1. Introduction
Light is particularly attractive for the control of biological functions because it provides highly accurate spatiotemporal resolution as an external trigger that does not lead to sample contamination [
1,
2,
3,
4,
5,
6]. Azobenzene has been shown to be a reversible manipulated light-response molecule [
7]. It is possible to control biological functions by specific wavelengths of light by the incorporation of azobenzene units into nucleic acids in proper structural positions [
8]. Such molecular photoswitches undergo a light-induced reversible change in their structure that results in a change in their properties, for example, geometry, polarity, flexibility or degree of conjugation, etc. These effects may be translated into changes in the functioning of biological systems, as already presented by the photocontrol of aptamer recognition [
9], RNA digestion [
10], enzyme activity [
11] and gene expression [
12].
Among the many operational strategies for biological function, azobenzene is an excellent choice because the photo-induced reversible trans-cis isomerization of azobenzene is accompanied by geometry, with large changes in considerable influence on the structure and activity of biomolecules [
13]. Azobenzene can be introduced into DNA, using the anticipant structure of azobenzene to regulate DNA ligature or departure [
14]. Azobenzene is affected by ultraviolet light, its structure changes from trans to cis, irradiation with visible light will change from cis to trans, and this reversible isomerization will affect the structure of DNA double strands. Based on this technique, Michael et al. designed two different types of azobenzene derivatives, actinic DNA walkers under the control of different light wavelengths [
15]. A machine-like DNA enzyme that digests RNA by photomodulating the topology of the enzyme’s active site has recently been reported [
16]. Modulation of substrate binding affinity is a simpler, more direct and easier-to-perform strategy for manipulating enzyme activity than modulation of the topology of the active site.
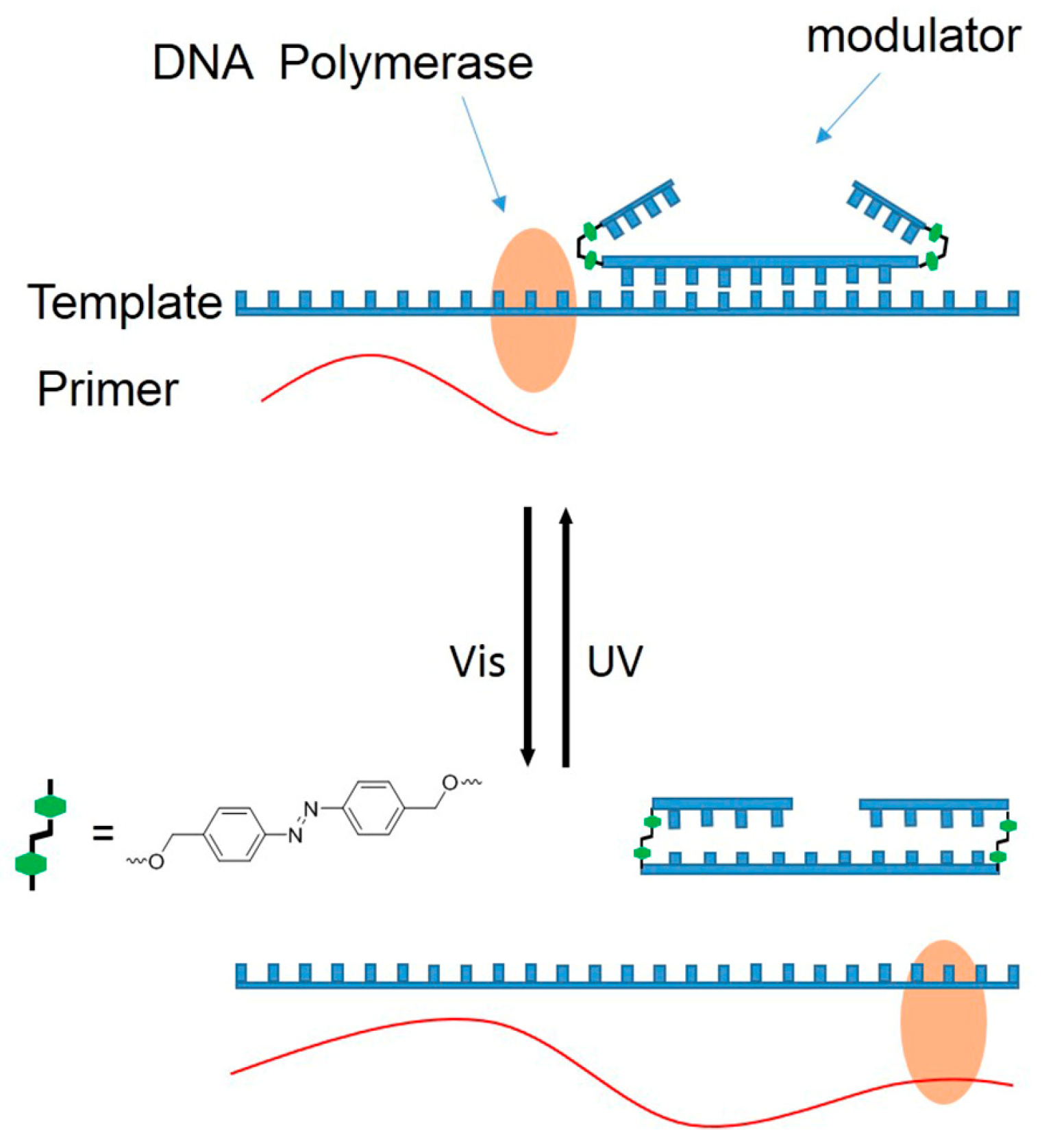
The pioneered application of azobenzene molecules as optical switches in nucleoside surrogates was in the photomodulation of DNA primer extension by DNA polymerase, and the modularity method is demonstrated by oligonucleotides bearing azobenzenes. Azobenzene is introduced into the phosphate backbone of the antisense oligodeoxynucleotides close to 5′, and this chain can form a stable duplex with the template when azobenzene is a trans form, forming an oligomer chain as a blocking unit, and the primer extension stops at the position of 5′-azobenzene. For the cis form, the stability of the oligomers is greatly reduced, exposing the template single strand, and primer extension can reach the end of the template [
17]. Azobenzene switches also quickly undergo cis- and trans- isomerization due to the influence of temperature. However, when trans-azobenzene is inserted into a base in the DNA duplex, the photoisomerization efficiency of trans- to cis-form is generally not high, and the method has so far been limited to short-modified oligonucleotides, which may have limited effects under physiological conditions [
18]. As a prominent example, tethering of azobenzene to DNA can be used for the photomodulation of RNase H assays [
19].
So far, the application of an optical switch as a nucleoside substitute in biological environments is relatively limited. This could be due to several factors. First of all, most optical switches are isomerized under ultraviolet light, which is harmful to organisms. In addition, photoisomerization efficiency is generally poor under physiological conditions. Finally, the free volume available for isomerization is limited at temperatures below oligonucleotide T
m (melting temperature), resulting in low photoisomerization efficiency under irradiation. Including any non-natural skeleton joints in long stranded RNA or DNA can be challenging because modifications can prevent primer extension of DNA polymerase. Therefore, the possibility of including azobenzene-modified nucleotides in genes by PCR is limited [
20,
21,
22]. Molecular photoswitches as nucleoside substitutes is a design that has important implications for future in vivo applications, initially in a closed state with antisense oligodeoxynucleotides (asODNs), which can silence disease genes caused by abnormal gene expression or normal gene overexpression.
We recently proposed the use of a dumbbell structure with azobenzene DNA as modulators. Given our previous research, two hairpins at two terminals of an antisense oligodeoxynucleotides (asODNs) form a dumbbell structure with azobenzene as hairpin loops (azODNs), which successfully controlled RNA digestion by reversibly photomodulating the hybridization of asODNs to target RNA molecules [
23]. Here, we hope to apply azODNs to regulate primer extensions by manipulating asODNs binding to template molecules artificially through the trans- and cis-structures of azobenzene. This method is very flexible and can synthesize fragment-length azODNs in vitro for the target gene sequence you want to study and even develop it for use on plasmids in cells. This will lay the foundation for exploring the mechanisms of action between substrates in the presence of polymerase.
In this study, according to the commonly used phosphoramide monomer synthesis method (
Figure S1), 2-Cyanoethyl-4-O-{[4-(4,4′-dimethoxytrityl)-O-methyl-diazenyl)]benzyl}-N,N′-diisopropylaminophosphoramidite is introduced into asODNs by using a DNA synthesizer. asODNs connected to azobenzene can be used to achieve primer extension on-off optical switches (
Figure 1). Herein, azODNs are designed to be 16 nt (Az1, Az2 and Az3), 18 nt (Az4, Az5 and Az6) and 20 nt (Az7, Az8 and Az9) long asODNs, with two azobenzene linked hairpin structures, and each hairpin contains inhibitory sensory chains of 4-mer (Az1, Az4 and Az5), 5-mer (Az2, Az5 and Az8) and 6-mer (Az3, Az6 and Az9) lengths at both ends of these ODNs (
Table 1). Then, photomodulation of the primer extension by Taq DNA polymerase are evaluated in gel shift assay. Thus, the azobenzene-based photoresponse ODNs can convert the light signal directly into DNA systems and this signal can control genetic information.
3. Discussion and Conclusions
We have synthesized and evaluated azo-containing hairpin nucleic acids and azo-containing azODNs, a new class of asODNs that can be photochemically controlled. We have shown that asODNs can be inactivated in an efficient manner to minimally inhibit primer extension by using UV light at 365 nm, and that reactivation by visible light can release asODNs to recover the polymerization reactions. We consider this to be an improvement over the traditional cage approach [
24]. Azobenzene has the potential to activate or close several times during treatment, whereas the traditional light cage releases its full load once activated and is not affected by light afterward. For example, the photocage group needs to be synthesized with the base of the nucleotide monomer. Once the NPOM is left by ultraviolet light, it cannot be restored to the state of modified nucleotide monomer, and NPOM is a reaction product after illumination, which may also have a side effect on organisms. In addition to the advantage that azobenzene can be switched on and off repeatedly, chemical synthesis of azobenzene is easier than the traditional photocage group NPOM. It has the added effect of not producing potentially serious by-products, such as reactive oxygen species (ROS), by removing photocage groups. Azobenzene has its own challenges. Thermal relaxation back to the more stable trans conformation limits its use as a long-term therapy (beyond two half-lives) [
25,
26]. The azobenzene moiety itself is also metabolized by thiol reduction or the metabolism of glutathione, which is present in millimolar quantities in cells and, therefore, its future effectiveness as a reversible tool needs to be fully investigated [
27]. In addition to UV illumination control, chemical groups can also be modified on azobenzene to introduce other control light sources, such as red light [
28] and green light [
29], which further expands the illumination sources and avoids the selection of light sources harmful to nucleic acids and organisms. These modifications also allow us to better control the time and space of asODNs and can be applied to time-sensitive dose control.
Other possible methods include incorporating multiple azobenzenes into the positive strand or even modifying the antisense strand. However, prolonged UV irradiation would lead to side effects, such as cell toxicity. For azobenzene-linked hairpin nucleic acids and dumbbell nucleic acids, the controllable irradiation time was required to generate the fully active molecule. In the isomerization study of azobenzene nucleic acid chains, samples were treated with 365 nm UV light, and by measuring the absorption value at 335 nm, the results showed that the change was very slow after 5 min, which was observed by oligonucleotides with azo. When determining the stability of asODNs, the Tm value of azobenzene nucleotides is significantly higher than that of natural nucleotides. The 4, 5 and 6 base protective chains linked by 4,4′-dihydroxymethylazobenzene coupling with lengths of 16 mer, 18 mer and 20 mer of azODNs were screened to adjust primer extension efficiency under UV illumination. These azODNs generally have a good photoregulation effect on elongation, especially for Az8 which has up to 9-fold photomodulation efficiency. We also achieved photoregulation in the reversibility of Az8 and Az9 by alternate irradiation with UV and visible light and concluded that the main effect of azobenzene modified DNA strand on the photocontrol behavior of primer elongation is because the enzyme inhibits elongation through the obstruction of template-strand formation during derivation.
In conclusion, we report here a new light-controlled extension technique because we can control primer extension reversibly with light. This new technology may provide the ability to reversibly control the activity of endogenous targets and will aid in the replication of disease genes. Another potential application of azODNs is their use as a biomolecular tool to detect the effects of gene replication in complex and/or related genes in real time, allowing for the smooth extension of several different genes controlled in real time by light. The photoactive modification of azobenzene on the template sequences involved in life activities can be used as a scientific tool in the future and guide the application of nucleic acid drugs. Our future work includes modifying azobenzene to improve biosecurity by using non-UV light to affect switching activity and using multiple light sources to develop logic and gate gene expression control.
4. Materials and Methods
Compound 1. 4,4′-bis(hydroxyethyl)−azobenzene: 6.0 g (26 mmol) 4−nitrobenzyl alcohol was added to 70 mL NaOH (5.7 M) aqueous solution, and 7.0 g (100 mmol) Zn power was added slowly. After addition of the materials, the solution was refluxed by stirring vigorously. The reaction mixture was filtered after one day, and the filtered solid was suspended in hot methanol many times until the azo components were dissolved completely. Air was bubbled into the azobenzene methanol solution and refluxed for 10 h. Upon concentration on vacuum evaporator, the left methanol solution was slowly cooled to give orange solid 3.2 g, with yield 50%. 1H NMR (DMSO, 400 M): δ 7.87 (d, 4H), 7.54 (d, 4H), 5.39 (t, 2H), 4.61 (d, 4H). 13C NMR (DMSO, 101 M): δ 105.84, 146.24, 127.09, 122.34, 62.43.
Compound 2. 4-hydroxymethyl-4′-O-(4,4′-dimethoxytrityl)-azobenzene 1.5 g (6.5 mmol) of 4,4′-bis(hydroxyethyl)-azobenzene was dissolved in 30 mL dry pyridine, and some pyridine was distilled to remove water residue in the solution. After cooling the solution, 2.1 g (6.5 mmol) 4,4′-dimethoxytrityl chloride was added with stirring vigorously. The reaction was monitored by TLC. The reaction mixture was concentrated and the residue was purified by silica gel chromatography with CH2Cl2/MeOH (volume ratio: 100/2) to give orange solids (2.0 g), yield 34%. 1H NMR (CDCl3, 400 M): δ 7.90 (m, 4H), 7.52 (m, 6H), 7.42 (m, 4H), 7.24 (m, 3H), 6.85 (m, 4H), 4.73 (s, 2H), 4.26 (s, 2H) 3.78 (s, 6H). 13C NMR (CDCl3, 101 M): δ 158.56, 152.05, 151.84, 144.99, 144.44, 142.58, 136.19, 130.1, 128.2, 127.93, 127.46, 127.34, 126.87, 122.98, 122.82, 113.22, 86.63, 65.31, 64.40, 55.24.
Compound 3. 2-Cyanoethyl-4-O-{[4-(4,4′-dimethoxytrityl)-O-methyl-diazenyl)]benzyl}-N,N′-diisopropylaminophosphoramidite to the solution of 4-hydroxymethyl-4′-O-(4,4′-dimethoxytrityl)-azobenzene (0.160 g, 0.29 mmol) dissolved in 2 mL dry acetonitrile, 0.120 g 2-cyanoethyl-N,N,N,N -tetraisopropylphosphora-diamidite and 21 mg (0.30 mmol) 1H-tetrazole were added. The mixture was stirred under N2, and the reaction was monitored by TLC (yield was estimated above 90%). After an hour, the solution was filtered using hydrophobic membrane filter and moved to a vial for application to DNA synthesis without purification, and completion of the reaction was identified by 31PNMR. 31PNMR (CDCl3, 162 M): δ 148.92, 148.77.
The synthesis of azobenzene modified DNA. The balance weighs a 35mg (1 μmol) CPG solid-phase support into a Universal-CPG synthetic column. Edit the oligonucleotide sequence of interest from 5′ to 3′ (from 3′ to 5′ during synthesis) depending on where the different phosphoramite monomers are loaded on the solid-phase synthesizer. All nucleic acid sequences are connected to the solid-phase CPG from the 3′ end to the 5′ end using the DNA/RNA solid-phase synthesizer by removing DMT, ETT activation, coupling, capping and oxidation steps according to conventional synthesis methods. The coupling time of ordinary DNA monomers is 120 s, and the coupling time of azobenzene derivative monomers is increased to 600 s. Until all phosphoramite monomers are successively coupled to the oligonucleotides of CPG, the solid phase of the target sequence is obtained. The average single-step yield is above 90%.
Purification of the azobenzene modified DNA. (1) Cut the solid phase. The synthesized oligonucleotides chain is connected to the CPG in a covalent bonding manner, transfer the solid phase CPG to a 2 mL centrifuge tube, add 1 mL of concentrated ammonia, hydrolyze at 50 °C for 8 h, remove the oligonucleotide from the solid phase and deprotect the group, then centrifuge with a centrifuge (2000 rpm), take the supernatant and concentrate it with a DNA concentrator until the ammonia water is completely dry (yellow solids can be seen at the bottom of the EP tube), add 200 μL of deionized water, and filtrate by membrane; (2) separation and purification. The crude DNA in this experiment was purified by Agilent reversed-phase C18 column (5 μm particle size, 9.6 mm × 250 mm) under liquid chromatography conditions: UV detection wavelength 260 nm and 335 nm dual-wavelength detection. Fluidity A is 50 mM TEAA, B is acetonitrile; gradient elution procedure: 0–10 min, 5–40% B; 40–45 min, 40–100% B; 45–50 min, 100% B; 50–55 min; 100–0% B; 55–60 min, 0% B; flow rate 1.0 mL/min; column temperature: 30 °C. Collect the target product according to the peak time of the absorbance at 260 nm and 335 nm of the UV detector.
Photoisomerization of DNA. Dissolve all sequences in 1× PBS to prepare a solution, heat in a metal bath at 95 °C for 2 min, and cool naturally to room temperature. The sample solution was processed for other operations then transferred to a quartz cuvette and irradiated with a UV lamp (365 nm, 10 W, trans to cis) for 1 min–5 min, absorbance was recorded with the Shimadzu UV/VIS spectrophotometer UV-2600 and graphed with the software Origin 2022b.
Thermodynamic stability. The nucleic acid sequences were dissolved in 1× PBS standard buffer and annealed then transferred to a Tm cuvette, and the air bubbles were drained and tightly closed for the experiment, and the melting temperature curve was determined by UV (365 nm, 10 W) or visible light for 10 min. The melting curve was obtained by measuring the UV absorption at 260 nm at gradient temperature (Shimadzu UV/Vis spectrophotometer UV-2600 and temperature controller programmed temperature rise of 1 °C/min), recording the device and differentially calculating the Tm value using the software Matlab program discrete function.
Primer extension Assays. Mix the template strand with molar amounts of azobenzene-modified nucleotides in 2 μL of 10×RNA Gerpol reaction solution (10 mM NaCl; 40 mM Tris, pH 7.8; 6 mM MgCl2; 2 mM spermidine and 10 mM DTT) solution, add 1 μL dNTP 95 °C annealing for 5 min, cool naturally to room temperature, perform parallel experiments, one set of visible light illumination, one set irradiated with an ultraviolet lamp (365 nm) for 5 min, followed by incubation under dark conditions for 10 min to fully bind the template strands and azODNs. After adding Taq DNA polymerase (50 U/μL) and primers, the reactants are gently vortexed and incubated at 37 °C for 20 min, 30 min and 40 min, respectively, to obtain the extension product. After the reaction is complete, add the same volume of loading buffer (90% deionized formamide, 25 mM EDTA, 0.02% bromophenol blue) and incubate at 90 °C for 5 min. Take 10 μL of sample and inject 1× of TBE buffer into a 20% denaturing polyacrylamide gel containing 7 M urea, 150 V for 2 h. DNA templates and product of DNA primer extension isolated on the gel analyzed by a gel imaging system. Efficiency of primer extension was determined by percentage of PCR products, by dividing the intensity of the band corresponding to full-length products by total intensity of primers and products in gel.
Kinetic data determination. Mix the template strand with a molar amount of Az9 in 2 μL of 10 × Taq buffer reaction solution (10 mM NaCl; 40 mM Tris, pH 7.8; 6 mM MgCl 2; 2 mM spermidine and 10 mM DTT) solution, add 1 μL dNTP 95 °C annealed for 5 min, naturally cooled to room temperature, then incubated under dark conditions, parallel experiments are performed, one group is irradiated with an ultraviolet lamp (365 nm) for 0 min to 60 min, one group is not irradiated with UV lamp, samples are taken every 10 min, Taq DNA polymerase (50 U/μL) and primers are added, the reactants are gently vortexed and incubated at 37 °C for 1 h to obtain an extension product, respectively. After the reaction is complete, add the same volume of loading buffer (90% deionized formamide, 25 mM EDTA, 0.02% bromophenol blue) and incubate at 90 °C for 5 min. Take 10 μL of sample and inject 1× of TBE buffer into a 20% denaturing polyacrylamide gel containing 7 M urea, 150 V for 2 h. DNA templates and primer products isolated on the gel are analyzed by a gel imaging system.
Quantify the percentage of DNA generated at time t, fit discrete value s using a nonlinear curve, exponential function y = y0 + AeR0×t to obtain kinetic data for elongation products in the presence of even azODNs and Taq polymerases, for light irradiation, where y is the proportion of PCR products generated at time t and R0 is the rate constant of PCR product formation. The R0 of the black line (without UV treatment) is 0.024 and the red line (UV treatment) is 0.0056. The elongation efficiency without UV treatment is almost 4.3 times that of UV treatment.
Reversible photomodulation assays. Mix the template strand with a molar amount of Az9 in 2 μL of 10 × Taq buffer reaction solution (10 mM NaCl; 40 mM Tris, pH 7.8; 6 mM MgCl 2; 2 mM spermidine and 10 mM DTT) solution, add 1 μL dNTP 95 °C annealed for 5 min, naturally cool to room temperature, then incubate the binding nucleic acid strands under dark conditions, perform parallel experiments, one group irradiates with UV (365 nm), one group does not irradiate with ultraviolet lamps, after adding Taq polymerase (50 U/μL) and primers, gently vortex the reactants and incubate at 37 °C, samples are taken every 5 min (UV lamp groups irradiated for 2 min after 12 min and 26 min) to obtain the extension product, respectively. After the reaction is complete, add the same volume of loading buffer (90% deionized formamide, 25 mM EDTA, 0.02% bromophenol blue) and incubate at 90 °C for 5 min. Take 10 μL of sample and inject 1× of TBE buffer into a 20% denaturing polyacrylamide gel containing 7 M urea, 150 V for 2 h. DNA templates and primer products isolated on the gel are analyzed by a gel imaging system.