Carbazole and Diketopyrrolopyrrole-Based D-A π-Conjugated Oligomers Accessed via Direct C–H Arylation for Opto-Electronic Property and Performance Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterizations
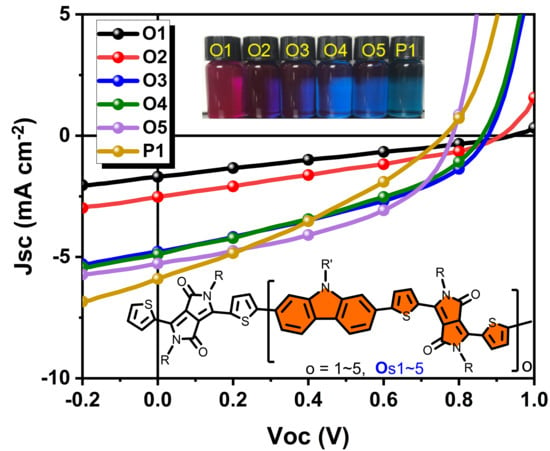
2.2. Opto-Electrochemical Property Study
2.3. BHJ OPV Performance Study
3. Materials and Methods
3.1. Measurements and Reagents
3.2. Synthesis of Os1~5
3.3. Synthesis of P1 ((Cz-DPP)n)
3.4. BHJ Device Fabrication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, Y. Molecular design of photovoltaic materials for polymer solar cells: Toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, A.; Hamid, Z.; Kosco, J.; Gasparini, N.; McCulloch, I. The bulk heterojunction in organic photovoltaic, photodetector, and photocatalytic applications. Adv. Mater. 2020, 32, 2001763. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Xiao, Z.; Du, X.; Zuo, C.; Li, Y.; Lv, M.; Yuan, Y.; Yi, C.; Hao, F.; Hua, Y.; et al. Progress of the key materials for organic solar cells. Sci. China Chem. 2020, 63, 758–765. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, X. Oligomer molecules for efficient organic photovoltaics. Acc. Chem. Res. 2016, 49, 175–183. [Google Scholar] [CrossRef]
- Hou, J.; Inganäs, O.; Friend, R.H.; Gao, F. Organic Solar Cells Based on Non-Fullerene Acceptors. Nat. Mater. 2018, 17, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, J.; Zhang, Z.-G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Squeo, B.M.; Ganzer, L.; Virgili, T.; Pasini, M. BODIPY-Based Molecules, a Platform for Photonic and Solar Cells. Molecules 2021, 26, 153. [Google Scholar] [CrossRef]
- Xie, L.; Tang, W.; Liu, Z.; Tang, W.; Yuan, Z.; Qin, Y.; Yan, L.; Zhu, X.; Zhu, W.; Wang, X. Effects of Side-Chain Engineering with the S Atom in Thieno[3,2-b]thiophene-porphyrin to Obtain Small-Molecule Donor Materials for Organic Solar Cells. Molecules 2021, 26, 6134. [Google Scholar] [CrossRef]
- Zou, X.; Wen, G.; Hu, R.; Dong, G.; Zhang, C.; Zhang, W.; Huang, H.; Dang, W. An Insight into the Excitation States of Small Molecular Semiconductor Y6. Molecules 2020, 25, 4118. [Google Scholar] [CrossRef]
- Ma, R.; Yan, C.; Fong, P.W.K.; Yu, J.; Liu, H.; Yin, J.; Huang, J.; Lu, X.; Yan, H.; Li, G. In situ and ex situ investigations on ternary strategy and co-solvent effects towards high-efficiency organic solar cells. Energy Environ. Sci. 2022, 15, 2479–2488. [Google Scholar] [CrossRef]
- Ma, R.; Yan, C.; Yu, J.; Liu, T.; Liu, H.; Li, Y.; Chen, J.; Luo, Z.; Tang, B.; Lu, X.; et al. High-Efficiency Ternary Organic Solar Cells with a Good Figure-of-Merit Enabled by Two Low-Cost Donor Polymers. ACS Energy Lett. 2022, 7, 2547–2556. [Google Scholar] [CrossRef]
- Ma, R.; Zhou, K.; Sun, Y.; Liu, T.; Kan, Y.; Xiao, Y.; Peña, T.A.D.; Li, Y.; Zou, X.; Xing, Z.; et al. Achieving high efficiency and well-kept ductility in ternary all-polymer organic photovoltaic blends thanks to two well miscible donors. Matter 2022, 5, 725–734. [Google Scholar] [CrossRef]
- Yao, S.; Yang, T.; Shen, X.; Li, T.; Huang, B.; Liu, H.; Lu, X.; Liu, T.; Zou, B. Realizing the efficiency-stability balance for all-polymer photovoltaic blends. J. Mater. Chem. C 2022, 10, 9723–9729. [Google Scholar] [CrossRef]
- Yao, S.; Huang, C.; Wang, Q.; Yang, T.; Shi, S.; Liu, Y.; Zhao, C.; Zhang, Z.; Shen, X.; Li, T.; et al. Tuning the Crystallinity and Phase Separation by Two-Step Annealing Enables Block Copolymer-Based Organic Solar Cells with 15% Efficiency. Solar RRL 2022, 6, 2200617. [Google Scholar] [CrossRef]
- Kan, B.; Li, M.; Zhang, Q.; Liu, F.; Wan, X.; Wang, Y.; Ni, W.; Long, G.; Yang, X.; Feng, H.; et al. A series of simple oligomer-like small molecules based on oligothiophenes for solution-processed solar cells with high efficiency. J. Am. Chem. Soc. 2015, 137, 3886–3893. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Liu, K.K.; Yan, J.; Wu, Z.; Liu, F.; Xiao, F.; Chang, Z.F.; Wu, H.B.; Gao, Y.; Russell, T.P. Series of multifluorine substituted oligomers for organic solar cells with efficiency over 9% and fill factor of 0.77 by combination thermal and solvent vapor annealing. J. Am. Chem. Soc. 2016, 138, 7687–7697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liang, Y.; Liu, F.; Sun, C.; Huang, X.; Xie, Z.; Huang, F.; Roncali, J.; Russell, T.P.; Cao, Y. Chain length dependence of the photovoltaic properties of monodisperse donor–acceptor oligomers as model compounds of polydisperse low band gap polymers. Adv. Funct. Mater. 2014, 24, 7538–7547. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, D.; Wu, Z.; Jia, T.; Lüer, L.; Tang, H.; Hong, L.; Zhang, J.; Zhang, K.; Brabec, C.J.; et al. Organic solar cells using oligomer acceptors for improved stability and efficiency. Nat. Energy 2022, 7, 1180–1190. [Google Scholar] [CrossRef]
- Yuan, L.; Lu, K.; Xia, B.; Zhang, J.; Wang, Z.; Wang, Z.; Deng, D.; Fang, J.; Zhu, L.; Wei, Z. Acceptor end-capped oligomeric conjugated molecules with broadened absorption and enhanced extinction coefficients for high-efficiency organic solar cells. Adv. Mater. 2016, 28, 5980–5985. [Google Scholar] [CrossRef]
- Wang, L.-H.; Chen, X.-J.; Ye, D.-N.; Liu, H.; Chen, Y.; Zhong, A.-G.; Li, C.-Z.; Liu, S.-Y. Pot- and atom-economic synthesis of oligomeric non-fullerene acceptors via C−H direct arylation. Polym. Chem. 2022, 13, 2351–2361. [Google Scholar] [CrossRef]
- Liu, H.; Tao, Y.D.; Wang, L.H.; Ye, D.N.; Huang, X.M.; Chen, N.; Li, C.Z.; Liu, S.Y. C-H Direct Arylation: A Robust Tool to Tailor the pi-Conjugation Lengths of Non-Fullerene Acceptors. ChemSusChem 2022, 15, e202200034. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.; Goto, E.; Ren, J.M.; McDearmon, B.; Kim, D.S.; Ochiai, Y. A versatile and efficient strategy to discrete conjugated oligomers. J. Am. Chem. Soc. 2017, 139, 13735–13739. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.-F.; Wang, L.-H.; Chen, Y.; Ye, D.-N.; Chen, L.; Wen, H.-R.; Liu, S.-Y. One-Pot Synthesis of 3- to 15-Mer π-Conjugated Discrete Oligomers with Widely Tunable Optical Properties. Chin. J. Chem. 2021, 39, 577–584. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, L.; Su, Z.; Zhang, X.-F.; Liu, H.; Wang, L.-H.; Huang, B.; Li, Z.; Liu, S.-Y. Hole Transfer Prompted by Viscous Oligomer Solid Additives in Non-Fullerene Bulk-Heterojunction Layers. ACS Appl. Polym. Mater. 2022, 4, 1940–1947. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Cheng, J.-Z.; Zhang, X.-F.; Liu, H.; Shen, Z.-Q.; Wen, H.-R. Single-step access to a series of D–A π-conjugated oligomers with 3–10 nm chain lengths. Polym. Chem. 2019, 10, 325–330. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, X.; Long, G. High Performance Photovoltaic Applications Using Solution-Processed Small Molecules. Acc. Chem. Res. 2013, 46, 2645–2655. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Liang, H.; He, T.; Xu, X.; Zhang, Y.; Ma, Z.; Wang, J.; Zhang, M.; Li, Q.; et al. Lowing the energy loss of organic solar cells by molecular packing engineering via multiple molecular conjugation extension. Sci. China Chem. 2022, 65, 1362–1373. [Google Scholar] [CrossRef]
- Carsten, B.; He, F.; Son, H.J.; Xu, T.; Yu, L. Stille Polycondensation for Synthesis of Functional Materials. Chem. Rev. 2011, 111, 1493–1528. [Google Scholar] [CrossRef]
- Stuart, D.R.; Fagnou, K. The Catalytic Cross-Coupling of Unactivated Arenes. Science 2007, 316, 1172–1175. [Google Scholar] [CrossRef]
- Bergman, R.G. C–H activation. Nature 2007, 446, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Osedach, T.P.; Andrew, T.L.; Bulovic, V. Effect of synthetic accessibility on the commercial viability of organic photovoltaics. Energy Environ. Sci. 2013, 6, 711–718. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Shi, M.-M.; Huang, J.-C.; Jin, Z.-N.; Hu, X.-L.; Pan, J.-Y.; Li, H.-Y.; Jen, K.-Y.A.; Chen, H. C–H activation: Making diketopyrrolopyrrole derivatives easily accessible. J. Mater. Chem. A 2013, 1, 2795–2805. [Google Scholar] [CrossRef]
- Okamoto, K.; Zhang, J.; Housekeeper, J.B.; Marder, S.R.; Luscombe, C.K. C–H Arylation Reaction: Atom Efficient and Greener Syntheses of π-Conjugated Small Molecules and Macromolecules for Organic Electronic Materials. Macromolecules 2013, 46, 8059–8078. [Google Scholar] [CrossRef]
- Pouliot, J.-R.; Grenier, F.; Blaskovits, J.T.; Beaupre, S.; Leclerc, M. Direct (Hetero)arylation Polymerization: Simplicity for Conjugated Polymer Synthesis. Chem. Rev. 2016, 116, 14225–14274. [Google Scholar] [CrossRef]
- Gobalasingham, N.S.; Thompson, B.C. Direct arylation polymerization: A guide to optimal conditions for effective conjugated polymers. Prog. Polym. Sci. 2018, 83, 135–201. [Google Scholar] [CrossRef]
- Tan, Z.-R.; Xing, Y.-Q.; Cheng, J.-Z.; Zhang, G.; Shen, Z.-Q.; Zhang, Y.-J.; Liao, G.; Chen, L.; Liu, S.-Y. EDOT-based conjugated polymers accessed via C–H direct arylation for efficient photocatalytic hydrogen production. Chem. Sci. 2022, 13, 1725–1733. [Google Scholar] [CrossRef]
- Stepek, I.A.; Itami, K. Recent Advances in C–H Activation for the Synthesis of π-Extended Materials. ACS Mater. Lett. 2020, 2, 951–974. [Google Scholar] [CrossRef]
- Mainville, M.; Leclerc, M. Direct (Hetero)arylation: A Tool for Low-Cost and Eco-Friendly Organic Photovoltaics. ACS Appl. Polym. Mater. 2021, 3, 2–13. [Google Scholar] [CrossRef]
- Chen, N.; Yang, L.-J.; Chen, Y.; Wu, Y.; Huang, X.-M.; Liu, H.; Xie, H.-Y.; Hu, L.; Li, Z.; Liu, S.-Y. PBDB-T Accessed via Direct C−H Arylation Polymerization for Organic Photovoltaic Application. ACS Appl. Polym. Mater. 2022, 4, 7282–7289. [Google Scholar] [CrossRef]
- Chen, T.-W.; Peng, K.-L.; Lin, Y.-W.; Su, Y.-J.; Ma, K.-J.; Hong, L.; Chang, C.-C.; Hou, J.; Hsu, C.-S. A chlorinated nonacyclic carbazole-based acceptor affords over 15% efficiency in organic solar cells. J. Mater. Chem. A 2020, 8, 1131. [Google Scholar] [CrossRef]
- Zou, Y.P.; Gendron, D.; Badrou-Aich, R.; Najari, A.; Tao, Y.; Leclerc, M. A High-Mobility Low-Bandgap Poly(2,7-carbazole) Derivative for Photovoltaic Applications. Macromolecules 2009, 42, 2891–2894. [Google Scholar] [CrossRef]
- Wienk, M.M.; Turbiez, M.; Gilot, J.; Janssen, R. Narrow-bandgap diketopyrrolopyrrole polymer solar cells: The effect of processing on the performance. Adv. Mater. 2008, 20, 2556–2560. [Google Scholar] [CrossRef]
- Kim, H.G.; Kang, B.; Ko, H.; Lee, J.; Shin, J.; Cho, K. Synthetic tailoring of solid-state order in diketopyrrolopyrrole-based copolymers via intramolecular noncovalent interactions. Chem. Mater. 2015, 27, 829–838. [Google Scholar] [CrossRef]




| π-CO/P1 | λmaxs (nm) | λmaxf (nm) | λonset (nm) | Egopt (eV) | HOMO (eV) | LUMO (eV) | ε (M−1·cm−1) |
|---|---|---|---|---|---|---|---|
| O1 | 598 | 575 | 708 | 1.75 | −5.47 | −3.99 | 6.3 × 104 |
| O2 | 603 | 614 | 725 | 1.71 | −5.41 | −3.70 | 1.2 × 105 |
| O3 | 645 | 644 | 749 | 1.66 | −5.39 | −3.73 | 1.9 × 105 |
| O4 | 650 | 638 | 756 | 1.64 | −5.37 | −3.74 | 2.8 × 105 |
| O5 | 652.5 | 704.5 | 761 | 1.63 | −5.35 | −3.72 | 3.8 × 105 |
| P1 | 658 | 696 | 776 | 1.60 | −5.32 | −3.72 | -- |
| BHJs | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| O1:PC70BM | 0.941 ± 0.001 | 1.67 ± 0.04 | 26.43 ± 0.07 | 0.41 ± 0.01 |
| O2:PC70BM | 0.887 ± 0.034 | 2.59 ± 0.10 | 29.00 ± 2.33 | 0.67 ± 0.05 |
| O3:PC70BM | 0.879 ± 0.003 | 4.23 ± 0.77 | 36.32 ± 2.97 | 1.36 ± 0.35 |
| O4:PC70BM | 0.861 ± 0.001 | 5.16 ± 0.24 | 36.83 ± 0.80 | 1.64 ± 0.11 |
| O5:PC70BM | 0.786 ± 0.004 | 4.85 ± 0.60 | 46.16 ± 1.13 | 1.76 ± 0.16 |
| P1:PC70BM | 0.747 ± 0.019 | 6.17 ± 0.07 | 40.93 ± 0.91 | 1.89 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Feng, L.; Zhang, K.; Liu, S.-Y. Carbazole and Diketopyrrolopyrrole-Based D-A π-Conjugated Oligomers Accessed via Direct C–H Arylation for Opto-Electronic Property and Performance Study. Molecules 2022, 27, 9031. https://doi.org/10.3390/molecules27249031
Zhang X, Feng L, Zhang K, Liu S-Y. Carbazole and Diketopyrrolopyrrole-Based D-A π-Conjugated Oligomers Accessed via Direct C–H Arylation for Opto-Electronic Property and Performance Study. Molecules. 2022; 27(24):9031. https://doi.org/10.3390/molecules27249031
Chicago/Turabian StyleZhang, Xiaofeng, Lingwei Feng, Kai Zhang, and Shi-Yong Liu. 2022. "Carbazole and Diketopyrrolopyrrole-Based D-A π-Conjugated Oligomers Accessed via Direct C–H Arylation for Opto-Electronic Property and Performance Study" Molecules 27, no. 24: 9031. https://doi.org/10.3390/molecules27249031