Density Functional Theory Study of the Point Defects on KDP (100) and (101) Surfaces
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
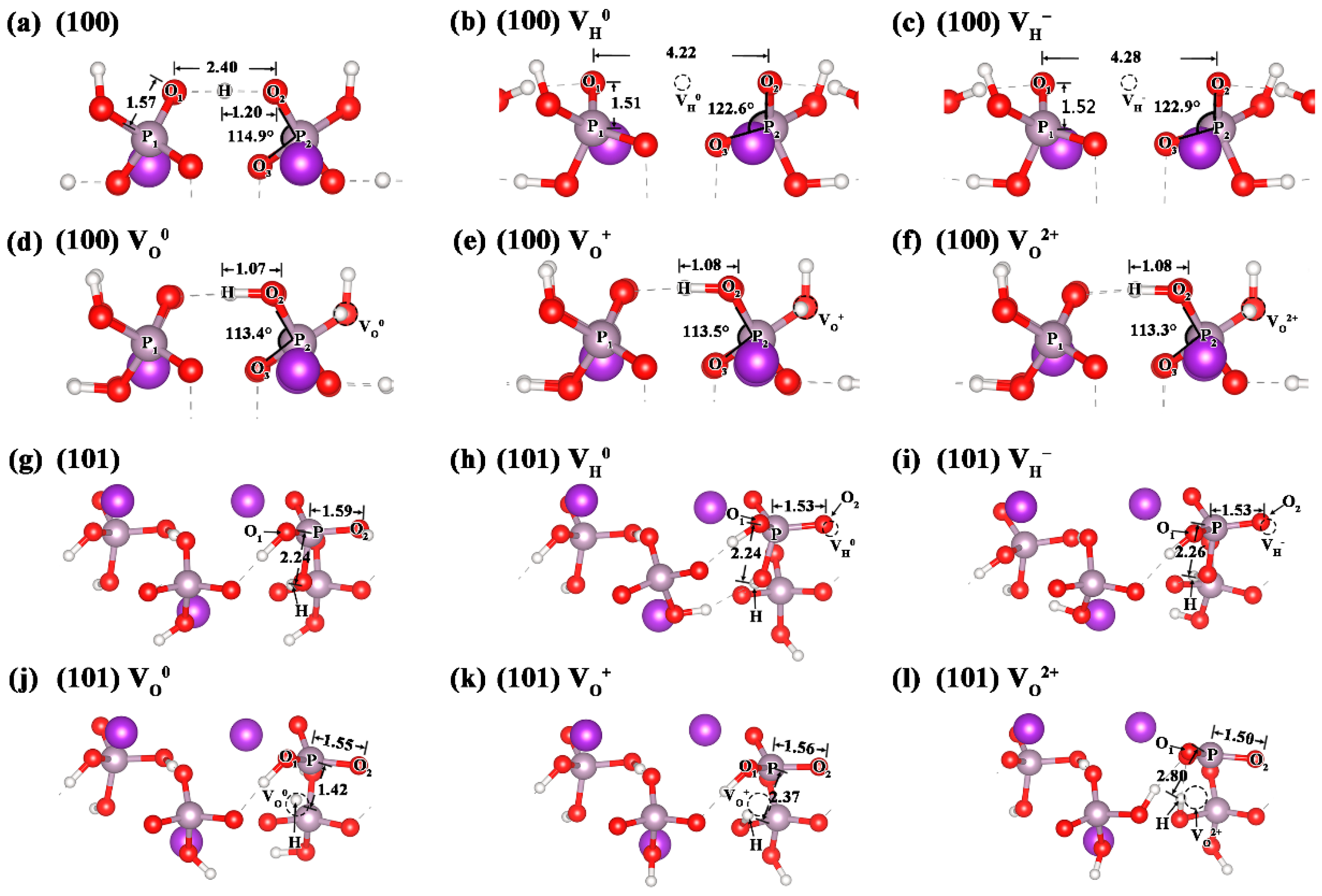
3.1. Stability and Structures of the Point Defects on the KDP Surfaces
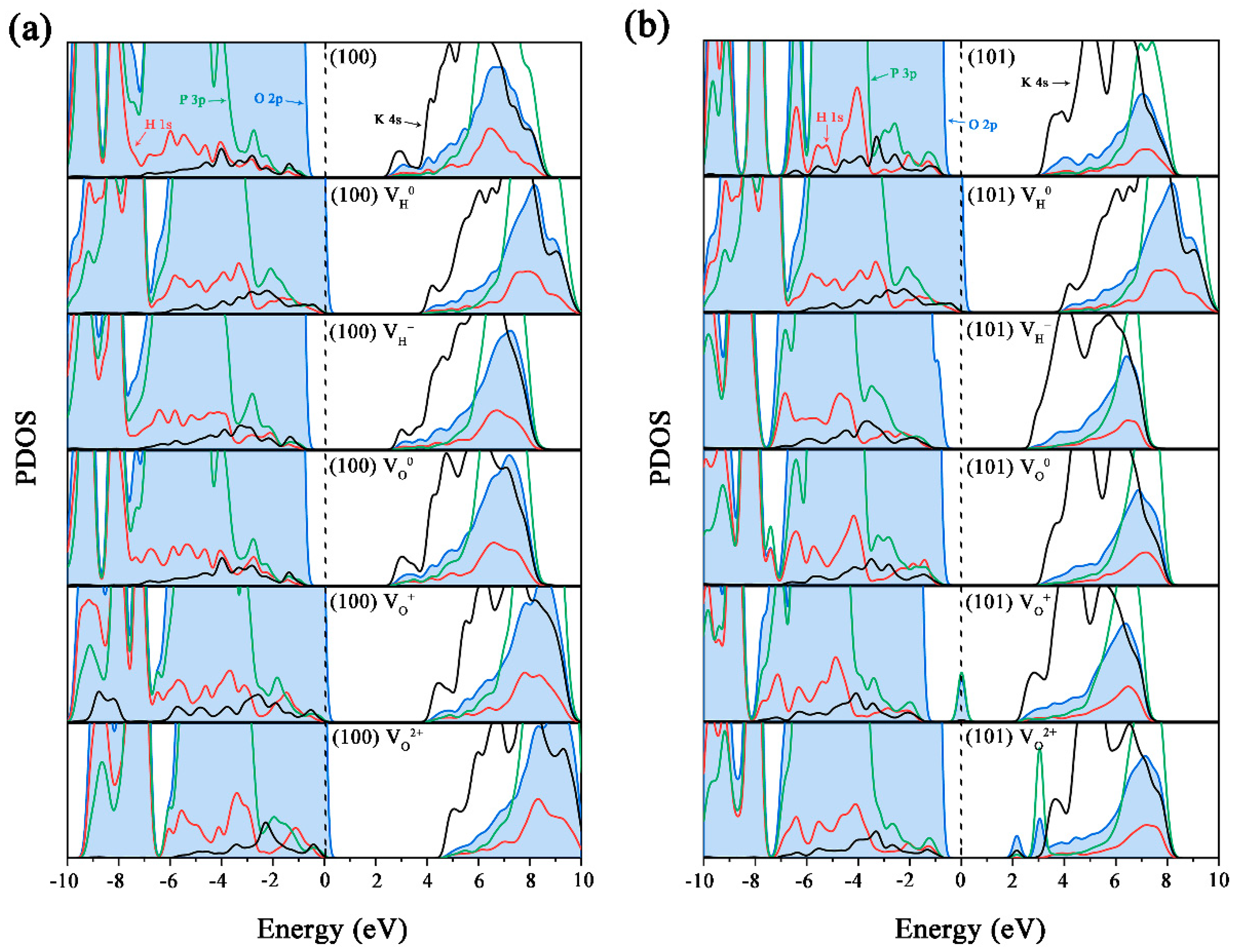
3.2. Electronic Structures of KDP Surfaces with Vacancy Defects
3.3. Effects of Acidic Environment on the Defective Surface of KDP Crystals
3.4. Effects of Alkaline Environment on the Defective Surface of KDP Crystals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashkovich, L.N. KDP-Family Single Crystals. Published under the Adam Hilger Imprint by IOP Publishing Ltd. 1991. Available online: https://www.amazon.co.uk/KDP-Family-Single-Crystals-Optoelectronics/dp/0750301058 (accessed on 17 November 2022).
- De Yoreo, J.J.; Burnham, A.K.; Whitman, P.K. Developing KH2PO4 and KD2PO4 crystals for the world’s most powerful laser. Int. Mater. Rev. 2002, 47, 113–152. [Google Scholar] [CrossRef]
- Nikogosyan, D. Nonlinear Optical Crystals: A Complete Survey; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Yin, H.W.; Li, M.W.; Zhou, C.; Song, J. KDP single crystal growth via three-dimensional motion growth method. Cryst. Res. Technol. 2016, 51, 491–497. [Google Scholar] [CrossRef]
- Chen, D.Y.; Wang, B.; Wang, H.; Zhu, X.Y.; Xu, Z.Y.; Zhao, Y.N.; Wang, S.H.; Ni, K.Z.; Zheng, L.L.; Zhang, H.; et al. Rapid growth of a long-seed KDP crystal. High Power Laser Sci. Eng. 2020, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fan, Q.C.; Gao, W.; Wang, C.; Ji, F. Novel abrasive-free jet polishing for Bulk single-crystal KDP with a low viscosity microemulsion. Sci. Rep. 2022, 12, 8346. [Google Scholar] [CrossRef]
- Cheng, Z.P.; Gao, H.; Liu, Z.Y.; Guo, D.M. Investigation of the trajectory uniformity in water dissolution ultraprecision continuous polishing of large-sized KDP crystal. Int. J. Extreme Manuf. 2020, 2, 045101. [Google Scholar] [CrossRef]
- Guillet, F.; Bertussi, B.; Surmin, A.; Duchateau, G. Effect of stress on laser damage and its relation with precursor defects in KDP/DKDP. In Proceedings of the 43rd Annual Laser Damage Symposium on Optical Materials for High Power Lasers, Natl Inst Stand & Technol, Boulder, CO, USA, 18–21 September 2011; Spie-Int Soc Optical Engineering: Boulder, CO, USA, 2011. [Google Scholar]
- Liu, C.S.; Kioussis, N.; Demos, S.G.; Radousky, H.B. Electron- or hole-assisted reactions of H defects in hydrogen-bonded KDP. Phys. Rev. Lett. 2003, 91, 015505. [Google Scholar] [CrossRef]
- Liu, C.S.; Hou, C.J.; Kioussis, N.; Demos, S.G.; Radousky, H.B. Electronic structure calculations of an oxygen vacancy in KH2PO4. Phys. Rev. B 2005, 72, 134110. [Google Scholar] [CrossRef]
- Liu, C.S.; Zhang, Q.; Kioussis, N.; Demos, S.G.; Radousky, H.B. Electronic structure calculations of intrinsic and extrinsic hydrogen point defects in KH2PO4. Phys. Rev. B 2003, 68, 224107. [Google Scholar] [CrossRef]
- Wang, K.P.; Fang, C.S.; Zhang, J.X.; Liu, C.S.; Boughton, R.I.; Wang, S.L.; Zhao, X. First-principles study of interstitial oxygen in potassium dihydrogen phosphate crystals. Phys. Rev. B 2005, 72, 184105. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Z.; Xiao, J.M.; Shao, H.Z.; Zhao, L.; Xu, M.; Zhuang, J. Atomic scale study of stress-induced misaligned subsurface layers in KDP crystals. Sci. Rep. 2019, 9, 10399. [Google Scholar] [CrossRef]
- Sui, T.T.; Lian, Y.F.; Xu, M.X.; Zhang, L.S.; Li, Y.L.; Zhao, X.; Xu, X.G.; Sun, X. Comparison of hydrogen vacancies in KDP and ADP crystals: A combination of density functional theory calculations and experiment. Phys. Chem. Chem. Phys. 2019, 21, 6186–6197. [Google Scholar] [CrossRef] [PubMed]
- Sui, T.T.; Wei, L.N.; Cao, X.Z.; Xu, M.X.; Zhang, L.S.; Zhao, X.; Chen, Z.X.; Li, Y.L.; Xu, X.G.; Sun, X. Comparison of oxygen vacancy and interstitial oxygen in KDP and ADP crystals from density functional theory calculations. Comput. Mater. Sci. 2020, 182, 109783. [Google Scholar] [CrossRef]
- Ding, W.Y.; Cheng, J.; Zhao, L.J.; Wang, Z.H.; Yang, H.; Liu, Z.C.; Xu, Q.; Wang, J.; Geng, F.; Chen, M.J. Determination of intrinsic defects of functional KDP crystals with flawed surfaces and their effect on the optical properties. Nanoscale 2022, 14, 10041–10050. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Soustov, L.V. Absorption in and laser damage to KDP and DKDP crystals. Sov. J. Quantum Electron. 1989, 19, 39–45. [Google Scholar] [CrossRef]
- Ogorodnikov, I.N.; Pustovarov, V.A.; Shul’gin, B.V.; Kuanyshev, V.T.; Satybaldieva, M.K. Low-temperature time-resolved vacuum ultraviolet luminescent spectroscopy of KH2PO4 crystals with defects. Opt. Spectrosc. 2001, 91, 224–231. [Google Scholar] [CrossRef]
- Ogorodnikov, I.N.; Kirm, M.; Pustovarov, V.A.; Cheremnykh, V.S. Low-temperature time-resolved vacuum ultraviolet spectroscopy of self-trapped excitons in KH2PO4 crystals. Opt. Spectrosc. 2003, 95, 385–389. [Google Scholar] [CrossRef]
- Koketai, T.; Tagayeva, B.; Tussupbekova, A.; Mussenova, E. A mechanism of formation of radicals in crystal KDP (KH2PO4). In Proceedings of the 17th International Conference on Luminescence and Optical Spectroscopy of Condensed Matter (ICL), Univ Wroclaw, Fac Law, Adm & Econ, Wroclaw, Poland, 13–18 July 2014; Elsevier Science Bv: Wroclaw, Poland, 2014; pp. 101–105. [Google Scholar]
- Wang, Y.; Shao, J.D.; Hu, G.H.; Liu, X.F.; Zhao, Y.N.; Zhu, M.P. Effects of molecular vibration on the formation of transient defects during high-power UV laser excitation. Opt. Laser Technol. 2021, 135, 106681. [Google Scholar] [CrossRef]
- Ogorodnikov, I.N.; Yakovlev, V.Y.; Shul’gin, B.V.; Satybaldieva, M.K. Transient optical absorption of hole polarons in ADP (NH4H2PO4) and KDP (KH2PO4) crystals. Phys. Solid State 2002, 44, 880–887. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Z.Q.; Wang, F.R.; Liu, H.J.; Sun, L.X.; Zhou, X.Y.; Ye, X.; Deng, Q.H.; Jiang, X.D.; Zheng, W.G.; et al. Initial damage and damage growth of KDP crystals induced by 355 nm pulse laser. Cryst. Res. Technol. 2018, 53, 1700269. [Google Scholar] [CrossRef]
- Wei, L.N.; Li, Y.; Jiang, X.Y.; Wu, P.C.; Zhao, X.; Zhang, L.S.; Liu, B.A.; Chai, X.X.; Xu, M.X.; Sun, X.; et al. Electron paramagnetic resonance spectroscopy and first-principles calculations of Cr3+ doped KDP crystals. CrystEngComm 2022, 24, 4948–4954. [Google Scholar] [CrossRef]
- Sui, T.T.; Wei, L.N.; Lian, Y.F.; Xu, M.X.; Zhang, L.S.; Li, Y.L.; Zhao, X.; Xu, X.G.; Sun, X. Structural stress and extra optical absorption induced by the intrinsic cation defects in KDP and ADP crystals: A theoretical study. CrystEngComm 2020, 22, 1962–1969. [Google Scholar] [CrossRef]
- Xu, D.L.; Xue, D.F. Chemical bond analysis of the crystal growth of KDP and ADP. J. Cryst. Growth 2006, 286, 108–113. [Google Scholar] [CrossRef]
- de Vries, S.A.; Goedtkindt, P.; Bennett, S.L.; Huisman, W.J.; Zwanenburg, M.J.; Smilgies, D.M.; De Yoreo, J.J.; van Enckevort, W.J.P.; Bennema, P.; Vlieg, E. Surface atomic structure of KDP crystals in aqueous solution: An explanation of the growth shape. Phys. Rev. Lett. 1998, 80, 2229–2232. [Google Scholar] [CrossRef]
- de Vries, S.A.; Goedtkindt, P.; Huisman, W.J.; Zwanenburg, M.J.; Feidenhans’l, R.; Bennett, S.L.; Smilgies, D.M.; Stierle, A.; De Yoreo, J.J.; van Enckevort, W.J.P.; et al. X-ray diffraction studies of potassium dihydrogen phosphate (KDP) crystal surfaces. J. Cryst. Growth 1999, 205, 202–214. [Google Scholar] [CrossRef]
- Liu, G.; Wang, S.; Gu, Q.; Ding, J.; Sun, Y.; Liu, W.; Zhu, S.; Liu, L. Numerical simulation of temperature field and velocity field of KDP crystal grown by traditional method. J. Synth. Cryst. 2013, 42, 1261–1266. [Google Scholar]
- Zhu, S.J.; Wang, S.L.; Ding, J.X.; Liu, G.X.; Wang, D.L.; Liu, L.; Li, W.D.; Huang, P.P.; Gu, Q.T.; Xu, X.G. Rapid growth and optical studies of KDP crystals with organic additives. J. Cryst. Growth 2014, 402, 48–52. [Google Scholar] [CrossRef]
- Liu, F.F.; Lisong, Z.; Yu, G.W.; Sun, X. Effect of pH value on the growth morphology of KH2PO4 crystal grown in defined crystallographic direction. Cryst. Res. Technol. 2015, 50, 164–170. [Google Scholar] [CrossRef]
- Li, W.D.; Li, Y.; Wang, S.L.; Cheng, W.Y. The relationship between the laser damaged threshold and step velocity in different supersaturation regions. RSC Adv. 2018, 8, 36453–36458. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, W.D.; Zhang, X.M.; Wang, D.; Wang, S.L.; Liu, G.X. Influence of supersaturation on step evolution behavior of high concentration Fe3+ poisoned KDP crystal. J. Cryst. Growth 2020, 533, 125483. [Google Scholar] [CrossRef]
- Zhong, D.-G.; Teng, B.; Zhang, G.-H.; Li, X.-B.; Lu, X.-D. Influence of pH on solubility and solution stability of KDP crystal. J Synth. Cryst. 2006, 35, 1209–1213. [Google Scholar]
- Badrouj, M.; Malekfar, R. The pH effects on the growth rate of KDP (KH2PO4) crystal by investigating Raman active lattice modes. J. Raman Spectrosc. 2007, 38, 1089–1096. [Google Scholar] [CrossRef]
- Min, C.; Mingwei, L.I.; Dong, F.U.; Hang, S.H.I.; Jiangtao, Y.U. KDP crystal growth in differing pH value. J. Chongqing Univ. Nat. Sci. Ed. 2008, 31, 809–814. [Google Scholar]
- Sun, X.; Xu, X.; Wang, Z.; Chen, H.; Li, Y.; Shao, Z.; Gao, Z.; Fang, C. Growth Condition on the Scatter Particles in KDP Crystal. Plezoelectrics Acoustooptics 2002, 24, 303–305. [Google Scholar]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Kohan, A.F.; Ceder, G.; Morgan, D.; Van de Walle, C.G. First-principles study of native point defects in ZnO. Phys. Rev. B 2000, 61, 15019–15027. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Behera, R.K.; Finnis, M.W.; Li, X.; Dickey, E.C.; Phillpot, S.R.; Sinnott, S.B. Prediction of high-temperature point defect formation in TiO2 from combined ab initio and thermodynamic calculations. Acta Mater. 2007, 55, 4325–4337. [Google Scholar] [CrossRef]
- Yamada, N.; Tanida, Y.; Murata, H.; Kondo, T.; Yoshida, S. Wide-Range-Tunable p-Type Conductivity of Transparent CuI1-xBrxAlloy. Adv. Funct. Mater. 2020, 30, 2003096. [Google Scholar] [CrossRef]
- Woo, Y.W.; Li, Z.; Jung, Y.-K.; Park, J.-S.; Walsh, A. Inhomogeneous Defect Distribution in Mixed-Polytype Metal Halide Perovskites. ACS Energy Lett. 2022, 8, 356–360. [Google Scholar] [CrossRef]
- Ren, X.; Xu, D.L.; Xue, D.F. Crystal growth of KDP, ADP, and KADP. J. Cryst. Growth 2008, 310, 2005–2009. [Google Scholar] [CrossRef]
- Lv, L.; He, X.B.; Wang, J.S.; Ruan, Y.J.; Ouyang, S.X.; Yuan, H.; Zhang, T.R. Charge localization to optimize reactant adsorption on KCu7S4/CuO interfacial structure toward selective CO2 electroreduction. Appl. Catal. B-Environ. 2021, 298, 120531. [Google Scholar] [CrossRef]
- Wang, W.H.; Shen, Y.Q.; Wang, X.O.; Zhou, Z.X.; Fei, W.D. Adsorption of water on the KNTN (001) surface: A density functional theory study. Appl. Surf. Sci. 2014, 298, 102–108. [Google Scholar] [CrossRef]
- Alam, M.W.; Farhan, M.; Souayeh, B.; Aamir, M.; Khan, M.S. Synthesis, Crystal Structure, Density Functional Theory (DFT) Calculations and Molecular Orbital Calculations of 4-Bromoanilinium Perchlorate Single Crystal. Crystals 2021, 11, 1070. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.L.; Liu, Y.; Li, H. DFT study of single water molecule adsorption on the (100) and (101) surfaces of KH2PO4. RSC Adv. 2017, 7, 26170–26178. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Shi, Z.G.; Huang, J.T.; Wang, P.T.; Yu, W.Y.; He, C.Z.; Zhang, Z.Y. Molecular adsorption properties of CH4 with noble metals doped onto oxygen vacancy defect of anatase TiO2 (101) surface: First-principles calculations. Appl. Surf. Sci. 2020, 514, 145900. [Google Scholar] [CrossRef]
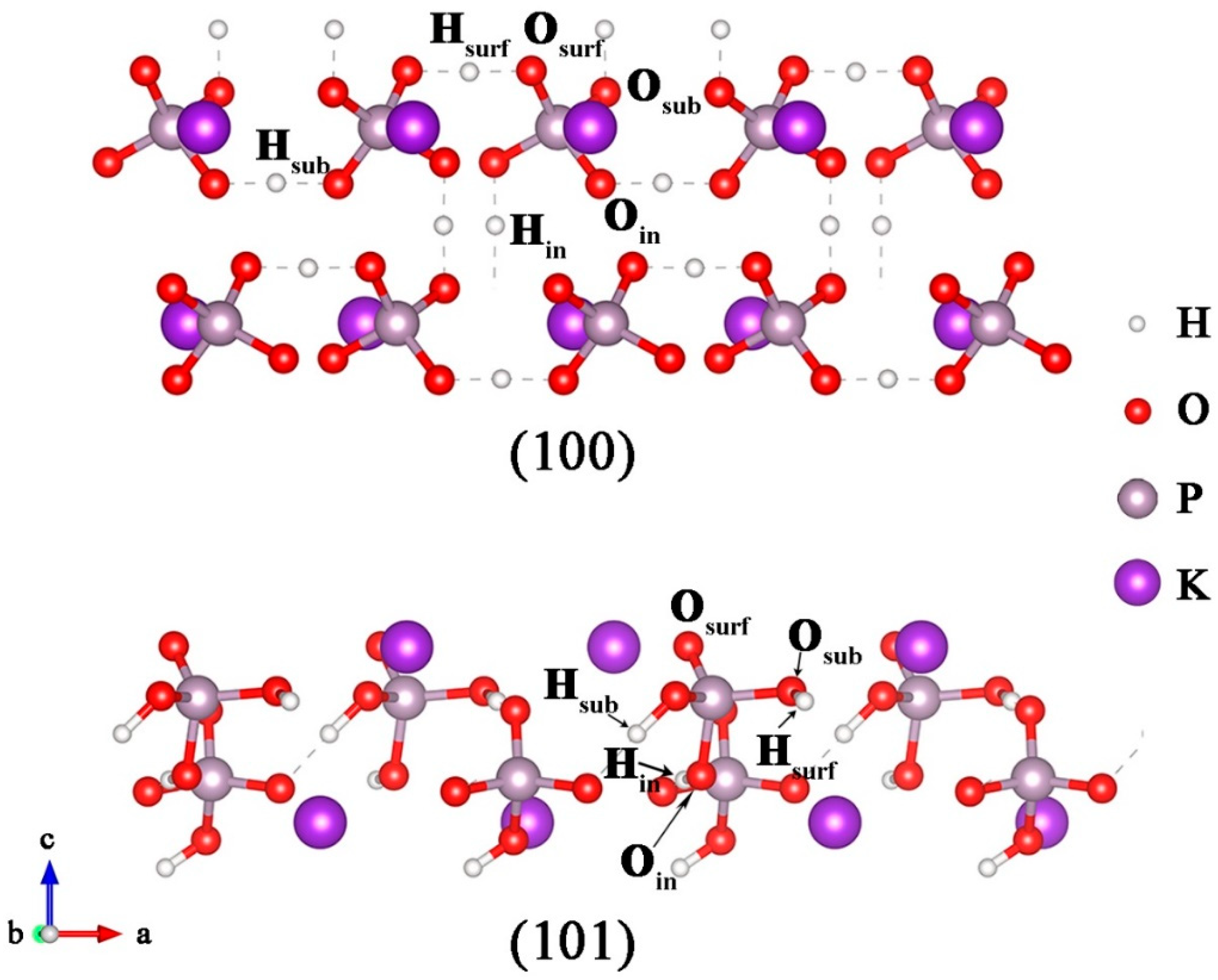




| Defect | VH | VO | ||||
|---|---|---|---|---|---|---|
| Position | Hsurf | Hsub | Hin | Osurf | Osub | Oin |
| (100) surface | 1.83 | 2.10 | 1.89 | 4.16 | 3.40 | 4.18 |
| (101) surface | 1.65 | 2.04 | 2.00 | 4.34 | 4.34 | 3.07 |
| KDP bulk | 2.84 | 4.48 | ||||
| Bond Length (Å) | Bond Angle | |||
|---|---|---|---|---|
| bond | H-O2 | O1-O2 | P1-O1 | O2-P2-O3 |
| (100) | 1.20 | 2.40 | 1.57 | 114.9° |
| (100), VH0 | - | 4.22 | 1.51 | 122.6° |
| (100), VH− | - | 4.28 | 1.52 | 122.9° |
| (100), VO0 | 1.07 | - | - | 113.4° |
| (100), VO+ | 1.08 | - | - | 113.5° |
| (100), VO2+ | 1.08 | - | - | 113.3° |
| bond | H-P | O1-O2 | P-O2 | O2-P-O3 |
| (101) | 2.24 | 2.50 | 1.59 | 104.2° |
| (101), VH0 | 2.24 | 2.48 | 1.53 | 105.5° |
| (101), VH− | 2.26 | 2.50 | 1.53 | 105.2° |
| (101), VO0 | 1.42 | 2.53 | 1.55 | 106.7° |
| (101), VO+ | 2.37 | 2.53 | 1.56 | 106.5° |
| (101), VO2+ | 2.80 | 2.58 | 1.50 | 118.9° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Li, Y.; Zhao, X. Density Functional Theory Study of the Point Defects on KDP (100) and (101) Surfaces. Molecules 2022, 27, 9014. https://doi.org/10.3390/molecules27249014
Zhao X, Li Y, Zhao X. Density Functional Theory Study of the Point Defects on KDP (100) and (101) Surfaces. Molecules. 2022; 27(24):9014. https://doi.org/10.3390/molecules27249014
Chicago/Turabian StyleZhao, Xiaoji, Yanlu Li, and Xian Zhao. 2022. "Density Functional Theory Study of the Point Defects on KDP (100) and (101) Surfaces" Molecules 27, no. 24: 9014. https://doi.org/10.3390/molecules27249014
APA StyleZhao, X., Li, Y., & Zhao, X. (2022). Density Functional Theory Study of the Point Defects on KDP (100) and (101) Surfaces. Molecules, 27(24), 9014. https://doi.org/10.3390/molecules27249014





