Copper-Catalyzed Asymmetric Sulfonylative Desymmetrization of Glycerol
Abstract
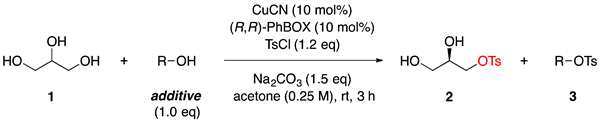
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
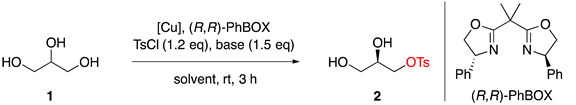
3.2. Copper-Catalyzed Asymmetric Desymmetrization of Glycerol
3.3. Large-Scale Experiment
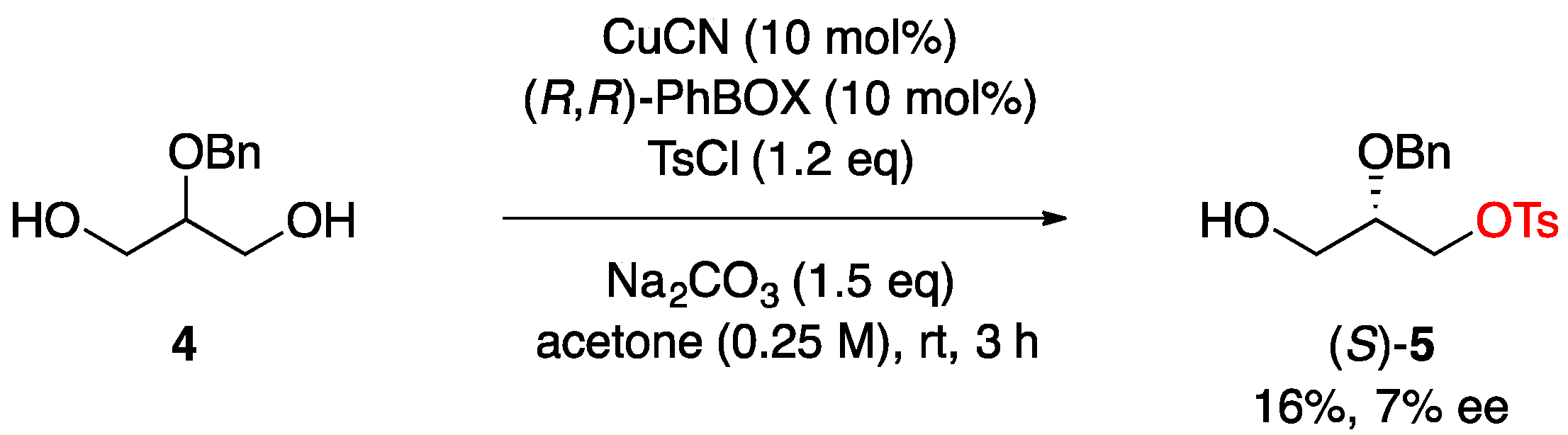
3.4. Copper-Catalyzed Asymmetric Desymmetrization of 2-O-Benzylglycerol
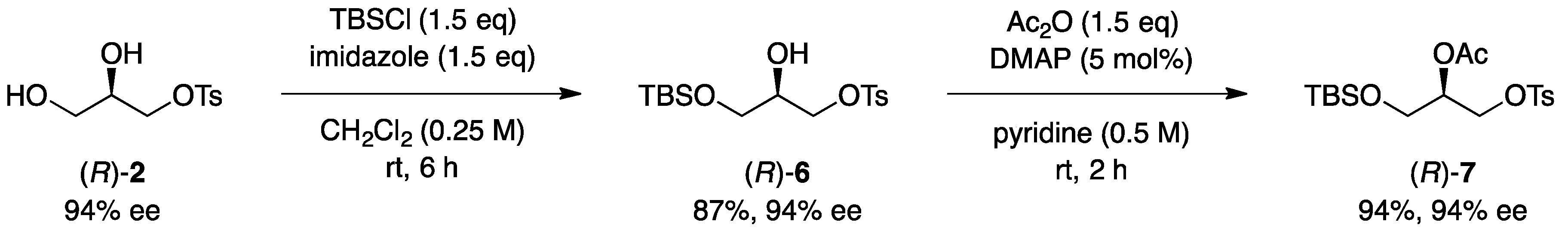
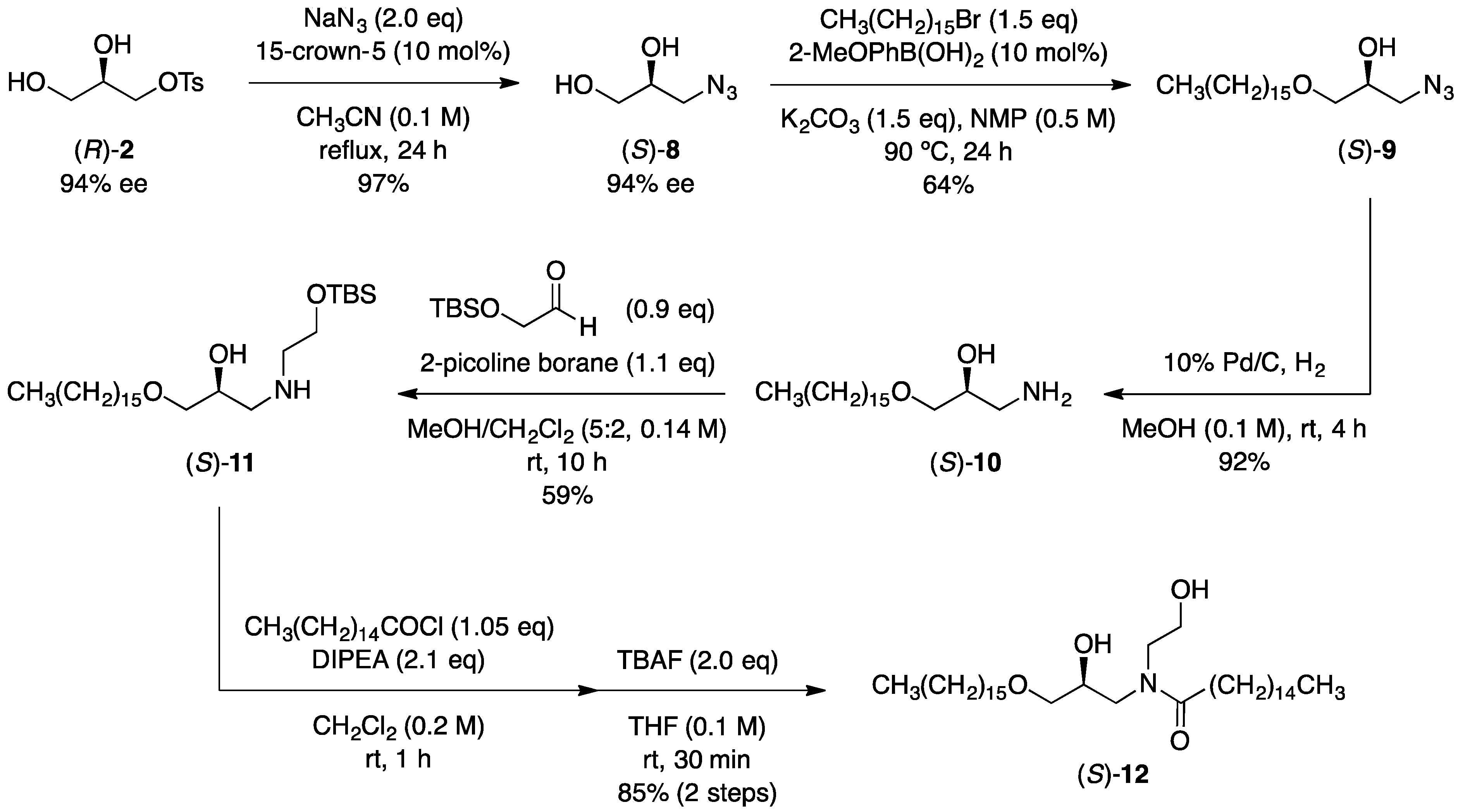
3.5. Synthesis of Optically Active Glycerol Derivatives
3.6. Synthesis of an Optically Active Synthetic Ceramide
3.7. Tosylation of Azide Diol (S)-8
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Karmakar, B.; Halder, G. Progress and future of biodiesel synthesis: Advancements in oil extraction and conversion technologies. Energy Convers. Manag. 2019, 182, 307–339. [Google Scholar] [CrossRef]
- Pasha, M.K.; Dai, L.; Liu, D.; Guo, M.; Du, W. An overview to process design, simulation and sustainability evaluation of biodiesel production. Biotechnol. Biofuels 2021, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; della Pina, C. From Glycerol to Value-Added Products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Beltramini, J.N.; Fan, Y.-X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1279. [Google Scholar] [CrossRef]
- Furuta, T.; Sakai, M.; Hayashi, H.; Asakawa, T.; Kataoka, F.; Fujii, S.; Suzuki, T.; Suzuki, Y.; Tanaka, K.; Fishkin, N.; et al. Design and synthesis of artificial phospholipid for selective cleavage of integral membrane protein. Chem. Commun. 2005, 4575–4577. [Google Scholar] [CrossRef] [PubMed]
- Andresen, T.L.; Jensen, S.S.; Madsen, R.; Jørgensen, K. Synthesis and Biological Activity of Anticancer Ether Lipids That Are Specifically Released by Phospholipase A2 in Tumor Tissue. J. Med. Chem. 2005, 48, 7305–7314. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, L.; Provencal, D.P.; Miller, T.A.; O’Bryan, C.; Langston, M.; Shen, M.; Bailey, D.; Sha, D.; Palmer, T.; et al. Process Research and Kilogram Synthesis of an Investigational, Potent MEK Inhibitor. Org. Process Res. Dev. 2012, 16, 1652–1659. [Google Scholar] [CrossRef]
- Tangherlini, G.; Torregrossa, T.; Agoglitta, O.; Köhler, J.; Melesina, J.; Sippl, W.; Holl, R. Synthesis and biological evaluation of enantiomerically pure glyceric acid derivatives as LpxC inhibitors. Bioorg. Med. Chem. 2016, 24, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pasunooti, K.K.; Peng, H.; Li, R.-J.; Shi, W.Q.; Liu, W.; Cheng, Z.; Head, S.A.; Liu, J.O. Design and Synthesis of Tetrazole- and Pyridine-Containing Itraconazole Analogs as Potent Angiogenesis Inhibitors. ACS Med. Chem. Lett. 2020, 11, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Tse, B. Total Synthesis of (−)-Galbonolide B and the Determination of Its Absolute Stereochemistry. J. Am. Chem. Soc. 1996, 118, 7094–7100. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Shiina, I.; Iwadare, H.; Saitoh, M.; Nishimura, T.; Ohkawa, N.; Sakoh, H.; Nishimura, K.; Tani, Y.; Hasegawa, M.; et al. Asymmetric Total Synthesis of Taxol®. Chem. Eur. J. 1999, 5, 121–161. [Google Scholar] [CrossRef]
- Byun, H.-S.; Sadlofsky, J.A.; Bittman, R. Enantioselective Synthesis of 3-Deoxy-(R)-sphingomyelin from (S)-1-(4’-Methoxyphenyl)glycerol. J. Org. Chem. 1998, 63, 2560–2563. [Google Scholar] [CrossRef]
- Reymond, S.; Cossy, J. Synthesis of migrastatin and its macrolide core. Tetrahedron 2007, 63, 5918–5929. [Google Scholar] [CrossRef]
- Yoshida, M.; Saito, K.; Kato, H.; Tsukamoto, S.; Doi, T. Total Synthesis and Biological Evaluation of Siladenoserinol A and its Analogues. Angew. Chem. Int. Ed. 2018, 57, 5147–5150. [Google Scholar] [CrossRef] [PubMed]
- Sigurjónsson, S.; Lúthersson, E.; Gudmundsson, H.G.; Haraldsdóttir, H.; Kristinsdóttir, L.; Haraldsson, G.G. Asymmetric Synthesis of Methoxylated Ether Lipids: A Glyceryl Glycidyl Ether Key Building Block Design, Preparation, and Synthetic Application. J. Org. Chem. 2022, 87, 12306–12314. [Google Scholar] [CrossRef]
- Lok, C.M.; Ward, J.P.; van Dorp, D.A. The synthesis of chiral glycerides starting from D- and L-serine. Chem. Phys. Lipids 1976, 16, 115–122. [Google Scholar]
- De Wilde, H.; De Clercq, P.; Vandewalle, M.; Röper, H. L-(S)-erythrulose a novel precursor to L-2,3-O-isopropylidene-C3 chirons. Tetrahedron Lett. 1987, 28, 4757–4758. [Google Scholar]
- Mikkilineni, A.B.; Kumar, P.; Abushanab, E. The Chemistry of L-Ascorbic and D-Isoascorbic Acids. 2. R and S Glyceraldehydes from a Common Intermediate. J. Org. Chem. 1988, 53, 6005–6009. [Google Scholar]
- Schmid, C.R.; Bryant, J.D.; Dowlatzedah, M.; Phillips, J.L.; Prather, D.E.; Schantz, R.D.; Sear, N.L.; Vianco, C.S. Synthesis of 2,3-O-Isopropylidene- D-Glyceraldehyde in High Chemical and Optical Purity: Observations on the Development of a Practical Bulk Process. J. Org. Chem. 1991, 56, 4056–4058. [Google Scholar]
- Doboszewski, B.; Herdewijn, P. Simple approach to 1-O-protected (R)- and (S)-glycerols from L- and D-arabinose for glycerol nucleic acids (GNA) monomers research. Tetrahedron Lett. 2011, 52, 3853–3855. [Google Scholar] [CrossRef]
- García-Urdiales, E.; Alfonso, I.; Gotor, V. Update 1 of: Enantioselective Enzymatic Desymmetrizations in Organic Synthesis. Chem. Rev. 2011, 111, PR110–PR180. [Google Scholar] [CrossRef] [PubMed]
- Chenault, H.K.; Chafin, L.F.; Liehr, S. Kinetic Chiral Resolutions of 1,2-Diols and Desymmetrization of Glycerol Catalyzed by Glycerol Kinase. J. Org. Chem. 1998, 63, 4039–4045. [Google Scholar] [CrossRef]
- Batovska, D.I.; Tsubota, S.; Kato, Y.; Asano, Y.; Ubukata, M. Lipase-mediated desymmetrization of glycerol with aromatic and aliphatic anhydrides. Tetrahedron Asymmetry 2004, 15, 3551–3559. [Google Scholar] [CrossRef]
- Caytan, E.; Cherghaoui, Y.; Barril, C.; Jouitteau, C.; Rabiller, C.; Remaud, G.S. Strategy for specific isotope ratio determination by quantitative NMR on symmetrical molecules: Application to glycerol. Tetrahedron Asymmetry 2006, 17, 1622–1624. [Google Scholar] [CrossRef]
- Franke, D.; Machajewski, T.; Hsu, C.-C.; Wong, C.-H. One-Pot Synthesis of L-Fructose Using Coupled Multienzyme Systems Based on Rhamnulose-1-phosphate Aldolase. J. Org. Chem. 2003, 68, 6828–6831. [Google Scholar] [CrossRef]
- Klibanov, A.M.; Alberti, B.N.; Marletta, M.A. Stereospecific Oxidation of Aliphatic Alcohols Catalyzed by Galactose Oxidase. Biochem. Biophys. Res. Commun. 1982, 108, 804–808. [Google Scholar] [CrossRef]
- Enríquez-García, Á.; Kündig, E.P. Desymmetrisation of meso-diols mediated by non-enzymatic acyl transfer catalysts. Chem. Soc. Rev. 2012, 41, 7803–7831. [Google Scholar] [CrossRef]
- Nájera, C.; Foubelo, F.; Sansano, J.M.; Yus, M. Enantioselective desymmetrization reactions in asymmetric catalysis. Tetrahedron 2022, 106–107, 132629. [Google Scholar] [CrossRef]
- Mizuta, S.; Sadamori, M.; Fujimoto, T.; Yamamoto, I. Asymmetric Desymmetrization of meso-1,2-Diols by Phosphinite Derivatives of Cinchona Alkaloids. Angew. Chem. Int. Ed. 2003, 42, 3383–3385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Rodrigo, J.; Hoveyda, A.H.; Snapper, M.L. Enantioselective silyl protection of alcohols catalysed by an amino-acid-based small molecule. Nature 2006, 443, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Mitra, A.W.; Hoveyda, A.H.; Snapper, M.L. Kinetic Resolution of 1,2-Diols through Highly Site- and Enantioselective Catalytic Silylation. Angew. Chem. Int. Ed. 2007, 46, 8471–8474. [Google Scholar] [CrossRef] [PubMed]
- Demizu, Y.; Matsumoto, K.; Onomura, O.; Matsumura, Y. Copper complex catalyzed asymmetric monosulfonylation of meso-vic-diols. Tetrahedron Lett. 2007, 48, 7605–7609. [Google Scholar] [CrossRef]
- Sun, X.; Worthy, A.D.; Tan, K.L. Scaffolding Catalysts: Highly Enantioselective Desymmetrization Reactions. Angew. Chem. Int. Ed. 2011, 50, 8167–8171. [Google Scholar] [CrossRef]
- Hamaguchi, N.; Kuriyama, M.; Onomura, O. Chiral copper-catalyzed asymmetric monoarylation of vicinal diols with diaryliodonium salts. Tetrahedron Asymmetry 2016, 27, 177–181. [Google Scholar] [CrossRef]
- Li, R.-Z.; Tang, H.; Yang, K.R.; Wan, L.-Q.; Zhang, X.; Liu, J.; Fu, Z.; Niu, D. Enantioselective Propargylation of Polyols and Desymmetrization of meso 1,2-Diols by Copper/Borinic Acid Dual Catalysis. Angew. Chem. Int. Ed. 2017, 56, 7213–7217. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Michimuko, C.; Yamaguchi, K.; Nakajima, M.; Sugiura, M. Selective Monoacylation of Diols and Asymmetric Desymmetrization of Dialkyl meso-Tartrates Using 2-Pyridyl Esters as Acylating Agents and Metal Carboxylates as Catalysts. J. Org. Chem. 2019, 84, 9313–9321. [Google Scholar] [CrossRef]
- Trost, B.M.; Mino, T. Desymmetrization of Meso 1,3- and 1,4-Diols with a Dinuclear Zinc Asymmetric Catalyst. J. Am. Chem. Soc. 2003, 125, 2410–2411. [Google Scholar] [CrossRef]
- Honjo, T.; Nakao, M.; Sano, S.; Shiro, M.; Yamaguchi, K.; Sei, Y.; Nagao, Y. Nonenzymatic Enantioselective Monoacetylation of Prochiral 2-Protectedamino-2-Alkyl-1,3-Propanediols Utilizing a Chiral Sulfonamide-Zn Complex Catalyst. Org. Lett. 2007, 9, 509–512. [Google Scholar] [CrossRef]
- Lee, J.Y.; You, Y.S.; Kang, S.H. Asymmetric Synthesis of All-Carbon Quaternary Stereocenters via Desymmetrization of 2,2-Disubstituted 1,3-Propanediols. J. Am. Chem. Soc. 2011, 133, 1772–1774. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Tan, C.K.; Chen, F.; Yeung, Y.-Y. Catalytic Asymmetric Bromoetherification and Desymmetrization of Olefinic 1,3-Diols with C2-Symmetric Sulfides. J. Am. Chem. Soc. 2014, 136, 5627–5630. [Google Scholar] [CrossRef] [PubMed]
- Zi, W.; Toste, F.D. Gold(I)-Catalyzed Enantioselective Desymmetrization of 1,3-Diols through Intramolecular Hydroalkoxylation of Allenes. Angew. Chem. Int. Ed. 2015, 54, 14447–14451. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, J. Enantioselective Medium-Ring Lactone Synthesis through an NHC-Catalyzed Intramolecular Desymmetrization of Prochiral 1,3-Diols. ACS Catal. 2017, 7, 7647–7652. [Google Scholar] [CrossRef]
- Yamamoto, K.; Tsuda, Y.; Kuriyama, M.; Demizu, Y.; Onomura, O. Copper-Catalyzed Enantioselective Synthesis of Oxazolines from Aminotriols via Asymmetric Desymmetrization. Chem. Asian. J. 2020, 15, 840–844. [Google Scholar] [CrossRef]
- Mandai, H.; Hironaka, T.; Mitsudo, K.; Suga, S. Acylative Desymmetrization of Cyclic meso-1,3-Diols by Chiral DMAP Derivatives. Chem. Lett. 2021, 50, 471–474. [Google Scholar] [CrossRef]
- Estrada, C.D.; Ang, H.T.; Vetter, K.-M.; Ponich, A.A.; Hall, D.G. Enantioselective Desymmetrization of 2-Aryl-1,3-Propanediols by Direct O-Alkylation with a Rationally Designed Chiral Hemiboronic Acid Catalyst That Mitigates Substrate Conformational Poisoning. J. Am. Chem. Soc. 2021, 143, 4162–4167. [Google Scholar] [CrossRef]
- Jung, B.; Hong, M.S.; Kang, S.H. Enantioselective Synthesis of Tertiary Alcohols by the Desymmetrizing Benzoylation of 2-Substituted Glycerols. Angew. Chem. Int. Ed. 2007, 46, 2616–2618. [Google Scholar] [CrossRef]
- Jung, B.; Kang, S.H. Chiral imine copper chloride-catalyzed enantioselective desymmetrization of 2-substituted 1,2,3-propanetriols. Proc. Natl. Acad. Sci. USA 2007, 104, 1471–1475. [Google Scholar] [CrossRef]
- You, Z.; Hoveyda, A.H.; Snapper, M.L. Catalytic Enantioselective Silylation of Acyclic and Cyclic Triols: Application to Total Syntheses of Cleroindicins D, F, and C. Angew. Chem. Int. Ed. 2009, 48, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Manville, N.; Alite, H.; Haeffner, F.; Hoveyda, A.H.; Snapper, M.L. Enantioselective silyl protection of alcohols promoted by a combination of chiral and achiral Lewis basic catalysts. Nat. Chem. 2013, 5, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Suganomata, Y.; Inoue, T.; Kuriyama, M.; Demizu, Y.; Onomura, O. Copper-Catalyzed Asymmetric Oxidative Desymmetrization of 2-Substituted 1,2,3-Triols. J. Org. Chem. 2022, 87, 6479–6491. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, J.; Asami, M.; Mukaiyama, T. An asymmetric synthesis of glycerol derivatives by the enantioselective acylation of prochiral glycerol. Chem. Lett. 1984, 13, 949–952. [Google Scholar] [CrossRef]
- Lewis, C.A.; Sculimbrene, B.R.; Xu, Y.; Miller, S.J. Desymmetrization of Glycerol Derivatives with Peptide-Based Acylation Catalysts. Org. Lett. 2005, 7, 3021–3023. [Google Scholar] [CrossRef]
- Trost, B.M.; Malhotra, S.; Mino, T.; Rajapaksa, N.S. Dinuclear Zinc-Catalyzed Asymmetric Desymmetrization of Acyclic 2-Substituted-1,3-Propanediols: A Powerful Entry into Chiral Building Blocks. Chem. Eur. J. 2008, 14, 7648–7657. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, A.; Umemura, S.; Ishihara, K. Desymmetrization of meso-Glycerol Derivatives Induced by L-Histidine-Derived Acylation Catalysts. Adv. Synth. Catal. 2011, 353, 1938–1942. [Google Scholar] [CrossRef]
- Giustra, Z.X.; Tan, K.L. The efficient desymmetrization of glycerol using scaffolding catalysis. Chem. Commun. 2013, 49, 4370–4372. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Tian, Y. Preparation of chiral glycerol sulfonate. Chinese Patent CN114394919A, 26 April 2022. [Google Scholar]
- Trost, B.M.; Older, C.M. A Convenient Synthetic Route to [CpRu(CH3CN)3]PF6. Organometallics 2002, 21, 2544–2546. [Google Scholar] [CrossRef]
- Hu, P.; Kan, J.; Su, W.; Hong, M. Pd(O2CCF3)2/Benzoquinone: A Versatile Catalyst System for the Decarboxylative Olefination of Arene Carboxylic Acids. Org. Lett. 2009, 11, 2341–2344. [Google Scholar] [CrossRef]
- Fu, Z.; Huang, S.; Su, W.; Hong, M. Pd-Catalyzed Decarboxylative Heck Coupling with Dioxygen as the Terminal Oxidant. Org. Lett. 2010, 12, 4992–4995. [Google Scholar] [CrossRef] [PubMed]
- Onomura, O.; Takemoto, Y.; Miyamoto, K.; Ito, M. Method for preparing optically active 1,2,3-triol monoesters in the presence of bisoxazoline ligands and copper compounds. World Intellectual Property Organization WO2015/072290A1, 21 May 2015. [Google Scholar]
- Yokose, U.; Ishikawa, J.; Morokuma, Y.; Naoe, A.; Inoue, Y.; Yasuda, Y.; Tsujimura, H.; Fujimura, T.; Murase, T.; Hatamochi, A. The ceramide [NP]/[NS] ratio in the stratum corneum is a potential marker for skin properties and epidermal differentiation. BMC Dermatol. 2020, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Fukasawa, J.; Iwai, H.; Sugai, I.; Yamashita, O.; Kawamata, A. Multilamellar Emulsion of Stratum Corneum Lipid –Formation Mechanism and its Skin Care Effects. J. Soc. Cosmet. Chem. Japan 1993, 27, 193–205. [Google Scholar] [CrossRef]
- Ishida, K. Development and Properties of the Optically Active Ceramides. Oleoscience 2004, 4, 105–116. [Google Scholar] [CrossRef][Green Version]
- Maki, T.; Ushijima, N.; Matsumura, Y.; Onomura, O. Catalytic monoalkylation of 1,2-diols. Tetrahedron Lett. 2009, 50, 1466–1468. [Google Scholar] [CrossRef]
- Boldwin, J.J.; Raab, A.W.; Mensler, K.; Arison, B.H.; McClure, D.E. Synthesis of (R)- and (S)-Epichlorohydrin. J. Org. Chem. 1978, 43, 4876–4878. [Google Scholar] [CrossRef]
- Tanabe, G.; Sakano, M.; Minematsu, T.; Matusda, H.; Yoshikawa, M.; Muraoka, O. Synthesis and elucidation of absolute stereochemistry of salaprinol, another thiosugar sulfonium sulfate from the ayurvedic traditional medicine Salacia prinoides. Tetrahedron 2008, 64, 10080–10086. [Google Scholar] [CrossRef]
- Kurimura, M.; Takemoto, M.; Achiwa, K. Synthesis of Optically Active Lipopeptide Analogs from Outer membrane of Escherichia coli. Chem. Pharm. Bull. 1991, 39, 2590–2596. [Google Scholar] [CrossRef]
- Tuin, A.W.; Palachanis, D.K.; Buizert, A.; Grotenbreg, G.M.; Spalburg, E.; de Neeling, A.J.; Mars-Groenendijk, R.H.; Noort, D.; van der Marel, G.A.; Overkleeft, H.S.; et al. Synthesis and Biological Evaluation of Novel Gramicidin S Analogues. Eur. J. Org. Chem. 2009, 4231–4241. [Google Scholar] [CrossRef]
- Paterson, I.; Delgado, O.; Florence, G.J.; Lyothier, I.; O’Brien, M.; Scott, J.P.; Sereinig, N. A Second-Generation Total Synthesis of (+)-Discodermolide: The Development of a Practical Route Using Solely Substrate-Based Stereocontrol. J. Org. Chem. 2005, 70, 150–160. [Google Scholar] [CrossRef]

 | |||||
| Entry | [Cu] | Base | Solvent | Yield (%) 2 | ee (%) 3 |
| 1 | Cu(OTf)2 | Na2CO3 | CH3CN | 91 | 83 |
| 2 | Cu(OTf)2 | K2CO3 | CH3CN | 62 | 65 |
| 3 4 | Cu(OTf)2 | Cs2CO3 | CH3CN | 16 | 47 |
| 4 4 | Cu(OTf)2 | pyridine | CH3CN | 41 | rac |
| 5 4 | Cu(OTf)2 | DIPEA | CH3CN | 51 | 43 |
| 6 | CuCl | Na2CO3 | CH3CN | 73 | 79 |
| 7 | CuBr | Na2CO3 | CH3CN | 89 | 84 |
| 8 | CuI | Na2CO3 | CH3CN | 80 | 87 |
| 9 | CuCN | Na2CO3 | CH3CN | 83 | 90 |
| 10 5 | CuCN | Na2CO3 | CH3CN | 82 | 90 |
| 11 5 | CuCN | Na2CO3 | acetone | 96 | 94 |
| 12 6 | CuCN | Na2CO3 | acetone | 93 | 89 |
| 13 5,7 | CuCN | Na2CO3 | acetone | 83 | 91 |
| 14 5,8 | CuCN | Na2CO3 | acetone | 88 | 93 |
| 15 5 | – | Na2CO3 | acetone | trace | – |
| 16 5,9 | CuCN | Na2CO3 | acetone | 3 | rac |
 | ||||
| Entry | Additive | Yield of 2 (%) 2 | ee of 2 (%) 3 | Yield of 3 (%) 2 |
| 1 | none | 96 | 94 | n.d. |
| 2 |  | 81 | 85 | n.d. |
| 3 |  | 93 | 94 | n.d. |
| 4 |  | 91 | 95 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, K.; Miyamoto, K.; Ueno, M.; Takemoto, Y.; Kuriyama, M.; Onomura, O. Copper-Catalyzed Asymmetric Sulfonylative Desymmetrization of Glycerol. Molecules 2022, 27, 9025. https://doi.org/10.3390/molecules27249025
Yamamoto K, Miyamoto K, Ueno M, Takemoto Y, Kuriyama M, Onomura O. Copper-Catalyzed Asymmetric Sulfonylative Desymmetrization of Glycerol. Molecules. 2022; 27(24):9025. https://doi.org/10.3390/molecules27249025
Chicago/Turabian StyleYamamoto, Kosuke, Keisuke Miyamoto, Mizuki Ueno, Yuki Takemoto, Masami Kuriyama, and Osamu Onomura. 2022. "Copper-Catalyzed Asymmetric Sulfonylative Desymmetrization of Glycerol" Molecules 27, no. 24: 9025. https://doi.org/10.3390/molecules27249025
APA StyleYamamoto, K., Miyamoto, K., Ueno, M., Takemoto, Y., Kuriyama, M., & Onomura, O. (2022). Copper-Catalyzed Asymmetric Sulfonylative Desymmetrization of Glycerol. Molecules, 27(24), 9025. https://doi.org/10.3390/molecules27249025







