A Facile Synthesis of 2-Oxazolines via Dehydrative Cyclization Promoted by Triflic Acid
Abstract
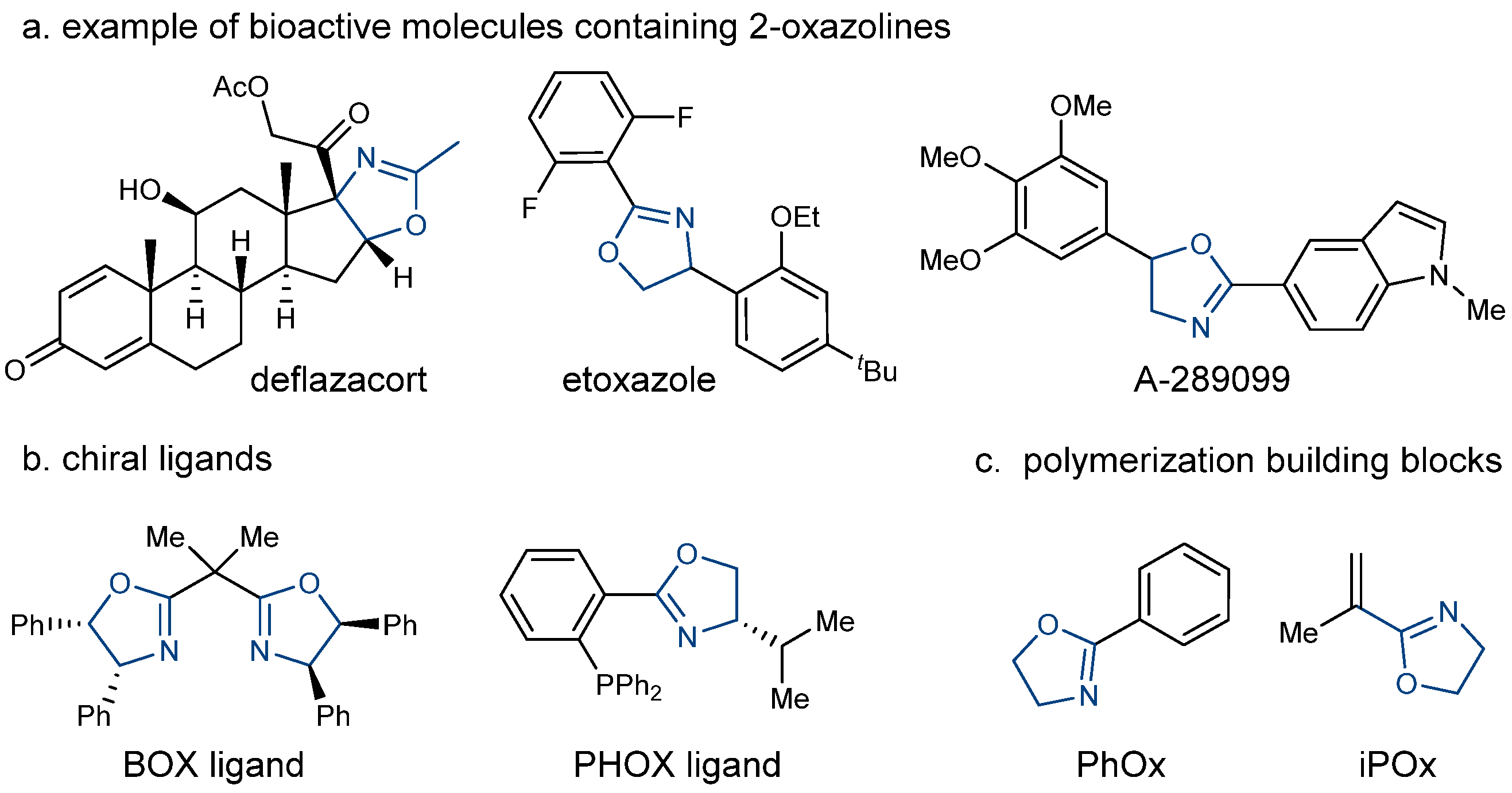
1. Introduction
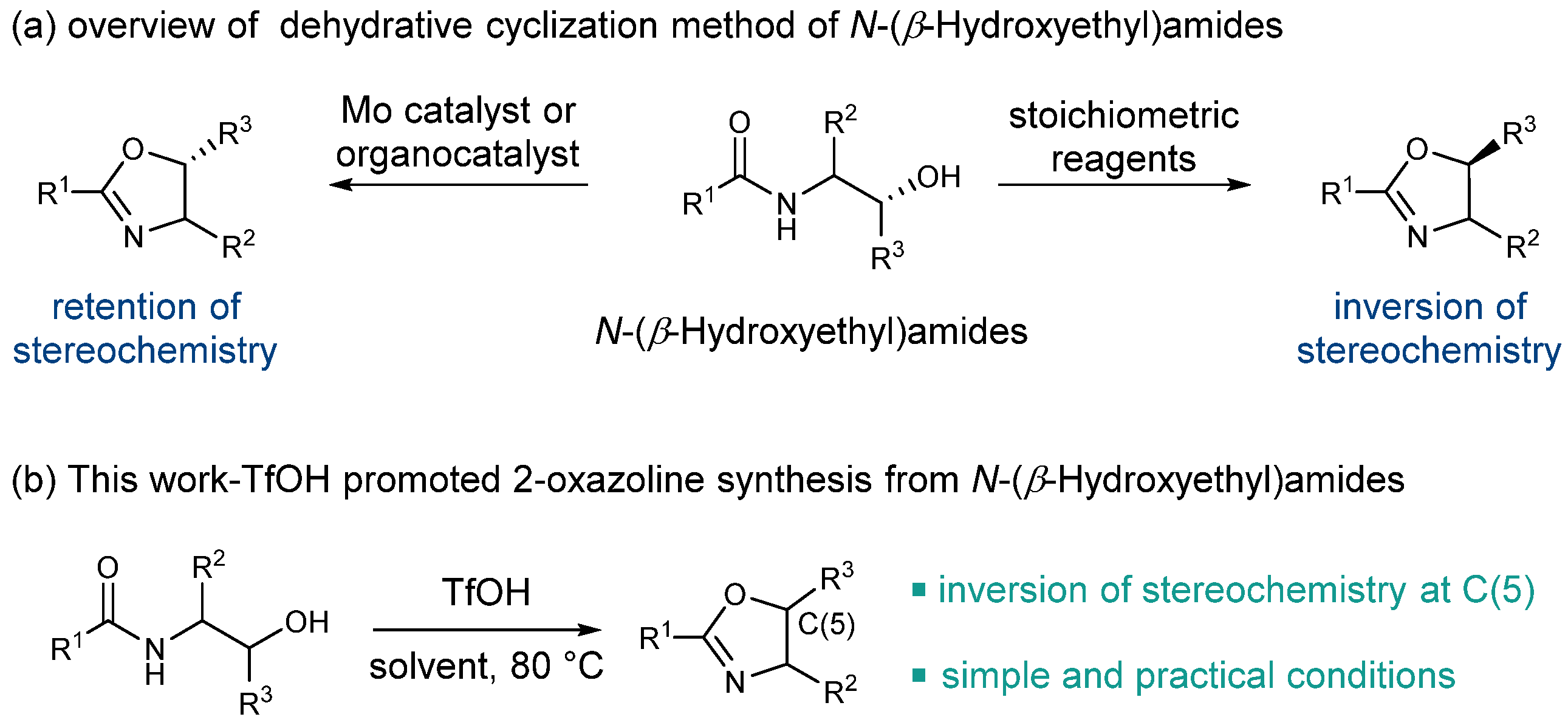
2. Results
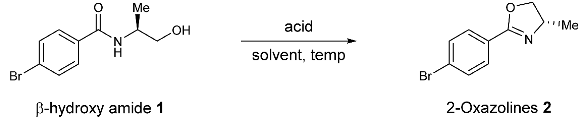
2.1. Optimization of the Reaction Conditions
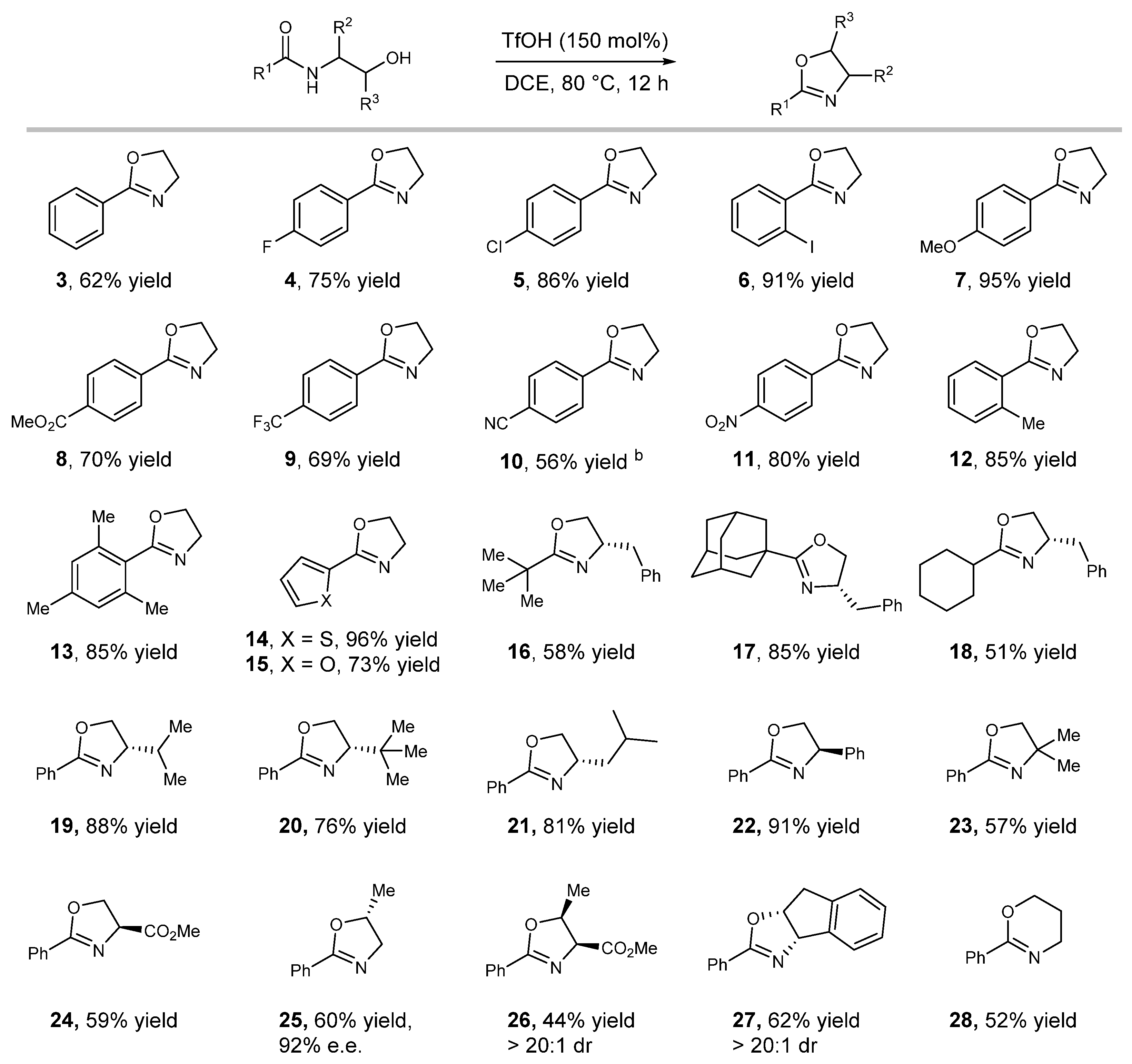
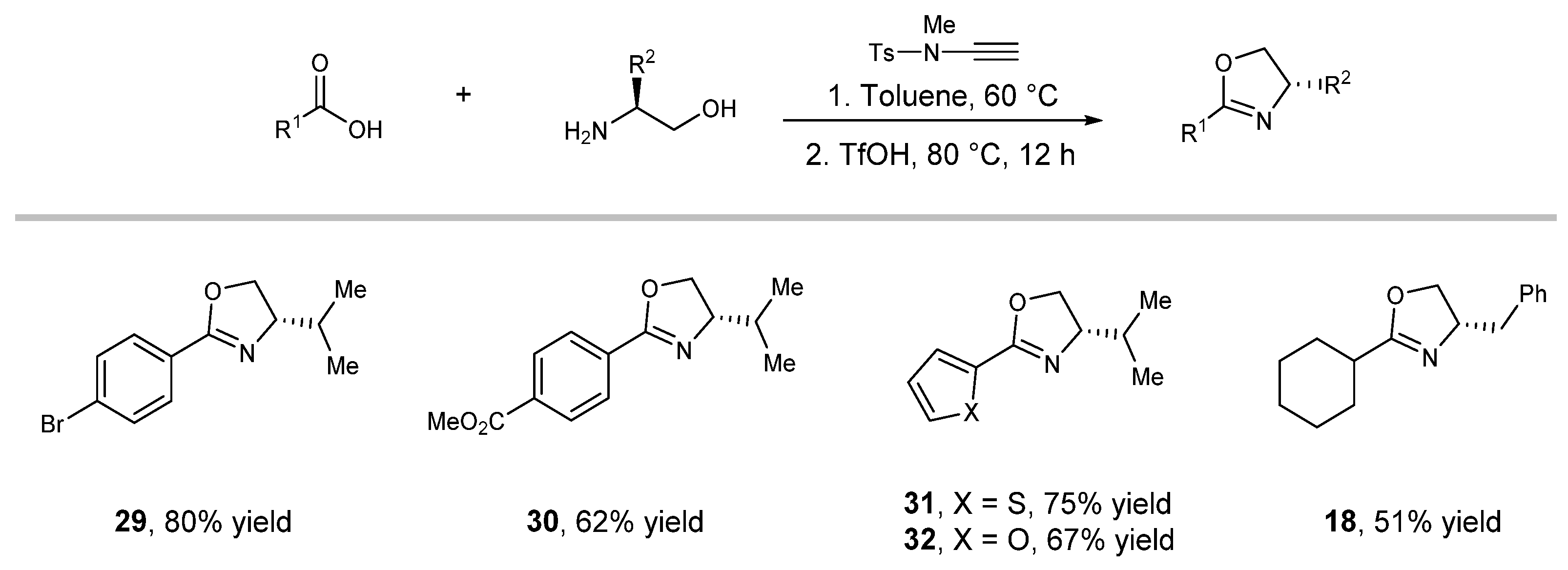
2.2. Substrate Scope Studies
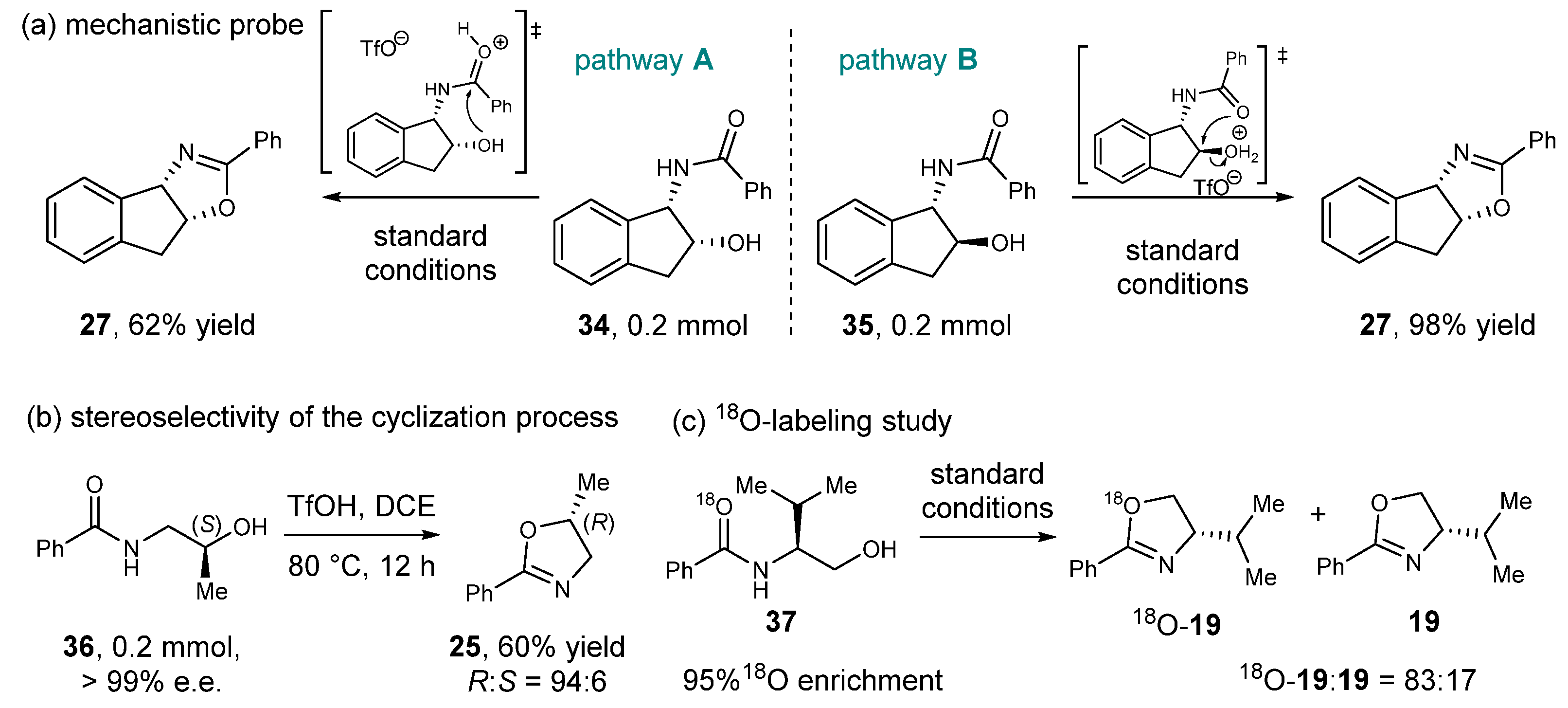
2.3. Control Experiments and Mechanistic Studies
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Inahashi, Y.; Iwatsuki, M.; Ishiyama, A.; Namatame, M.; Nishihara-Tsukashima, A.; Matsumoto, A.; Hirose, T.; Sunazuka, T.; Yamada, H.; Otoguro, K.; et al. Spoxazomicins A–C, Novel Antitrypanosomal Alkaloids Produced by an Endophytic Actinomycete, Streptosporangium oxazolinicum K07-0460T. J. Antibiot. 2011, 64, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Salomon, C.E.; Aldrich, C.C. Total Synthesis and Biological Evaluation of Transvalencin Z. J. Nat. Prod. 2012, 75, 1037–1043. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marson, C.M.; Matthews, C.J.; Atkinson, S.J.; Lamadema, N.; Thomas, N.S.B. Potent and Selective Inhibitors of Histone Deacetylase-3 Containing Chiral Oxazoline Capping Groups and a N-(2-Aminophenyl)-benzamide Binding Unit. J. Med. Chem. 2015, 58, 6803–6818. [Google Scholar] [CrossRef] [PubMed]
- Tyler, A.R.; Mosaei, H.; Morton, S.; Waddell, P.G.; Wills, C.; McFarlane, W.; Gray, J.; Goodfellow, M.; Errington, J.; Allenby, N.; et al. Structural Reassignment and Absolute Stereochemistry of Madurastatin C1 (MBJ-0034) and the Related Aziridine Siderophores: Madurastatins A1, B1, and MBJ-0035. J. Nat. Prod. 2017, 80, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, K.A.; Saunders, M.A.; Zhang, Y.; Tran, T.; Elshahawi, S.I.; Ponomareva, L.V.; Wang, X.; Zhang, J.; Copley, G.C.; Sunkara, M.; et al. Spoxazomicin D and Oxachelin C, Potent Neuroprotective Carboxamides from the Appalachian Coal Fire-Associated Isolate Streptomyces sp. RM-14-6. J. Nat. Prod. 2017, 80, 2–11. [Google Scholar] [CrossRef]
- Mohr, J.F.; Baldeweg, F.; Deicke, M.; Morales-Reyes, C.F.; Hoffmeister, D.; Wichard, T. Frankobactin Metallophores Produced by Nitrogen-Fixing Frankia Actinobacteria Function in Toxic Metal Sequestration. J. Nat. Prod. 2021, 84, 1216–1225. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Liu, Y.; Wang, Q. Highly Efficient Synthesis and Acaricidal and Insecticidal Activities of Novel Oxazolines with N-Heterocyclic Substituents. J. Agric. Food Chem. 2021, 69, 3601–3606. [Google Scholar] [CrossRef]
- Luxenhofer, R.; Han, Y.; Schulz, A.; Tong, J.; He, Z.; Kabanov, A.V.; Jordan, R. Poly(2-oxazoline)s as Polymer Therapeutics. Macromol. Rapid Commun. 2012, 33, 1613–1631. [Google Scholar] [CrossRef]
- Salgarella, A.R.; Zahoranová, A.; Srámková, P.; Majerčíková, M.; Pavlova, E.; Luxenhofer, R.; Kronek, J.; Lacík, I.; Ricotti, L. Investigation of Drug Release Modulation from Poly(2-oxazoline) Micelles through Ultrasound. Sci. Rep. 2018, 8, 9893–9906. [Google Scholar] [CrossRef]
- Sedlacek, O.; Lava, K.; Verbraeken, B.; Kasmi, S.; De Geest, B.G.; Hoogenboom, R. Unexpected Reactivity Switch in the Statistical Copolymerization of 2-Oxazolines and 2-Oxazines Enabling the One-Step Synthesis of Amphiphilic Gradient Copolymers. J. Am. Chem. Soc. 2019, 141, 9617–9622. [Google Scholar] [CrossRef]
- Wu, Y.-C.M.; Swager, T.M. Living Polymerization of 2-Ethylthio-2-oxazoline and Postpolymerization Diversification. J. Am. Chem. Soc. 2019, 141, 12498–12501. [Google Scholar] [CrossRef]
- Sahn, M.; Weber, C.; Schubert, U.S. Poly(2-oxazoline)-Containing Triblock Copolymers: Synthesis and Applications. Polym. Rev. 2019, 59, 240–279. [Google Scholar] [CrossRef]
- Park, J.-R.; Sarwat, M.; Bolle, E.C.L.; de Laat, M.A.; Van Guyse, J.F.R.; Podevyn, A.; Hoogenboom, R.; Dargaville, T.R. Drug–polymer Conjugates with Dynamic Cloud Point Temperatures Based on Poly(2-oxazoline) Copolymers. Polym. Chem. 2020, 11, 5191–5199. [Google Scholar] [CrossRef]
- Sedlacek, O.; Hoogenboom, R. Drug Delivery Systems Based on Poly(2-Oxazoline)s and Poly(2-Oxazine)s. Adv. Ther. 2020, 3, 1900168. [Google Scholar] [CrossRef]
- Zhou, M.; Qian, Y.; Xie, J.; Zhang, W.; Jiang, W.; Xiao, X.; Chen, S.; Dai, C.; Cong, Z.; Ji, Z.; et al. Poly(2-Oxazoline)-Based Functional Peptide Mimics: Eradicating MRSA Infections and Persisters while Alleviating Antimicrobial Resistance. Angew. Chem. Int. Ed. 2020, 59, 6412–6419. [Google Scholar] [CrossRef] [PubMed]
- Kline, T.; Andersen, N.H.; Harwood, E.A.; Bowman, J.; Malanda, A.; Endsley, S.; Erwin, A.L.; Doyle, M.; Fong, S.; Harris, A.L.; et al. Potent, Novel in Vitro Inhibitors of the Pseudomonas aeruginosa Deacetylase LpxC. J. Med. Chem. 2002, 45, 3112–3129. [Google Scholar] [CrossRef]
- Pirrung, M.C.; Tumey, L.N.; McClerren, A.L.; Raetz, C.R.H. High-Throughput Catch-and-Release Synthesis of Oxazoline Hydroxamates. Structure–Activity Relationships in Novel Inhibitors of Escherichia coli LpxC: In Vitro Enzyme Inhibition and Antibacterial Properties. J. Am. Chem. Soc. 2003, 125, 1575–1586. [Google Scholar] [CrossRef]
- Carmeli, S.; Moore, R.E.; Patterson, G.M.L.; Corbett, T.H.; Valeriote, F.A. Tantazoles, Unusual Cytotoxic Alkaloids from the Blue-green Alga Scytonema Mirabile. J. Am. Chem. Soc. 1990, 112, 8195–8197. [Google Scholar] [CrossRef]
- Prinsep, M.R.; Moore, R.E.; Levine, I.A.; Patterson, G.M.L. Westiellamide, a Bistratamide-Related Cyclic Peptide from the Blue-Green Alga Westiellopsis prolifica. J. Nat. Prod. 1992, 55, 140–142. [Google Scholar] [CrossRef]
- Kim, M.Y.; Vankayalapati, H.; Shin-ya, K.; Wierzba, K.; Hurley, L.H.J. Telomestatin, a Potent Telomerase Inhibitor That Interacts Quite Specifically with the Human Telomeric Intramolecular G-Quadruplex. J. Am. Chem. Soc. 2002, 124, 2098–2099. [Google Scholar] [CrossRef]
- Bode, B.H.; Irschik, H.; Wenzel, S.C.; Reichenbach, H.; Müller, R.; Höfle, G. The Leupyrrins: A Structurally Unique Family of Secondary Metabolites from the Myxobacterium Sorangium cellulosum. J. Nat. Prod. 2003, 66, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Lizos, D.E.; Kim, D.W.; Schlawe, D.; de Noronha, R.G.; Longbottom, D.A.; Rodriquez, M.; Bucci, M.; Cirino, G. Total Synthesis and Biological Evaluation of Halipeptins A and D and Analogues. J. Am. Chem. Soc. 2006, 128, 4460–4470. [Google Scholar] [CrossRef] [PubMed]
- Gant, T.G.; Meyers, A.I. The Chemistry of 2-oxazolines (1985–present). Tetrahedron 1994, 50, 2297–2360. [Google Scholar] [CrossRef]
- Byrne, C.M.; Church, T.L.; Kramer, J.W.; Coates, G.W. Catalytic Synthesis of β3-Amino Acid Derivatives from α-Amino Acids. Angew. Chem. Int. Ed. 2008, 47, 3979–3983. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, Z.-W.; Shen, P.-X.; Zhao, H.-W.; Shi, Z.-J. Development of Modifiable Bidentate Amino Oxazoline Directing Group for Pd-Catalyzed Arylation of Secondary C-H Bonds. Chem. Eur. J. 2015, 21, 7389–7393. [Google Scholar] [CrossRef]
- Shang, M.; Wang, M.-M.; Saint-Denis, T.G.; Li, M.-H.; Dai, H.-X.; Yu, J.-Q. Copper-Mediated Late-Stage Functionalization of Heterocycle-Containing Molecules. Angew. Chem. Int. Ed. 2017, 56, 5317–5321. [Google Scholar] [CrossRef] [PubMed]
- Vorbrüggen, H.; Krolikiewicz, K. A Simple Synthesis of Δ2-oxazines, Δ2-oxazines, Δ2-thiazolines and 2-Substituted Benzoxazoles. Tetrahedron 1993, 49, 9353–9372. [Google Scholar] [CrossRef]
- Marrero-Terrero, A.L.; Loupy, A. Synthesis of 2-Oxazolines from Carboxylic Acids and α, α, α-Tris(hydroxymethyl)methylamine under Microwaves in Solvent-Free Conditions. Synlett 1996, 1996, 245–246. [Google Scholar] [CrossRef]
- Kangani, C.O.; Kelley, D.E. One Pot Direct Synthesis of Amides or Oxazolines from Carboxylic Acids Using Deoxo-Fluor Reagent. Tetrahedron Lett. 2005, 46, 8917–8920. [Google Scholar] [CrossRef]
- Zhou, P.; Blubaum, J.E.; Burns, C.T.; Natale, N.R. The Direct Synthesis of 2-Oxazolines from Carboxylic Esters using Lanthanide Chloride as Catalyst. Tetrahedron Lett. 1997, 38, 7019–7020. [Google Scholar] [CrossRef]
- Zhou, H.; Zeng, X.; Ding, L.; Xie, Y.; Zhong, G. Triflic Acid Catalyzed Formal [3 + 2] Cycloaddition of Donor–Acceptor Oxiranes and Nitriles: A Facile Access to 3-Oxazolines. Org. Lett. 2015, 17, 2385–2387. [Google Scholar] [CrossRef] [PubMed]
- Bolm, C.; Weickhardt, K.; Zehnder, M.; Ranff, T. Synthesis of Optically Active Bis(2-oxazolines): Crystal Structure of a 1,2-Bis(2-oxazolinyl)benzene ZnCl2 Complex. Chem. Ber. 1991, 124, 1173–1180. [Google Scholar] [CrossRef]
- Mohammadpoor-Baltork, I.; Khosropour, A.R.; Hojati, S.F. A Novel and Chemoselective Synthesis of 2-Aryloxazolines and Bis-oxazolines Catalyzed by Bi(III) Salts. Synlett 2005, 18, 2747–2750. [Google Scholar] [CrossRef]
- Cai, A.-J.; Zheng, Y.; Ma, J.-A. Copper-Triggered Three-Component Reaction of CF3CHN2, Nitriles, and Aldehydes: Highly Diastereoselective Synthesis of CF3-substituted Oxazolines and Vicinal Amino Alcohols. Chem. Commun. 2015, 51, 8946–8949. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Sawamura, M.; Ito, Y. Asymmetric Synthesis Catalyzed by Chiral Ferrocenyl Phosphine Transition Metal Complexes. 10 gold(i)-Catalyzed Asymmetric Aldol Reaction of Isocyanoacetate. Tetrahedron 1992, 48, 1999–2012. [Google Scholar] [CrossRef]
- Badiang, J.G.; Aubé, J. One-Step Conversion of Aldehydes to Oxazolines and 5,6-Dihydro-4H-1,3-oxazines Using 1,2- and 1,3-Azido Alcohols. J. Org. Chem. 1996, 61, 2484–2487. [Google Scholar] [CrossRef]
- Ishihara, M.; Togo, H. Direct Oxidative Conversion of Aldehydes and Alcohols to 2-imidazolines and 2-oxazolines Using Molecular Iodine. Tetrahedron 2007, 63, 1474–1480. [Google Scholar] [CrossRef]
- Minakata, S.; Nishimura, M.; Takahashi, T.; Oderaotoshi, Y.; Komatsu, M. Direct Asymmetric Synthesis of Oxazolines from Olefins Using a Chiral Nitridomanganese Complex: A Novel Three-component Coupling Leading to Chiral Oxazolines. Tetrahedron Lett. 2001, 42, 9019–9022. [Google Scholar] [CrossRef]
- Minakata, S.; Morino, Y.; Ide, T.; Oderaotoshi, Y.; Komatsu, M. Direct Synthesis of Oxazolines from Olefins and Amides Using t-BuOI. Chem. Commun. 2007, 31, 3279–3281. [Google Scholar] [CrossRef]
- Wu, F.; Alom, N.-E.; Ariyarathna, J.P.; Naβ, J.; Li, W. Regioselective Formal [3+2] Cycloadditions of Urea Substrates with Activated and Unactivated Olefins for Intermolecular Olefin Aminooxygenation. Angew. Chem. Int. Ed. 2019, 58, 11676–11680. [Google Scholar] [CrossRef]
- Wu, F.; Kaur, N.; Alom, N.-E.; Li, W. Chiral Hypervalent Iodine Catalysis Enables an Unusual Regiodivergent Intermolecular Olefin Aminooxygenation. JACS Au 2021, 1, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Mumford, E.M.; Hemric, B.N.; Denmark, S.E. Catalytic, Enantioselective Syn-Oxyamination of Alkenes. J. Am. Chem. Soc. 2021, 143, 13408–13417. [Google Scholar] [CrossRef]
- Roush, D.M.; Patel, M.M. A Mild Procedure for the Preparation of 2-oxazolines. Synth. Commun. 1985, 15, 675–679. [Google Scholar] [CrossRef]
- Galéotti, N.; Montagne, C.; Poncet, J.; Jouin, P. Formation of Oxazolines and Thiazolines in Peptides by the Mitsunobu Reaction. Tetrahedron Lett. 1992, 33, 2807–2810. [Google Scholar] [CrossRef]
- Wipf, P.; Fritch, P.C. Total Synthesis and Assignment of Configuration of Lissoclinamide 7. J. Am. Chem. Soc. 1996, 118, 12358–12367. [Google Scholar] [CrossRef]
- Phillips, A.J.; Uto, Y.; Wipf, P.; Reno, M.J.; Williams, D.R. Synthesis of Functionalized Oxazolines and Oxazoles with DAST and Deoxo-Fluor. Org. Lett. 2000, 2, 1165–1168. [Google Scholar] [CrossRef]
- Plant, A.; Stieber, F.; Scherkenbeck, J.; Losel, P.; Dyker, H. Syntheses of Analogues of the Insect Neuropeptide Proctolin Containing an Oxazole Ring as an Amide Bond Replacement. Org. Lett. 2001, 3, 3427–3430. [Google Scholar] [CrossRef]
- Doi, T.; Yoshida, M.; Shin-ya, K.; Takahashi, T. Total Synthesis of (R)-Telomestatin. Org. Lett. 2006, 8, 4165–4167. [Google Scholar] [CrossRef]
- Ying, Y.; Hong, J. Synthesis of Brasilibactin A and Confirmation of Absolute Configuration of β-hydroxy Acid Fragment. Tetrahedron Lett. 2007, 48, 8104–8107. [Google Scholar] [CrossRef]
- Liyanage, W.; Weerasinghe, L.; Strong, R.K.; Del Valle, J.R. Synthesis of Carbapyochelins via Diastereoselective Azidation of 5-(ethoxycarbonyl) Methylproline Derivatives. J. Org. Chem. 2008, 73, 7420–7423. [Google Scholar] [CrossRef] [PubMed]
- Castellano, S.; Kuck, D.; Sala, M.; Novellino, E.; Lyko, F.; Sbardella, G. Constrained Analogues of Procaine as Novel Small Molecule Inhibitors of DNA Methyltransferase-1. J. Med. Chem. 2008, 51, 2321–2325. [Google Scholar] [CrossRef]
- Pouliot, M.; Angers, L.; Hamel, J.; Paquin, J. Synthesis of 2-oxazolines and Related N-containing Heterocycles Using [Et2NSF2] BF4 as a Cyclodehydration Agent. Tetrahedron Lett. 2012, 53, 4121–4123. [Google Scholar] [CrossRef]
- Mollo, M.C.; Orelli, L.R. Microwave-Assisted Synthesis of 2-Aryl-2-oxazolines, 5,6-Dihydro-4H-1,3-oxazines, and 4,5,6,7-Tetrahydro-1,3-oxazepines. Org. Lett. 2016, 18, 6116–6119. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, A.; Kondo, R.; Ishihara, K. Molybdenum Oxides as Highly Effective Dehydrative Cyclization Catalysts for the Synthesis of Oxazolines and Thiazolines. Org. Lett. 2005, 7, 1971–1974. [Google Scholar] [CrossRef]
- Sakakura, A.; Umemura, S.; Kondo, R.; Ishihara, K. Dehydrative Cyclization Catalyzed by the Combination of Molybdenum (VI) Oxides and Benzoic Acids: First Synthesis of the Antitumour Substance BE-70016. Adv. Synth. Catal. 2007, 349, 551–555. [Google Scholar] [CrossRef]
- Rodriguez del Rey, F.O.; Floreancig, P.E. Synthesis of Nitrogen-Containing Heterocycles through Catalytic Dehydrative Cyclization Reactions. Org. Lett. 2021, 23, 150–154. [Google Scholar] [CrossRef]
- Movahed, F.S.; Foo, S.W.; Mori, S.; Ogawa, S.; Saito, S. Phosphorus-Based Organocatalysis for the Dehydrative Cyclization of N-(2-hydroxyethyl)amides into 2-Oxazolines. J. Org. Chem. 2022, 87, 243–257. [Google Scholar] [CrossRef] [PubMed]
- de Benneville, P.L.; Luskin, L.S.; Sims, H.J. Transesterification of Methyl Methacrylate with Amino Alcohols. Preparation of a Primary Aminoalkyl Methacrylate and 2-Isopropenyl-4,4-dimethyloxazoline. J. Org. Chem. 1958, 17, 1355–1357. [Google Scholar] [CrossRef]
- Hu, L.; Xu, S.; Zhao, Z.; Yang, Y.; Peng, Z.; Yang, M.; Wang, C.; Zhao, J. Ynamides as Racemization-free Coupling Reagents for Amide and Peptide Synthesis. J. Am. Chem. Soc. 2016, 138, 13135–13138. [Google Scholar] [CrossRef]
 | ||||
|---|---|---|---|---|
| Entry | Acid (Equiv.) | Solvent | Temperature | Yield b |
| 1 | MsOH (1.0) | DCE | 80 °C | 16 |
| 2 | TFA (1.0) | DCE | 80 °C | 9 |
| 3 | TfOH (1.0) | DCE | 80 °C | 89 |
| 4 | TfOH (0.2) | DCE | 80 °C | 14 |
| 5 | TfOH (0.5) | DCE | 80 °C | 29 |
| 6 | TfOH (1.2) | DCE | 80 °C | 94 |
| 7 | TfOH (1.5) | DCE | 80 °C | 96 (88) c |
| 8 | TfOH (2.0) | DCE | 80 °C | 86 |
| 9 | TfOH (1.5) | Toluene | 80 °C | 92 |
| 10 | TfOH (1.5) | PhCF3 | 80 °C | 86 |
| 11 | TfOH (1.5) | CH3CN | 80 °C | 95 |
| 12 | TfOH (1.5) | DCE | 70 °C | 91 |
| 13 | TfOH (1.5) | DCE | 60 °C | 85 |
| 14 | TfOH (1.5) | DCE | 25 °C | <5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Huang, C.; Jia, J.; Wu, F.; Ni, F. A Facile Synthesis of 2-Oxazolines via Dehydrative Cyclization Promoted by Triflic Acid. Molecules 2022, 27, 9042. https://doi.org/10.3390/molecules27249042
Yang T, Huang C, Jia J, Wu F, Ni F. A Facile Synthesis of 2-Oxazolines via Dehydrative Cyclization Promoted by Triflic Acid. Molecules. 2022; 27(24):9042. https://doi.org/10.3390/molecules27249042
Chicago/Turabian StyleYang, Tao, Chengjie Huang, Jingyang Jia, Fan Wu, and Feng Ni. 2022. "A Facile Synthesis of 2-Oxazolines via Dehydrative Cyclization Promoted by Triflic Acid" Molecules 27, no. 24: 9042. https://doi.org/10.3390/molecules27249042
APA StyleYang, T., Huang, C., Jia, J., Wu, F., & Ni, F. (2022). A Facile Synthesis of 2-Oxazolines via Dehydrative Cyclization Promoted by Triflic Acid. Molecules, 27(24), 9042. https://doi.org/10.3390/molecules27249042





