I.; et al. Ursolic acid exerts anti-cancer activity by suppressing vaccinia-related kinase 1-mediated damage repair in lung cancer cells. Sci. Rep. 2015, 5, 14570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassi, E.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Manoussakis, M.; Moutsatsou, P. Ursolic acid, a naturally occurring triterpenoid, demonstrates anticancer activity on human prostate cancer cells. J. Cancer Res. Clin. Oncol. 2007, 133, 493–500. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Rajendran, P.; Li, F.; Nema, T.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; Ho, P.C.; et al. Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. J. Mol. Med. 2011, 89, 713–727. [Google Scholar] [CrossRef]
- Gai, W.T.; Yu, D.P.; Wang, X.S.; Wang, P.T. Anti-cancer e_ect of ursolic acid activates apoptosis through ROCK/PTEN mediated mitochondrial translocation of cofilin-1 in prostate cancer. Oncol. Lett. 2016, 12, 2880–2885. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, X.; Lu, Z.; Yuet, W.J.; Zhou, L.L.; Fung, K.P.; Wu, P.; Wu, S. Ursolic acid induces doxorubicin-resistant HepG2 cell death via the release of apoptosis-inducing factor. Cancer Lett. 2010, 298, 128–138. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, P.; Jin, F.; Yao, C.; Zhang, G.; Zang, T.; Ai, X. Ursolic acid induces ER stress response to activate ASK1-JNK signaling and induce apoptosis in human bladder cancer T24 cells. Cell. Signal. 2013, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, G.; Zhang, K.; Zhou, Y.; Li, X.; Xu, W.; Zhang, H.; Shao, Y.; Zhang, Z.; Sun, W. Cyclooxygenase-2 mediated synergistic effect of ursolic acid in combination with paclitaxel against human gastric carcinoma. Oncotarget 2017, 8, 92770–92777. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.S.; Moon, A. Ursolic acid inhibits the invasive phenotype of SNU-484 human gastric cancer cells. Oncol. Lett. 2015, 9, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Yadav, V.R.; Kannappan, R.; Aggarwal, B.B. Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors. J. Biol. Chem. 2016, 291, 16924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Lin, C.; Hua, C.; Jou, Y.; Liao, C.; Chang, Y.; Wan, L.; Huang, S.; Hour, M.; Lin, C. Cis-3-O-p-hydroxycinnamoyl ursolic acid induced ROS-dependent p53-mediated mitochondrial apoptosis in oral cancer cells. Biomol. Ther. 2019, 27, 54–62. [Google Scholar] [CrossRef]
- Jiang, K.; Chi, T.; Li, T.; Zheng, G.; Fan, L.; Liu, Y.; Chen, X.; Chen, S.; Jia, L.; Shao, J. A smart pH-responsive nano-carrier as a drug delivery system for the targeted delivery of ursolic acid: Suppresses cancer growth and metastasis by modulating P53/MMP-9/PTEN/CD44 mediated multiple signaling pathways. Nanoscale 2017, 9, 9428–9439. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.H.; Song, G.; Kim, D.; Jeong, Y.; Liu, K.; Chung, Y.; Oh, S. Ursolic acid and its natural derivative corosolic acid suppress the proliferation of APC-mutated colon cancer cells through promotion of β-catenin degradation. Food Chem. Toxicol. 2014, 67, 87–95. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Tian, D.; Liu, Y.; Nian, W.; Zou, X.; Chen, Q.; Zhou, L.; Deng, Z.; He, B. Ursolic acid inhibits proliferation and induces apoptosis by inactivating Wnt/β-catenin signaling in human osteosarcoma cells. Int. J. Oncol. 2016, 49, 1973–1982. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Jin, H.; Meng, R.Y.; Kim, D.Y.; Liu, Y.C.; Chai, O.H.; Park, B.H.; Kim, S. Activating hippo pathway via Rassf1 by ursolic acid suppresses the tumorigenesis of gastric cancer. Int. J. Mol. Sci. 2019, 20, 4709. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Hyung; Lee, I. A. The mechanism of action of ursolic acid as a potential anti-toxoplasmosis agent, and its immunomodulatory effects. Pathogens 2019, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Pathak, A.K.; Bhutani, M.; Nair, A.S.; Kwang, S.A.; Chakraborty, A.; Kadara, H.; Guha, S.; Sethi, G.; Aggarwal, B.B. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol. Cancer Res. 2007, 5, 943–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhao, C.; Jou, D.; Lü, J.; Zhang, C.; Lin, L.; Lin, J. Ursolic acid inhibits the growth of colon cancer-initiating cells by targeting STAT3. Anticancer Res. 2013, 33, 4279–4284. [Google Scholar] [PubMed]
- Liu, T.; Ma, H.; Shi, W.; Duan, J.; Wang, Y.; Zhang, C.; Li, C.; Lin, J.; Li, S.; Lv, J.; et al. Inhibition of STAT3 signaling pathway by ursolic acid suppresses growth of hepatocellular carcinoma. Int. J. Oncol. 2017, 51, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wei, G.; Si, D.; Liu, C. Quantitation of ursolic acid in human plasma by ultra performance liquid chromatography tandem mass spectrometry and its pharmacokinetic study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, N.K.N.; Ong, T.H.; Kumar, A.P.; Lun, C.K.; Ho, P.C.; Wong, P.T.H.; Hui, K.M.; Sethi, G. Ursolic Acid Inhibits the Initiation, Progression of Prostate Cancer and Prolongs the Survival of TRAMP Mice by Modulating Pro-Inflammatory Pathways. PLoS ONE 2012, 7, e32476. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Qian, Z.; Yan, Z.; Zhao, C.; Wang, H.; Ying, G. A phase I pharmacokinetic study of ursolic acid nanoliposomes in healthy volunteers and patients with advanced solid tumors. Int. J. Nanomed. 2012, 8, 129–136. [Google Scholar]
- Bahia, S.B.B.; Reis, W.J.; Jardim, G.A.; Souto, F.T.; de Simone, C.A.; Gatto, C.C.; Menna-Barreto, R.F.; de Castro, S.L.; Cavalcanti, B.C.; Pessoa, C.; et al. Molecular hybridization as a powerful tool towards multitarget quinoidal systems: Synthesis, trypanocidal and antitumor activities of naphthoquinone-based 5-iodo-1,4-disubstituted-, 1,4- and 1,5-disubstituted-1,2,3-triazoles. MedChemComm 2016, 7, 1555–1563. [Google Scholar] [CrossRef]
- Bosquesi, P.L.; Melo, T.R.F.; Vizioli, E.O.; dos Santos, J.L.; Chung, M.C. Anti-inflammatory drug design using a molecular hybridization approach. Pharmaceuticals 2011, 4, 1450–1474. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. Molecular Hybridization Tools in the Development of Furoxan-Based NO-Donor Prodrugs. ChemMedChem 2017, 12, 622–638. [Google Scholar] [CrossRef]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef]
- Panda, S.S.; Tran, Q.L.; Rajpurohit, P.; Pillai, G.G.; Thomas, S.J.; Bridges, A.E.; Capito, J.E.; Thangaraju, M.; Lokeshwar, B.L. Design, Synthesis, and Molecular Docking Studies of Curcumin Hybrid Conjugates as Potential Therapeutics for Breast Cancer. Pharmaceuticals 2022, 15, 451. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.S.; Girgis, A.S.; Thomas, S.J.; Capito, J.E.; George, R.F.; Salman, A.; El-Manawaty, M.A.; Samir, A. Synthesis, pharmacological profile and 2D-QSAR studies of curcumin-amino acid conjugates as potential drug candidates. Eur. J. Med. Chem. 2020, 196, 112293. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.S.; Asiri, A.M.; Elagawany, M.; Buchanan, D.D.; Torkian, B.; Bathala, K.; Thomas, S.J.; Capito, J.E.; Arshad, M.N.; Al-Romaizan, A.N. Efficient synthesis of pyrazinoic acid hybrid conjugates. SynOpen 2017, 1, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Tangutur, A.D.; Kumar, D.; Krishna, V.; Kantevari, S. Microtubule targeting agents as cancer chemotherapeutics: An overview of molecular hybrids as stabilizing and destabilizing agents. Curr. Top. Med. Chem. 2017, 17, 2523–2537. [Google Scholar] [CrossRef]
- Viegas-Junior, C.; Danuello, A.; da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A.M. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef]
- Thien, D.D.; Nguyen, T.T.; Thien, D.G.; Nguyen, T.H.A.; Van Sung, T. Synthesis and Cytotoxic Activity of Ursolic Acid Derivatives. Z. Naturforsch. B 2013, 68, 201–206. [Google Scholar] [CrossRef]
- da Silva, E.F.; de Vargas, A.S.; Willig, J.B.; de Oliveira, C.B.; Zimmer, A.R.; Pilger, D.A.; Buffon, A.; Gnoatto, S.C.B. Synthesis and antileukemic activity of an ursolic acid derivative: A potential co-drug in combination with imatinib. Chem. Biol. Interact. 2021, 344, 109535. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Zhang, H.; Yue, J.; Sun, Y.; Zhang, X.; Zhao, Y. Synthesis of triterpenoid derivatives and their anti-tumor and anti-hepatic fibrosis activities. Nat. Prod. Res. 2020, 34, 766–772. [Google Scholar] [CrossRef]
- Fontana, G.; Bruno, M.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Spinella, A.; Rosselli, S. Cytotoxicity of oleanolic and ursolic acid derivatives toward hepatocellular carcinoma and evaluation of NF-κB involvement. Bioorg. Chem. 2019, 90, 103054. [Google Scholar] [CrossRef]
- Ma, C.-M.; Cai, S.-Q.; Cui, J.-R.; Wang, R.-Q.; Tu, P.-F.; Hattori, M.; Daneshtalab, M. The cytotoxic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2005, 40, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, H.I.; Radwan, M.O.; Ozturk, S.E.; Ulusoy, N.G.; Sozer, E.; Ellakwa, D.E.; Ocak, Z.; Can, M.; Ali, T.F.S.; Abd-Alla, H.; et al. Design, synthesis and biological evaluation of pentacyclic triterpene derivatives: Optimization of anti-ABL kinase activity. Molecules 2019, 24, 3535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, H.-Y.; Huang, A.-M.; Wei, B.-L.; Gan, K.-H.; Hour, T.-C.; Yang, S.-C.; Pu, Y.-S.; Lin, C.-N. Ursolic acid derivatives induce cell cycle arrest and apoptosis in NTUB1 cells associated with reactive oxygen species. Bioorg. Med. Chem. 2009, 17, 7265–7274. [Google Scholar] [CrossRef] [PubMed]
- Baglin, I.; Poumaroux, A.; Nour, M.; Tan, K.; Mitaine-Offer, A.C.; Lacaille-Dubois, M.A.; Chauffert, B.; Cave, C. New Ursolic and Betulinic Derivatives as Potential Cytotoxic Agents. J. Enzym. Inhib. Med. Chem. 2003, 18, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.-K.; Yu, Z.; Chen, F.-L.; Li, F.; Li, W.-Y.; Guo, Y.-H. Synthesis and evaluation of ursolic acid derivatives as potent cytotoxic agents. Bioorg. Med. Chem. Lett. 2012, 22, 2488–2493. [Google Scholar] [CrossRef]
- Tian, T.; Liu, X.; Lee, E.-S.; Sun, J.; Feng, Z.; Zhao, L.; Zhao, C. Synthesis of novel oleanolic acid and ursolic acid in C-28 position derivatives as potential anticancer agents. Arch. Pharm. Res. 2017, 40, 458–468. [Google Scholar] [CrossRef]
- Liu, M.-C.; Yang, S.-J.; Jin, L.-H.; Hu, D.-Y.; Xue, W.; Song, B.-A.; Yang, S. Synthesis and cytotoxicity of novel ursolic acid derivatives containing an acyl piperazine moiety. Eur. J. Med. Chem. 2012, 58, 128–135. [Google Scholar] [CrossRef]
- Liu, M.-C.; Yang, S.-J.; Jin, L.-H.; Hu, D.-Y.; Xue, W.; Yang, S. Synthesis and evaluation as potential antitumor agents of novel ursolic acid derivatives. Med. Chem. Res. 2016, 25, 2267–2279. [Google Scholar] [CrossRef]
- Chi, K.-Q.; Wei, Z.-Y.; Wang, K.-S.; Wu, J.; Chen, W.-Q.; Jin, X.-J.; Piao, H.-R. Design, synthesis, and evaluation of novel ursolic acid derivatives as HIF-1α inhibitors with anticancer potential. Bioorg. Chem. 2017, 75, 157–169. [Google Scholar] [CrossRef]
- Pattnaik, B.; Lakshmi, J.K.; Kavitha, R.; Jagadeesh, B.; Bhattacharjee, D.; Jain, N.; Mallavadhani, U.V. Synthesis, structural studies, and cytotoxic evaluation of novel ursolic acid hybrids with capabilities to arrest breast cancer cells in mitosis. J. Asian Nat. Prod. Res. 2017, 19, 260–271. [Google Scholar] [CrossRef]
- Semenova, M.D.; Popov, S.A.; Golubeva, T.S.; Baev, D.S.; Shults, E.E.; Turks, M. Synthesis and Cytotoxicity of Sulfanyl, Sulfinyl and Sulfonyl Group Containing Ursane Conjugates with 1,3,4-Oxadiazoles and 1,2,4-Triazoles. ChemistrySelect 2021, 6, 6472–6477. [Google Scholar] [CrossRef]
- Khazanov, V.A.; Manuylova, A.V.; Gogvadze, V.; Spivak, A.Y. Mitochondria-targeted betulinic and ursolic acid derivatives: Synthesis and anticancer activity. MedChemComm 2017, 8, 1934–1945. [Google Scholar]
- Saraswat, B.P.; Visen, K.; Agarwal, D.P. Ursolic acid isolated from Eucaliptus tereticornis protects against ethanol toxicity in isolated rat hepatocytes. Phytother. Res. 2000, 14, 163–166. [Google Scholar]
- Popov, S.A.; Semenova, M.D.; Baev, D.S.; Frolova, T.S.; Shestopalov, M.A.; Wang, C.; Qi, Z.; Shults, E.E.; Turks, M. Synthesis and cytotoxicity of hybrids of 1,3,4- or 1,2,5-oxadiazoles tethered from ursane and lupane core with 1,2,3-triazole. Steroids 2020, 162, 108698. [Google Scholar] [CrossRef]
- Shao, J.-W.; Dai, Y.-C.; Xue, J.-P.; Wang, J.-C.; Lin, F.-P.; Guo, Y.-H. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur. J. Med. Chem. 2011, 46, 2652–2661. [Google Scholar] [CrossRef]
- Toni, C.; Denner, T.C.; Hoenke, S.; Kraft, O.; Deigner, H.-P.; Al-Harrasi, A.; Csuk, R. Hydroxyethylamide substituted triterpenoic acids hold good cytotoxicity for human tumor cells. Results Chem. 2022, 4, 100371. [Google Scholar]
- Wiemann, J.; Heller, L.; Csuk, R. Targeting cancer cells with oleanolic and ursolic acid derived hydroxamates. Bioorg. Med. Chem. Lett. 2016, 26, 907–909. [Google Scholar] [CrossRef]
- Meng, Y.; Song, Y.; Yan, Z.; Xia, Y. Synthesis and in vitro Cytotoxicity of Novel Ursolic Acid Derivatives. Molecules 2010, 15, 4033–4040. [Google Scholar] [CrossRef] [Green Version]
- Bai, K.-K.; Chen, F.-L.; Yu, Z.; Zheng, Y.-Q.; Li, Y.-N.; Guo, Y.-H. Synthesis of [3β-acetoxy-urs-12-en-28-oyl]-1-monoglyceride and investigation on its anti tumor effects against BGC-823. Bioorg. Med. Chem. 2011, 19, 4043–4050. [Google Scholar] [CrossRef]
- Quail, D.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Kroemer, G.; Pouyssegur, J. Tumor cell metabolism: Cancer’s Achilles’ heel. Cancer Cell 2008, 13, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, 5473. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Cassim, S.; Vučetić, M.; Ždralević, M.; Pouyssegur, J. Warburg and Beyond: The Power of Mitochondrial Metabolism to Collaborate or Replace Fermentative Glycolysis in Cancer. Cancers 2020, 12, 1119. [Google Scholar] [CrossRef] [PubMed]
- Cassim, S.; Raymond, V.A.; Lacoste, B.; Lapierre, P.; Bilodeau, M. Metabolite profiling identifies a signature of tumorigenicity in hepatocellular carcinoma. Oncotarget 2018, 9, 26868–26883. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Yang, X.; Xie, J.; Xiang, L.; Li, Y.; Ou, M.; Chi, T.; Liu, Z.; Yu, S.; Gao, Y.; et al. UP12, a novel ursolic acid derivative with potential for targeting multiple signaling pathways in hepatocellular carcinoma. Biochem. Pharmacol. 2015, 93, 151–162. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Jiang, W.; Ou, M.; Chen, Y.; Xu, Y.; Wu, Q.; Zheng, Q.; Wu, F.; Wang, L.; et al. Synthesis and Biological Evaluation of Novel Ursolic acid Derivatives as Potential Anticancer Prodrugs. Chem. Biol. Drug Des. 2015, 86, 1397–1404. [Google Scholar] [CrossRef]
- Hua, S.-X.; Huang, R.-Z.; Ye, M.-Y.; Pan, Y.-M.; Yao, G.-Y.; Zhang, Y.; Wang, H.-S. Design, synthesis and in vitro evaluation of novel ursolic acid derivatives as potential anticancer agents. Eur. J. Med. Chem. 2015, 95, 435–452. [Google Scholar] [CrossRef]
- Friedrich, S.; Serbian, I.; Hoenke, S.; Wolfram, R.K.; Csuk, R. Synthesis and cytotoxic evaluation of malachite green derived oleanolic and ursolic acid piperazineamides. Med. Chem. Res. 2020, 29, 926–933. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Huang, R.-Z.; Zhang, J.; Guo, T.; Zhang, M.-T.; Huang, X.-C.; Zhang, B.; Liao, Z.-X.; Sun, J.; Wang, H.-S. Discovery of antitumor ursolic acid long-chain diamine derivatives as potent inhibitors of NF-κB. Bioorg. Chem. 2018, 79, 265–276. [Google Scholar] [CrossRef]
- Kahnt, M.; Fischer, L.; Al-Harrasi, A.; Csuk, R. Ethylenediamine Derived Carboxamides of Betulinic and Ursolic Acid as Potential Cytotoxic Agents. Molecules 2018, 23, 2558. [Google Scholar] [CrossRef] [Green Version]
- Blanchet, M.; Borselli, D.; Brunel, J.M. Polyamine derivatives: A revival of an old neglected scaffold to fight resistant Gram-negative bacteria? Future Med. Chem. 2016, 8, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Nowotarski, S.L.; Woster, P.M.; Casero, R.A. Polyamines and cancer: Implications for chemotherapy and chemoprevention. Expert Rev. Mol. Med. 2013, 15, e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spivak, A.; Khalitova, R.; Nedopekina, D.; Dzhemileva, L.; Yunusbaeva, M.; Odinokov, V.; D’yakonov, V.; Dzhemilev, U. Synthesis and Evaluation of Anticancer Activities of Novel C-28 Guanidine-Functionalized Triterpene Acid Derivatives. Molecules 2018, 23, 3000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, S.A.; Semenova, M.D.; Baev, D.S.; Frolova, T.S.; Shults, E.E.; Wang, C.; Turks, M. Synthesis of cytotoxic urs-12-ene- and 28-norurs-12-ene- type conjugates with amino- and mercapto-1,3,4-oxadiazoles and mercapto-1,2,4-triazoles. Steroids 2020, 153, 108524. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Dar, B.A.; Majeed, R.; Hamid, A.; Bhat, B.A. Synthesis and biological evaluation of ursolic acid-triazolyl derivatives as potential anti-cancer agents. Eur. J. Med. Chem. 2013, 66, 238–245. [Google Scholar] [CrossRef]
- Meng, Y.-Q.; Zhang, L.-F.; Liu, D.Y.; Liu, L.-W.; Zhang, Y.; Zhao, Y. Synthesis and antitumor activity evaluation of novel ursolic acid derivatives. J. Asian Nat. Prod. Res. 2016, 18, 280–288. [Google Scholar] [CrossRef]
- Meng, Y.-Q.; Xu, C.-D.; Yu, T.-T.; Li, W.; Li, Q.-W.; Li, X.-X. Synthesis and antitumor activity evaluation of ursolic acid derivatives. J. Asian Nat. Prod. Res. 2020, 22, 359–369. [Google Scholar] [CrossRef]
- Zhao, C.-H.; Zhang, C.-L.; Shi, J.-J.; Hou, X.-Y.; Feng, B.; Zhao, L.-X. Design, synthesis, and biofunctional evaluation of novel pentacyclic triterpenes bearing O-[4-(1-piperazinyl)-4-oxo-butyryl moiety as antiproliferative agents. Bioorg. Med. Chem. Lett. 2015, 25, 4500–4504. [Google Scholar] [CrossRef] [PubMed]
- Kahnt, M.; Hoenke, S.; Fischer, L.; Al-Harrasi, A.; Csuk, R. Synthesis and Cytotoxicity Evaluation of DOTA-Conjugates of Ursolic Acid. Molecules 2019, 24, 2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.-H.; Zhang, Z.-H.; Li, M.-Y.; Wei, Z.-Y.; Jin, X.-J.; Piao, H.-R. Synthesis and evaluation of the HIF-1α inhibitory activities of novel ursolic acid tetrazole derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.S.; Wang, R.; Salvador, J.A.R.; Jing, Y. Synthesis of novel ursolic acid heterocyclic derivatives with improved abilities of antiproliferation and induction of p53, p21waf1 and NOXA in pancreatic cancer cells. Bioorg. Med. Chem. 2012, 20, 5774–5786. [Google Scholar] [CrossRef]
- Leal, A.S.; Wang, R.; Salvador, J.A.R.; Jing, Y. Semisynthetic Ursolic Acid Fluorolactone Derivatives Inhibit Growth with Induction of p21waf1 and Induce Apoptosis with Upregulation of NOXA and Downregulation of c-FLIP in Cancer Cells. ChemMedChem 2012, 7, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Dar, B.A.; Lone, A.M.; Shah, W.A.; Qurishi, M.A. Synthesis and screening of ursolic acid-benzylidine derivatives as potential anti-cancer agents. Eur. J. Med. Chem. 2016, 111, 26–32. [Google Scholar] [CrossRef]
- Gu, W.; Hao, Y.; Zhang, G.; Wang, S.-F.; Miao, T.-T.; Zhang, K.-P. Synthesis, in vitro antimicrobial and cytotoxic activities of new carbazole derivatives of ursolic acid. Bioorg. Med. Chem. Lett. 2015, 25, 554–557. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Yang, Z.-H.; Li, A.-L.; Liu, Q.-S.; Sun, Y.; Gu, W. Design, synthesis, anticancer activity and mechanism studies of novel 2-amino-4-arylpyrimidine derivatives of ursolic acid. New J. Chem. 2022, 46, 2335–2350. [Google Scholar] [CrossRef]
- Gu, W.; Jin, X.-Y.; Li, D.-D.; Wang, S.-F.; Tao, X.-B.; Chen, H. Design, synthesis and in vitro anticancer activity of novel quinoline and oxadiazole derivatives of ursolic acid. Bioorg. Med. Chem. Lett. 2017, 27, 4128–4132. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.-H.; Zhang, L.-H.; Jin, X.-J.; Ma, J.; Piao, H.-R. Design, synthesis, and screening of novel ursolic acid derivatives as potential anti-cancer agents that target the HIF-1α pathway. Bioorg. Med. Chem. Lett. 2019, 29, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, H.P.; Costa, P.E.; Reis, A.; Stern, A. Nitric Oxide: Protein tyrosine phosphorylation and protein S-nitrosylation in cancer. Biomed. J. 2015, 38, 380–388. [Google Scholar] [PubMed]
- Ishima, Y.; Kragh-Hansen, U.; Maruyama, T.; Otaqiri, M. Poly-S-nitrosated albumin as a safe and effective multifunctional antitumor agent: Characterization, biochemistry and possible future therapeutic applications. BioMed Res. Int. 2013, 2013, 353892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; He, B.; Yuan, H.; Feng, G.; Chen, F.; Wu, A.; Zhang, L.; Lin, H.; Zhuo, Z.; Wang, T. Synthesis and antitumor evaluation in vitro of NO-donating ursolic acid-benzylidene derivatives. Chem. Biodivers. 2019, 16, e1900111. [Google Scholar] [CrossRef]
- He, B.; Zhu, Z.; Chen, F.; Zhang, R.; Chen, W.; Zhang, T.; Wang, T.; Lei, J. Synthesis and antitumor potential of new arylidene ursolic acid derivatives via caspase-8 activation. Arch. Pharm. 2021, 354, 2000448. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Wu, W.-Y.; Liu, Q.-S.; Sun, T.; Gu, W. Synthesis, anticancer evaluation and mechanism studies of novel indolequinone derivatives of ursolic acid. Bioorg. Chem. 2021, 109, 104705. [Google Scholar] [CrossRef]
- Cui, Z.L.; Liu, Z.; Zeng, J.X.; Zhang, S.L.; Chen, L.; Zhang, G.; Xu, W.; Song, L.; Guo, X. TRIM59 promotes gefitinib resistance in EGFR mutant lung adenocarcinoma cells. Life Sci. 2019, 224, 23–32. [Google Scholar] [CrossRef]
- Onmaz, D.E.; Abusoglu, S.; Unlu, A.; Dagli, B.M.; Bagci, M.; Tok, O.; Abusoglu, G. Determination of serum imatinib and its’ metabolite in patients chronic myeloid leukemia. Clin. Chim. Acta 2019, 497, 120–124. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, M.; Liu, Y.; Sun, L.; Lu, B.; Zhao, L. Synthesis, characterization and in vitro anti-proliferative effects of pentacyclic triterpenoids. Med. Chem. Res. 2021, 30, 2055–2068. [Google Scholar] [CrossRef]
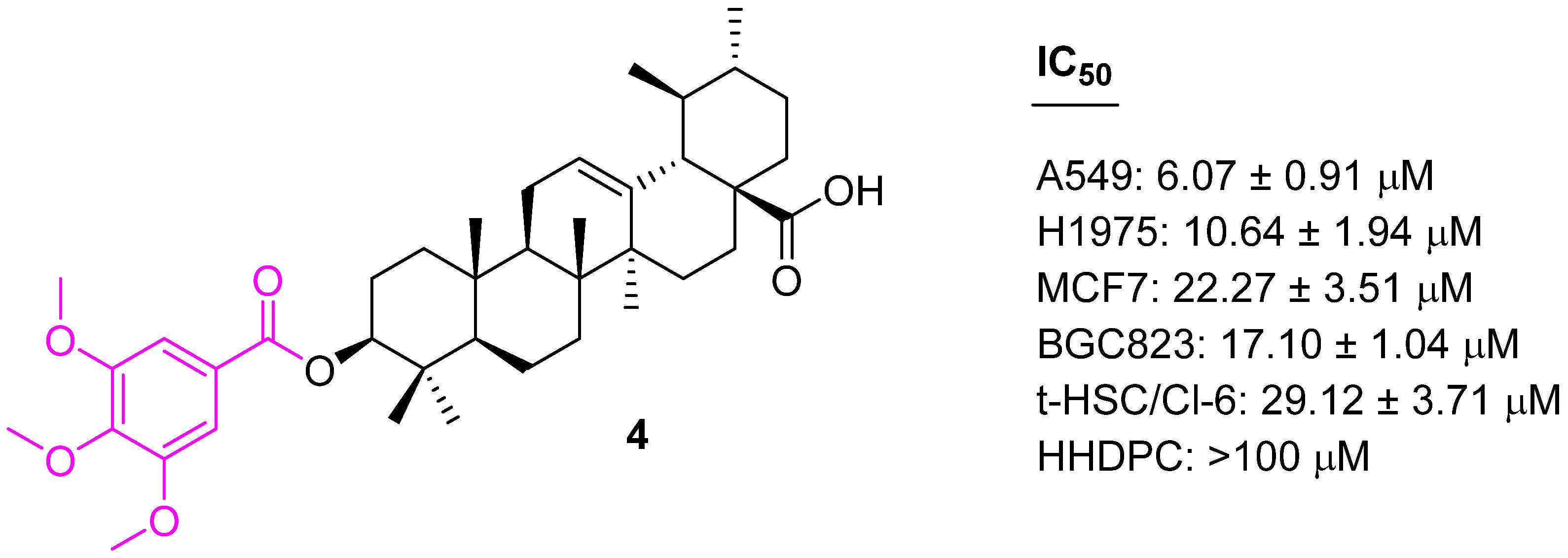
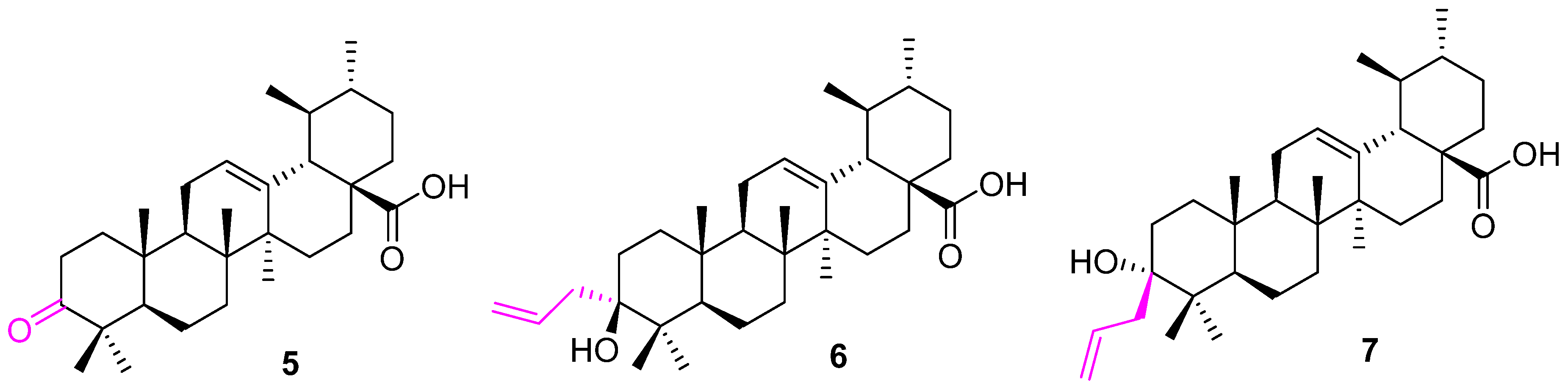

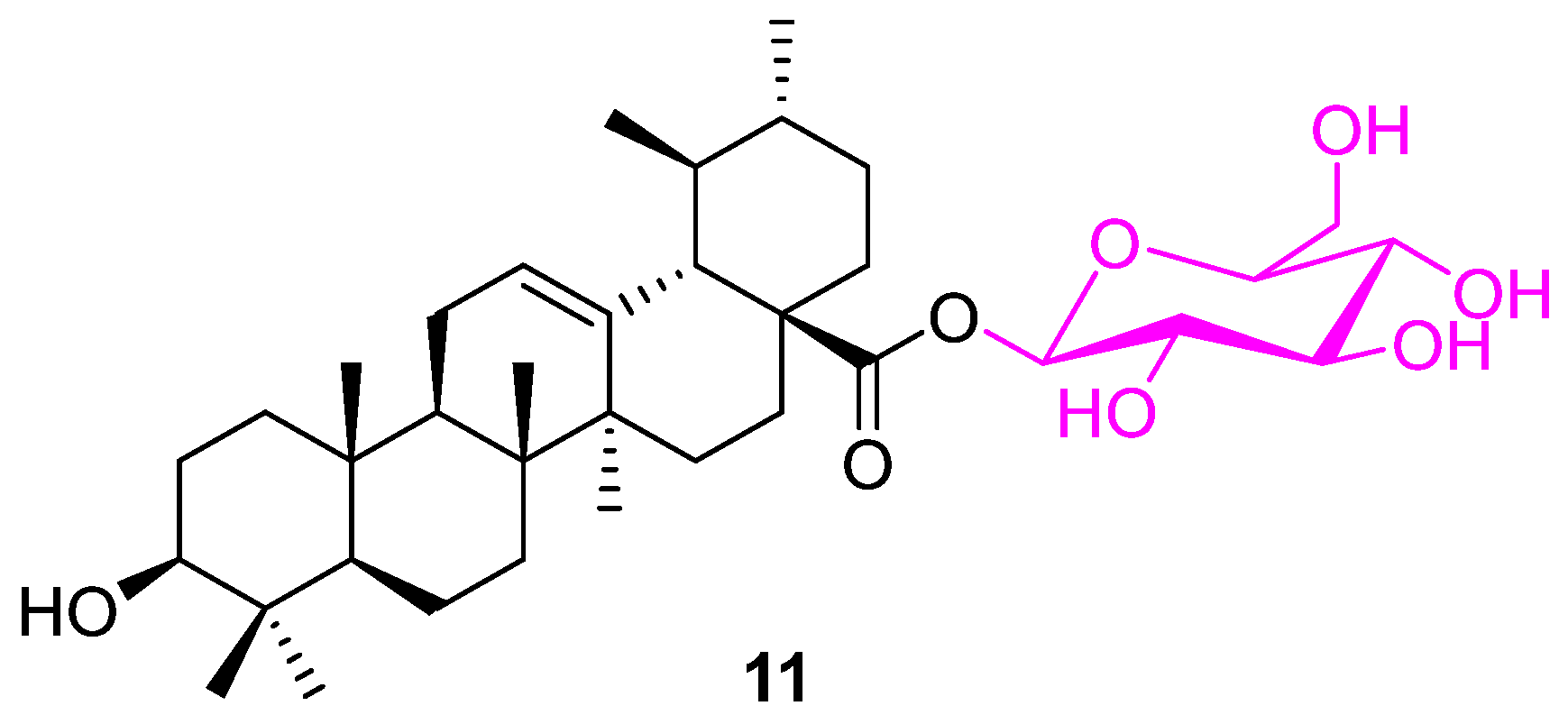

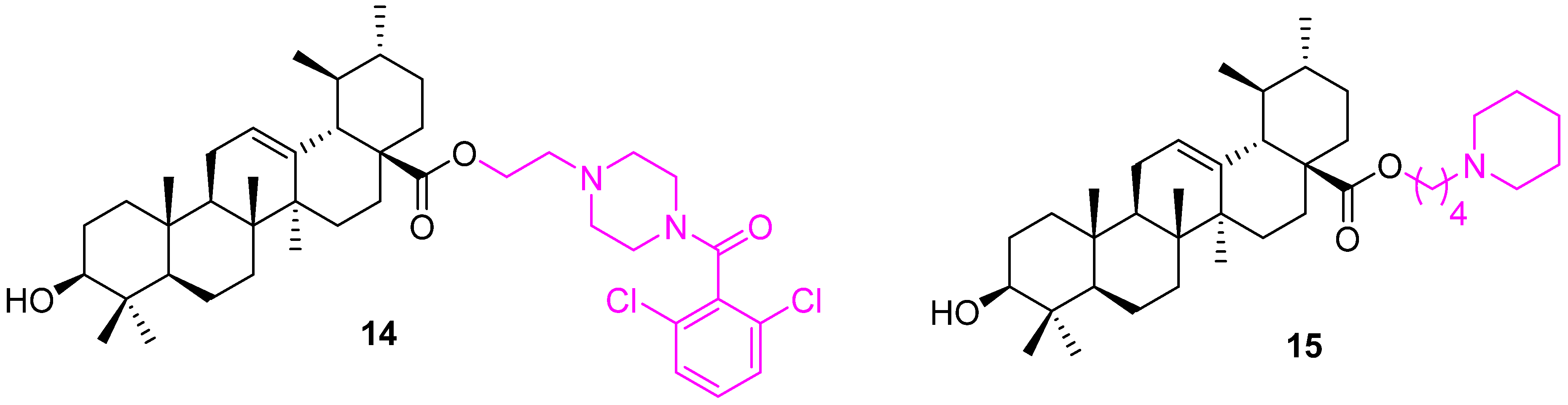
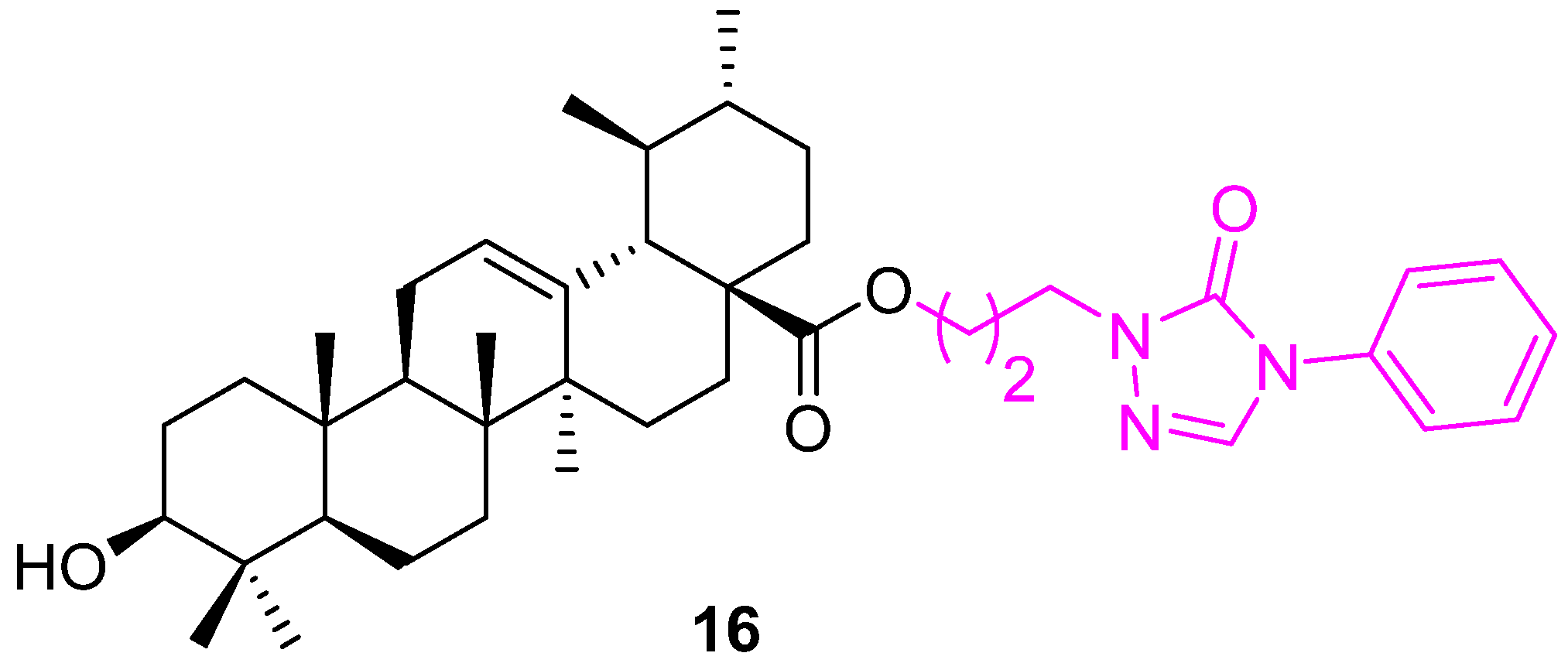
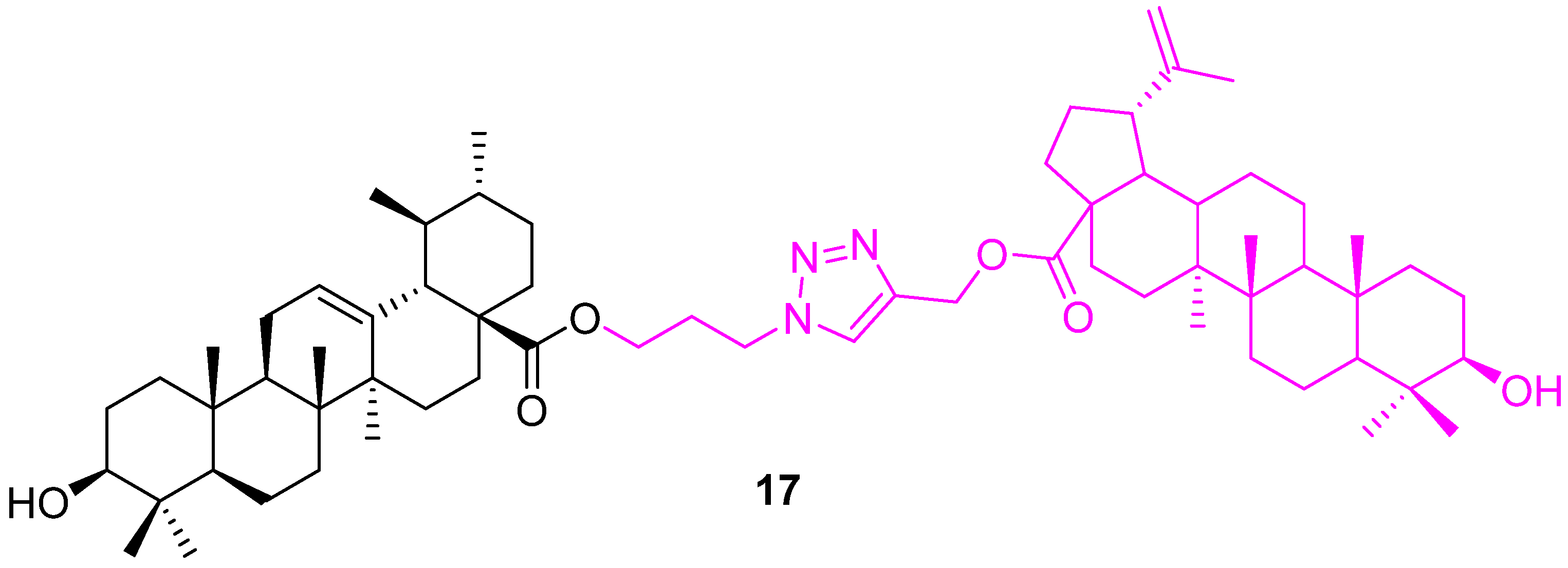



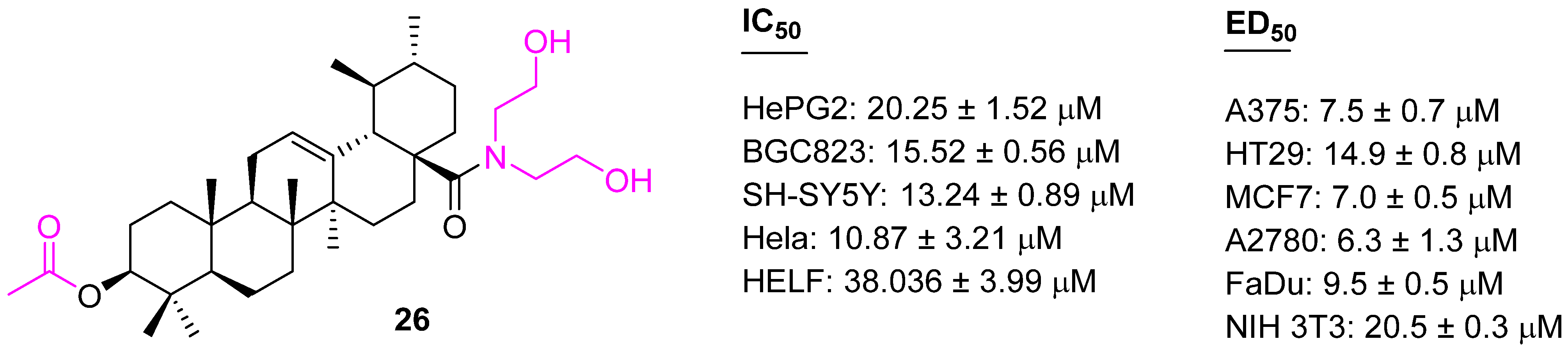
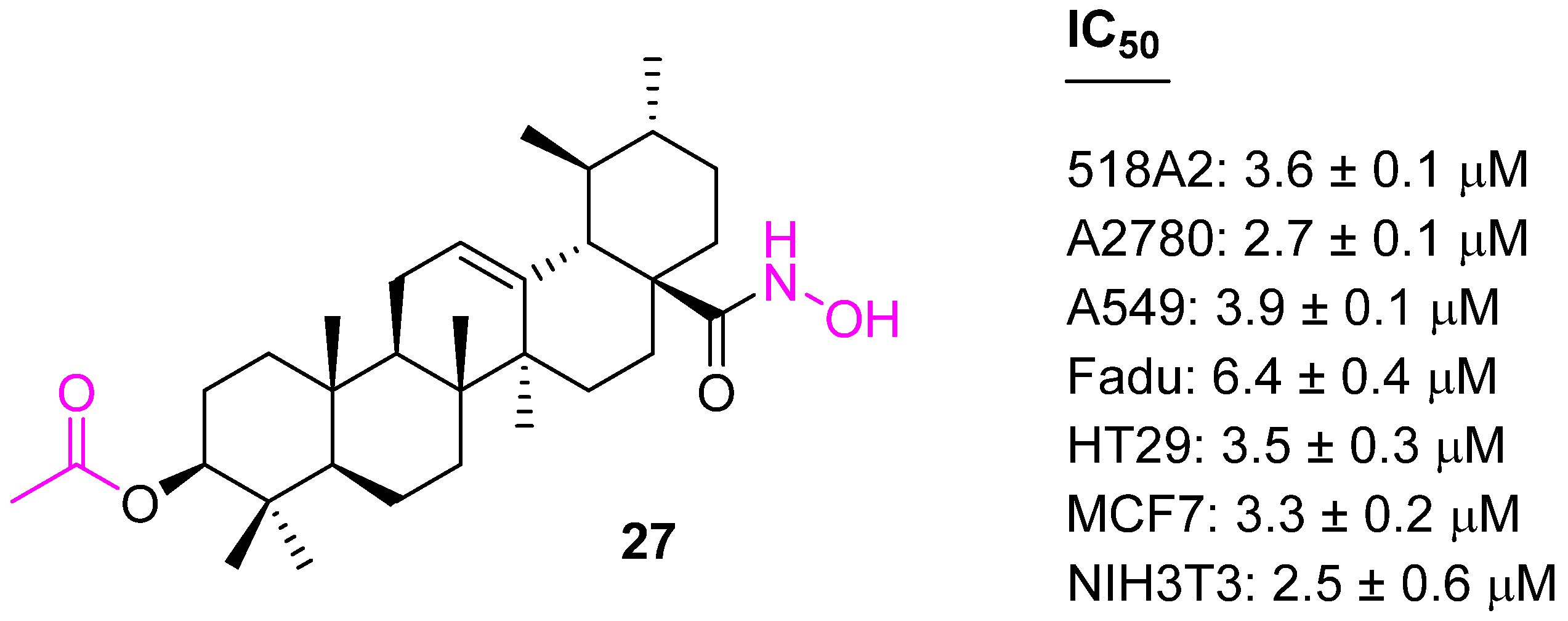

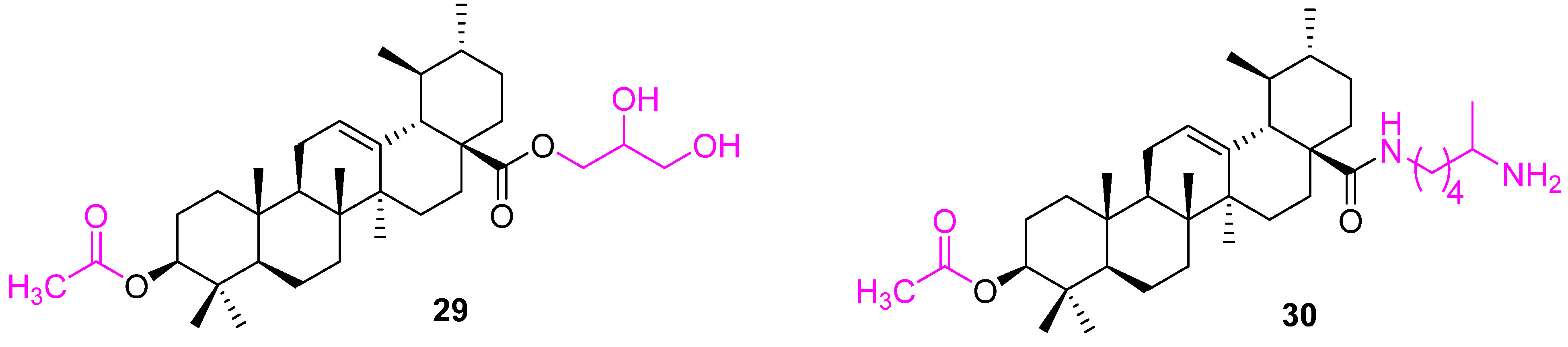
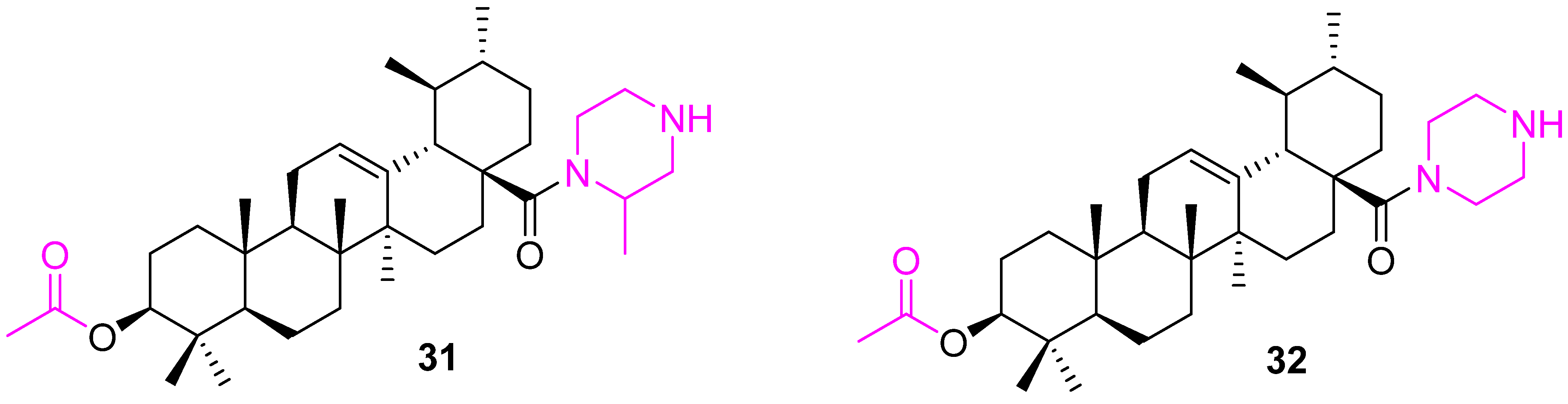
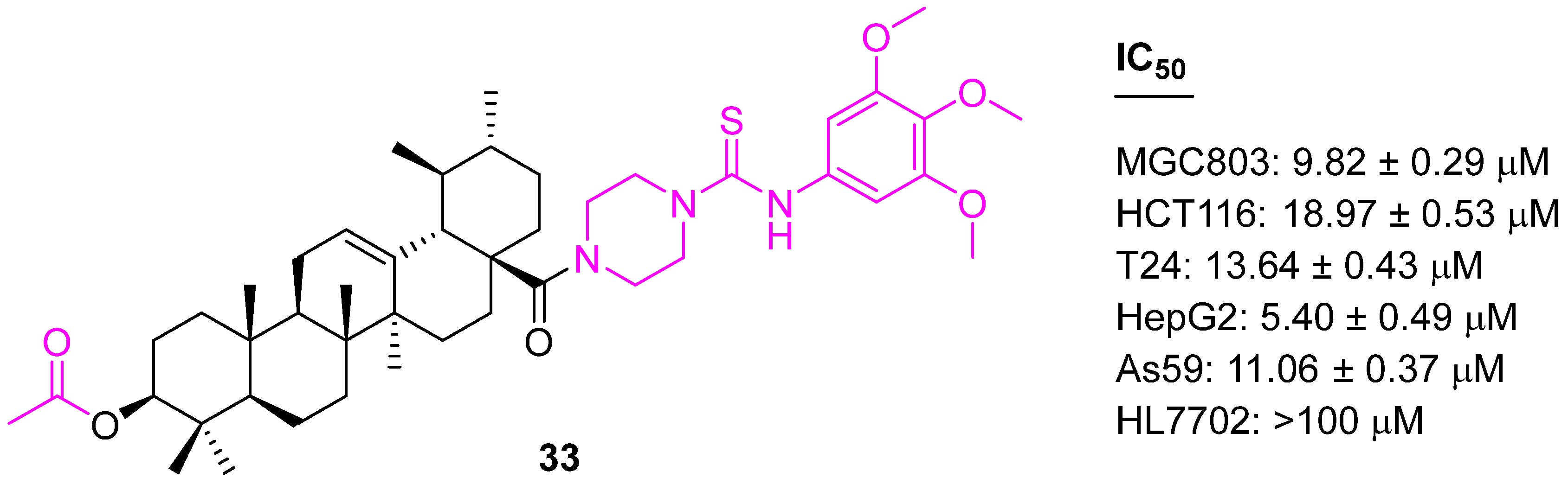
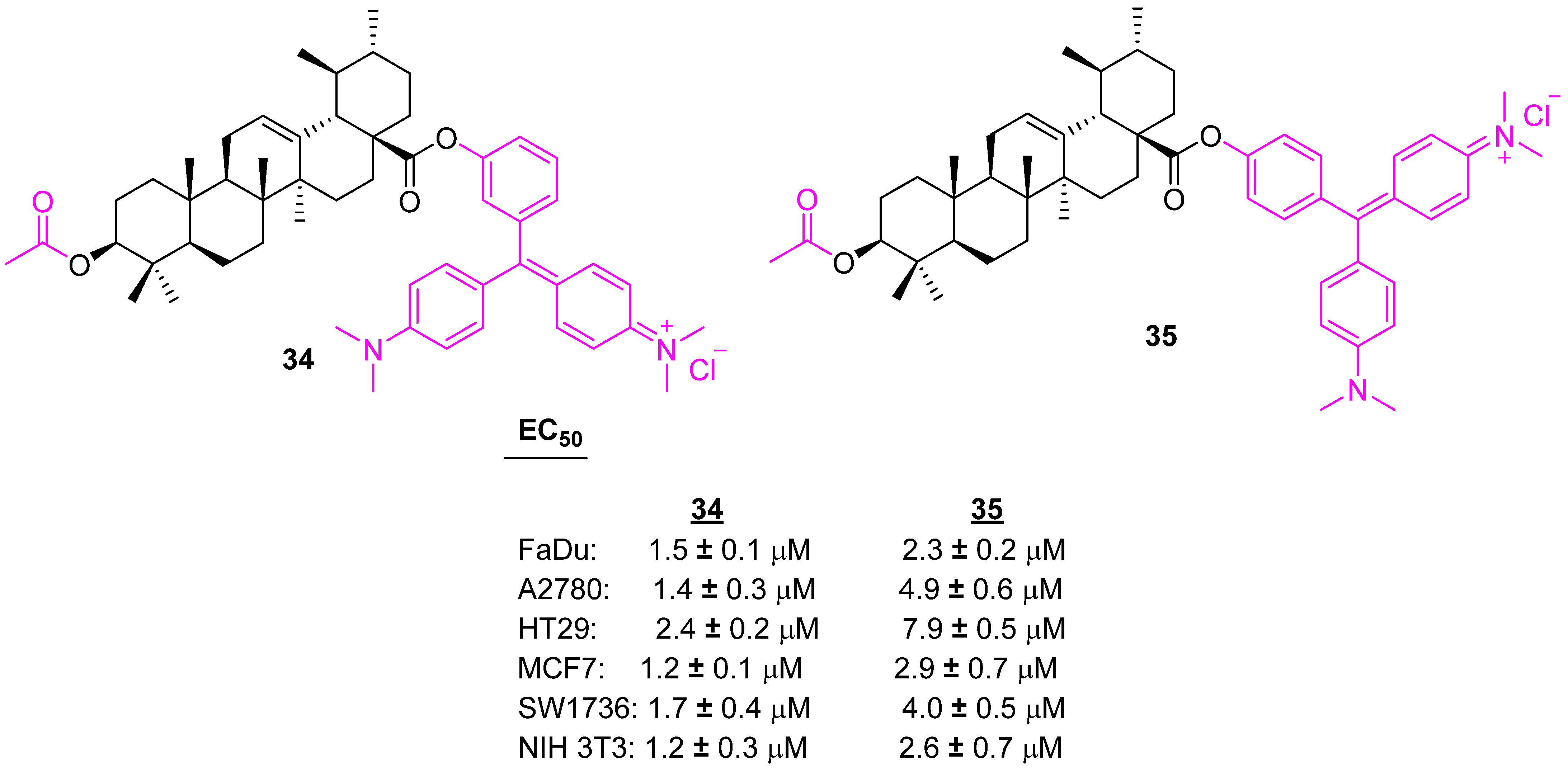


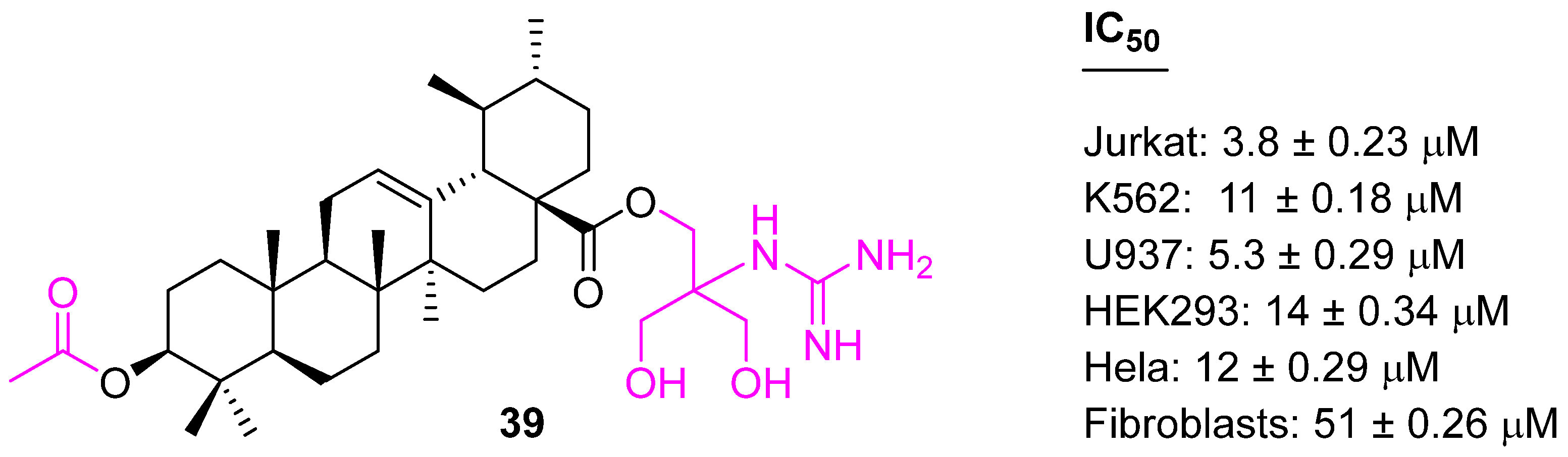
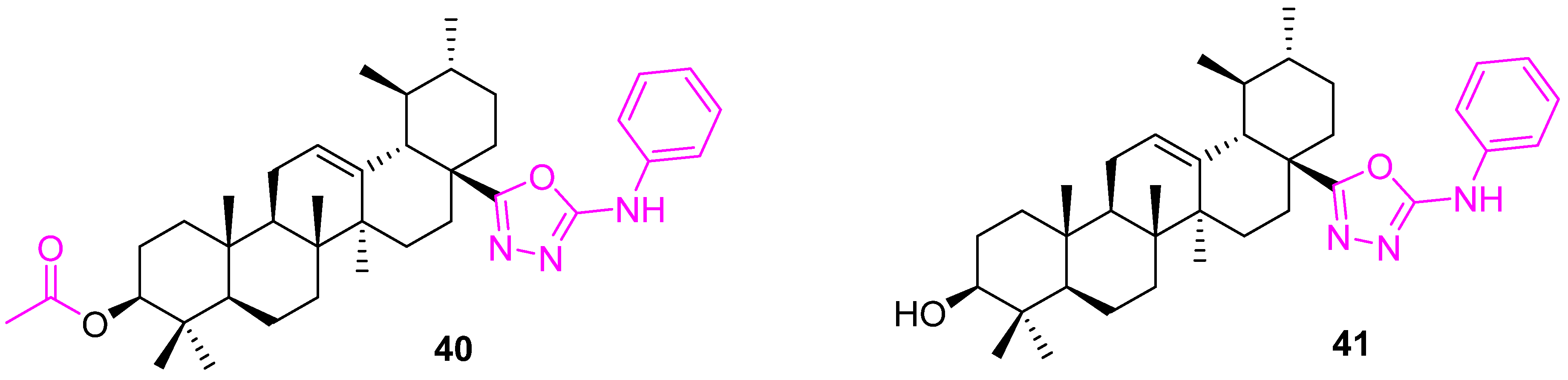
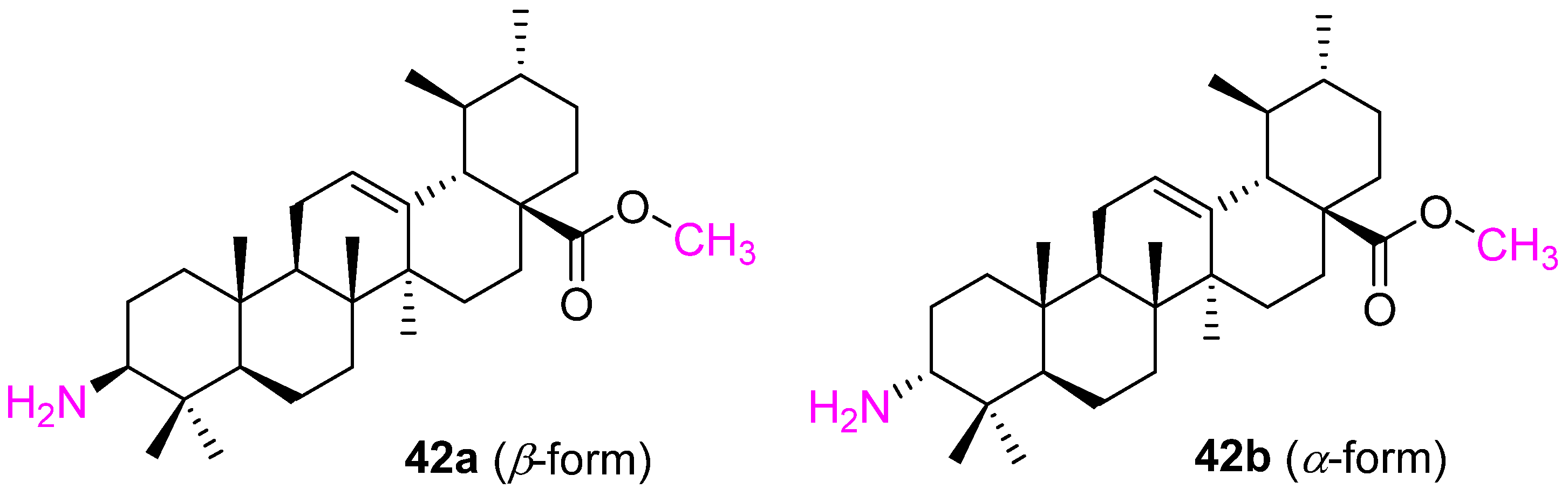
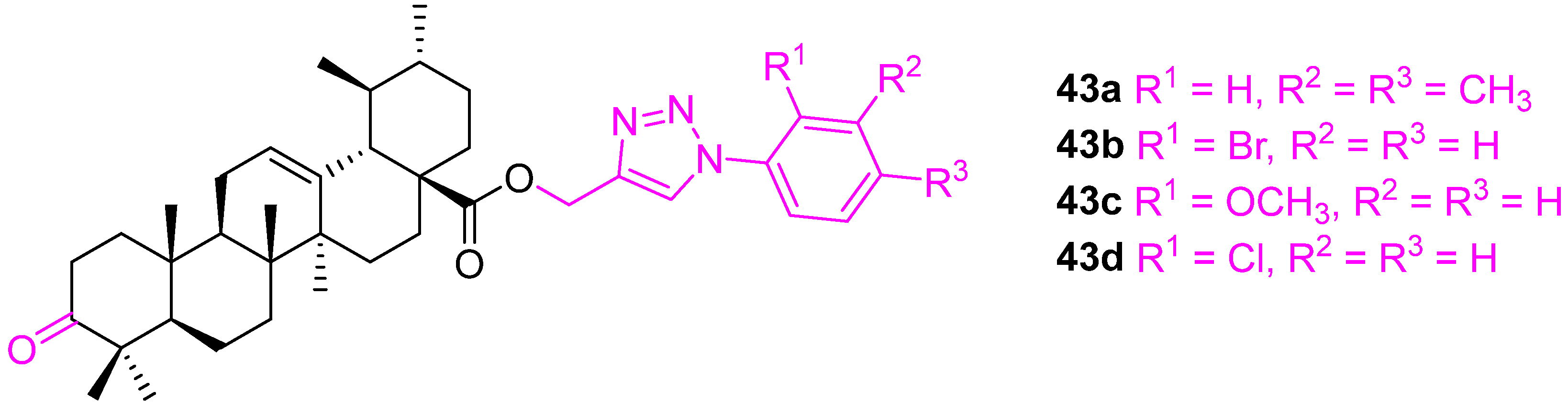
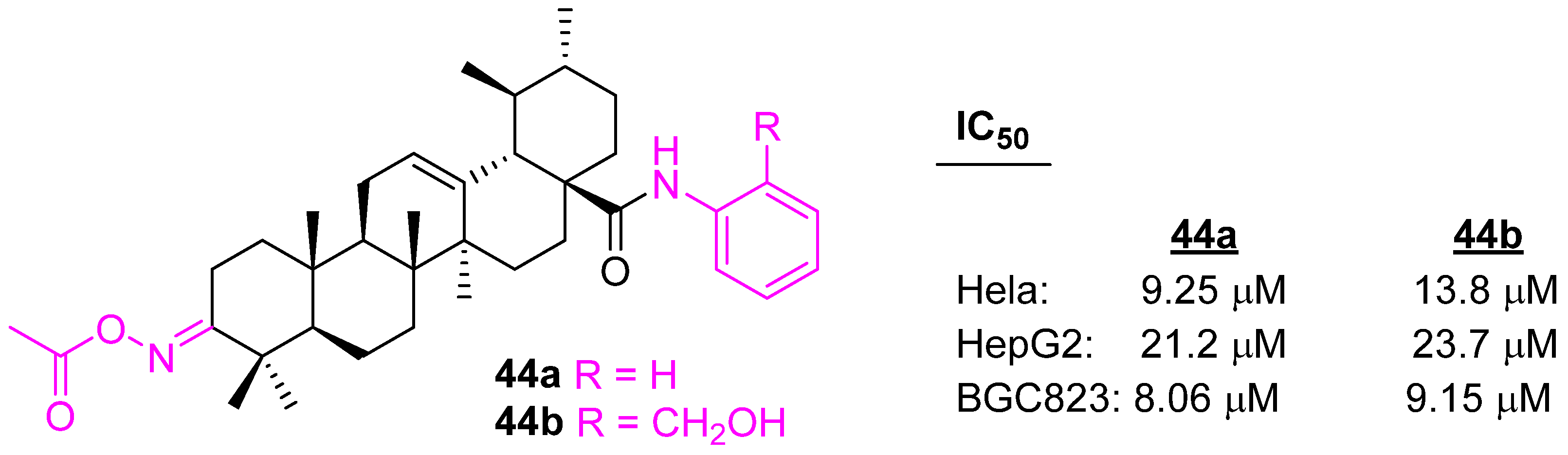

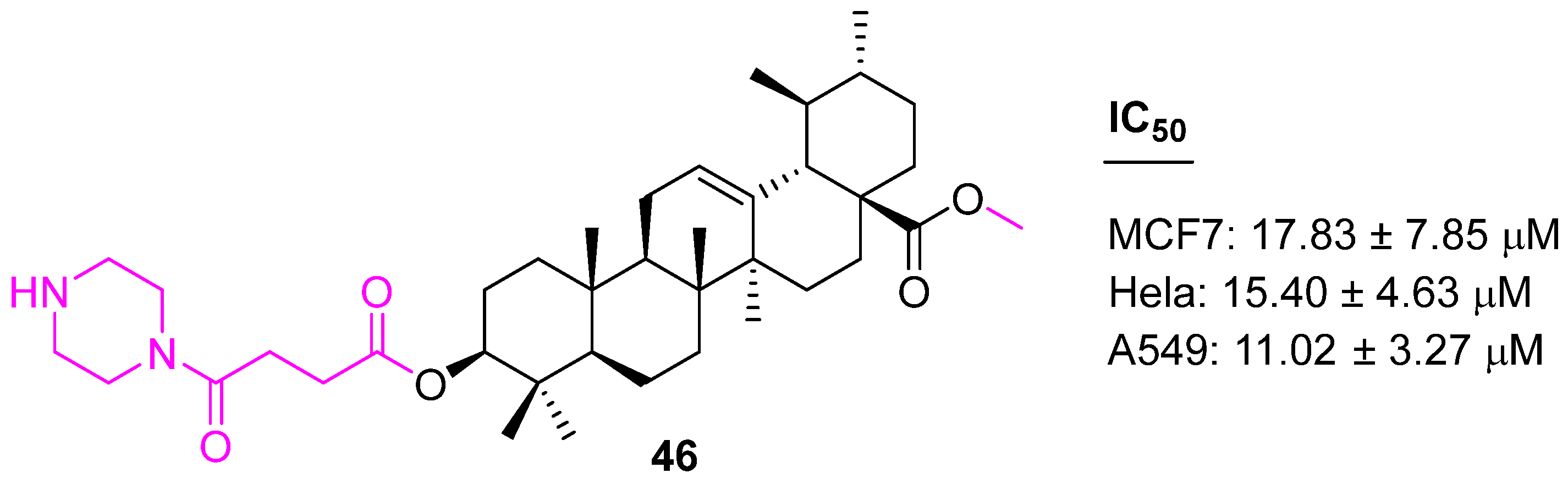
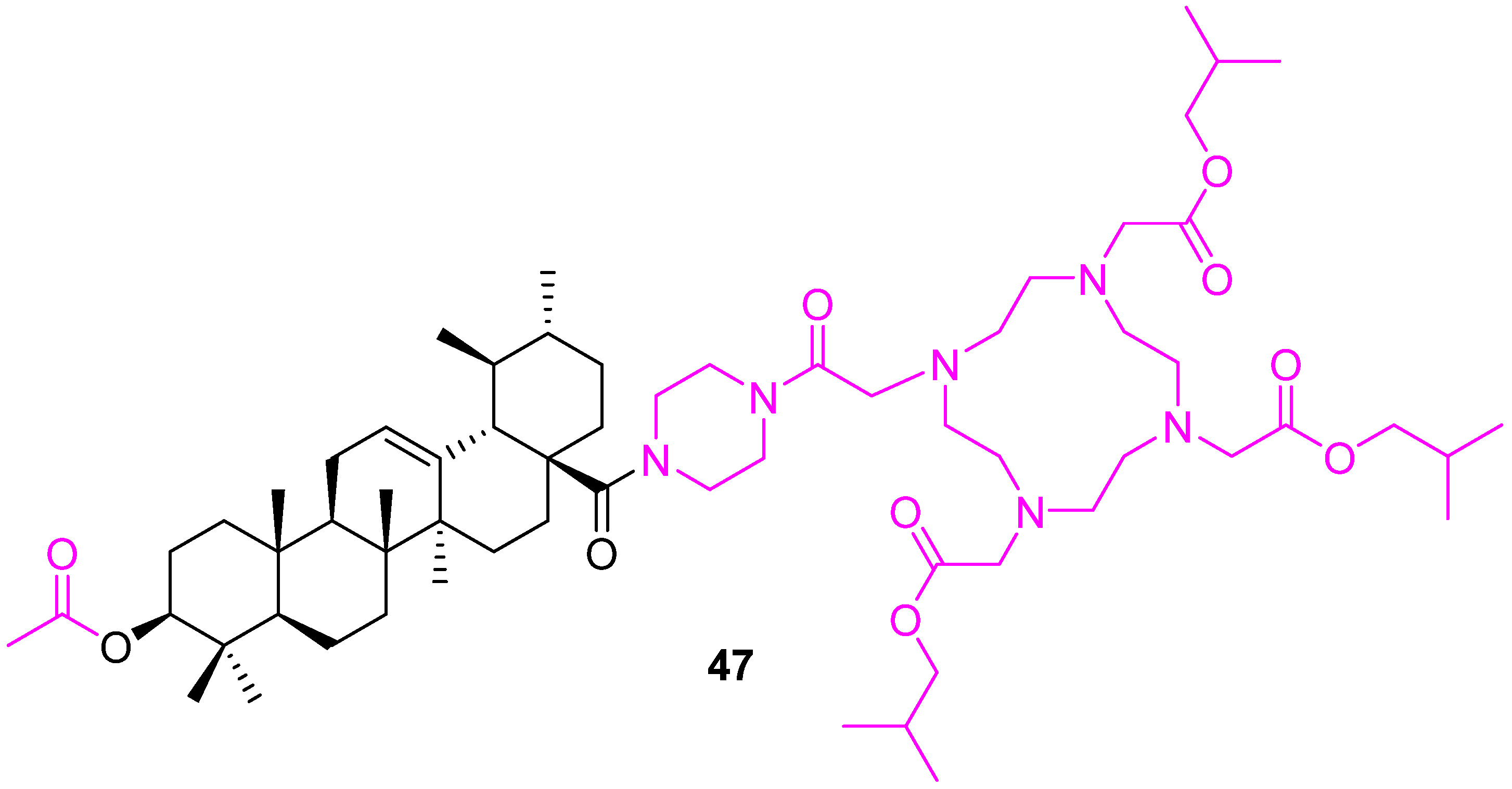
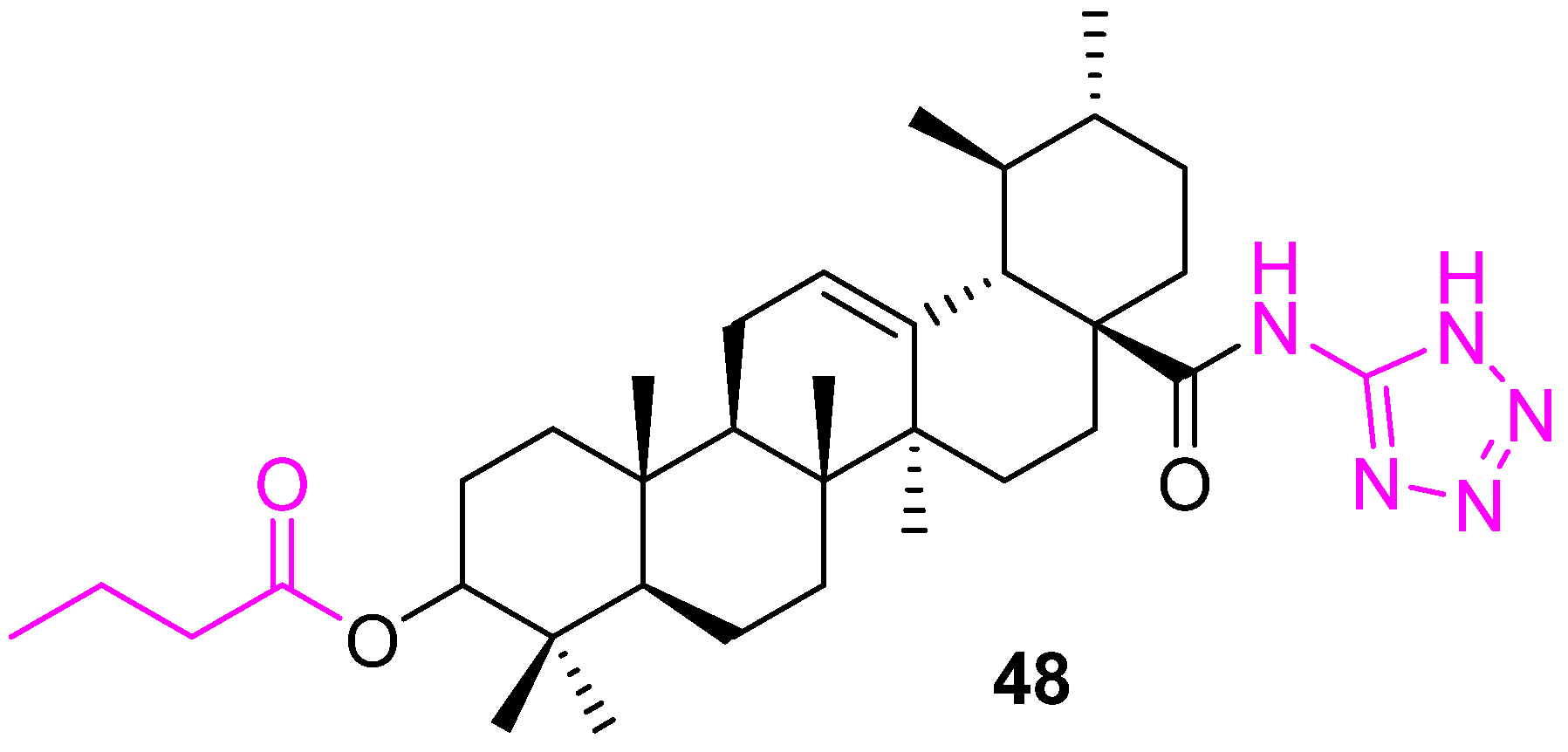

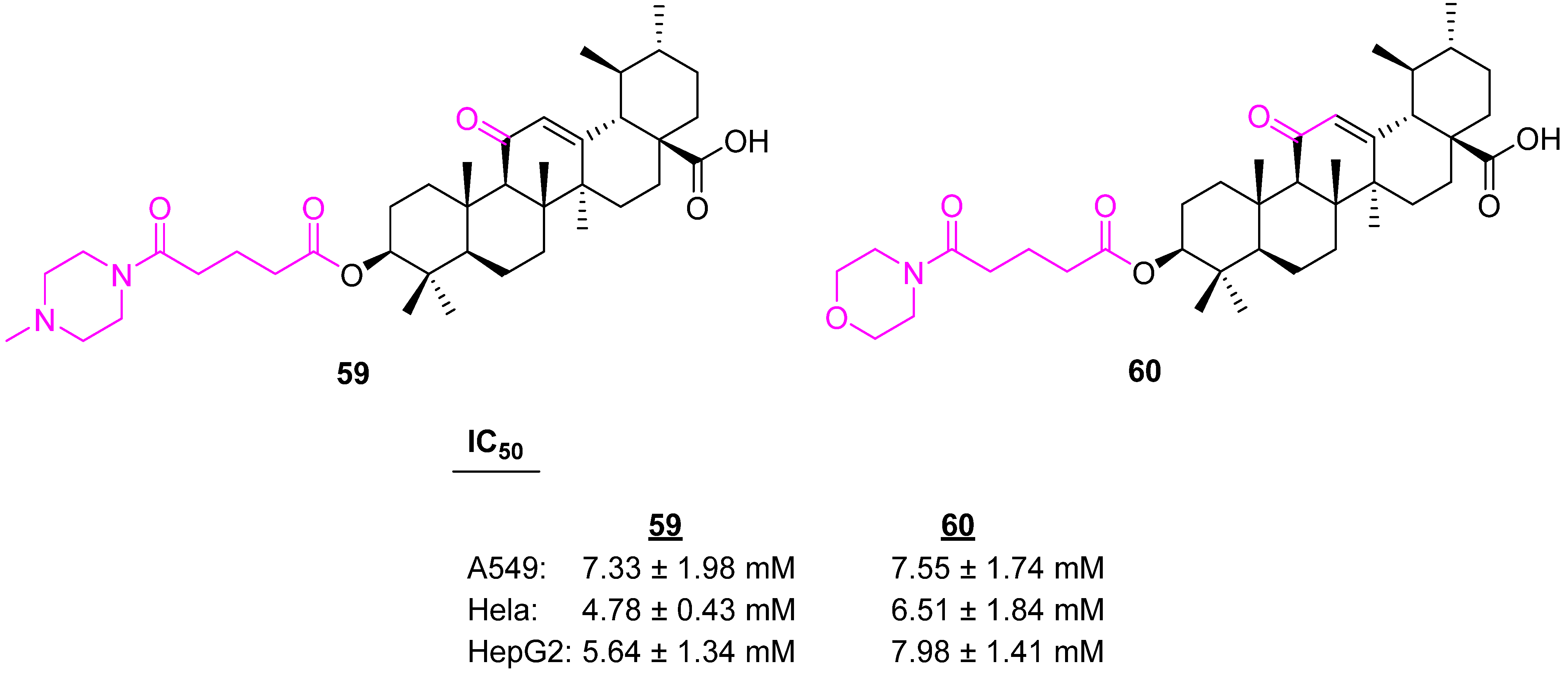
| Compound | Pathway/Mechanism | Regulation | Cell Cycle Arrest (Phase) | Animal Study | [Ref] |
|---|---|---|---|---|---|
| 3 | Activation of caspases-8, -9, and -3 | Apoptosis NA | Sub G1 & G2/M | no | [68] |
| 4 | Activation of p53 | Bcl2↓ MDM2↓ Bax↑ | Sub G1 & G1 | no | [69] |
| 6 | NF−κB | NA | NA | no | [70] |
| Compound | Pathway/Mechanism | Regulation | Cell Cycle Arrest (Phase) | Animal Study | [Ref] |
|---|---|---|---|---|---|
| 9 | Inhibition of extracellular signal-regulated kinase: ERK | NA | NA | no | [72] |
| 10 | Increased production of reactive oxygen species (ROS) | apoptosis | Sub G1 | no | [73] |
| 12 | NA | NA | G2/M | no | [76] |
| 14 | DNA cleavage | NA | NA | no | [77] |
| 16 | Inhibition of HIF-1α transcriptional activity | HIF-1α↓ mRNA↓ | G1 | no | [79] |
| 17 | Mitotic catastrophe | NA | G2/M | no | [80] |
| Compd. | Immortalized Human Fibroblasts | MCF7 | U-87 MG | A549 | HepG2 |
|---|---|---|---|---|---|
| Doxorubicin | 3.33 ± 0.67 | 4.51 ± 1.12 | 2.05 ± 0.22 | 6.17 ± 1.17 | 10.02 ± 1.67 |
| UA | 74.9 ± 4.58 | 74.9 ± 4.58 | 74.9 ± 4.58 | 74.9 ± 4.58 | 74.9 ± 4.58 |
| 21a | >100 | >100 | >100 | >100 | >100 |
| 21b | >100 | >100 | >100 | >100 | >100 |
| 21c | >100 | >100 | >100 | >100 | >100 |
| 22a | 25.85 ± 3.04 | 22.9 ± 10.02 | 29.71 ± 6.52 | >100 | 12.89 ± 2.63 |
| 22b | 10.41 ± 1.25 | 1.55 ± 0.08 | >100 | >100 | >100 |
| 23a | 82.59 ± 16.87 | 26.23 ± 8.76 | >100 | >100 | 35.58 ± 8.83 |
| 23b | >100 | >100 | >100 | >100 | >100 |
| 24a | 74.9 ± 4.58 | >100 | >100 | 40.26 ± 7.55 | >100 |
| 24b | >100 | >100 | >100 | >100 | >100 |
| 24c | >100 | >100 | >100 | >100 | >100 |
| 24d | >100 | >100 | >100 | >100 | >100 |
| 25 | 48.35 ± 11.47 | >100 | 81.67 ± 2.89 | >100 | >100 |
| Compound | A549 | MCF7 | HTC116 | THP1 | FR2 |
|---|---|---|---|---|---|
| UA | 33 ± 0.03 | 37 ± 0.07 | 42 ± 0.08 | 9.1 ± 0.07 | 31 ± 0.08 |
| 43a | 0.5 ± 0.05 | 5.5 ± 0.08 | <0.1 ± 0.09 | 0.9 ± 0.02 | 10 ± 0.04 |
| 43b | 2.9 ± 0.05 | <0.1 ± 0.05 | 15 ± 0.06 | <0.1 ± 0.03 | 69 ± 0.05 |
| 43c | <0.1 ± 0.001 | <0.1 ± 0.09 | 0.3 ± 0.001 | <0.1 ± 0.001 | >50 ± 4.1 |
| 43d | 0.15 ± 0.01 | <0.1 ± 0.001 | 9.1 ± 0.1 | <0.1 ± 0.001 | >50 ± 3.9 |
| Compound | Pathway/Mechanism | Regulation | Cell Cycle Arrest (Phase) | Animal Study | [Ref] |
|---|---|---|---|---|---|
| 26 | Activation of caspase-3 | Apoptosis | S | yes | [85] |
| 29 | Activation of the caspase-3 mitochondria pathway | Bcl2↓ Survivin↓ | NA | yes | [89] |
| 31 | Activation of caspases-8, -9, and -3 | Bcl2↓ HKII↓ Bax↑ p53↑ | S G2/M | yes | [96] |
| 32 | NA | NA | G0/G1 | no | [97] |
| 33 | Activation of caspases-8, -9, and -3 Increased production of reactive oxygen species (ROS) | Bcl2↓ | G1 | no | [98] |
| 36 | NF-κB | NA | G1 | no | [100] |
| Compound | Pathway/Mechanism | Regulation | Cell Cycle Arrest (Phase) | Animal Study | [Ref] |
|---|---|---|---|---|---|
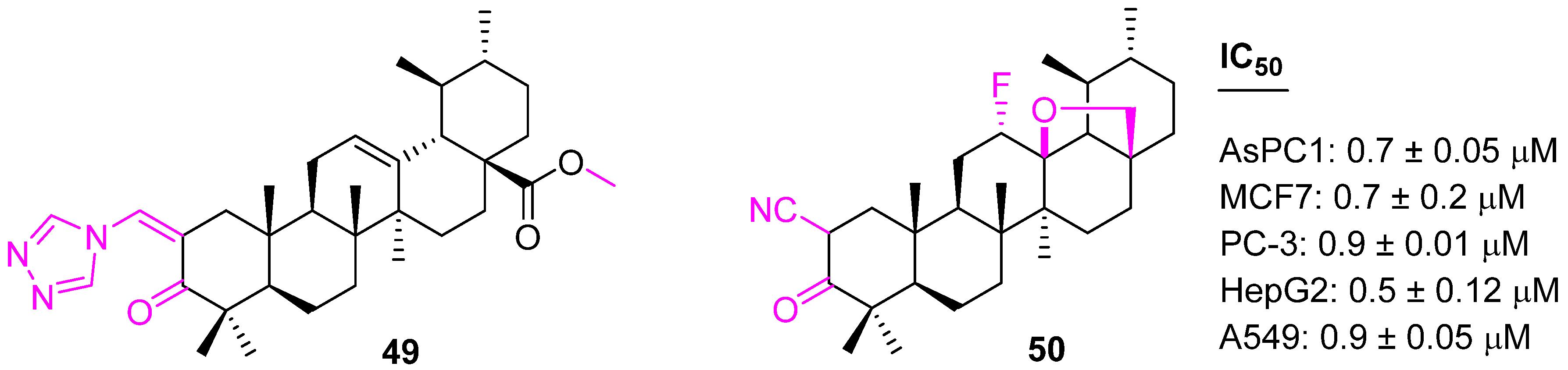
| 49 | NA | c-FLIP↓ NOXA↑ p21waf1↑ | G1 | no | [115] |
| 50 | NA | c-FLIP↓ NOXA↑ p21waf1↑ | G1 | no | [115] |
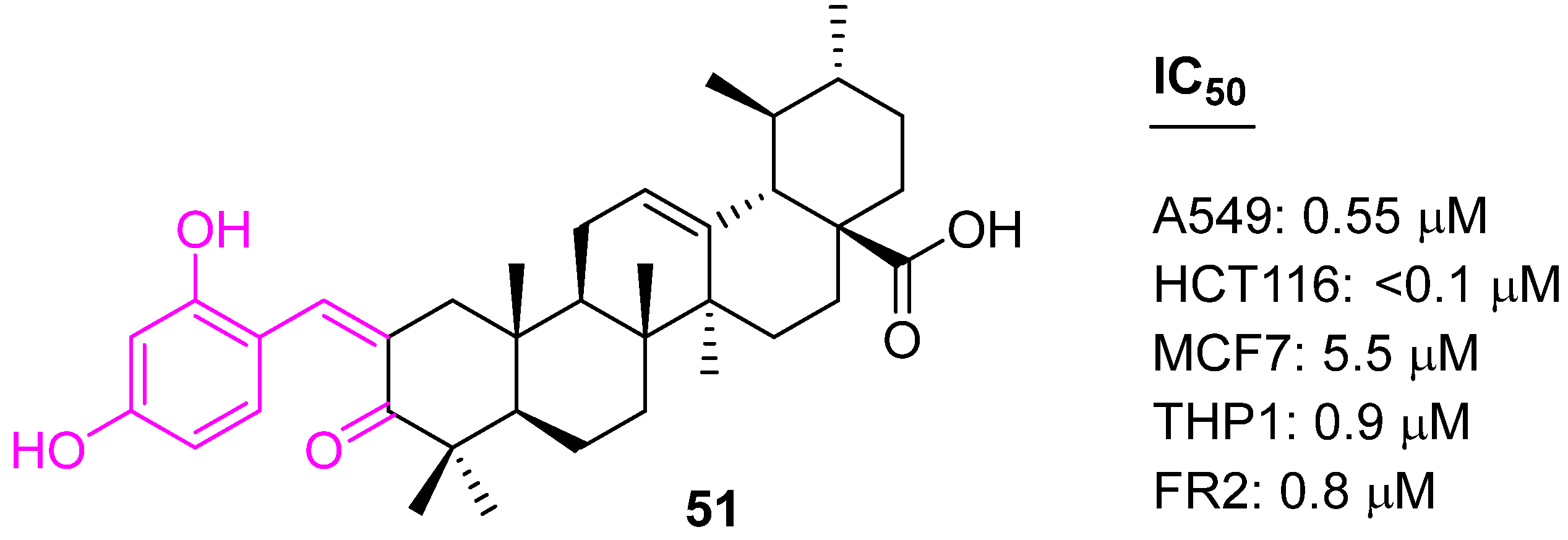
| 51 | Activation of caspases-8, -9, and -3 | G1 | no | [116] | |
| 53 | Activation of caspase cascade RAS/Raf/MEK/ERK PI3K/AKT/mTOR Increased production of reactive oxygen species (ROS) | Bcl-2↓ Bax↑ | S | no | [118] |
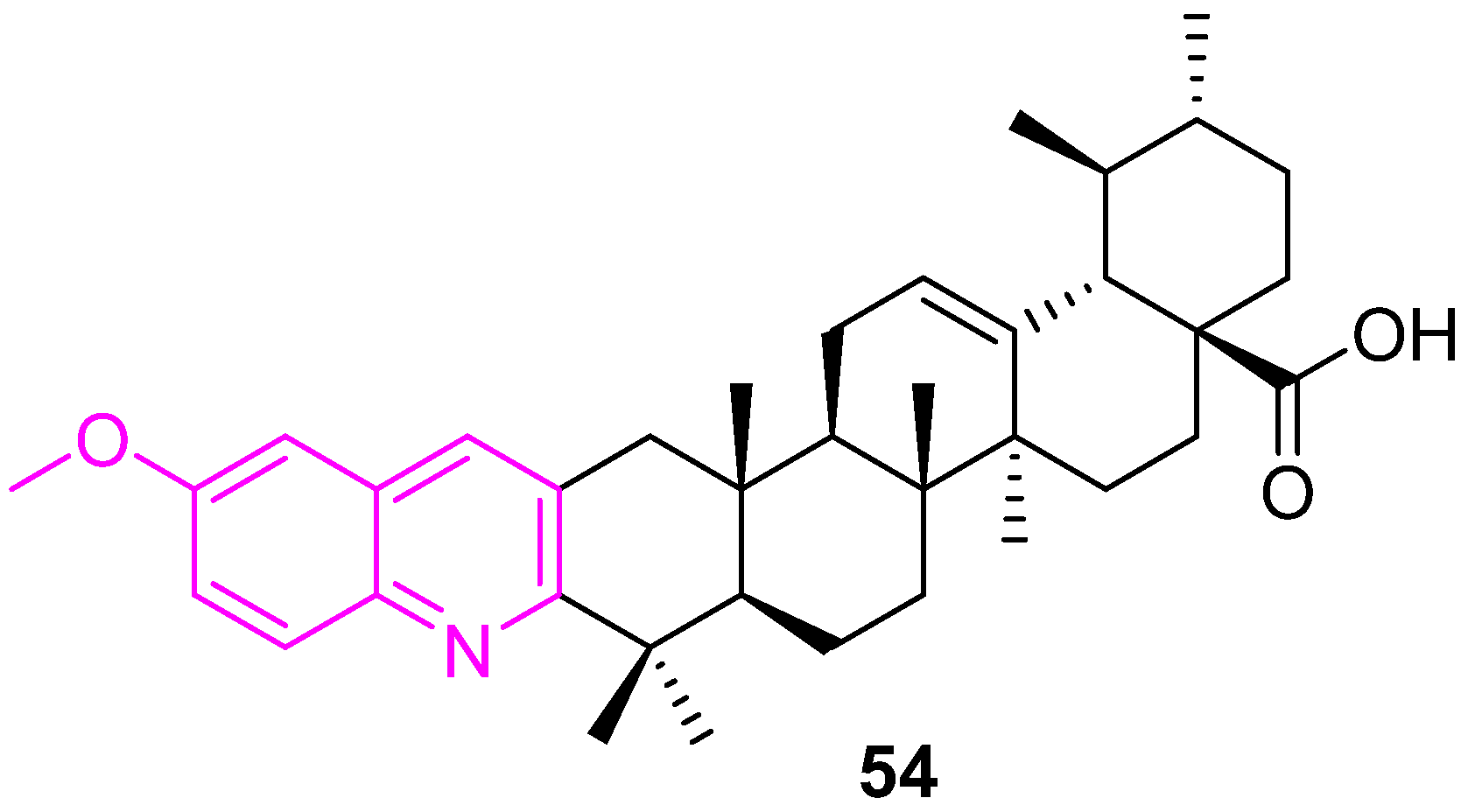
| 54 | NA | NA | G0/G1 | no | |
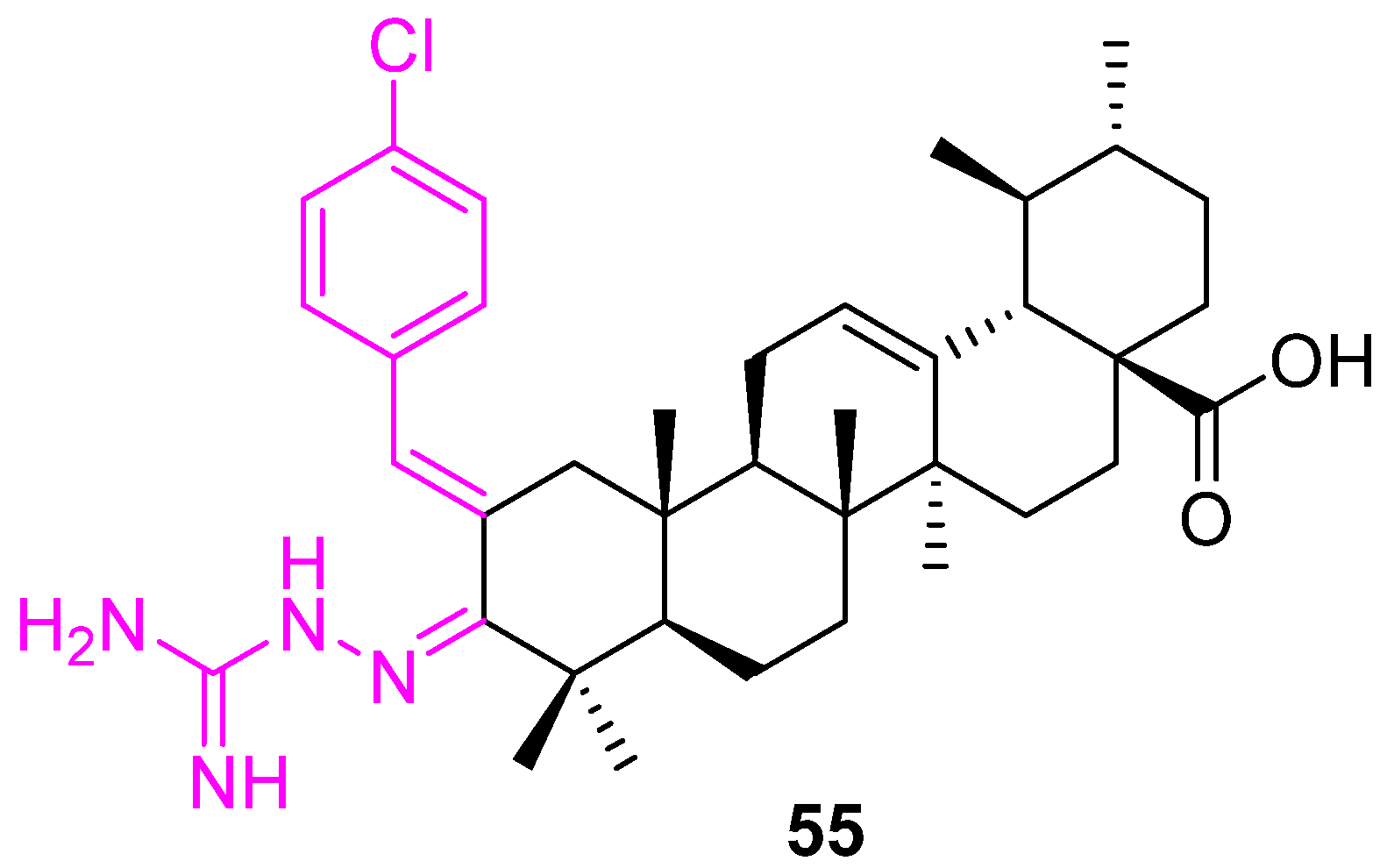
| 55 | VEGF | HIF-1α↓ | NA | no | |
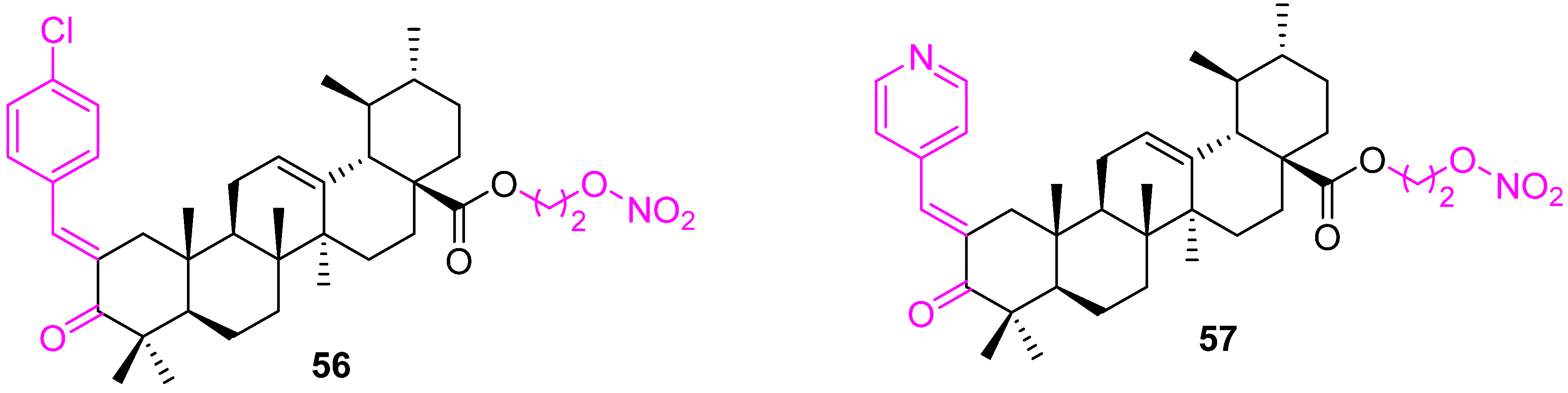
| 56 | Caspase activation Increased production of reactive oxygen species (ROS) | Bcl2↓ Bax↑ | G1 | no | [124] |
| 57 | Caspase activation Increased production of reactive oxygen species (ROS) | Bcl2↓ Bax↑ | G1 | no | [124] |
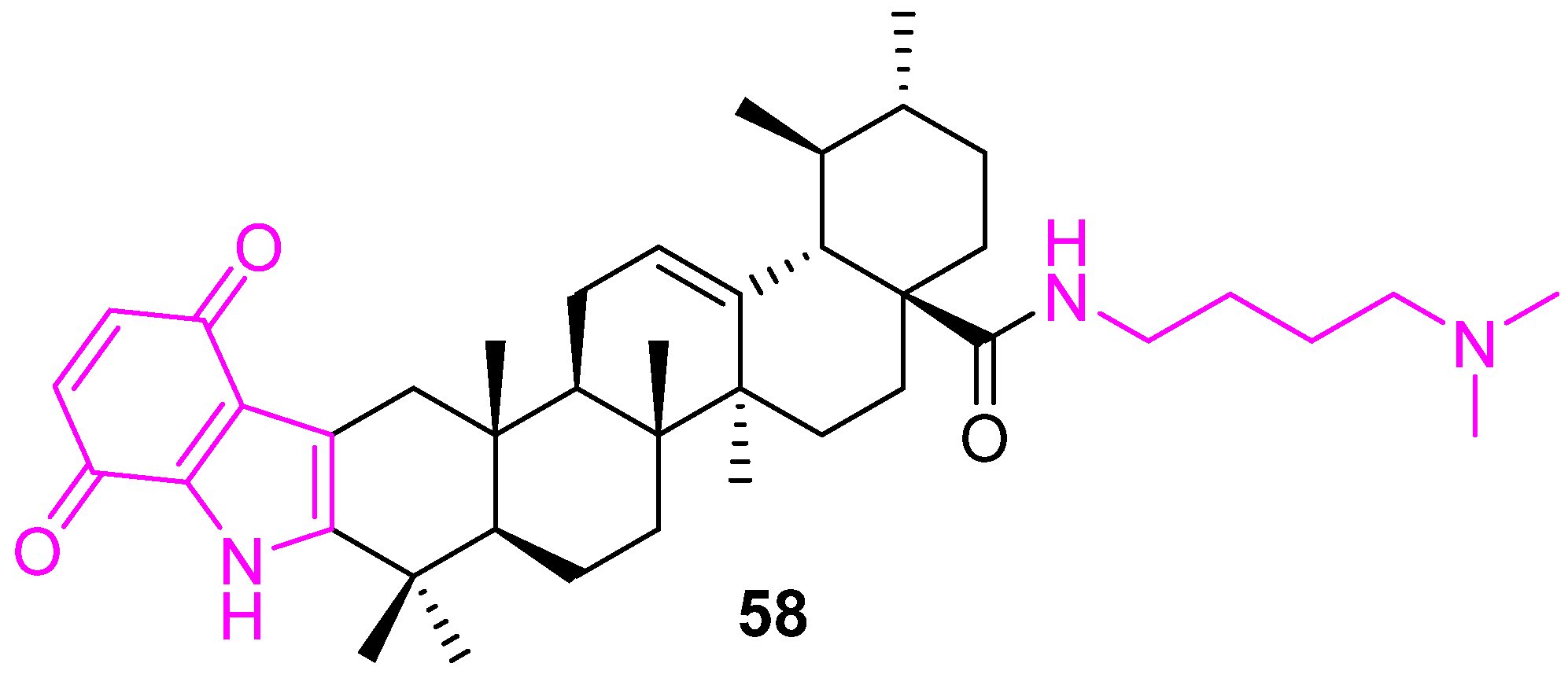
| 58 | PI3K/AKT/mTOR Increased production of reactive oxygen species (ROS) | Bcl2↓ PARP↑ Bax↑ caspase-3/9↑ | S | no | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, S.S.; Thangaraju, M.; Lokeshwar, B.L. Ursolic Acid Analogs as Potential Therapeutics for Cancer. Molecules 2022, 27, 8981. https://doi.org/10.3390/molecules27248981
Panda SS, Thangaraju M, Lokeshwar BL. Ursolic Acid Analogs as Potential Therapeutics for Cancer. Molecules. 2022; 27(24):8981. https://doi.org/10.3390/molecules27248981
Chicago/Turabian StylePanda, Siva S., Muthusamy Thangaraju, and Bal L. Lokeshwar. 2022. "Ursolic Acid Analogs as Potential Therapeutics for Cancer" Molecules 27, no. 24: 8981. https://doi.org/10.3390/molecules27248981
APA StylePanda, S. S., Thangaraju, M., & Lokeshwar, B. L. (2022). Ursolic Acid Analogs as Potential Therapeutics for Cancer. Molecules, 27(24), 8981. https://doi.org/10.3390/molecules27248981








