Non-Covalent Reactions Supporting Antiviral Development
Abstract
:1. Introduction
2. Antiviral
3. Solid State Engineering
3.1. Interactions Supporting Solid Engineering Approaches to Pharmaceutical Development
3.2. Multicomponent Systems Preparation Methods
3.3. Multicomponent System Characterization
4. Antiviral Multicomponent System
| No. | Advantage categories | Multicomponent | Structure | Advantages | Preparation methods | Ref. |
|---|---|---|---|---|---|---|
| 1 | Increasing solubility and dissolution rate | Arbidol-maleic acid | 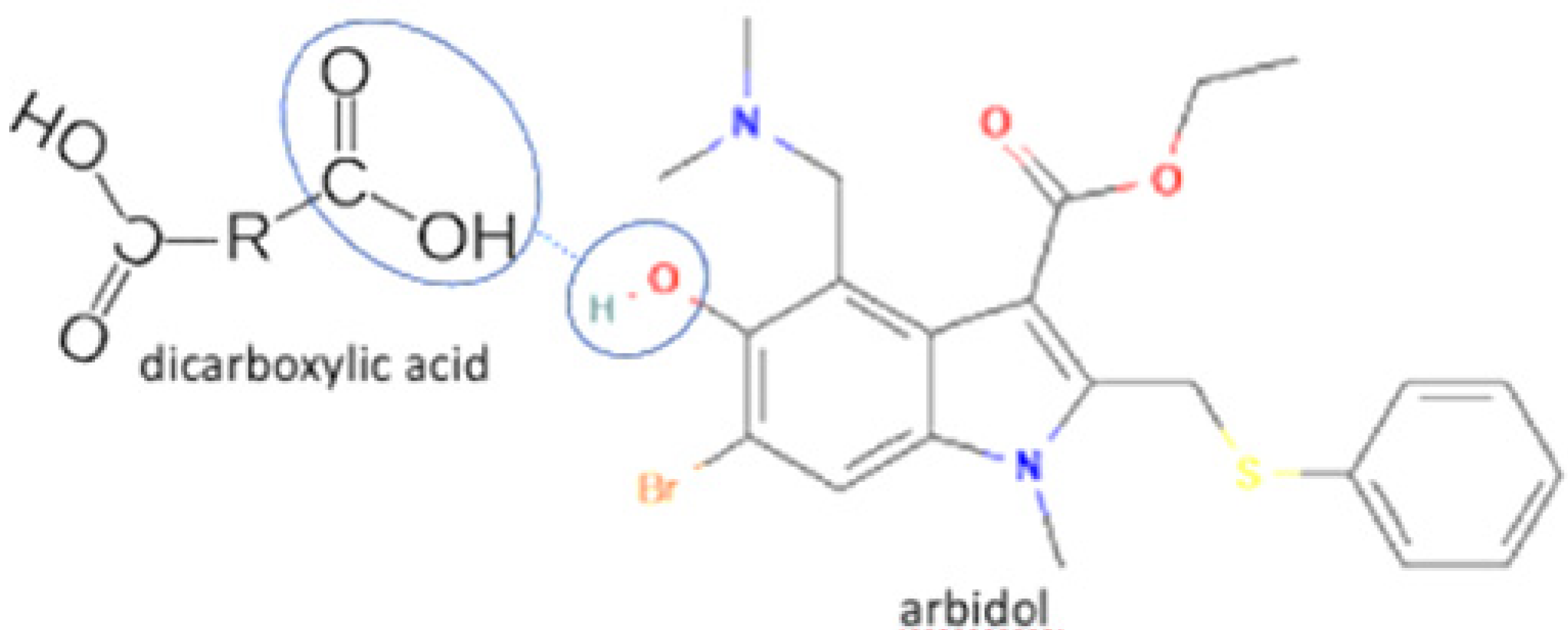 | Increasing the solubility and dissolution of arbidol alone | Slow evaporation | [72] |
| 2 | Arbidol-fumaric acid | Increasing the solubility and dissolution of arbidol alone | Slow evaporation | [72] | ||
| 3 | Favipiravir-piperazine | 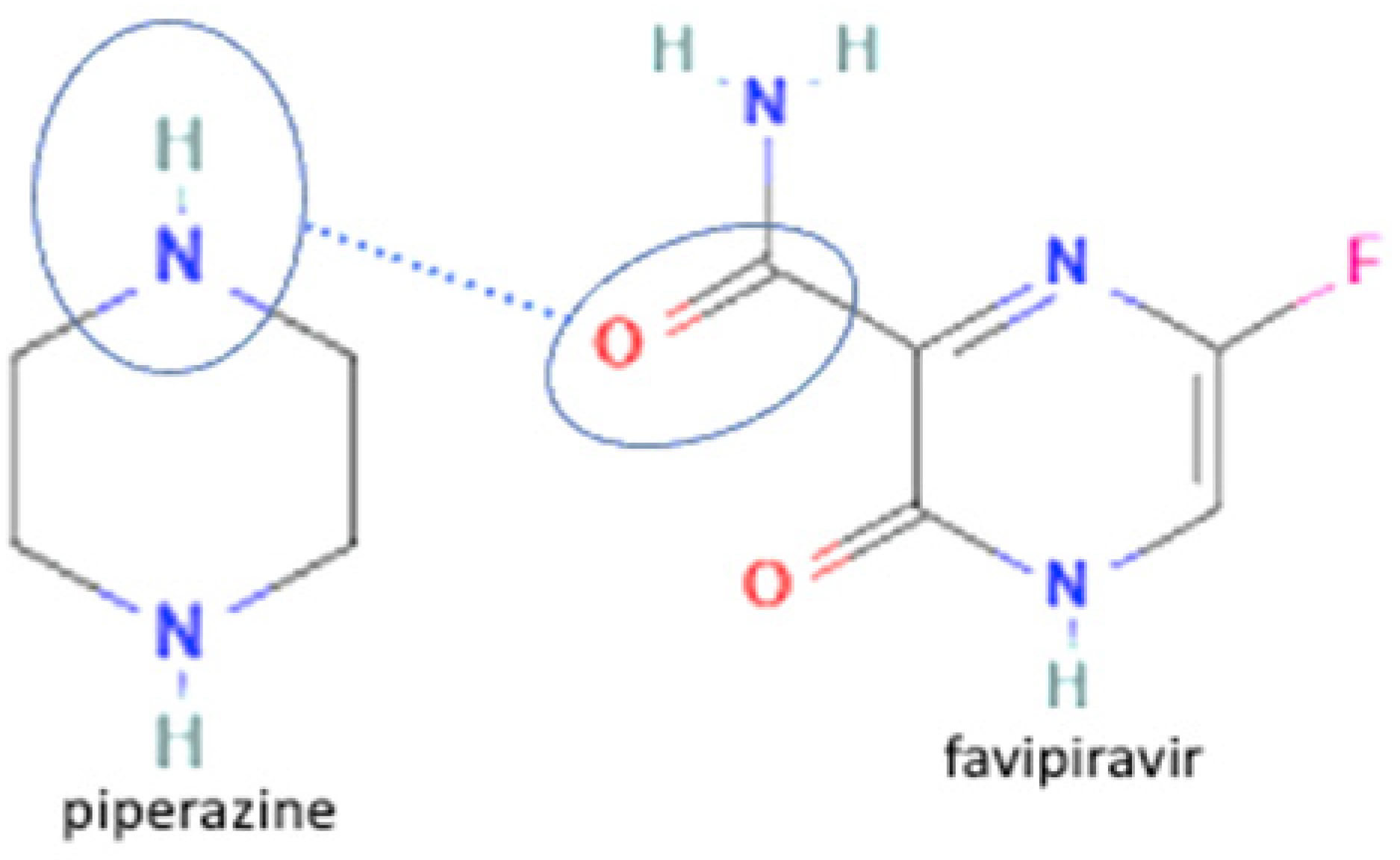 | Enhanced the solubility of favipiravir in pH 6.8 by 1.6-fold and enhanced the tabletability of favipiravir | Slow evaporation | [131] | |
| 4 | Favipiravir-4 dihydroxy benzoic acid |  | Solubility improvement of favipiravir in distilled water | Liquid assisted grinding | [134] | |
| 5 | Favipiravir-gallic acid | Solubility improvement of favipiravir in buffer phosphate pH 7 | Liquid assisted grinding | [134] | ||
| 6 | Favipiravir-4 amino benzoic acid | Solubility improvement of favipiravir in distilled water and in buffer phosphate pH 7 | Liquid assisted grinding | [134] | ||
| 7 | Curcumin-ascorbic acid | 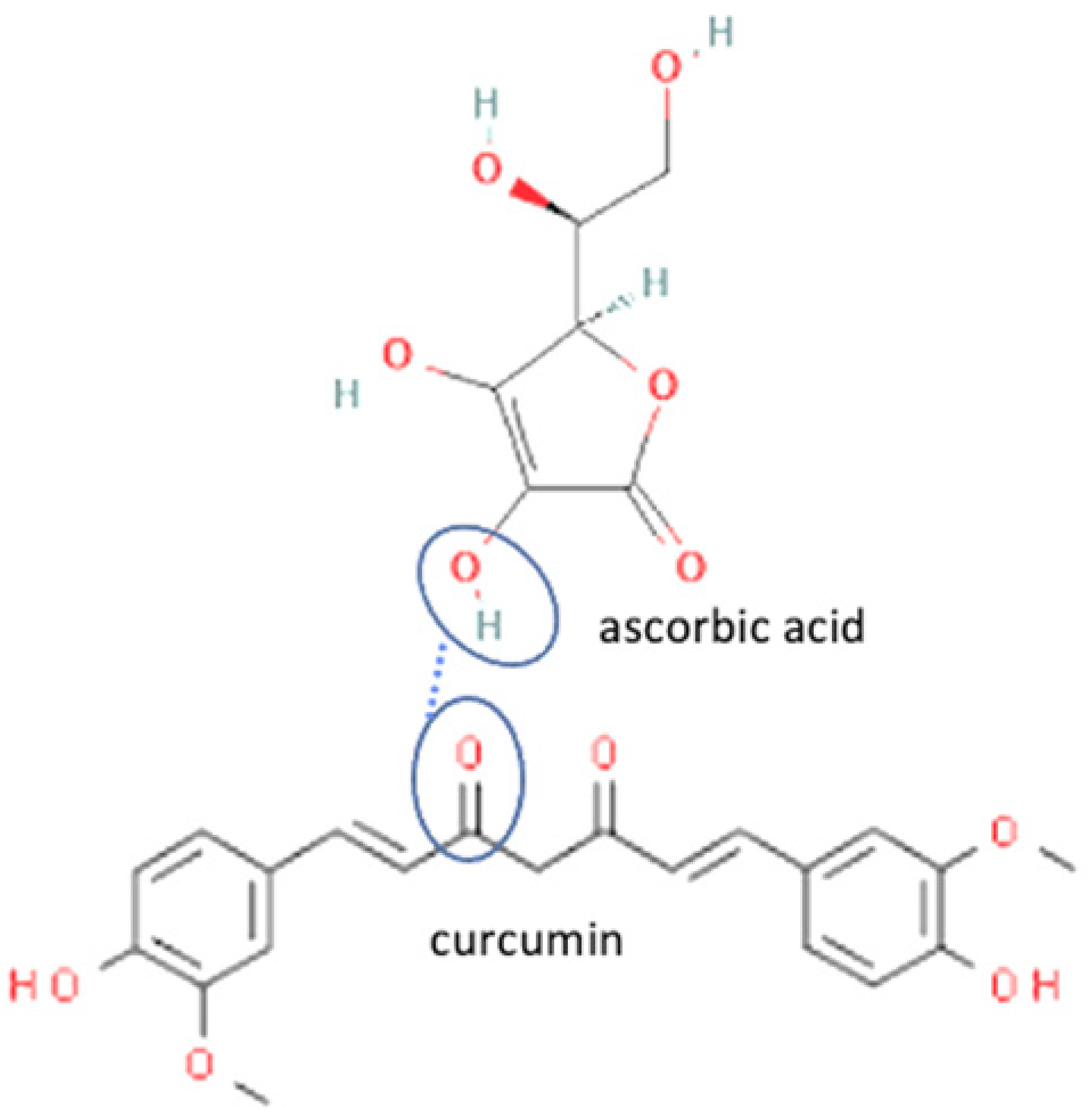 | Enhanced the aqueous solubility of curcumin in distilled water, pH 1.2, and pH 6.8 by 576, 10, and 9 fold, respectively. Enhanced the dissolution profile of neat curcumin | Solvent evaporation | [132] | |
| 8 | Abacavir-oxalic acid | 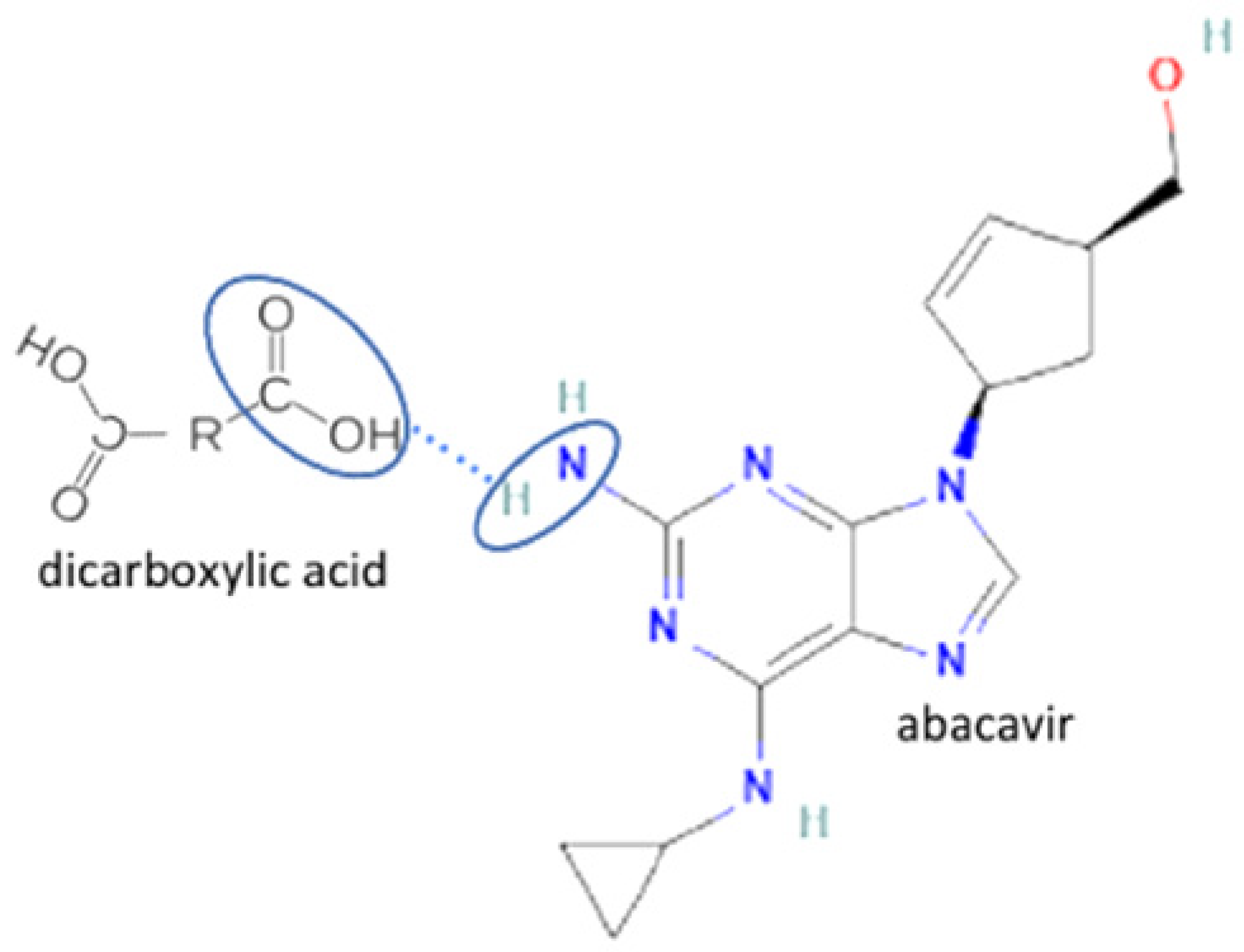 | Enhancing the aqueous solubility and dissolution rate of abacavir | Solvent evaporation | [135] | |
| 9 | Abacavir-glutaric acid | Enhancing the aqueous solubility and dissolution rate of abacavir | Solvent evaporation | [135] | ||
| 10 | Curcumin-tyramine | 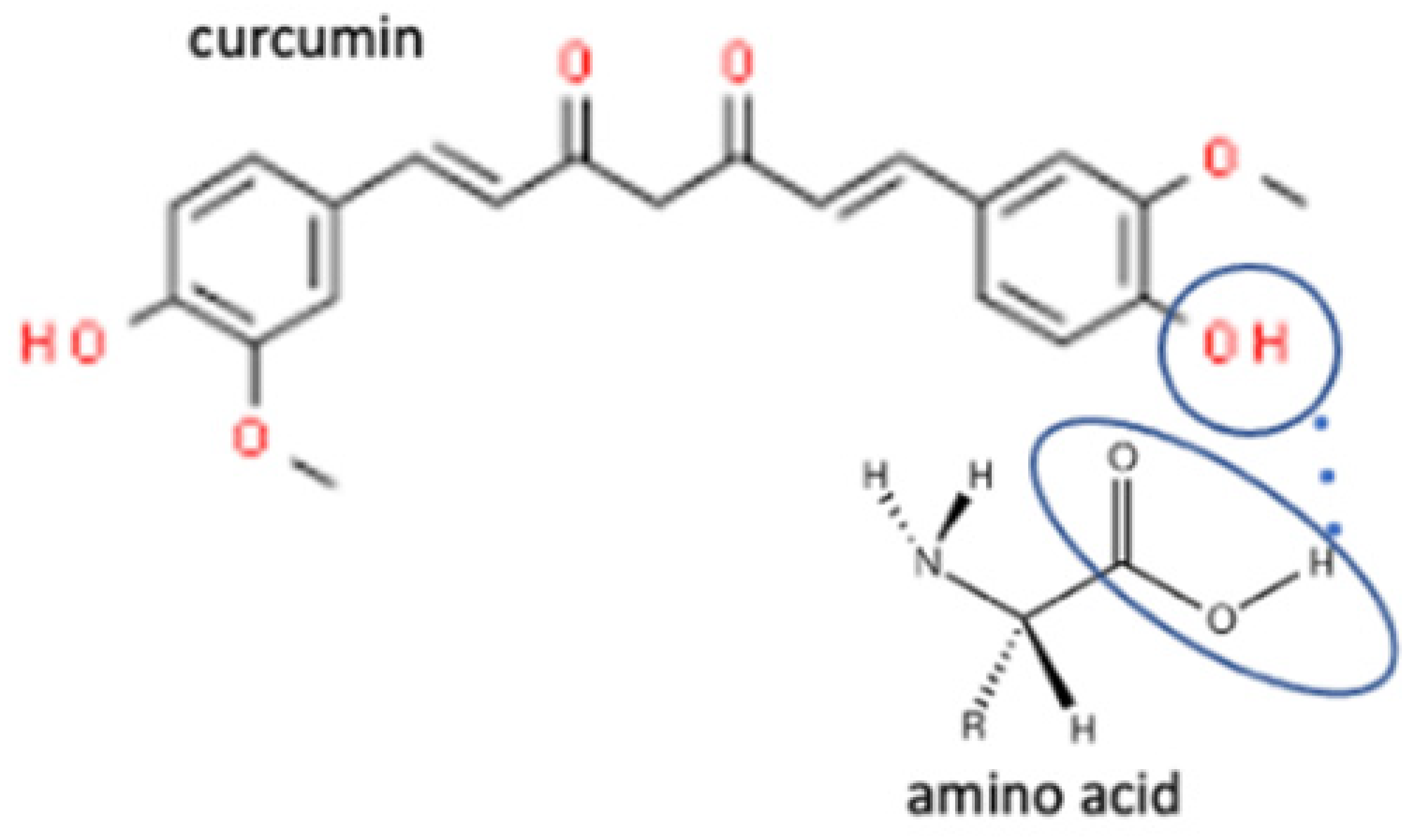 | Increasing the solubility by 18.6 times in a 40% ethanol medium of curcumin | Liquid assisted grinding | [132] | |
| 11 | Curcumin-tryptamine | Increasing the solubility by 6.3 times in a 40% ethanol medium of curcumin | Liquid assisted grinding | [132] | ||
| 12 | Curcumin-arginine | Increasing the solubility by 9.1 times in a 40% ethanol medium of curcumin | Neat grinding | [132] | ||
| 13 | Curcumin-asparagine | Increasing the solubility by 1 time in a 40% ethanol medium of curcumin | Neat grinding | [132] | ||
| 14 | Curcumin-glutamine | Increasing the solubility by 1.1 times in a 40% ethanol medium of curcumin | Neat grinding | [132] | ||
| 15 | Curcumin-lysine | Increasing the solubility by 1.1 times in 40% ethanol medium of curcumin | Neat grinding | [132] | ||
| 16 | Curcumin-histidine | Increasing the solubility by 4.2 times in 40% ethanol medium of curcumin | Neat grinding | [132] | ||
| 17 | Curcumin-citrulline | Increasing the solubility by 1.1 times in 40% ethanol medium of curcumin | Neat grinding | [132] | ||
| 18 | Quercetin-succinic acid | 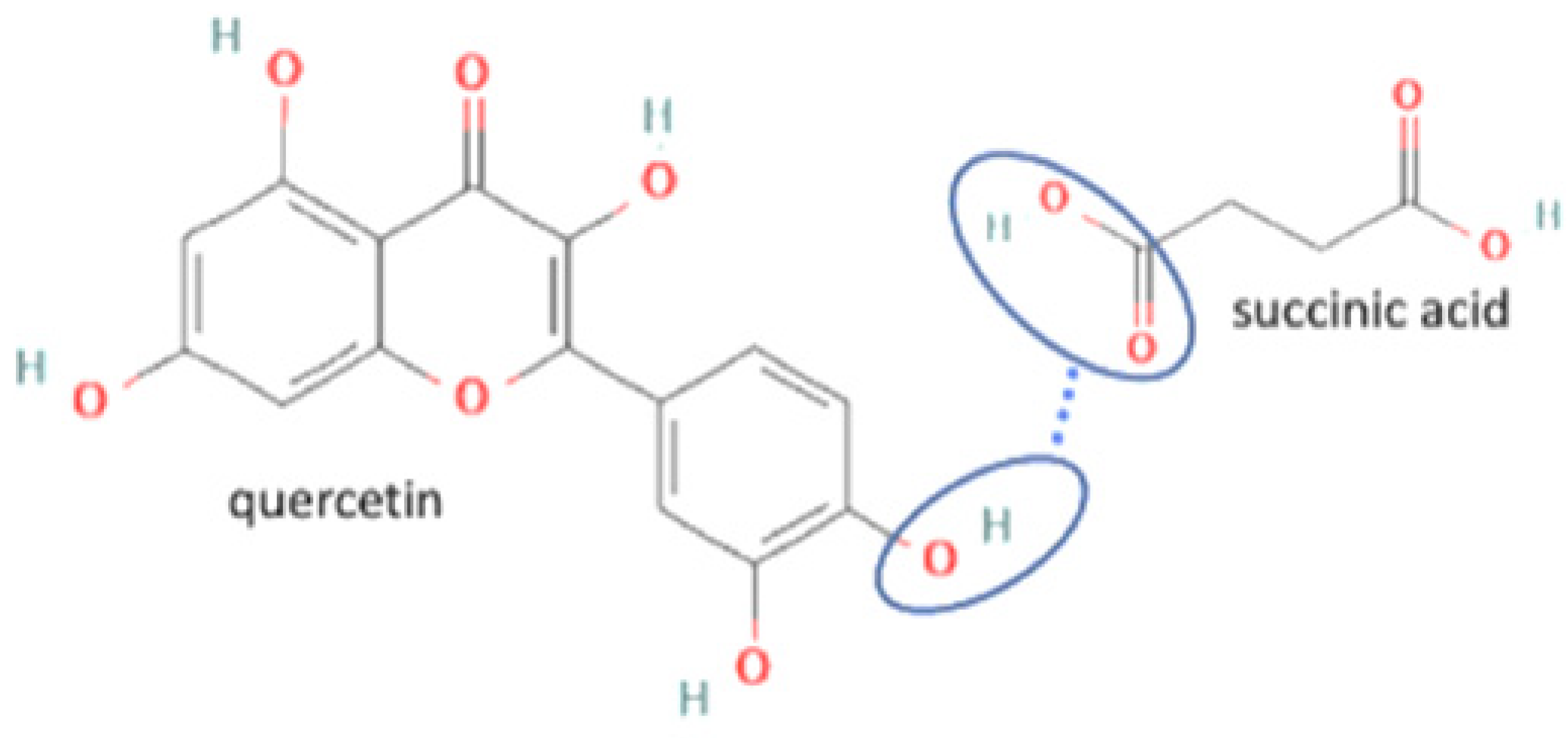 | Increasing the solubility and dissolution rate by 16.2 and 1.25 times, respectively of quercetin alone | Liquid assisted grinding | [136] | |
| 19 | Quercetin-isonicotinamide | 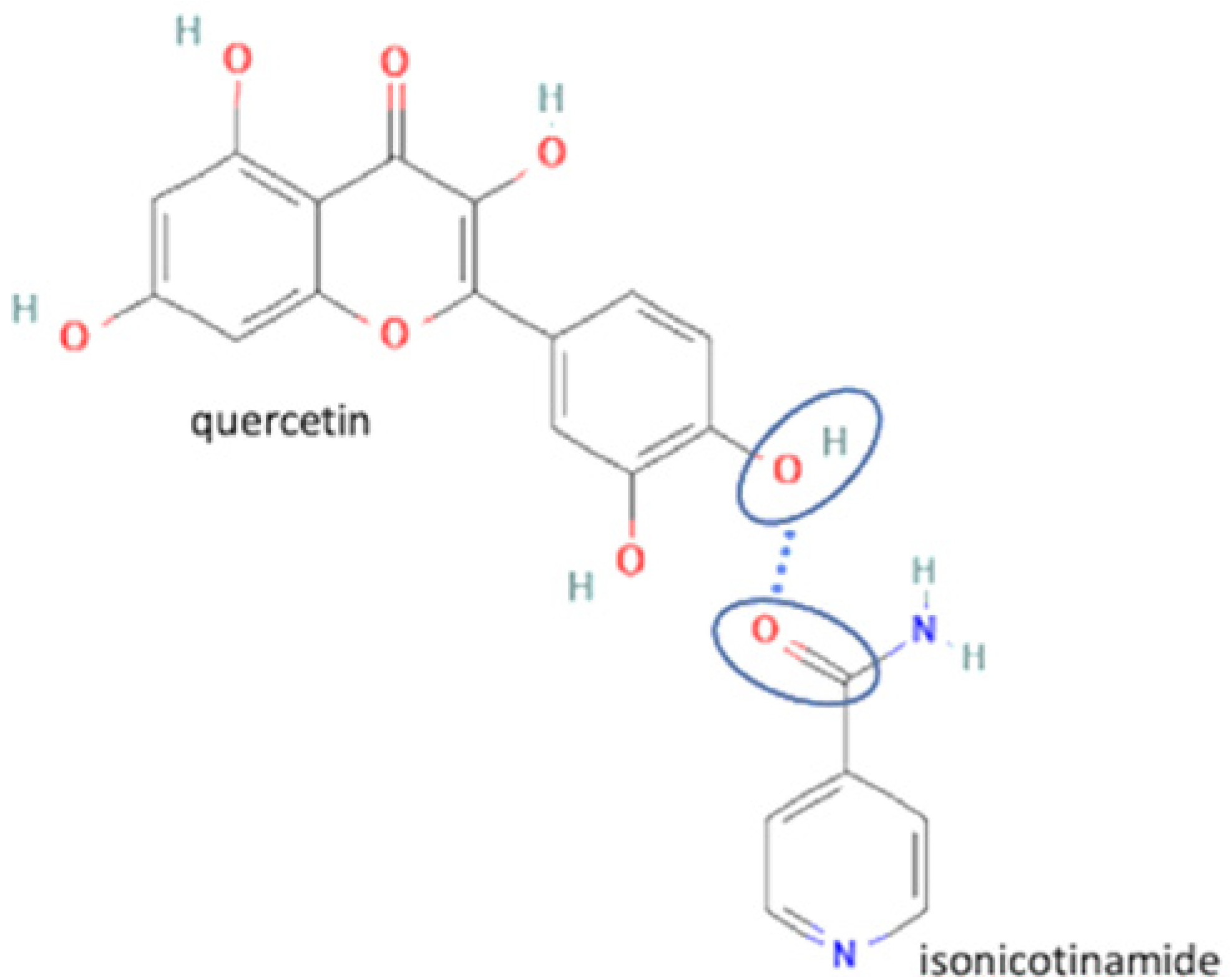 | Dissolution profile improvement of quercetin alone | Solvent evaporation | [137] | |
| 20 | Sulfathiazole-amantadine |  | Solubility improvement, diffusion improvement, dissolution improvement (2x), antibacterial activity improvement, | Liquid-assisted grinding followed by solvent evaporation | [51] | |
| 21 | Arbidol-succinic acid |  | Solubility improvement (7x in pH1.2), dissolution profile improvement | Liquid-assisted grinding followed by solvent evaporation | [72] | |
| 22 | Arbidol-salicylic acid | 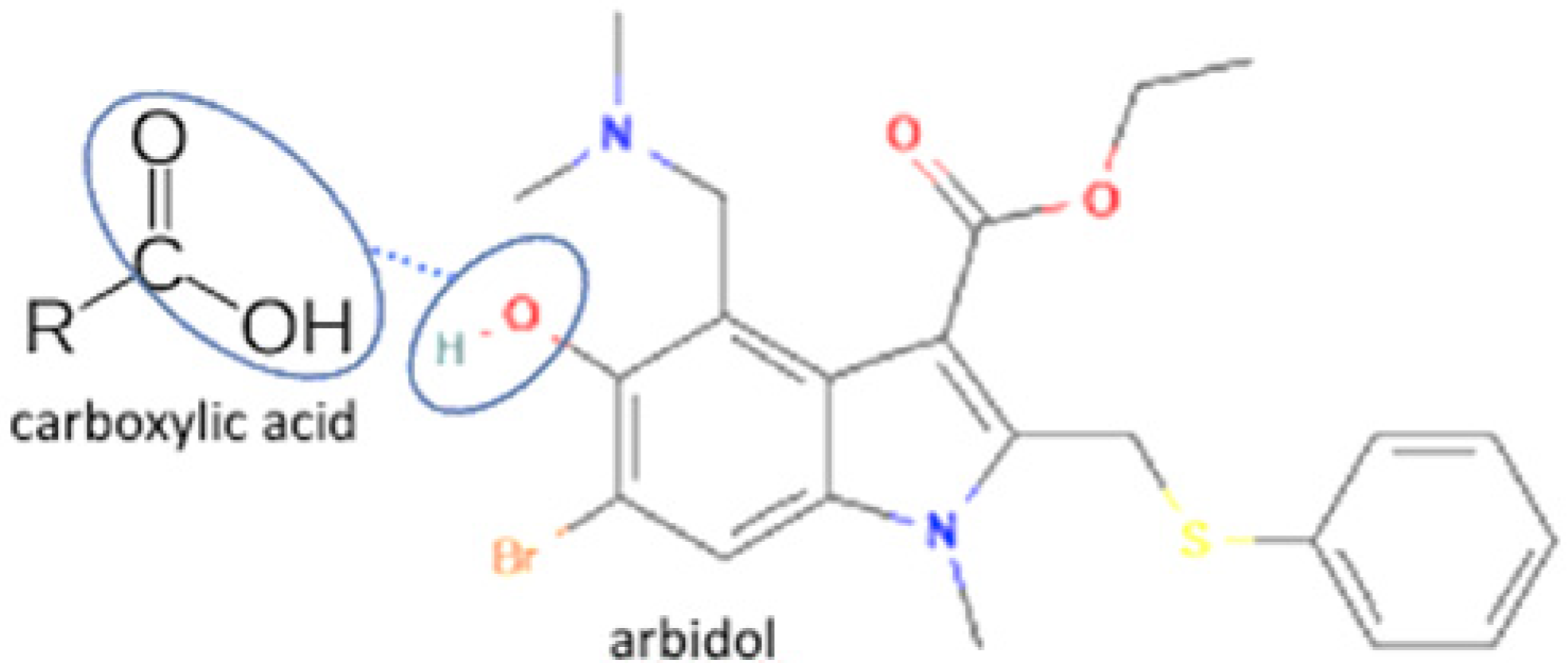 | Solubility improvement (3x), lower energy | Slow evaporation | [40] | |
| 23 | Arbidol-benzoic acid | Solubility improvement (3x), lower energy | Slow evaporation | [40] | ||
| 24 | Acyclovir-gallic acid |  | Solubility and dissolution rate improvement | Cogrinding | [138] | |
| 25 | Efavirenz-oxalic acid |  | Lower melting point, new hydrogen bond addition, solubility improvement | Cogrinding | [139] | |
| 26 | Efavirenz-glutaric acid | Solubility improvement, improvement of drug release | Spray and freeze drying | [62] | ||
| 27 | Efavirenz-citric acid | 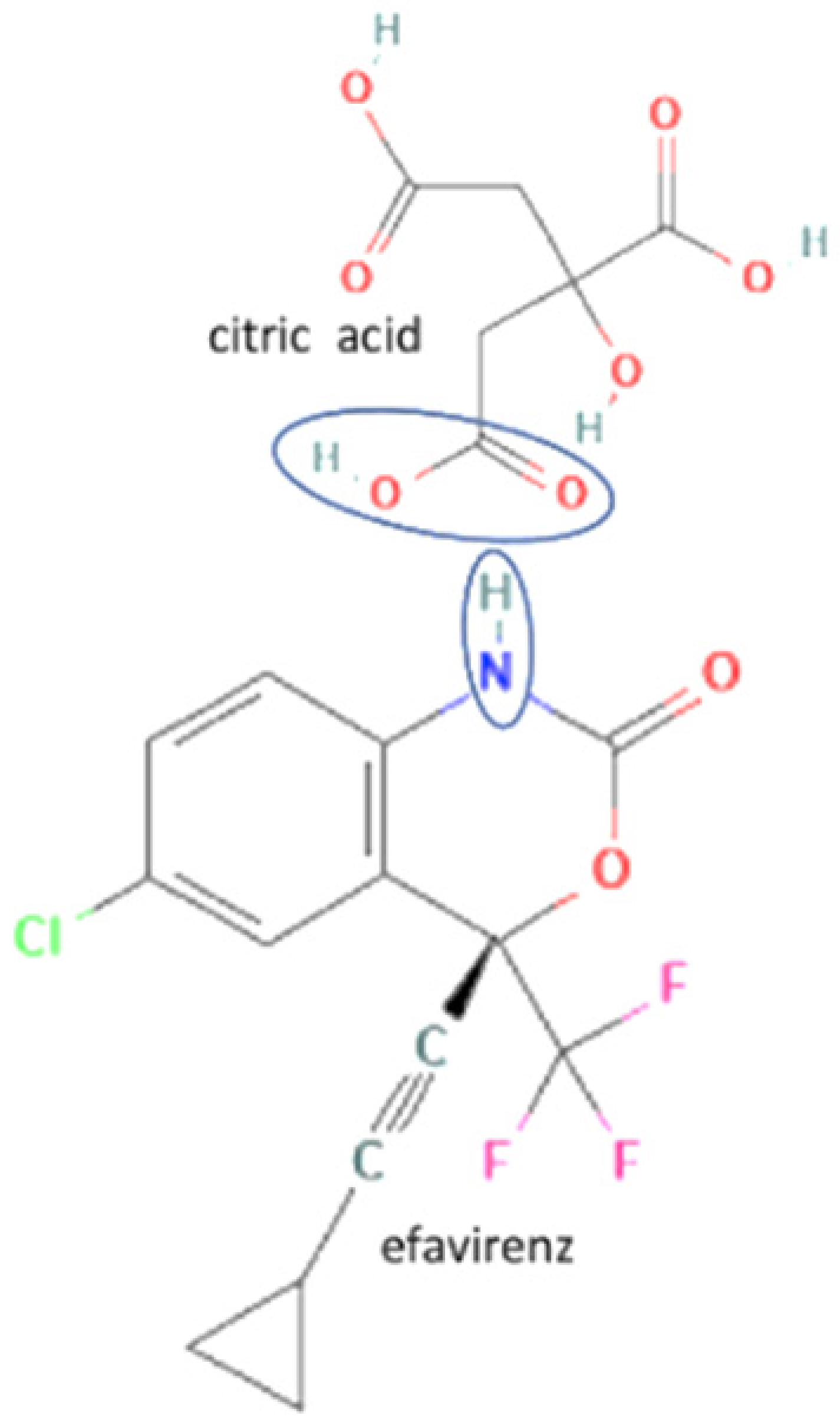 | Lower melting point, new hydrogen bond addition, solubility improvement | Cogrinding | [139] | |
| 28 | Acyclovir-tartaric acid | 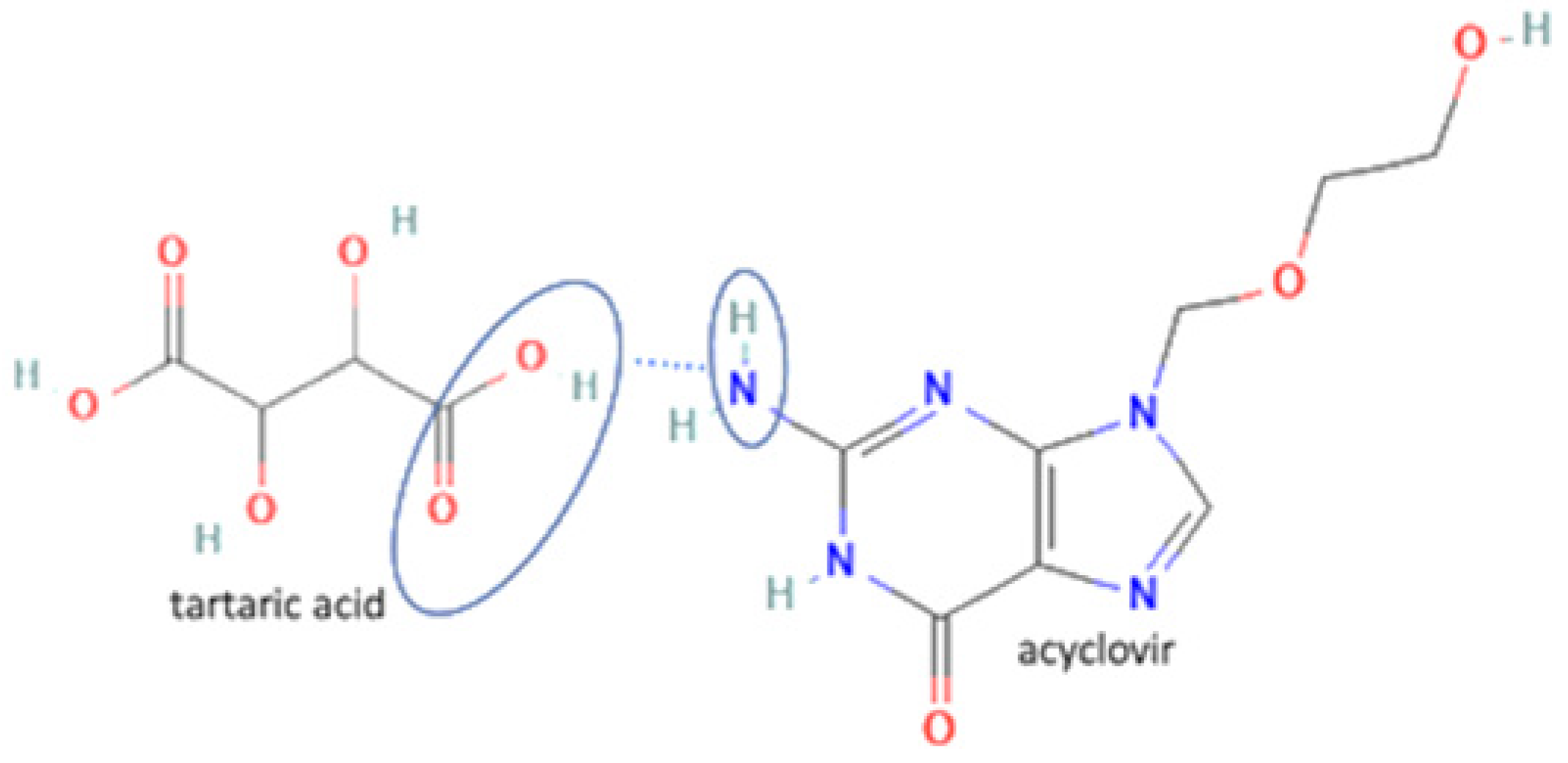 | Solubility improvement | Solution evaporation and grinding technique | [140] | |
| 29 | Etravirine-adipic acid | 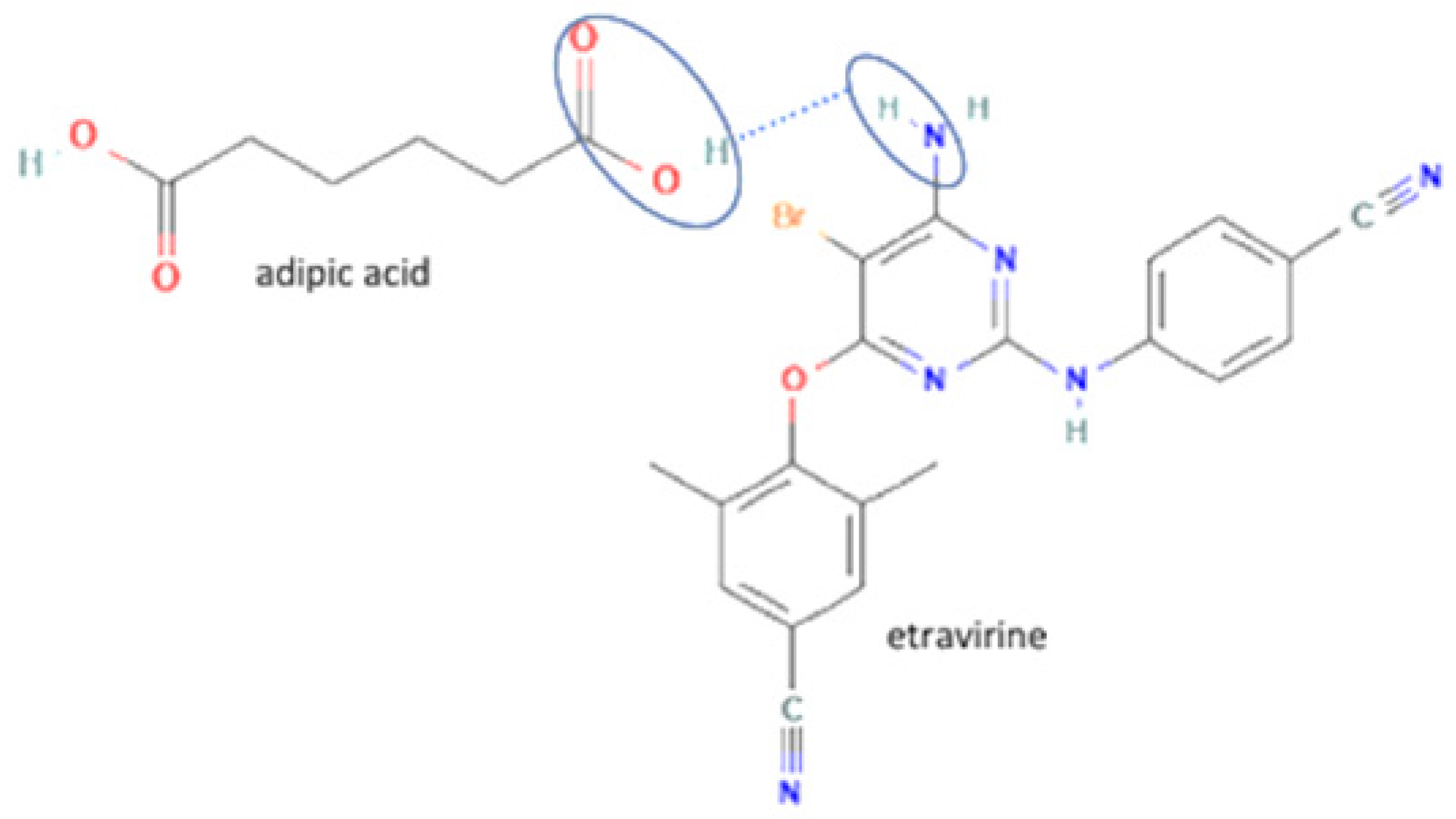 | Solubility improvement, improvement of drug release | Solvent evaporation | [141] | |
| 30 | Penciclovir-3.5 dihydroxy benzoic acid | 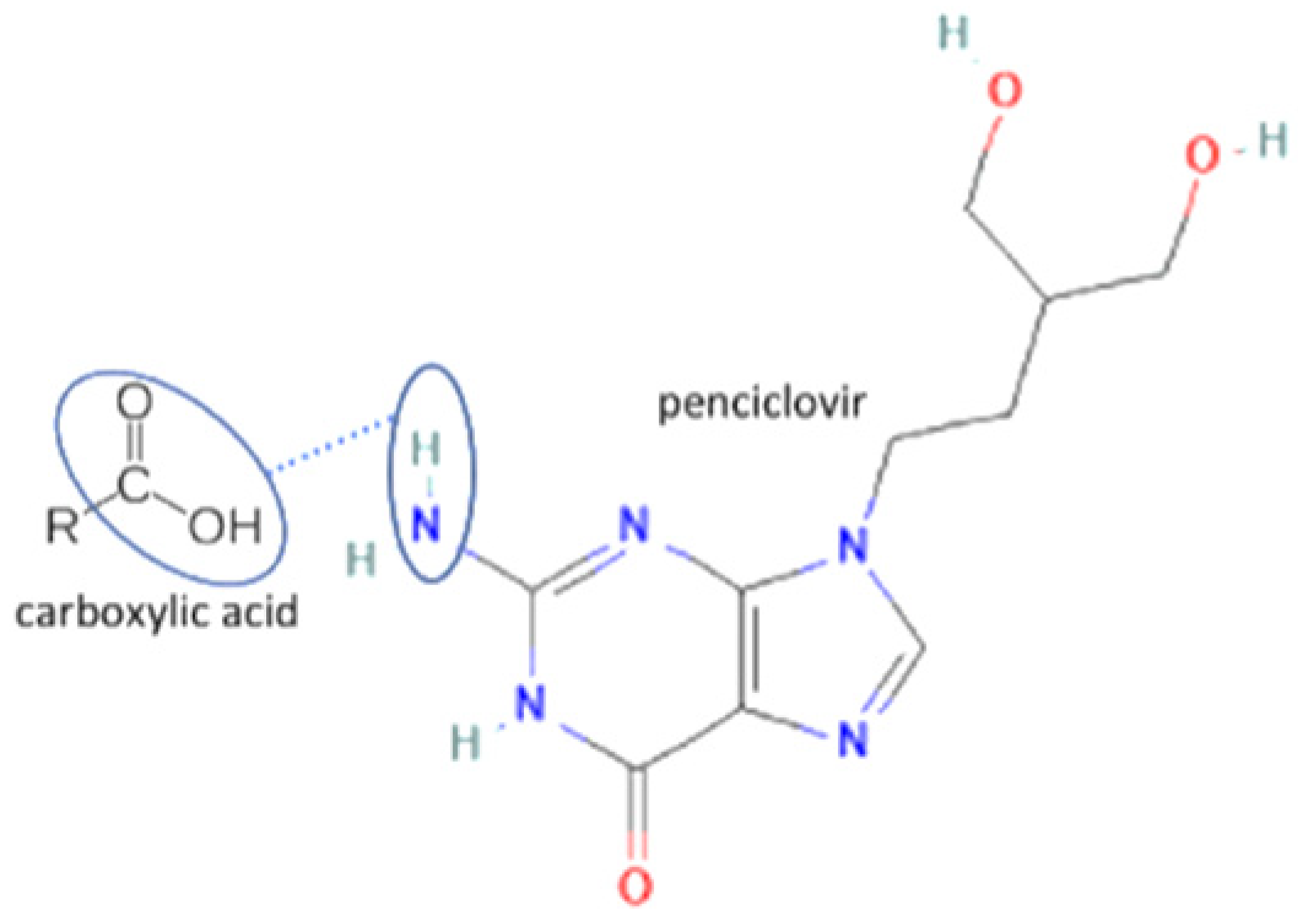 | Solubility improvement, hydrogen bond addition, maintaining stability | Neat grinding | [55] | |
| 31 | Penciclovir-gallic acid | Solubility improvement, hydrogen bond addition, maintaining stability | Neat grinding | [55] | ||
| 32 | Penciclovir-4 hydroxycinnamic acid | Solubility improvement, hydrogen bond addition, maintaining stability | Neat grinding | [55] | ||
| 33 | Ritonavir-nicotinamide |  | Solubility improvement (3–4 x) | Liquid assisted grinding | [142] | |
| 34 | Ritonavir-succinic acid | 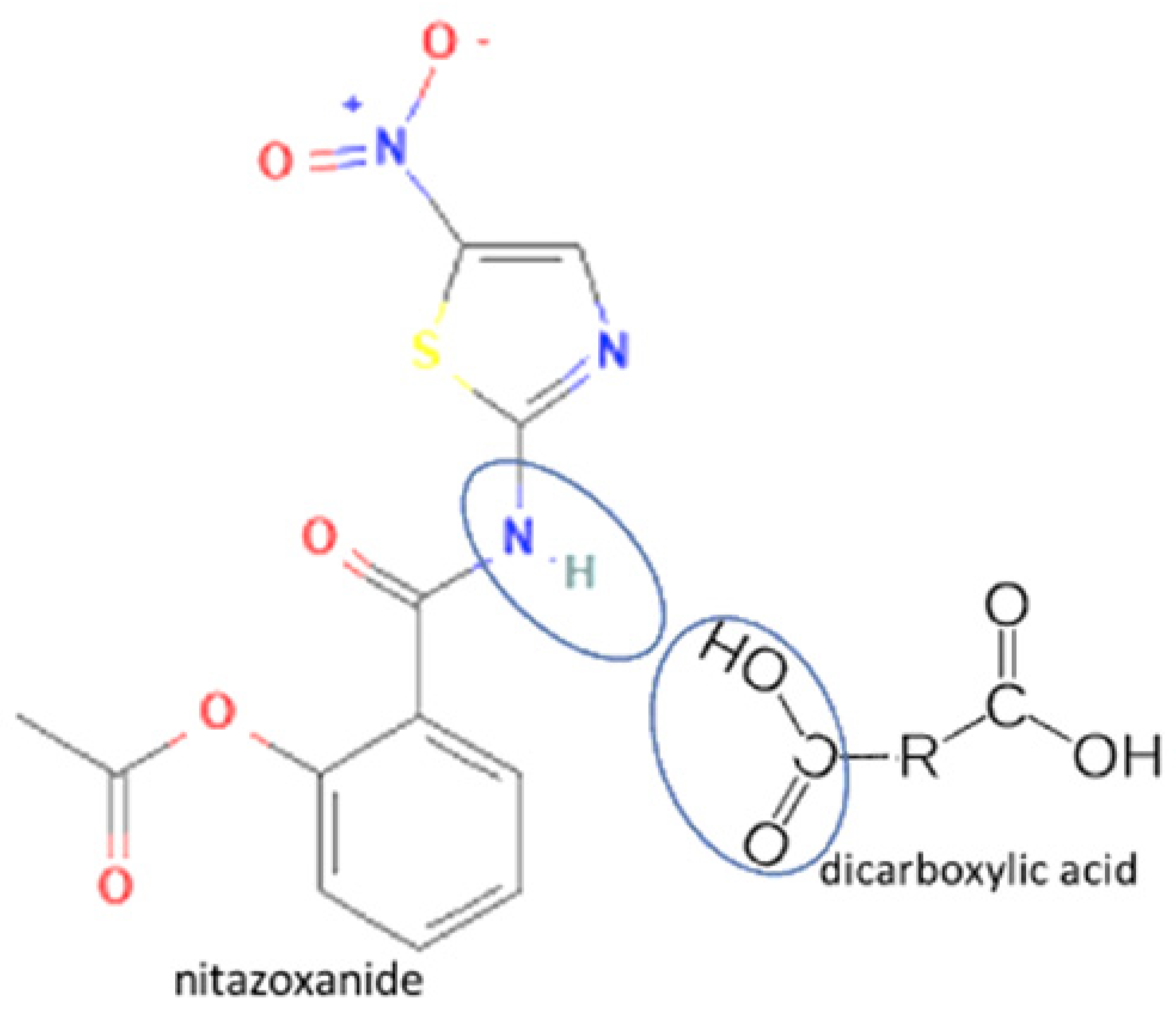 | Solubility improvement (3–4 x) | Liquid assisted grinding | [142] | |
| 35 | Ritonavir-adipic acid | Solubility improvement (3–4 x) | Liquid assisted grinding | [142] | ||
| 36 | Ritonavir-d-alanine | 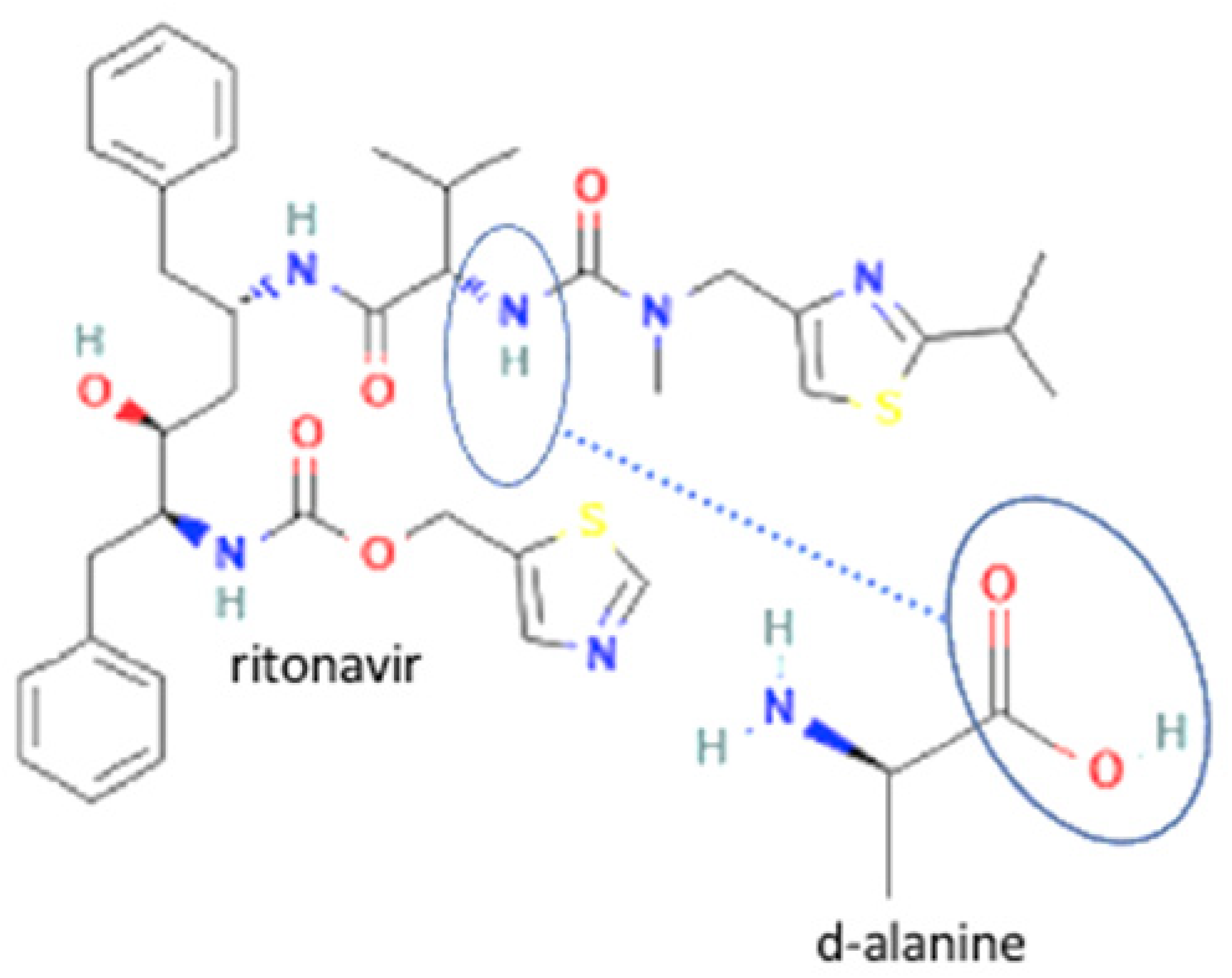 | Solubility improvement (3–4 x) | Liquid assisted grinding | [142] | |
| 37 | Nevirapine-saccharin | 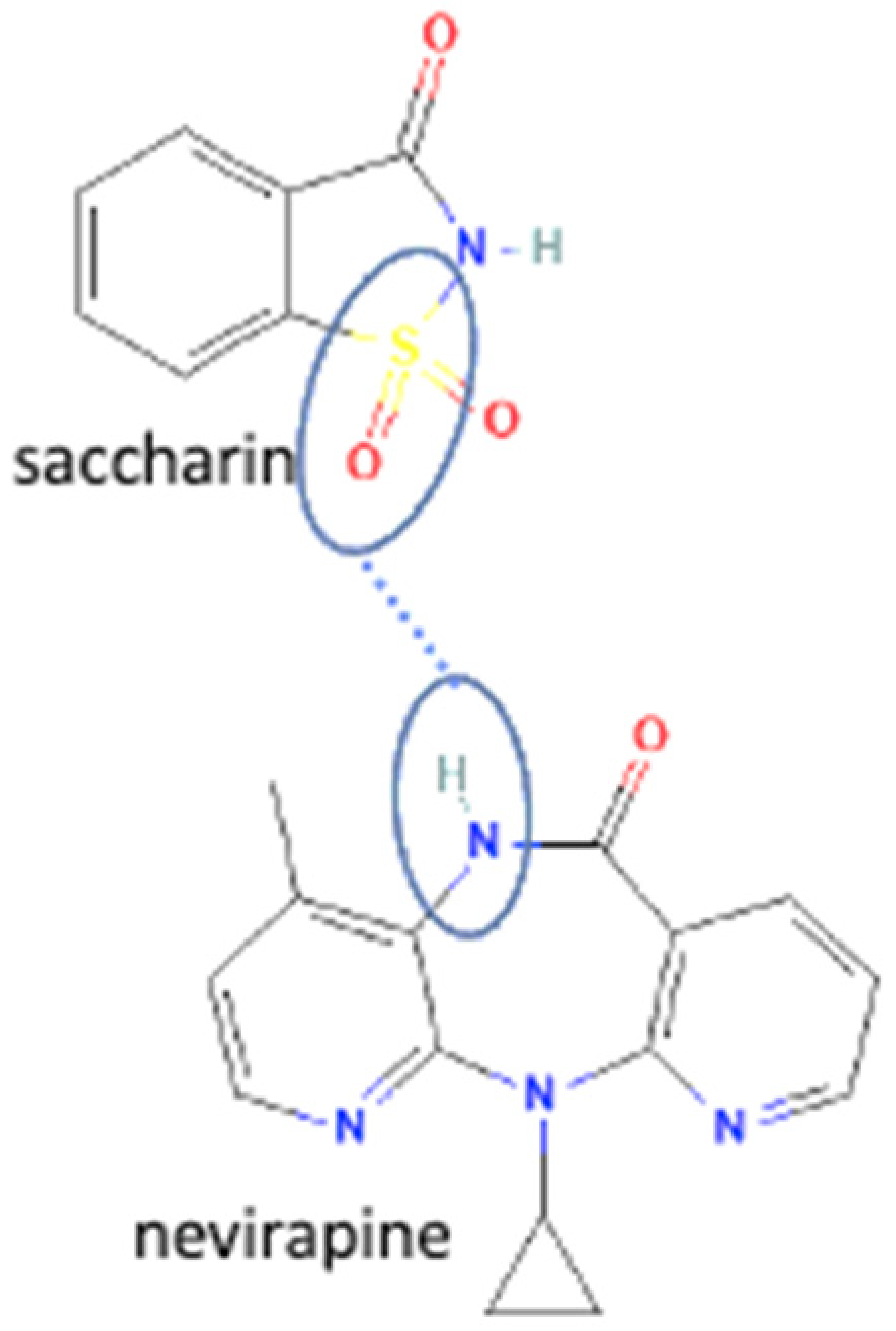 | Hydrogen bond formation [95], dissolution rate enhancement | kneading, solution crystallization, antisolvent addition, and solvent drop grinding | [83] | |
| 39 | Nevirapine-tartaric acid | 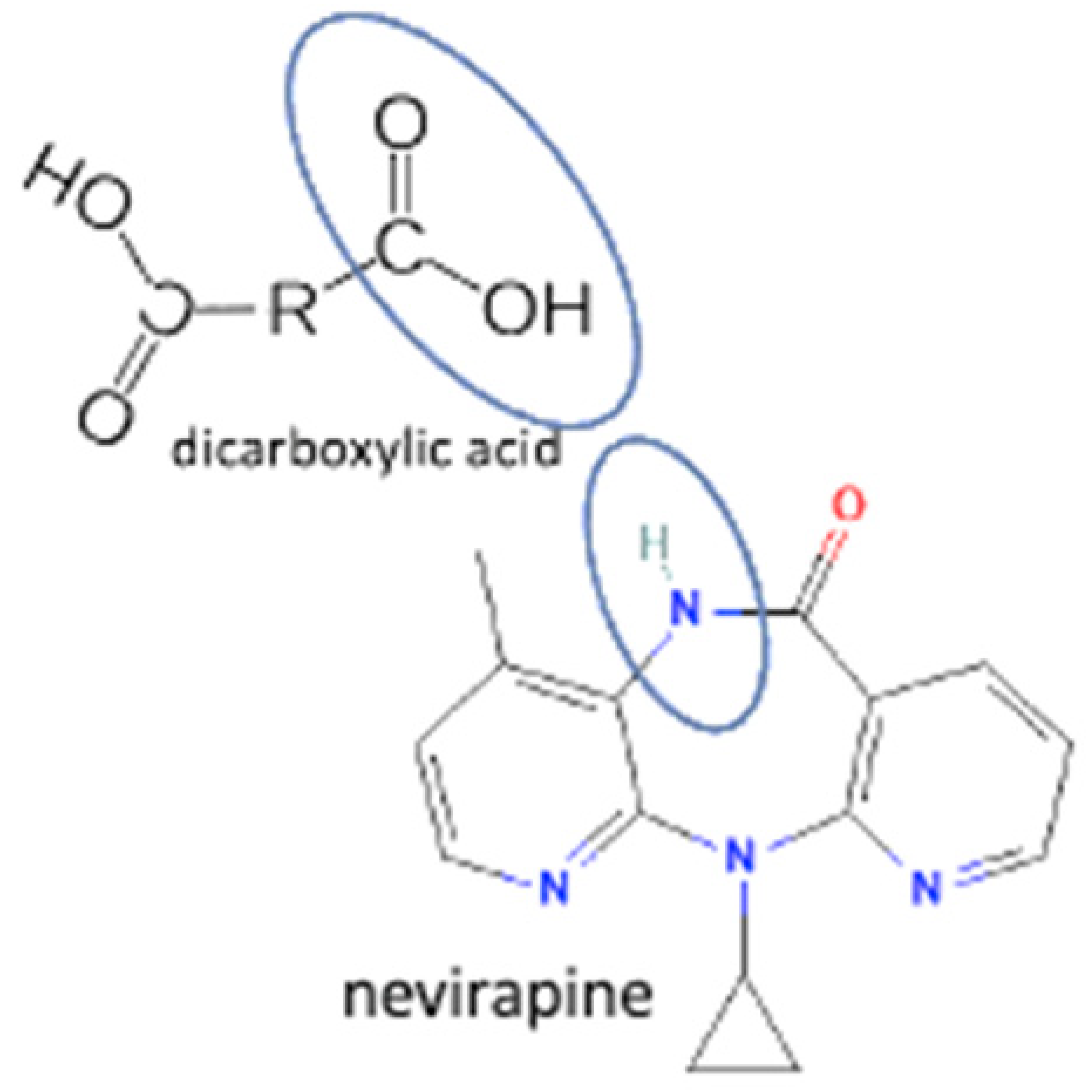 | Hydrogen bond formation, dissolution rate enhancement | liquid-assisted grinding | [83] | |
| 40 | Nevirapine-maleic acid | Hydrogen bond formation, dissolution rate enhancement | liquid-assisted grinding | [83] | ||
| 41 | Nevirapine -glutaric acid | Hydrogen bond formation, dissolution rate enhancement | liquid-assisted grinding | [83] | ||
| 42 | Nevirapine-salicylic acid | Hydrogen bond formation, dissolution rate enhancement | kneading, solution crystallization, antisolvent addition, and solvent drop grinding | [83] | ||
| 43 | Nevirapine -3 hydroxybenzoic acid | 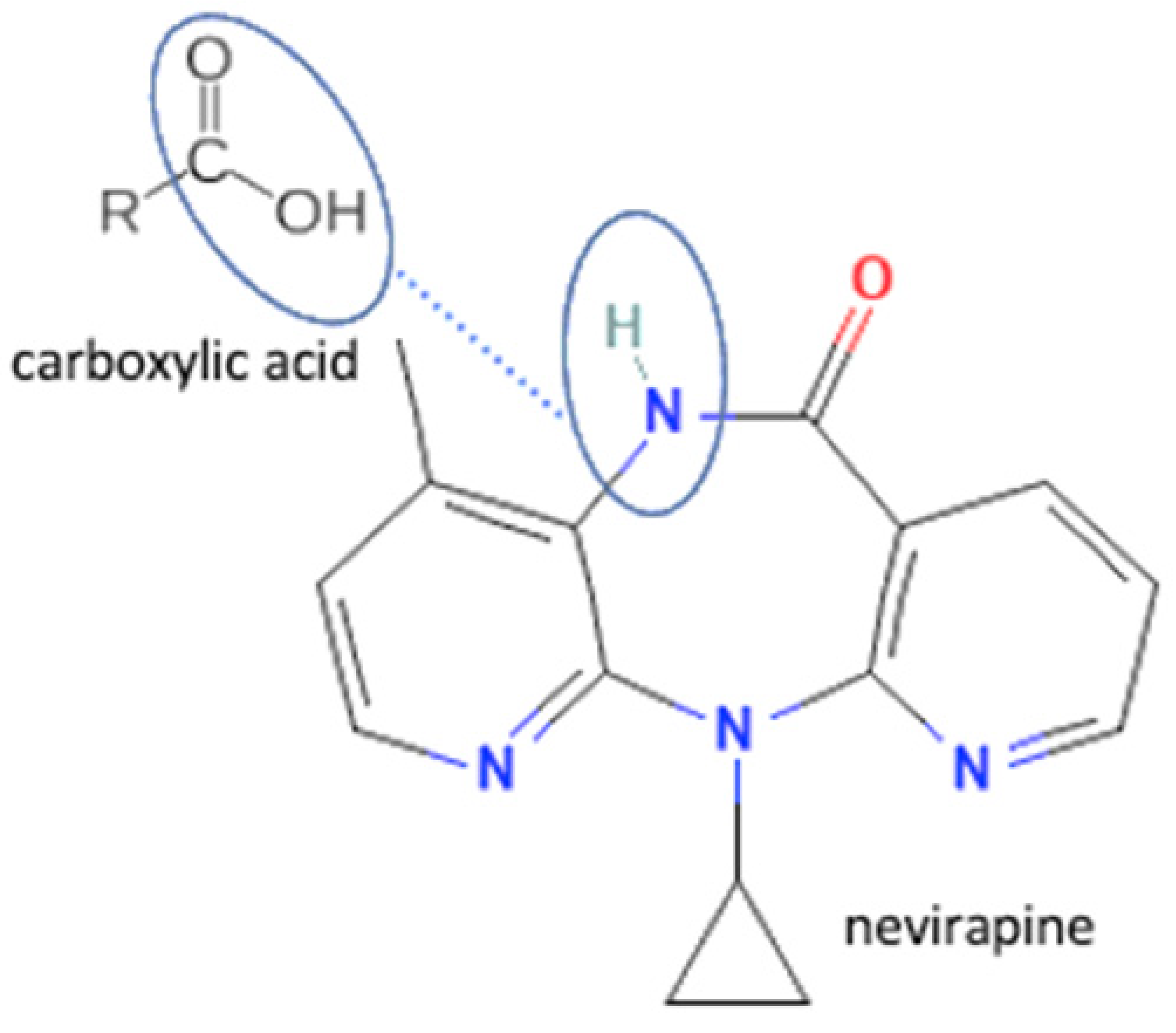 | Hydrogen bond formation, dissolution rate enhancement | liquid-assisted grinding | [83] | |
| 44 | Nevirapine -4 hydroxybenzoic acid | Hydrogen bond formation, dissolution rate enhancement | liquid-assisted grinding (LAG) | [83] | ||
| 45 | Nevirapine -theophylline | 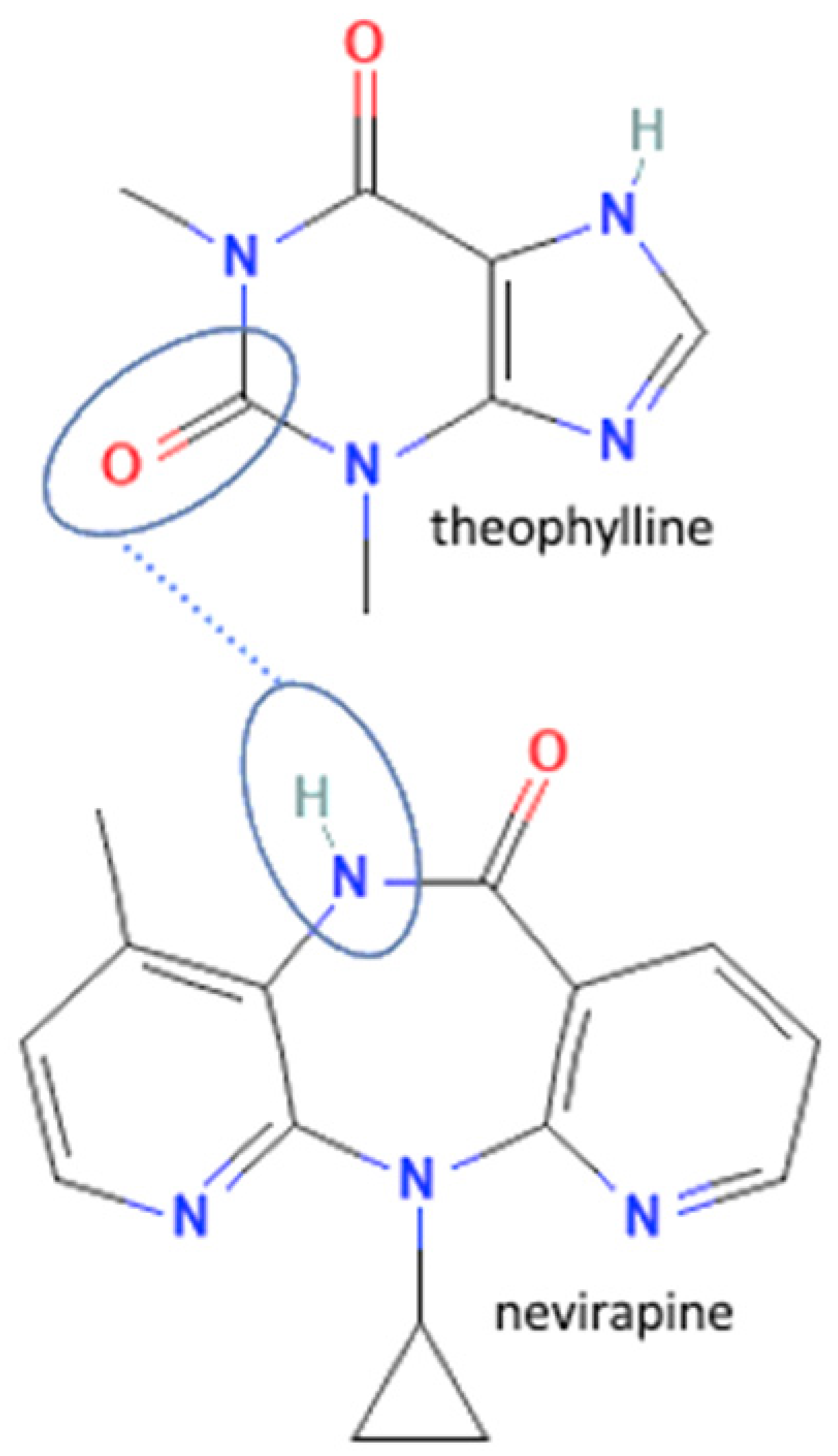 | Hydrogen bond formation, dissolution rate enhancement | liquid-assisted grinding | [83] | |
| 46 | Nevirapine -caffeine | 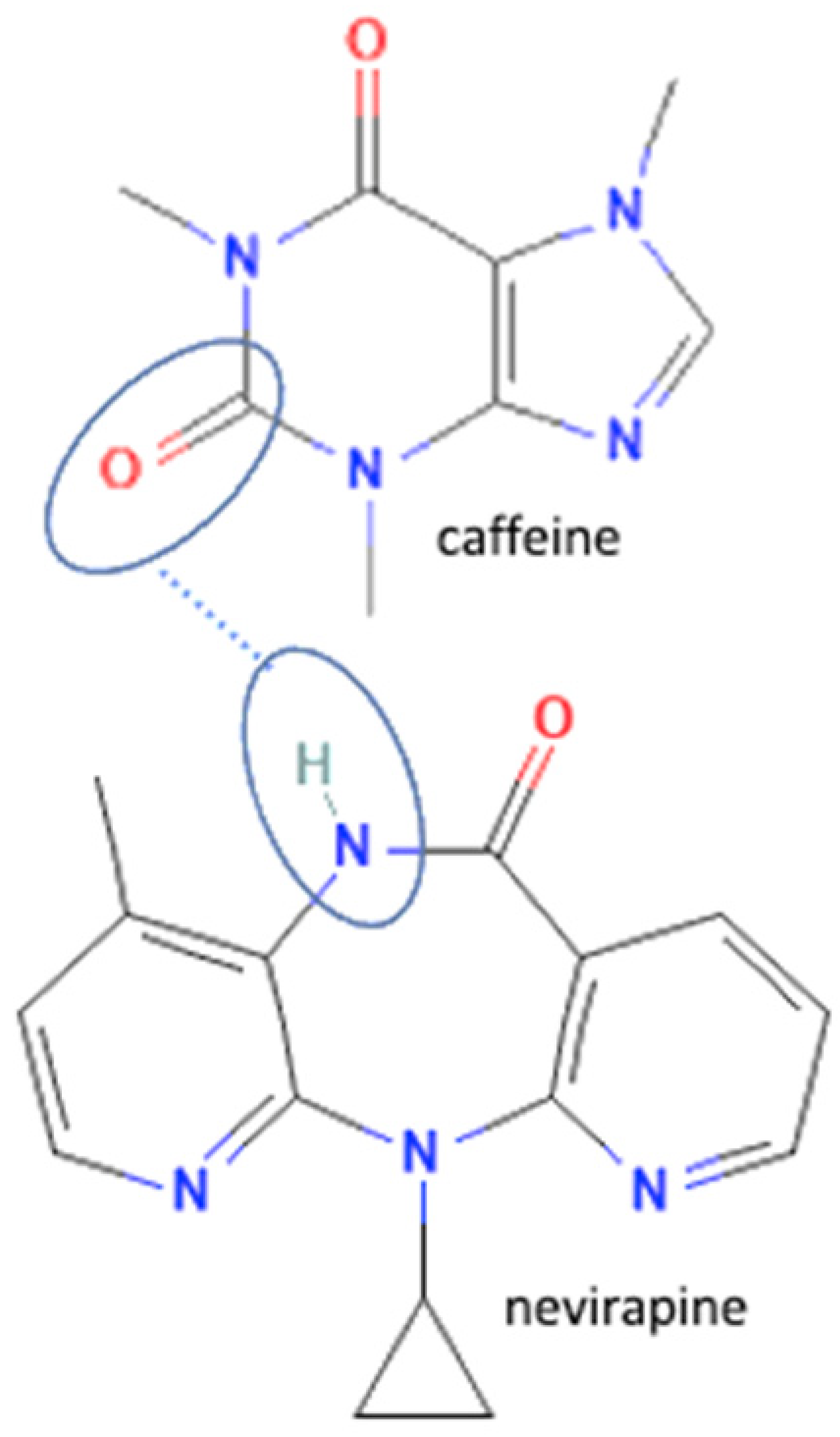 | Hydrogen bond formation, dissolution rate enhancement | liquid-assisted grinding | [83] | |
| 47 | Nitazoxanide-glutaric acid | 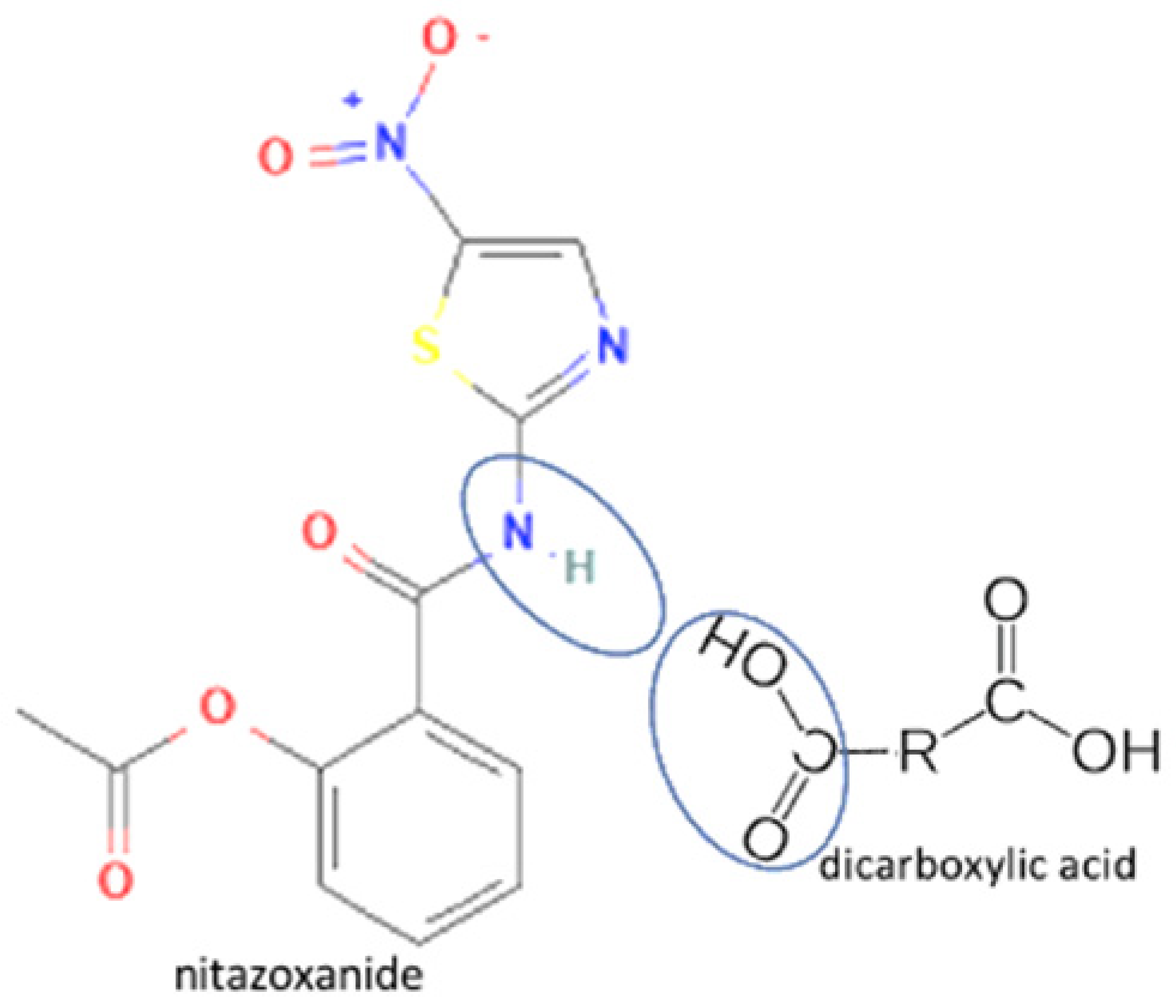 | Dissolution properties enhancement [97] | Neat grinding | [77] | |
| 48 | Nitazoxanide -succinic acid | Dissolution properties enhancement [97] | Neat grinding | [77] | ||
| 49 | Increasing stability | Efavirenz-tartaric acid |  | Enhanced the solubility by 1.8-fold and dissolution rate by 1.4 times of efavirenz alone, also increasing stability | [143] | |
| 50 | Efavirenz-adipic acid | Enhanced the solubility by 1.2-fold and dissolution rate by 1.2 times of efavirenz alone, also increasing stability | Slow evaporation | [143] | ||
| 51 | Penciclovir-3.5 hydroxybenzoic acid | 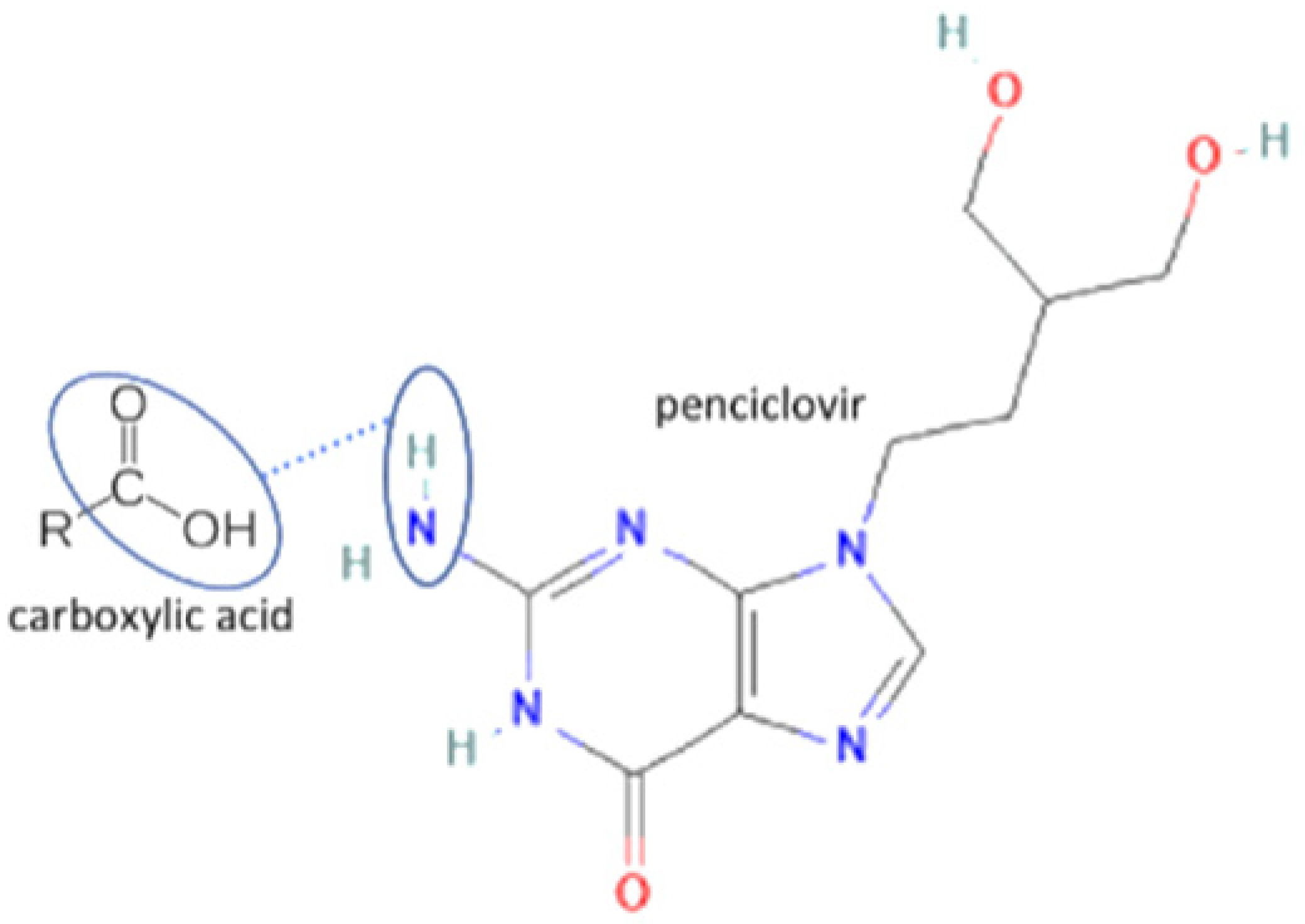 | Increasing the solubility by 129% and increasing the stability of penciclovir | Slurry method | [55] | |
| 52 | Penciclovir-gallic acid | Increasing the solubility by 29% and increasing the stability of penciclovir | Liquid-assisted grinding and slurry | [55] | ||
| 53 | Dipyridamole-tartaric acid | 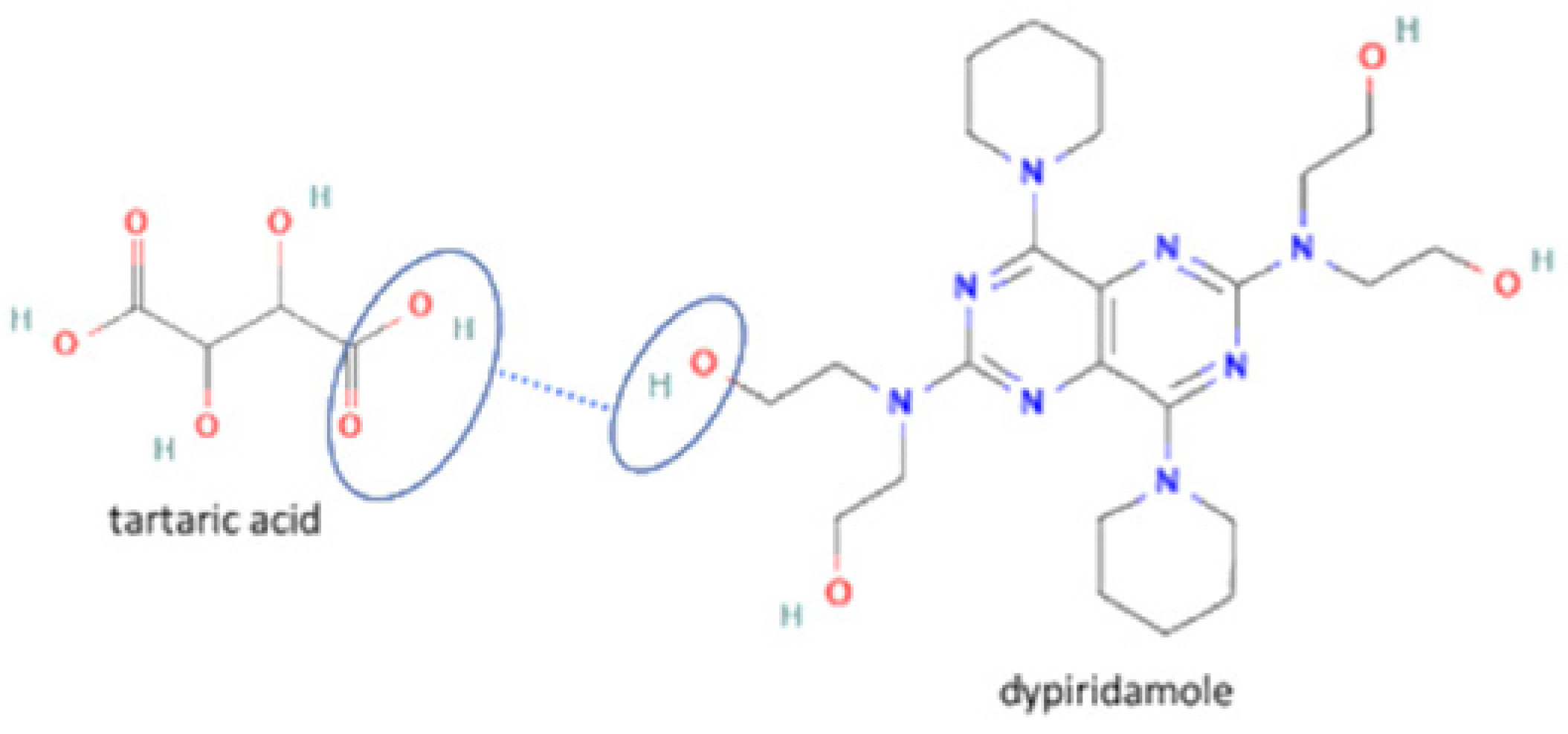 | Solubility and stability improvement of dipyridamole alone | Liquid assisted grinding | [144] | |
| 54 | Adefovir-gallic acid | 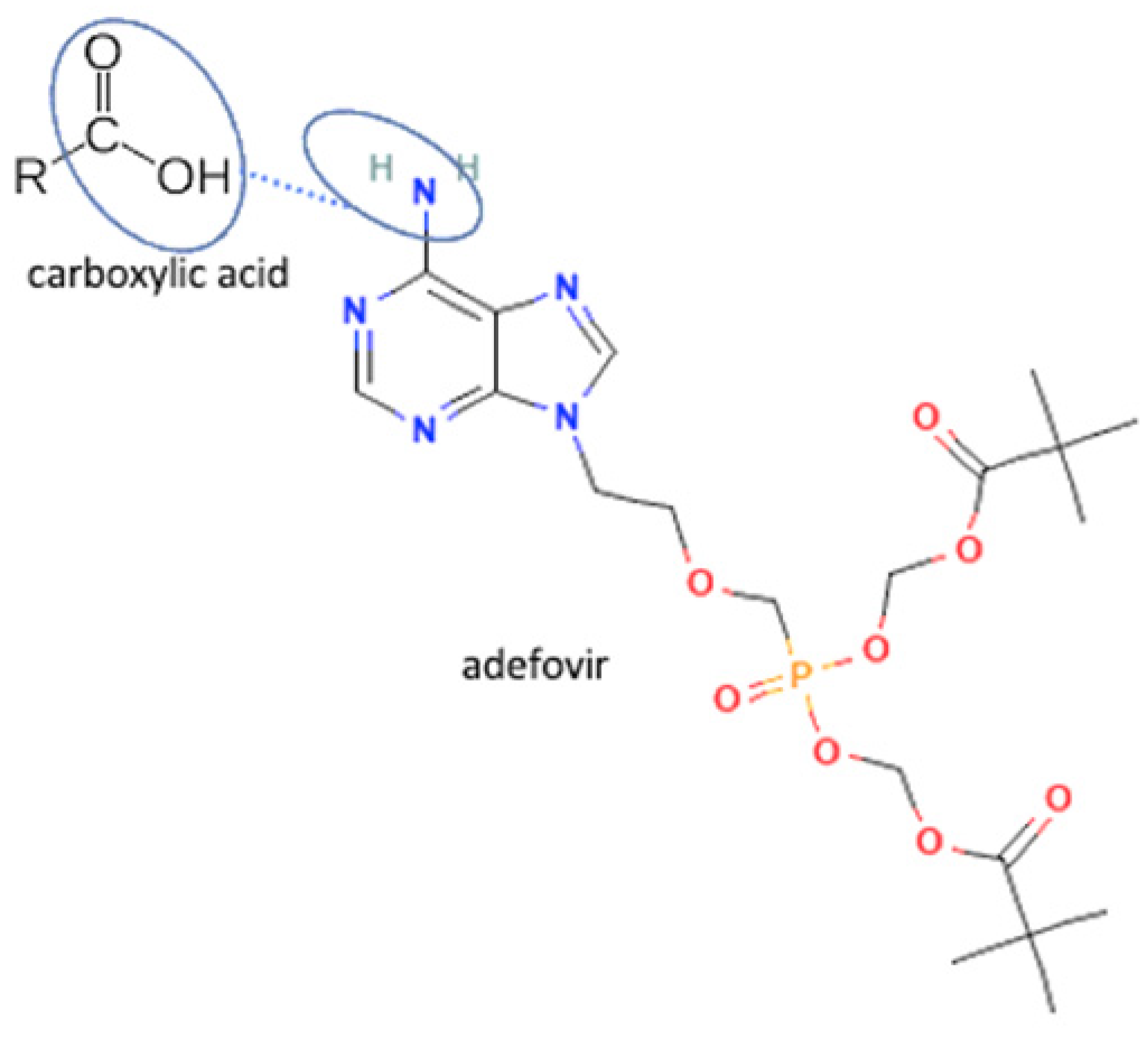 | Stability improvement | Liquid assisted grinding | [37] | |
| 55 | Adefovir-salicylate | Stability improvement | Slow evaporation | [37] | ||
| 56 | Adefovir-saccharin | 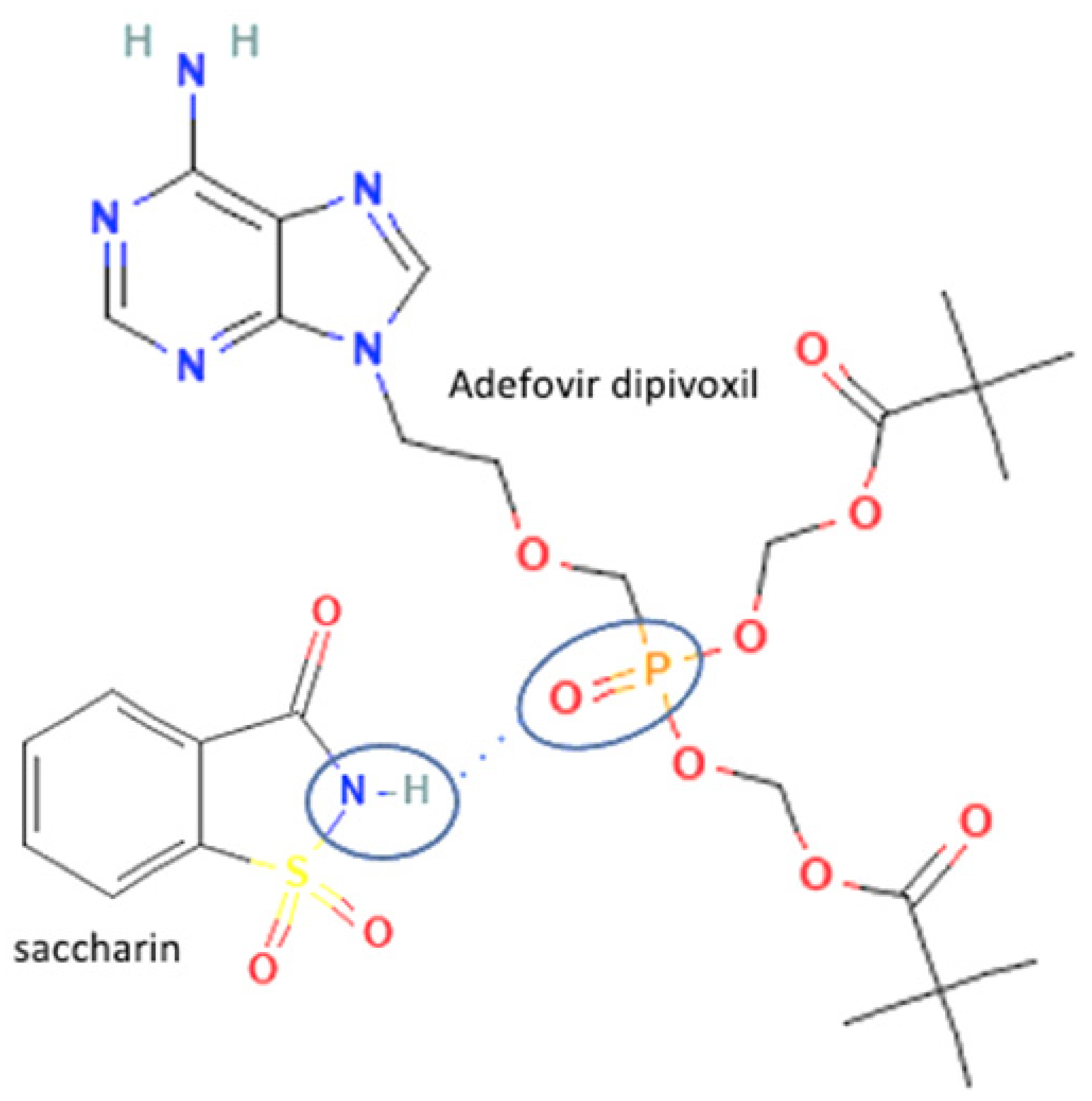 | Shelf-life enhancement of adevofir | Slow evaporation | [37] | |
| 57 | Acyclovir-fumaric acid | 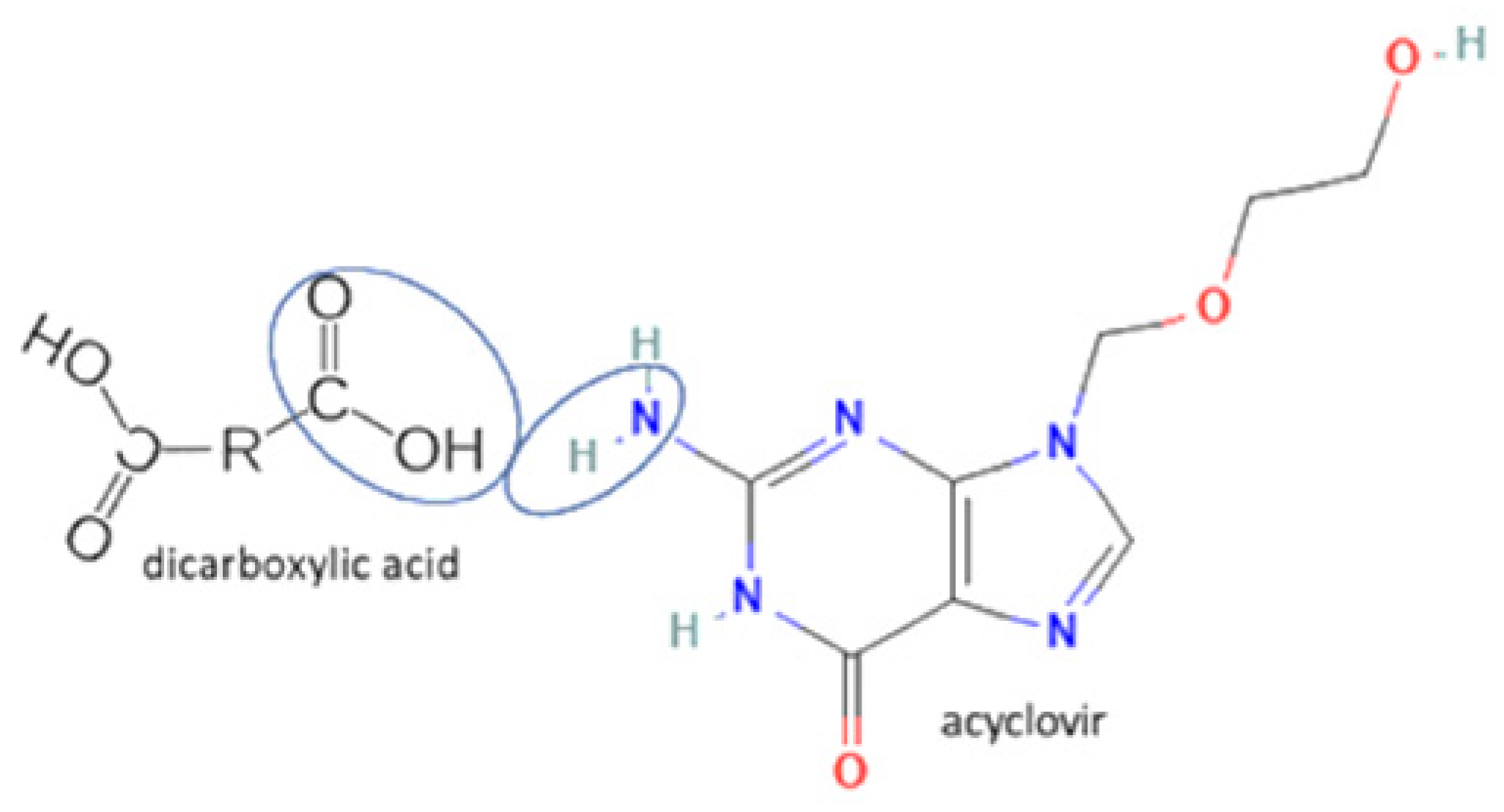 | Cocrystal stability improvement, solubility improvement, dissolution profile improvement. | Solution evaporation and grinding technique | [140] | |
| 58 | Acyclovir-maleic acid | Cocrystal stability improvement, solubility improvement acyclovir release from the higher crystal | Solution evaporation and grinding technique | [140] | ||
| 59 | Lamivudine-theophylline (polymorph 1) | 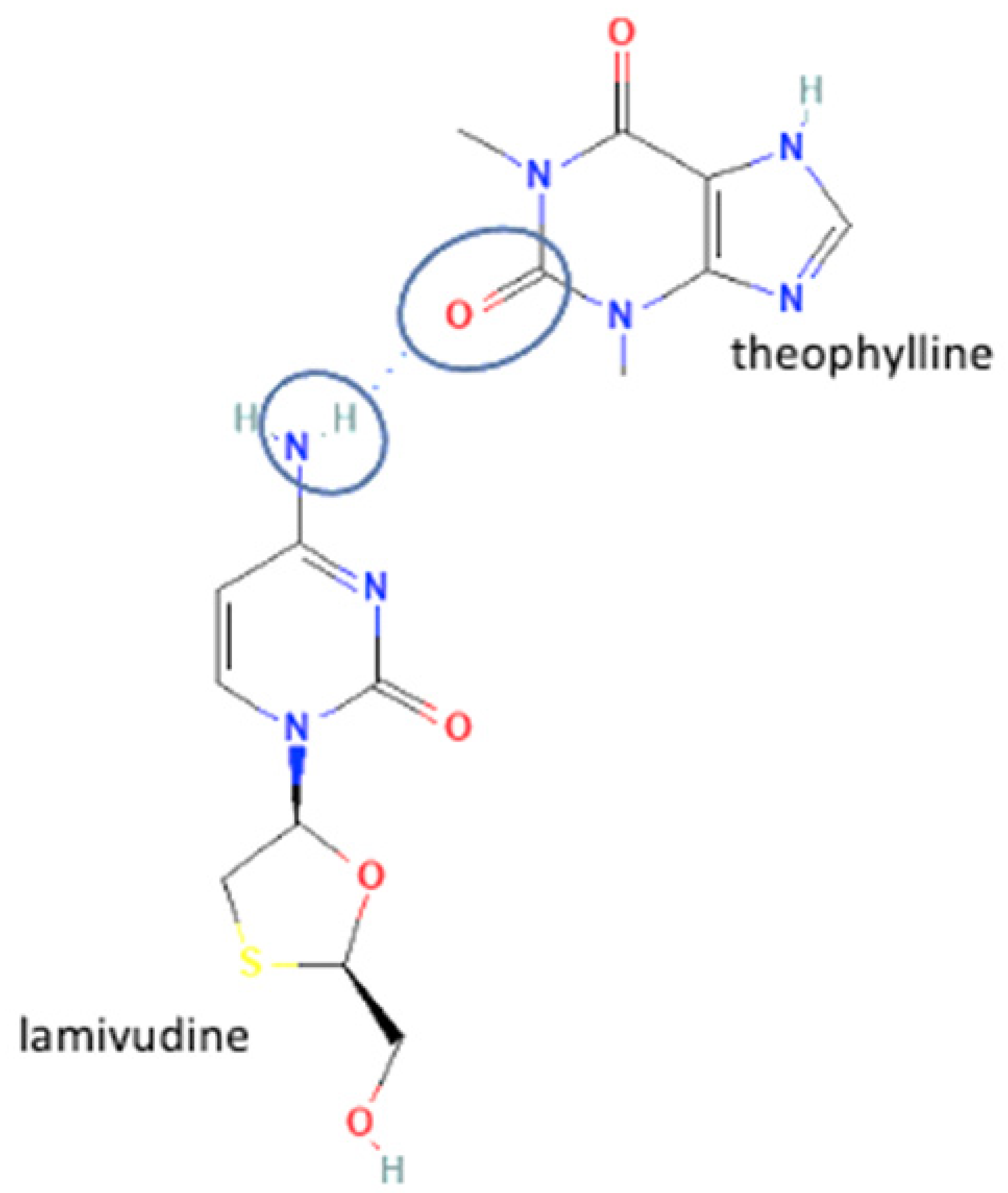 | Maintaining the stability of lamivudine | Neat grinding and liquid-assisted grinding | [62] | |
| 60 | Zidovudine-picric acid | 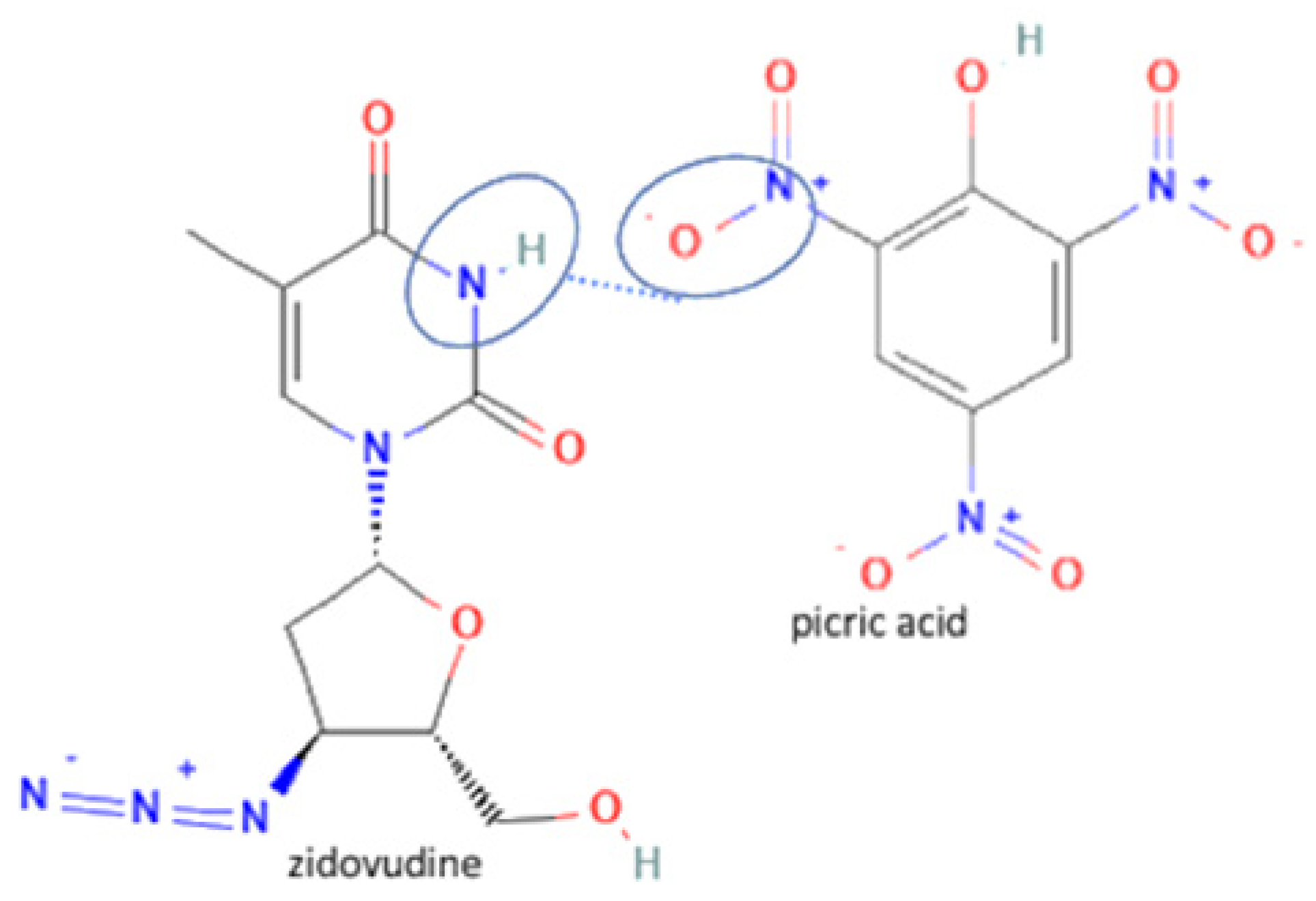 | Stability improvement at 129 °C | Slow evaporation | [62] | |
| 61 | Increasing stability toward moisture | Favipiravir-theophylline | 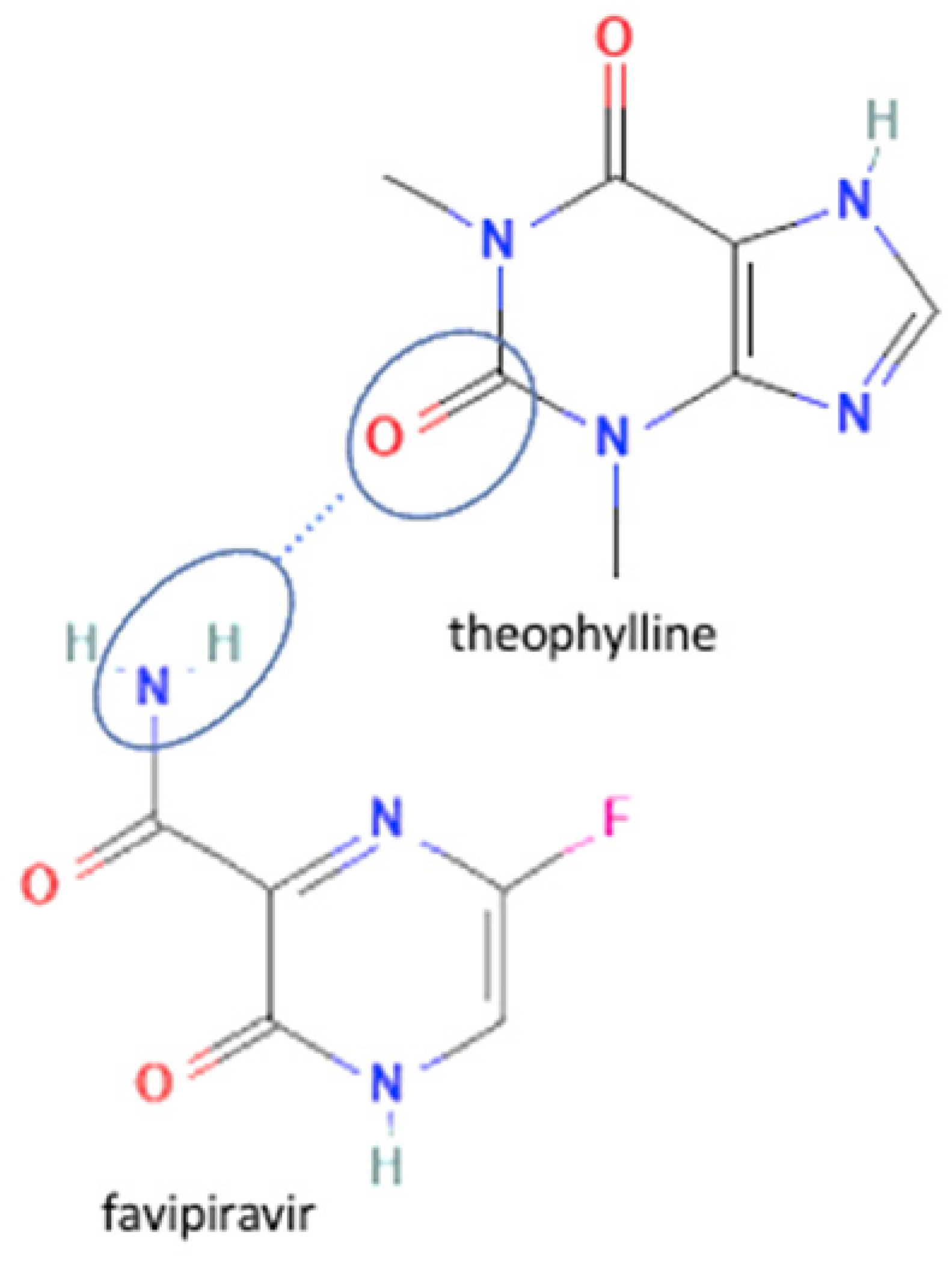 | Solubility improvement of favipiravir in distilled water and buffer phosphate pH 7, stability improvement towards moisture of favipiravir | Liquid assisted grinding | [131] |
| 62 | Increasing bioavailability | Amantadine hydrochloride-resveratrol |  | Increasing solubility and bioavailability 152 and 9.64 times compared to resveratrol alone, also achieving a synergistic antiviral efficacy. | Liquid-assisted grinding and solvent ultrasonic | [145] |
| 63 | Ribavirin-3.5 dihydroxy benzoic acid | 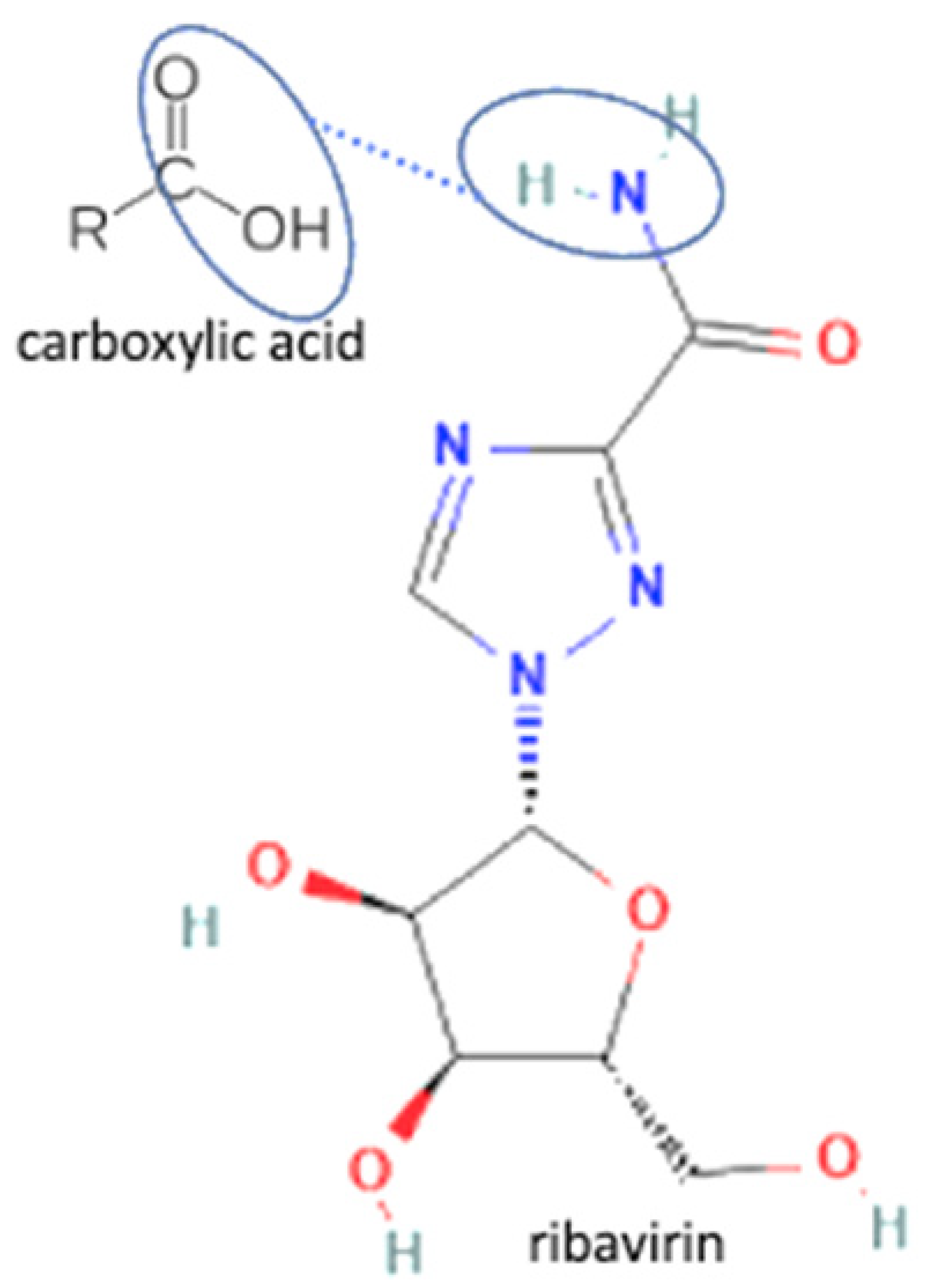 | Enhance drug release of riboflavin | Slurry method | [146] | |
| 64 | Ribavirin-gallic acid | Enhance drug release of riboflavin | Slurry method | [146] | ||
| 65 | Ribavirin-barbituric acid |  | Enhance drug release of riboflavin | Slurry method | [146] | |
| 66 | Emodin-nicotinamide acid |  | Drug release enhancement, stability towards humidity, and high-temperature stability improvement of emodin | Hot melt extrusion | [70] | |
| 67 | Increasing Penetrability | Sulfathiazole-amantadine hydrochloride | 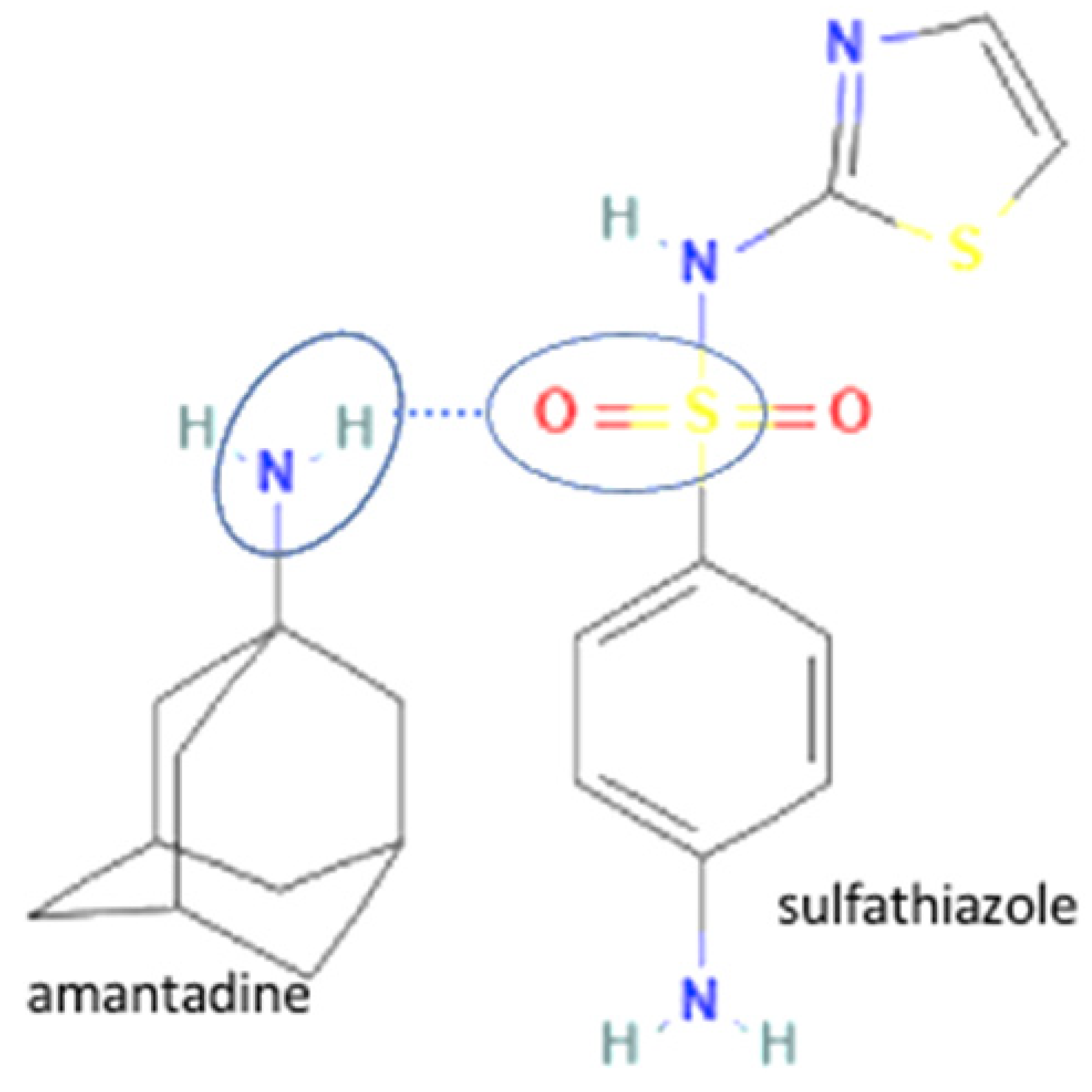 | Water solubility improvement 1.83–5.23 times and 2-fold enhancement in penetrability of sulfathiazole alone | Liquid-assisted grinding and solvent evaporation | [147] |
| 68 | Increasing antiviral efficacy | Amantadine hydrochloride-ferulic acid |  | Dissolution improvement of amantadine hydrochloride 2–3 folds and enhanced the antiviral effects with a combination index <1 | Liquid-assisted grinding and solvent evaporation | [148] |
| 69 | Increasing permeability | Favipiravir-saccharin |  | Enhanced the permeability and tablet ability of favipiravir | Liquid assisted grinding | [134] |
| 70 | Favipiravir-5 fluorouracil | 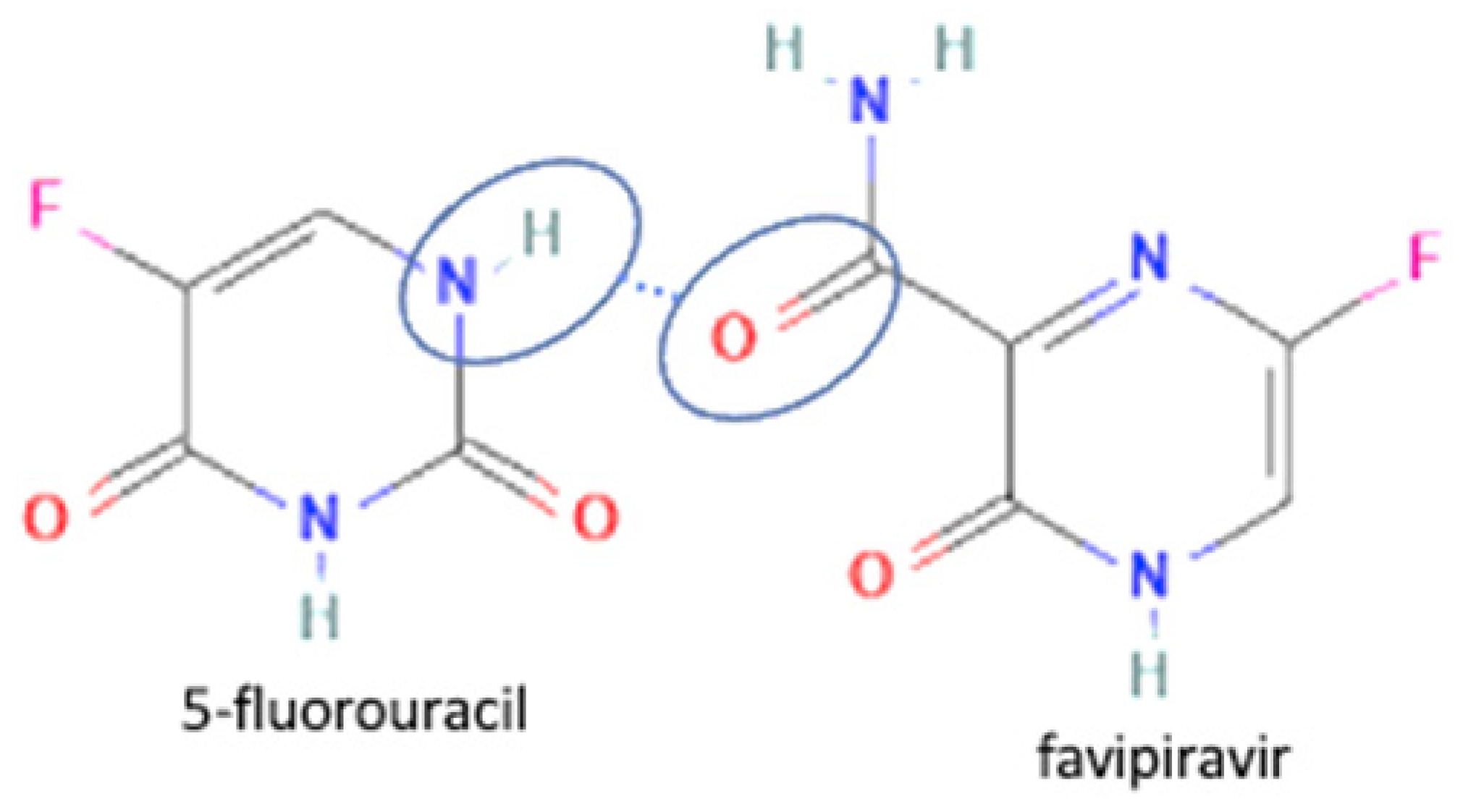 | Enhanced the permeability and tablet ability of favipiravir | Liquid assisted grinding | [134] | |
| 71 | Abacavir-azelaic acid |  | Enhancing the aqueous solubility and permeability rate of abacavir | Solvent evaporation | [135] | |
| 72 | Abacavir-suberic acid | Enhancing the aqueous solubility and permeability rate of abacavir | Solvent evaporation | [135] | ||
| 73 | Amantadine-sulfamethoxazole |  | Permeability and dissolution improvement over the bulk drug and the bacterial activity of sulfamethoxazole are getting stronger. | Liquid-assisted grinding and solvent evaporation | [148] | |
| 74 | Increasing powder properties | Efavirenz-fumaric acid | 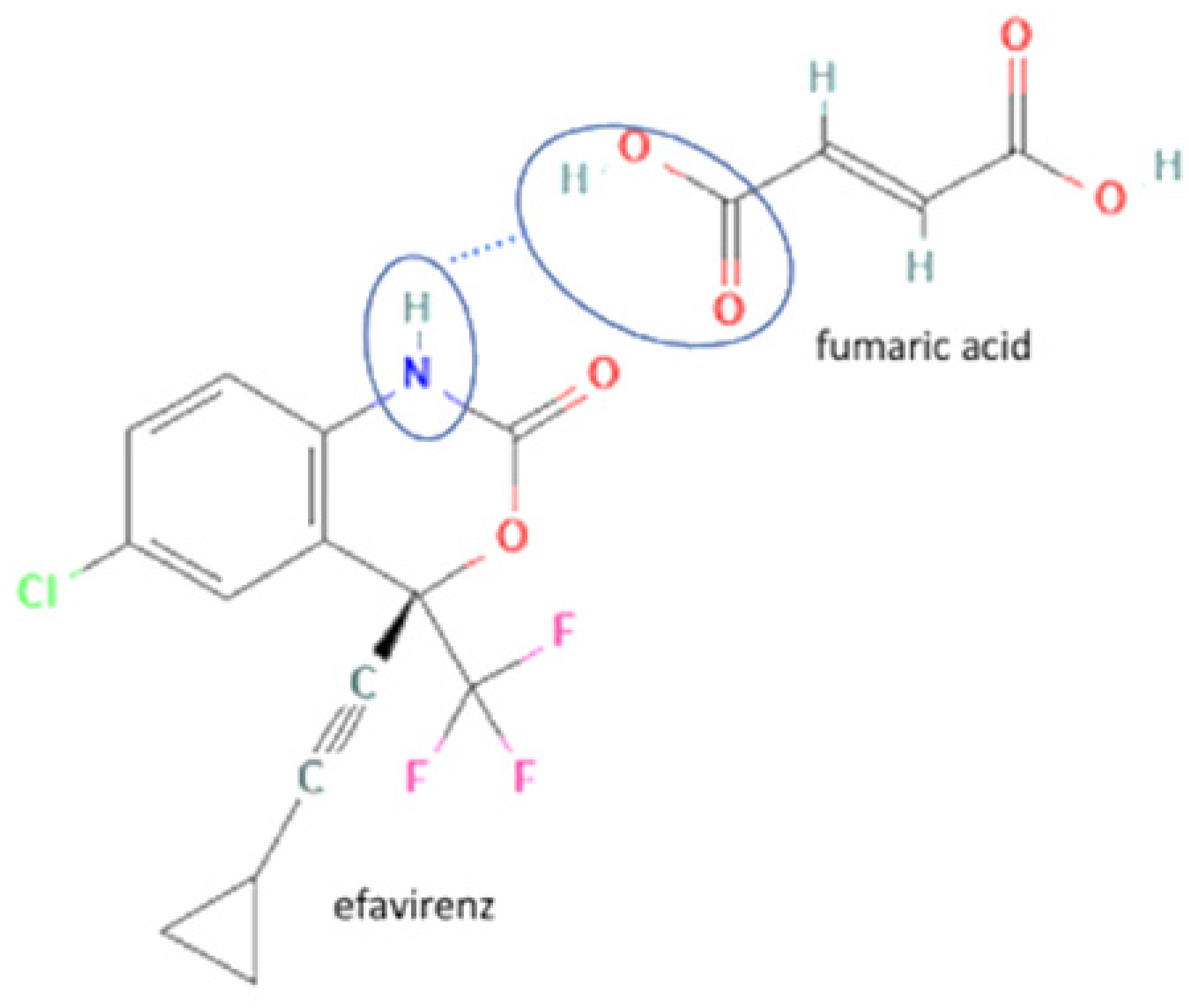 | Increasing the powder flow properties, solubility, and dissolution profile of efavirenz. | Neat grinding | [149] |
| 75 | Decreasing hygroscopicity | Penciclovir-4 hydroxycinnamic acid | 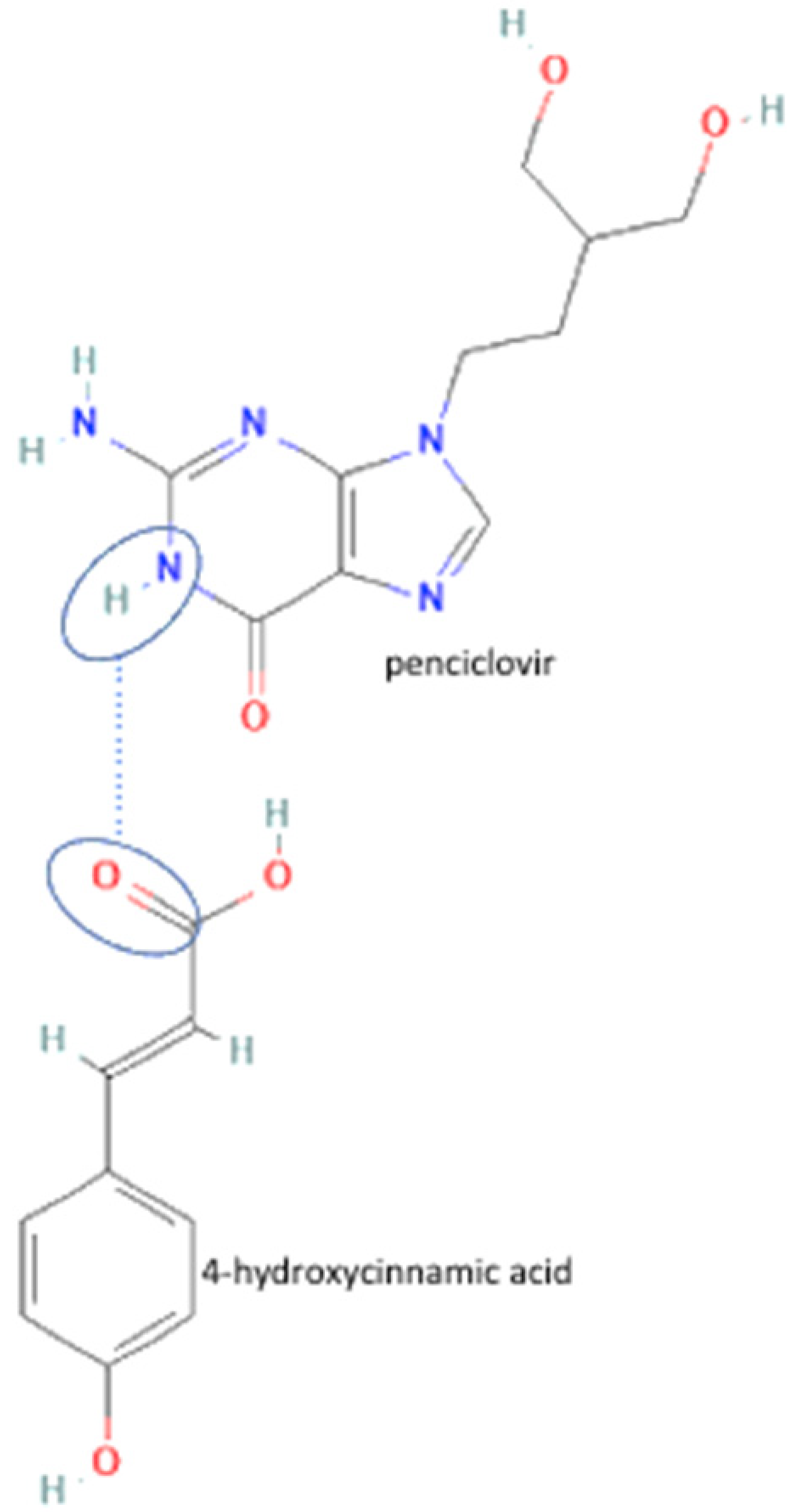 | Decreasing hygroscopicity of penciclovir | Liquid-assisted grinding and slurry | [55] |
| 76 | Adefovir-maleic acid |  | Decreasing hydration of adefovir | Slow evaporation | [37] | |
| 77 | Crystal energy improvement | Arbidol-salicylic acid-CHCl3 | 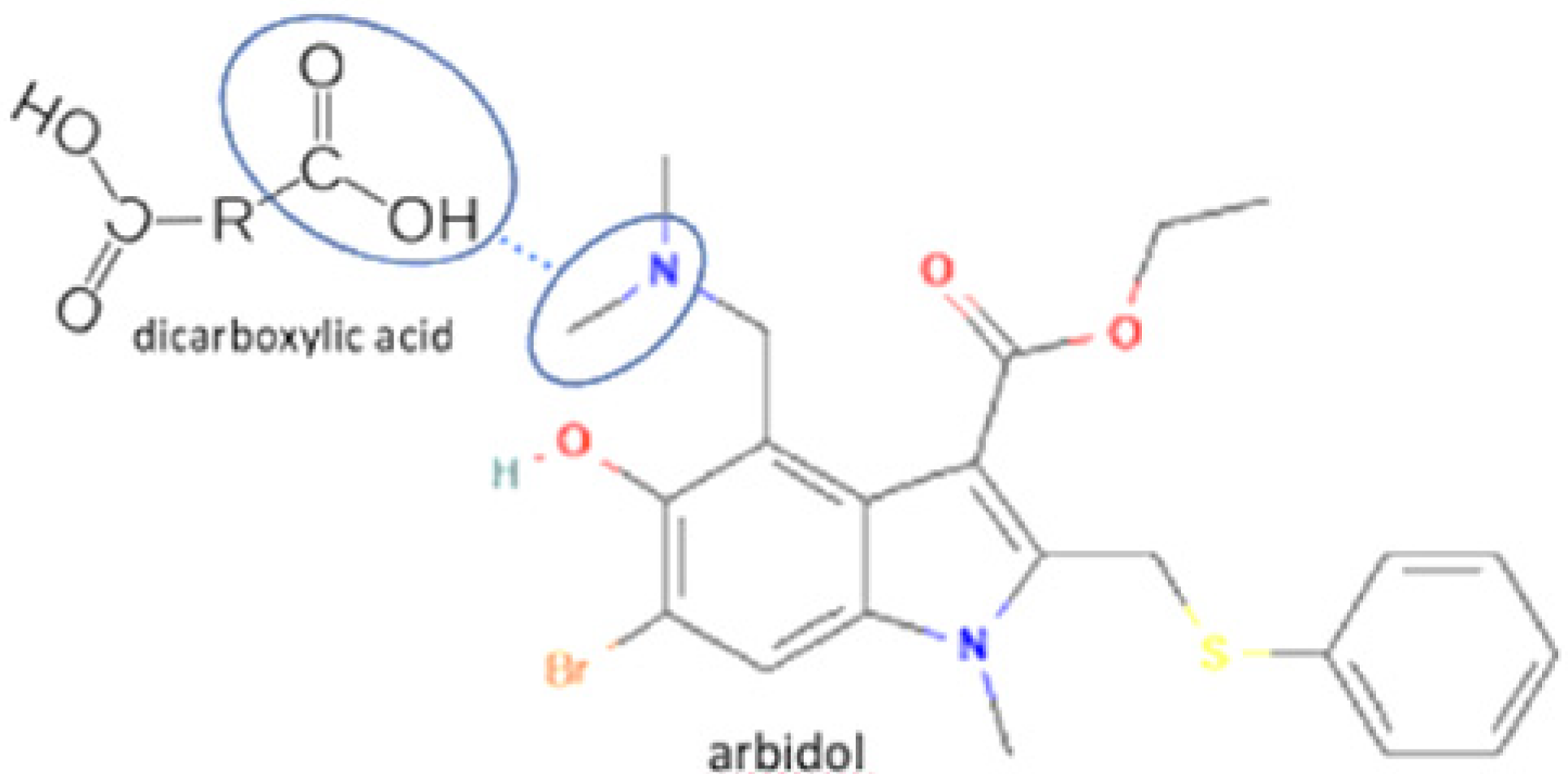 | Lowering crystal energy to gain superior crystal energy, stronger solvent bond | Cooling cocrystallization | [40] |
| 78 | Arbidol-maleic acid | Lowering crystal energy to gain superior crystal energy, stronger solvent bond | Cooling cocrystallization | [40] | ||
| 79 | Arbidol-gallic acid | Lowering crystal energy to gain superior crystal energy, stronger solvent bond | Cooling cocrystallization | [40] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perles, J. Characterisation and Study of Compounds by Single Crystal X-ray Diffraction. Crystals 2020, 10, 934. [Google Scholar] [CrossRef]
- Cedric, S.G.; Felipe, V.R.; Kamilla, R.R.; Arthur, E.K. Multicomponent reactions for thee synthesis of bioactive compounds: A review. Curr. Org. Synth. 2019, 16, 855–899. [Google Scholar]
- Bolla, G.; Chernyshev, V.; Nangia, A. Acemetacin cocrystal structures by powder x-ray diffraction. Int. Euro. J. 2017, 4, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Budziak-Wieczorek, I.; Maciolek, U. Synthesis and characterization of a (-)-epicatechin and barbituric acid cocrystal: Single crystal x-ray diffraction and vibrational spectroscopic stduies. ACS Omega 2021, 6, 8199–8209. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.O.; Voronin, A.P.; Drozd, K.V.; Volkova, T.V.; Vasilev, N.; Batov, D.; Churakov, A.V.; Perlovich, G.L. Extending the Range of Nitrofurantoin Solid Forms: Effect of Molecular and Crystal Structure on Formation Thermodynamics and Physicochemical Properties. Cryst. Growth Des. 2022, 22, 2569–2586. [Google Scholar] [CrossRef]
- Li, S.; Yu, T.; Tian, Y.; McCoy, P.C.; Jones, S.D.; Andrews, P.G. Mechanochemical Synthesis of Pharmaceutical Cocrystal Suspensions via Hot Melt Extrusion: Feasibility Studies and Physicochemical Characterization Molecular. Pharmaceutics 2016, 13, 3054–3068. [Google Scholar] [CrossRef] [Green Version]
- Sanphui, P.; Tothadi, S.; Ganguly, S.; Desiraju, R.G. Salt and Cocrystals of Sildenafil with Dicarboxylic Acids: Solubility and Pharmacokinetic Advantage of the Glutarate Salt. Mol. Pharm. 2013, 10, 4687–4697. [Google Scholar] [CrossRef]
- Yunxia, Y.; Huihui, N.; Shiying, X.; Yingwa, G.; Xiangxiang, W. Solubility and dissolution rate of progesterone cocrystals using 4-fluorobenzoic acid and 2-hydroxy-6-naphthoic acid as coformers. J. Cryst. Growth 2022, 585, 126601. [Google Scholar] [CrossRef]
- Nugrahani, I.; Fisandra, F.; Horikawa, A.; Uekusa, H. New Sodium Mefenamate–Nicotinamide Multicomponent Crystal Development to Modulate Solubility and Dissolution: Preparation, Structural, and Performance Study. J. Pharm. Sci. 2021, 110, 3246–3260. [Google Scholar] [CrossRef]
- Nugrahani, I.; Tjengal, B.; Gusdinar, T.; Horikawa, A.; Uekusa, H. A Comprehensive Study of a New 1.75 Hydrate of Ciprofloxacin Salicylate: SCXRD Structure Determination, Solid Characterization, Water Stability, Solubility, and Dissolution Study. Crystals 2020, 10, 349. [Google Scholar] [CrossRef]
- Nugrahani, I.; Utami, D.; Ibrahima, I.; Nugraha, P.Y.; Uekusa, H. Zwitterionic cocrystal of diclofenac and l-proline: Structure determination, solubility, kinetics of cocrystallization, and stability study. Europ. J. Pharm. Sci. 2018, 117, 168–176. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Q.; Zhang, Z.; Ding, Q.; Mei, X. Improving Stability of Vitamin B5 Through Double Salt Formation. Cryst. Growth 2021, 21, 4997–5005. [Google Scholar] [CrossRef]
- Lecoq, H. Discovery of the first virus, the tobacco mosaic virus: 1892 or 1898? Comptes Rendus L’academie Sci. Ser. III Sci. Vie 2001, 324, 929–933. [Google Scholar]
- Wen, L.; Xia, N.; Chen, X.; Li, Y.; Hong, Y.; Liu, J.Y.; Wang, Z.; Liu, Y.J. Activity of antibacterial, antiviral, anti-inflammatory in compounds andrographolide salt. Euro. J. Pharm. 2014, 740, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.L.C.S.; Fernandes, P.R.; Charpentier, D.M.; Horst, H.J.; Caires, J.F.; Marlus, C. Co-crystals of non-steroidal anti-inflammatory drugs (NSAIDs): Insight toward formation, methods, and drug enhancement. Particuology 2021, 58, 227–241. [Google Scholar] [CrossRef]
- Chen, L.; Hu, C.; Hood, M.; Zhang, X.; Zhang, L.; Kan, J.; Du, J. A Novel Combination of Vitamin C, Curcumin and Glycyrrhizic Acid Potentially Regulates Immune and Inflammatory Response Associated with Coronavirus Infections: A Perspective from System Biology Analysis. MPI 2020, 12, 1193. [Google Scholar] [CrossRef]
- Maeno, Y.; Fukami, T.; Kawahata, M.; Yamaguchi, K.; Tagami, T.; Ozeki, T.; Suzuki, T.; Tomono, K. Novel pharmaceutical cocrystal consisting of paracetamol and trimethylglycine, a new promising cocrystal former. Int. J. Pharm. 2014, 473, 179–186. [Google Scholar] [CrossRef]
- Hiendrawan, S.; Veriansyah, B.; Widjojokusumo, E.; Soewandhi, S.N.; Wikarsa, S.; Tjandrawinata, R.R. Physicochemical and mechanical properties of paracetamol cocrystal with 5-nitroisophthalic acid. Int. J. Pharm. 2016, 497, 106–113. [Google Scholar] [CrossRef]
- Gadade, D.D.; Kulkarni, D.A.; Rathi, P.B.; Pekamwar, S.S.; Joshi, S.S. Solubility enhancement of lornoxicam by crystal engineering. J. Pharm. Sci. 2017, 79, 277–286. [Google Scholar] [CrossRef]
- Bhatt, P.M.; Azim, Y.; Thakur, T.S.; Desiraju, G.R. Co-crystals of the anti-HIV drugs lamivudine and zidovudine. Cryst. Growth Des. 2009, 9, 951–957. [Google Scholar] [CrossRef]
- Blazecka, P.G.; Che, D.; McPhail, C.L.; Rey, A.W. Salt form and cocrystal of adevofit dipivoxil and processes for preparation thereof. United States Patent US 7,935,817 B2, 3 May 2011. [Google Scholar]
- Ferreira, L.T.; Alarcon, R.T.; Perpetuo, G.L.; Bannach, G. Investigation and characterization by TG/DTG-DTA and DSC of the fusion of riboflavin, and its interaction with the antibiotic norfloxacin in the screening of cocrystal. J. Therm. Anal. Cal. 2019, 136, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Pi, J.; Wang, S.; Li, W.; Kebebe, D.; Zhang, Y.; Zhang, B.; Qi, D.; Guo, P.; Liu, Z. A nano-crystal strategy to improve the dissolution rate and oral bioavailability of baicalein. Asian J. Pharm. 2019, 14, 154–164. [Google Scholar]
- Clercq, E.D. Antiviral drugs in current clinical use. J. Clin. Virol. 2004, 30, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Clercq, E.D.; Li, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vardanyan, R.; Hruby, V. Antiviral Drugs: Synthesis of Best-Seller Drugs; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Mahajan, S.; Choudhary, S.; Kumar, P.; Tomar, S. Antiviral strategies targeting host factors and mechanisms obliging +ssRNA viral pathogens. Bioorg. Med. Chem. 2021, 46, 116356. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kumar, P.; Vyas, R.K.; Pandit, P.; Dalai, A.K. Occurance and removal of antiviral drugs in environment: A review. Water Air Soil Pollut. 2013, 224, 1410. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 135398513, Acyclovir. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acyclovir (accessed on 4 December 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 563244, Indinavir. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Indinavir#section=Structures (accessed on 4 December 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 4463, Nevirapine. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nevirapine#section=2D-Structure (accessed on 4 December 2022).
- Le Pevelen, D.D.; Tranter, G.E. Encyclopedia of Spectroscopy and Spectrometry: FT-IR and Raman Spectroscopies, Polymorphism Applications, 3rd ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 750–761. [Google Scholar]
- Duan, W.; Liu, B.; Gong, N.; Famulari, A.; Guo, F. Polymorphs and Transformations of the Solid Forms of Organic Salts of 5-Sulfosalicylic Acid and Isonicotinamide. Cryst. Growth Des. 2020, 20, 7606–7614. [Google Scholar] [CrossRef]
- Grothe, E.; Meekes, H.; Vlieg, E.; Horst, J.H.; Gelder, R. Solvates, Salts, and Cocrystals: A Proposal for a Feasible Classification System. Cryst. Growth Des. 2016, 16, 3237–3243. [Google Scholar] [CrossRef] [Green Version]
- Caira, M.R. Handbook of Thermal Analysis and Calorimetry: Polymorphism; Elsevier Science B.V: Amsterdam, The Netherlands, 2008; Volume 5, Chapter 16; pp. 597–629. [Google Scholar]
- Babu, R.K.; Rao, K.V.; Kumar, N.Y.; Polireddy, K.; Subbaiah, V.K.; Bhaskar, M.; Lokanatha, V.; Raju, N.C. Identification of substituted [3, 2-a] pyrimidines as selective antiviral agents: Molecular modeling study. Antivir. Res. 2012, 95, 118–127. [Google Scholar] [CrossRef]
- Lin, R.Z.; Sun, P.J.; Tao, Q.; Yao, J.; Chen, J.M.; Lu, T.B. Mechanism study on stability enhancement of adefovir dipivoxil by cocrystallization: Degradation kinetics and structure-stability correlation. Eur. J. Pharm. Sci. 2016, 85, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Deb, P.K.; Abed, S.N.; Maher, H.; Al-Aboudi, A.; Paradkar, A.; Bandopadhyay, S.; Tekade, R.K. Drug Delivery Systems: Arosols in Pharmaceutical Product Development; Academic Press: Cambridge, MA, USA, 2020; Chapter 11; pp. 521–577. [Google Scholar]
- Pathak, K. Advance and Challenges in Phamaceutical Technology: Effective Formulation Strategies for Poorly Water Soluble Drugs; Academic Press: Cambridge, MA, USA, 2021; Chapter 6; pp. 181–228. [Google Scholar]
- Surov, A.O.; Manin, A.N.; Churakov, V.; Perlovich, L. New solid forms of the antiviral drug arbidol: Crystal structures, thermodynamic stability, and solubility. Mol. Pharm. 2015, 12, 4154–4165. [Google Scholar] [CrossRef] [PubMed]
- Aitipamula, S.; Tan, B.H. Pharmaceutical co-crystals: Crystal engineering and applications. In Multi-Component Crystals: Synthesis, Concepts, Function; Tiekink, E.R., Schpector, J.Z., Eds.; De Gruyter: Berlin, Germany, 2018; pp. 1–27. [Google Scholar]
- Duggirala, N.K.; Perry, M.L.; Almarsson, O.; Zaworotko, M.J. Pharmaceutical cocrystals: Along the path to improved medicine. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Zhang, Y.; Na, B.; Liu, Z.; Zhou, L.; Hao, H. Solubility Determination of Nicotinamide and Its Application for the Cocrystallization with Benzoic Acid. J. Chem. Eng. Data 2018, 63, 4157–4165. [Google Scholar] [CrossRef]
- Nanjwade, V.K.; Manvi, F.V.; Manvi, S.A.; Basavaraj, K.N.; Maste, M.M. New trends in the co-crystallization of active pharmaceutical ingridients. J. Appl. Pharm. Sci. 2011, 1, 1–5. [Google Scholar]
- Nugrahani, I. Sustainable pharmaceutical preparation methods and solid state analysis supporting green pharmacy. Pharm. Anal. 2020, 17, 969–982. [Google Scholar] [CrossRef]
- Nugrahani, I.; Utami, D.; Permana, B.; Ibrahim, S. Development of the NSAID-L-Proline amino acid. J. Appl. Pharm. Sci. 2018, 8, 57–63. [Google Scholar]
- Kuminek, G.; Cao, F.; Rocha, A.B.O.; Cardoso, S.G.; Hornedo, N.R. Cocrystal to facilitate delivery of poorly soluble compoundsbeyond-rule-of-5. Adv. Drug Deliv. Rev. 2016, 101, 143–166. [Google Scholar] [CrossRef] [Green Version]
- Golbedaghi, R.; Fausto, R. Coordination aspects in Schiff bases cocrystal. Polyhedron 2018, 115, 1–12. [Google Scholar] [CrossRef]
- Kent, C.; Rao Khandavili, U.B.; Alfarisi, A.; Hanna-Brown, M.; McSweeney, S.; Lawrence, S.; Fitzpatrick, D. Tracking cocrystallization of active pharmaceutical ingridients with benzoic acids coformer using broadband acoustic resonance dissolution spectroscopy. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Barikah, K.Z. Traditional and novel methods for cocrystal formation: A mini review. Syst. Rev. Pharm. 2018, 9, 79–82. [Google Scholar] [CrossRef]
- Wang, L.Y.; Bu, F.Z.; Li, Y.T.; Wu, Z.Y.; Yan, C.W. A Sulfathiazole-Amantadine Hydrochloride Cocrystal: The First Codrug Simultaneously Comprising Antiviral and Antibacterial Components. Cryst. Growth Des. 2020, 20, 3236–3246. [Google Scholar] [CrossRef]
- Thakuria, R.; Sarma, D. Drug-drug and drug-neutraceutical cocrystal/salt as alternative medicine for combination therapy: A crystal engineering approach. Crystals 2018, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Linberg, K.; Ali, N.Z.; Etter, M.; Michalchuk, A.A.L.; Rdemann, K.; Emmerling, F. Comparative study of the ionic cocrystal NaX. Cryst. Growth Des. 2019, 19, 4293–4299. [Google Scholar] [CrossRef]
- Utami, D.; Nugrahani, I.; Ibrahim, S. Mefenamic Acid-Nicotinamide Co-Crystal Synthesized By Using Melt Crystallization Method And Its Solubility Study. Asian J. Pharm. Clin. Res. 2017, 10, 135–139. [Google Scholar]
- Yuan, Z.J.; Dai, X.L.; Huang, Y.L.; Lu, T.B.; Chen, J.M. Cocrystals of Penciclovir with Hydroxybenzoic Acids: Synthesis, Crystal Structures, and Physicochemical Evaluation. Cryst. Growth Des. 2020, 20, 4108–4119. [Google Scholar] [CrossRef]
- Likhitha, U.; Narayana, B.; Sarojini, B.K.; Kumar, S.M.; Lobo, A.G.; Karthick, T. A study on interwoven hydrogen bonding interactions in new zidovudine-picric acid (1:1) cocrystal through single crystal XRD, spectral and computational methods. J. Mol. Struct. 2020, 1211, 128052. [Google Scholar] [CrossRef]
- Nugrahani, I.; Asyarie, S.; Soewandhi, S.N.; Ibrahim, S. Chemical-Physical Interaction Identification in Amoxicillin Trihydrate-Clavulanate Potassium Antibiotic Combination with Solvent Calorimetry and Recrystallization. Pharm. Mag. 2007, 4. [Google Scholar]
- Melo, A.; Amorim, I.; Cirqueira, M. Toward Novel Solid state Forms of the Anti-HIV Drug Efavirenz: From Low Screening Success to Cocrystals Engineering Strategies and Discovery of a New Polymorph. Cryst. Growth Des. 2013, 13, 1558–1569. [Google Scholar] [CrossRef]
- Nugrahani, I.; Laksana, A.N.; Uekusa, H.; Oyama, H. New organic salt from levofloxacin-citric acid: What is the impact on the stability and antibiotic potency? Molecules 2022, 27, 2166. [Google Scholar] [CrossRef]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D. Creating cocrystals: A review of pharmaceutical cocrystal preparation routes and applications. Cryst. Growth. Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef] [Green Version]
- Holaň, J.; Ridvan, L.; Billot, P.; Štepánek, F. Design of co-ćrystallization processes with regard to particle size distribution. Chem. Eng. Sci. 2015, 128, 36–43. [Google Scholar] [CrossRef]
- Jaywant, P.; Purima, A. Development of efavirenz cocrystals from stoichiometric solutions by spray drying technology. Mater. Today Proc. 2016, 3, 1742–1751. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Patel, B.; Day, G.M.; Jones, W. Cocrystallization by freeze-drying: Preparation of novel multicomponent crystal forms. Cryst. Growth Des. 2013, 13, 4599–4606. [Google Scholar] [CrossRef] [Green Version]
- Mazivilaa, A.; Castro, R.; Leitão, J. At-line green synthesis monitoring, new pharmaceutical co-crystals lamivudine:theophylline polymorph I and Il, quantification of polymorph I among its API using FI-IR spectroscopy and MCR-ALS. J. Pharm. Biomed. Anal. 2019, 30, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Nugrahani, I.; Utami, D.; Nugraha, Y.P.; Uekusa, H.; Hasianna, R.; Darusman, A.A. Cocrystal construction between the athyl etil with parent drug of diclofenac structural, stability, and anti-inflamatory study. Heliyon 2019, 5, e02946. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; Choi, I.; Kim, I.W. Liquid-assisted grinding to prepare a cocrystal of adefovir dipivoxil thermodynamically less stable than its neat phase. Crystals 2015, 5, 583–591. [Google Scholar] [CrossRef] [Green Version]
- Nugrahai, I.; Jessica, A.M. Amino Acids as the Potential Co-Former for Co-Crystal Development: A Review. Molecules 2021, 26, 3279. [Google Scholar] [CrossRef]
- Gunaam, A.; Suresh, K.; Nangia, A. Salts and salt cocrystals of the antibacterial drug perfloxacin. Cryst. Growth Des. 2018, 18, 2824–2835. [Google Scholar] [CrossRef]
- Wilson, J.M.; Newcombe, R.J.; Denaro, A.R. Experiments in Physical Chemistry, 2nd ed.; Experiment 10; Pergamon Press: Oxford, UK, 2016. [Google Scholar]
- Park, B.; Yoon, W.; Yun, J.; Ban, E.; Yun, H.; Kim, A. Emodin-nicotinamide (1:2) cocrystal identified by thermal screening to improve emodin solubility. Int. J. Pharm. 2019, 557, 26–35. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.; Kim, I.W. Structures and physical properties of the cocrystals of adefovir dipivoxil with dicarboxylic acids. J Cryst. Growth 2013, 373, 59–63. [Google Scholar] [CrossRef]
- Manin, A.N.; Surov, A.O.; Churakov, A.V.; Perlovich, G.L. Crystalstructures, thermal analysis, and dissolution behavior of new solid forms of the antiviral drug arbidol with dicarboxylic acids. Crystals 2015, 5, 650–669. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, P.A.; Almeida, A.C.D.; dos Santos, E.C.; Junior, R.D.; Ferreira, F.F.; Kogawa, A.C.; Caires, F.J. A norfloxacin-nicotinic acid cocrystal: Mechanochemical synthesis, thermal and structural characterization, and solubility assays. Thermochim. Acta 2020, 694, 178782. [Google Scholar] [CrossRef]
- Nugrahani, I.; Komara, W.S.; Horikawa, A.; Uekusa, H. Composing Novel Diclofenac Potassium and l-Proline Salt Cocrystal as a Strategy to Increase Solubility and Dissolution. J. Pharm. Sci. 2020, 109, 3423–3438. [Google Scholar] [CrossRef]
- Nugrahani, I.; Kumalasari, R.A.; Auli, W.N.; Horikawa, A.; Uekusa, H. Salt Cocrystal of Diclofenac Sodium-L-Proline: Structural, Pseudopolymorphism, and Pharmaceutics Performance Study. Pharmaceutics 2020, 12, 690. [Google Scholar] [CrossRef]
- Nugrahani, I.; Parwati, D.R. Challenges and Progress in Nonsteroidal Anti-Inflammatory Drugs Co-Crystal Development. Molecules 2021, 26, 4185. [Google Scholar] [CrossRef]
- Salas-Zúñiga, R.; Rodríguez-Ruiz, C.; Höpfl, H.; Morales-Rojas, H.; Sánchez-Guadarrama, O.; Rodríguez-Cuamatzi, P.; Herrera-Ruiz, D. Dissolution Advantage of Nitazoxanide Cocrystals in the Presence of Cellulosic Polymers. Pharmaceutics 2020, 12, 23. [Google Scholar] [CrossRef] [Green Version]
- Guru, P. Spectral studies of some complexes with chloramphenicol. Int. J. Chemtech Res. 2011, 3, 119–121. [Google Scholar]
- Nugrahani, I.; Khalida, F. Green method for acetaminophen and ibuprofen simultaneous assay in the combination tablet using FTIR. Int. J. Appl. Pharm. 2018, 10, 77–83. [Google Scholar]
- Nugrahani, I.; Dillen, N. Rapid assay development of diclofenac sodium coated tablet assay using FTIR compared to HPLC method. Int. J. Appl. Pharm. 2018, 10, 43–50. [Google Scholar] [CrossRef]
- Nugrahani, I.; Sulistya, S. The rapid and green hair analysis method development using FTIR for papaverine HCl determination. Int. J. Appl. Pharm. 2019, 11, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Nugrahani, I.; Mussadah, M.V. Development and validation analysis of acyclovir tablet content determination method using FTIR. Int. J. Appl. Pharm. 2016, 8, 43–47. [Google Scholar]
- Samsodien, H.; Bapoo, M.; Doms, T.I. FTIR, Dissolution and Anti-viral Activity of Nevirapine Co-crystals. Pharm. Anal. Acta 2017, 8, 561. [Google Scholar]
- Elbagerma, M.A.; Edwards, H.G.M.; Munshi, T.; Hargreaves, M.D.; Matousek, P.; Scowen, I.J. Characterization of new cocrystals by rama spectroscopy, powder x-ray diffraction, differential scanning calorimetry, and transmission raman spectroscopy. Cryst. Growth Des. 2010, 10, 2360–2371. [Google Scholar] [CrossRef]
- Ohta, R.; Ueno, Y.; Ajito, K. Raman spectroscopy of pharmaceutical cocrystals in nanosized pores of mesoporous silica. Anal. Sci. 2017, 33, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, J.B.; Guglietta, G.W. Terahertz spectroscopy. Anal. Chem. 2011, 83, 4342–4368. [Google Scholar] [CrossRef] [PubMed]
- Naftaly, M.; Miles, R.E. In: Terahertz Time-Domain Spectroscopy for Material Characterization. Proc. IEEE 2007, 95, 1658–1665. [Google Scholar] [CrossRef]
- Beard, M.C.; Turner, G.M.; Schmuttenmaer, C.A. Terahertz spectroscopy. J. Phys. Chem. B. 2002, 106, 7146–7159. [Google Scholar] [CrossRef]
- Coutaz, J. Terahertz Time-Domain Spectroscopy: Principles and Recent Developments. In Proceedings of the XXXIth URSI General Assembly and Scientific Symposium (URSI GASS), Beijing, China, 16–23 August 2014; p. 1. [Google Scholar]
- García-García, E.; Diez, E.; Meziani, Y.M.; Velázquez-Pérez, J.E.; Calvo-Gallcao, J. Proceedings of the 2013 Spanish Conference on Electron Devices, Valladolid, Spain, 12 February 2013–14 February 2013; pp. 199–202.
- Ciccarelli, C.; Joyce, H.; Robinson, J.; Kholid, F.N.; Hamara, D.; Kar, S.; Jeonc, K.-R. Terahertz Time-Domain Spectroscopy. Sci. Video Protoc. 2020, 1, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Neu, J.; Schmuttenmaer, C.A. Tutorial: An introduction to terahertz time domain spectroscopy (THz-TDS). J. Appl. Phys. 2018, 124, 231101. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.M.; Arnold, M.A. Terahertz time-domain spectroscopy of solid samples: Principles, applications, and challenges. Appl. Spectrosc. Rev. 2011, 46, 636–679. [Google Scholar] [CrossRef]
- Taday, P.F. Applications of terahertz spectroscopy to pharmaceutical sciences. Philos. Trans.-Royal Soc. Math. Phys. Eng. Sci. 2004, 362, 351–363. [Google Scholar] [CrossRef]
- Han, P.Y.; Tani, M.; Usami, M.; Kono, S.; Kersting, R.; Zhang, X.-C. A direct comparison between terahertz time–domain spectroscopy and far-infrared Fourier transform spectroscopy. J. Appl. Phys. 2001, 89, 2357. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, A. Novel Characterization of Materials Using THz Spectroscopic Techniques. PhD Dissertation, The State University of New Jersey, Newark, NJ, USA, 2006. [Google Scholar]
- Tishmack, P.A.; Bugay, D.E.; Byrn, S.R. Solid state nuclear magnetic resonance spectroscopy--pharmaceutical applications. J. Pharm. Sci. 2003, 92, 441–474. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.K. Applications of solid state NMR to pharmaceutical polymorphism and related matters. J. Pharm. Pharmacol. 2007, 59, 225–239. [Google Scholar] [CrossRef]
- Geppi, M.; Mollica, G.; Borsacchi, S.; Veracini, C.A. Solid state NMR studies of pharmaceutical systems. Appl. Spectrosc. Rev. 2008, 43, 202–302. [Google Scholar] [CrossRef]
- Vogt, F.G. Evolution of solid state NMR in pharmaceutical analysis. Future Med. Chem. 2010, 2, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Berendt, R.T.; Sperger, D.M.; Isbester, P.K.; Munson, E.J. Solid state NMR spectroscopy in pharmaceutical research and analysis. TrAC Trends Analyt. Chem. 2006, 25, 977–984. [Google Scholar] [CrossRef]
- Vogt, F.G.; Williams, G.R. Advanced approaches to effective solid-state analysis: X-ray diffraction, vibrational spectroscopy and solid-state NMR. Am. Pharm. Rev. 2010, 7, 58–65. [Google Scholar] [CrossRef]
- Offerdahl, T.J.; Salsbury, J.S.; Dong, Z.; Grant, D.J.W.; Schroeder, S.A.; Prakash, I.; Gorman, E.M.; Barich, D.H.; Munson, E.J. Quantitation of crystalline and amorphous forms of anhydrous neotame using 13C CPMAS NMR spectroscopy. J. Pharm. Sci. 2005, 94, 2591–2605. [Google Scholar] [CrossRef] [PubMed]
- Lefort, R.; De Gusseme, A.; Willart, J.F.; Danède, F.; Descamps, M. Solid state NMR and DSC methods for quantifying the amorphous content in solid dosage forms: An application to ball-milling of trehalose. Int. J. Pharm. 2004, 280, 209–219. [Google Scholar] [CrossRef]
- Farrer, B.T.; Peresypkin, A.; Wenslow, R.M. Quantitation of crystalline material within a liquid vehicle using 1H/19F CP/MAS NMR. J. Pharm. Sci. 2007, 96, 264–267. [Google Scholar] [CrossRef]
- Virtanen, T.; Maunu, S.L. Quantitation of a polymorphic mixture of an active pharmaceutical ingredient with solid state (13)C CPMAS NMR spectroscopy. Int. J. Pharm. 2010, 394, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Pisklak, M.; Pisklak, D.M.; Wawer, I. Application of 13C CPMAS NMR for qualitative and quantitative characterization of carvedilol and its commercial formulations. J. Pharm. Sci. 2012, 101, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Gao, P. Determination of the composition of delavirdine mesylate polymorph and pseudopolymorph mixtures using 13C CP/MAS NMR. Pharm. Res. 1996, 13, 1095–1104. [Google Scholar] [CrossRef]
- Pugliese, A.; Hawarden, L.E.; Abraham, A.; Tobyn, M.; Blanc, F. Solid state nuclear magnetic resonance studies of hydroxypropylmethylcellulose acetyl succinate polymer, a useful carrier in pharmaceutical solid dispersions. Magn. Reson. Chem. 2019, 58, 1036–1048. [Google Scholar] [CrossRef]
- Brus, J.; Urbanova, M.; Sedenkova, I.; Brusova, H. New perspectives of 19F MAS NMR in the characterization of amorphous forms of atorvastatin in dosage formulations. Int. J. Pharm. 2011, 409, 62–74. [Google Scholar] [CrossRef]
- Kimura, K.; Hirayama, F.; Arima, H.; Uekama, K. Solid state 13C nuclear magnetic resonance spectroscopic study on amorphous solid complexes of tolbutamide with 2-hydroxypropyl-alpha- and -beta-cyclodextrins. Pharm. Res. 1999, 16, 1729–1734. [Google Scholar] [CrossRef]
- Forster, A.; Apperley, D.; Hempenstall, J.; Lancaster, R.; Rades, T. Investigation of the physical stability of amorphous drug and drug/polymer melts using variable temperature solid state NMR. Pharmazie 2003, 58, 761–762. [Google Scholar] [PubMed]
- Schachter, D.M.; Xiong, J.; Tirol, G.C. Solid state NMR perspective of drug-polymer solid solutions: A model system based on poly(ethylene oxide). Int. J. Pharm. 2004, 281, 89–101. [Google Scholar] [CrossRef]
- Brittain, H.G.; Morris, K.R.; Bugay, D.E.; Thakur, A.B.; Serajuddin, A.T. Solid state NMR and IR for the analysis of pharmaceutical solids: Polymorphs of fosinopril sodium. J. Pharm. Biomed. Anal. 1993, 11, 1063–1069. [Google Scholar] [CrossRef]
- Pham, T.N.; Watson, S.A.; Edwards, A.J.; Chavda, M.; Clawson, J.S.; Strohmeier, M.; Vogt, F.G. Analysis of amorphous solid dispersions using 2D solid state NMR and (1)H T(1) relaxation measurements. Mol. Pharm. 2010, 7, 1667–1691. [Google Scholar] [CrossRef]
- Masuda, K.; Tabata, S.; Sakata, Y.; Hayase, T.; Yonemochi, E.; Terada, K. Comparison of molecular mobility in the glassy state between amorphous indomethacin and salicin based on spin-lattice relaxation times. Pharm. Res. 2005, 22, 797–805. [Google Scholar] [CrossRef]
- Luthra, S.A.; Utz, M.; Gorman, E.M.; Pikal, M.J.; Munson, E.J.; Lubach, J.W. Carbon-deuterium rotational-echo double-resonance NMR spectroscopy of lyophilized aspartame formulations. J. Pharm. Sci. 2012, 101, 283–290. [Google Scholar] [CrossRef]
- Yoshioka, S.; Aso, Y.; Kojima, S. The effect of excipients on the molecular mobility of lyophilized formulations, as measured by glass transition temperature and NMR relaxation-based critical mobility temperature. Pharm. Res. 1999, 16, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Suihko, E.J.; Forbes, R.T.; Apperley, D.C. A solid state NMR study of molecular mobility and phase separation in co-spray-dried protein-sugar particles. Eur. J. Pharm. Sci. 2005, 25, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowska, Z.; Grzybowska, K.; Adrjanowicz, K.; Kaminski, K.; Paluch, M.; Hawelek, L.; Wrzalik, R.; Dulski, M.; Sawicki, W.; Mazgalski, J.; et al. Study of the amorphous glibenclamide drug: Analysis of the molecular dynamics of quenched and cryomilled material. Mol. Pharm. 2010, 7, 1692–1707. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.K. NMR studies of organic polymorphs and solvates. Analyst Lond. 2006, 131, 351–373. [Google Scholar] [CrossRef]
- Wenslow, R.M. 19F solid state NMR spectroscopic investigation of crystalline and amorphous forms of a selective muscarinic M3 receptor antagonist, in both bulk and pharmaceutical dosage form samples. Drug Dev. Ind. Pharm. 2002, 28, 555–561. [Google Scholar] [CrossRef]
- Fujii, K.; Toyota, K.; Sekine, A.; Uekusa, H.; Nugrahani, I.; Asyarie, S.; Soewandhi, N.S.; Ibrahim, S. Potassium clavulanate. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, 985–986. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sun, J. Application of X-ray diffraction and electron crystallography for solving complex structure problems. Acc. Chem. Res. 2017, 50, 2737–2745. [Google Scholar] [CrossRef]
- Srirambhatla, V.K.; Kraft, A.; Watt, S.; Powell, A.V. Crystal design approaches for the synthesis of paracetamol co-crystals. Cryst. Growth Des. 2012, 12, 4870–4879. [Google Scholar] [CrossRef]
- Nugrahani, I.; Pertiwi, E.A.; Putra, O.K. Theophylline-Na-Saccharine single crystal isolation for its structure determination. Int. J. Pharm. Pharm. Sci. 2015, 7, 15–24. [Google Scholar]
- Zhang, J.P.; Liao, P.Q.; Zhou, H.L.; Lin, R.B.; Chen, X.M. Single-crystal X-ray diffraction studies on structural transformations of porous coordination polymers. Chem. Soc. Rev. 2014, 43, 5789–5814. [Google Scholar] [CrossRef]
- Stevenson, R.; De Bo, G. Controlling reactivity by geometry in retro-diels–alder reactions under tension. J. Am. Chem. Soc. 2017, 139, 16768–16771. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.N.; Weng, J.; Ip, I.; Chen, R.; Lakerveld, R.; Telford, R.; Blagedm, N.; Scowen, I.J.; Chow, S.F. Rational development of a carrier-free dry powder inhalation formulation for respiratory viral infections via quality by design: A drug-drug cocrystal of favipiravir and theophylline. Pharmaceutics 2022, 14, 300. [Google Scholar]
- Gunnam, A.; Nangia, A.K. Solubility improvement of curcumin with amino acids. Sci. Eng. Res. Board 2021, 23, 3398–3410. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 35370, Zidovudine. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Zidovudine (accessed on 4 December 2022).
- Deka, P.; Gogoi, D.; Althubeiti, K.; Rao, D.R.; Thakuria, R. Mechanosynthesis, characterization, and physicochemical property investigation of favipiravir cocrystal with theophylline and GRAS coformers. Cryst. Growth Des. 2021, 21, 4417–4425. [Google Scholar] [CrossRef]
- Ji, X.; Wu, D.; Li, C.; Li, J.; Sun, Q.; Chang, D.; Yin, Q.; Zhou, L.; Xie, C.; Gong, J.; et al. Enhanced solubility, dissolution, and permeability of abacavir by salt and cocrystal formation. Cryst. Growth Des. 2022, 22, 428–440. [Google Scholar] [CrossRef]
- Athiyah, U.; Kusuma, P.A.; Tutik, T.; Maria, L.A.D.; Isadiartuti, D.; Paramita, D.P.; Setyawan, D. Crystal engineering of quercetin by liquid assisted grinding method. J. Teknol. 2019, 81(1), 39–45. [Google Scholar] [CrossRef] [Green Version]
- Wisudyaningsih, B.; Setyawan, D.; Siswandono. Co-crystallization of quercetin and isonicotinamide using solvent evaporation method. Trop. J. Pharm. Res. 2021, 18, 697–702. [Google Scholar] [CrossRef]
- Bruni, G.; Maietta, M.; Maggi, L. Preparation and physicochemical: Characterization of acyclovir cocrystals with improved dissolution properties. J. Pharm. Sci. 2013, 102, 4079–4086. [Google Scholar] [CrossRef]
- Chadha, R.; Saini, A.; Arora, P.; Chanda, S.; Jain, D.V.S. Cocrystals of efavirenz with selected coformers: Preparation and characterization. Int. J. Pharm. Sci 2012, 4, 244–250. [Google Scholar]
- Sarkar, A.; Rohani, S. Cocrystals of acyclovir with promising physicochemical properties. J. Pharm. Sci. 2015, 104, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Rajput, L.; Sanphui, P.; Desiraju, G.R. New solid forms of the anti-HIV drug etravirine: Salts, cocrystals, and solubility. Cryst. Growth Des. 2013, 13, 3681–3690. [Google Scholar] [CrossRef]
- Shah, K.; Borhade, S.; Londhe, V. Utilization of co-crystallization for solubility enhancement of a poorly soluble antiretroviral drug-ritonavir. Int. J. Pharm. Sci. 2004, 6, 556–558. [Google Scholar]
- Gowda, B.H.J.; Nechipadappu, S.K.; Shankar, S.J.; Chavali, M.; Paul, K.; Ahmed, M.G.; Sanjana, A.; Shanthala, H.K. Pharmaceutical cocrystals of efavirenz: Towards the improvement of solubility, dissolution rate and stability. Mater. Today Proc. 2022, 51, 394–402. [Google Scholar] [CrossRef]
- Gawade, A.; Kuchekar, A.; Soldhane, S.; Baheti, A. Improvement of physicochemical and solubility of dipyridamole by cocrystallization technology. J. Drug Deliv. Ther. 2021, 11, 43–48. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhao, M.Y.; Bu, F.Z.; Niu, Y.Y.; Yu, Y.M.; Li, Y.T.; Yan, C.W.; Wi, Z.Y. Cocrystallization of amantadine hydrochloride with resveratrol: The first drug-nutraceutical cocrystal displaying synergistic antiviral activity. Cryst. Growth Des. 2021, 21, 2763–2776. [Google Scholar] [CrossRef]
- Chen, J.M.; Li, S.; Lu, T.B. Pharmaceutical cocrystals of ribavirin with reduced release rates. Cryst. Growth Des. 2014, 14, 6399–6408. [Google Scholar] [CrossRef]
- Wang, L.Y.; Niu, Y.Y.; Zhao, M.Y.; Yu, Y.M.; Li, Y.T.; Wu, Z.Y.; Yan, C.W. Supramolecular self-assembly of amantadine hydrochloride with ferulic acid via dual optimization strategy establishes a precedent of synergistic antiviral drug-phenolic acid neutraceutical cocrystal. Analyst 2021, 146, 3988–3999. [Google Scholar] [CrossRef] [PubMed]
- Wanga, Y.L.; Bua, Z.F.; Yu, M.Y.; Niu, Y.Y.; Lia, T.Y.; WeiYan, W.U.; Wua, Y.Z. A novel crystalline molecular salt of sulfamethoxazole and amantadine hybridizing antiviral-antibacterial dual drugs with optimal in vitro/vivo pharmaceutical properties. Euro. J. Pharm. Sci. 2021, 163, 105883. [Google Scholar] [CrossRef] [PubMed]
- Gadade, D.D.; Pekamwar, S.S.; Shirsat, M.D. Crystal engineering of antiviral agent efavirenz for solubility enhancement. Drug Deliv. Thera. 2018, 8, 86–91. [Google Scholar] [CrossRef]

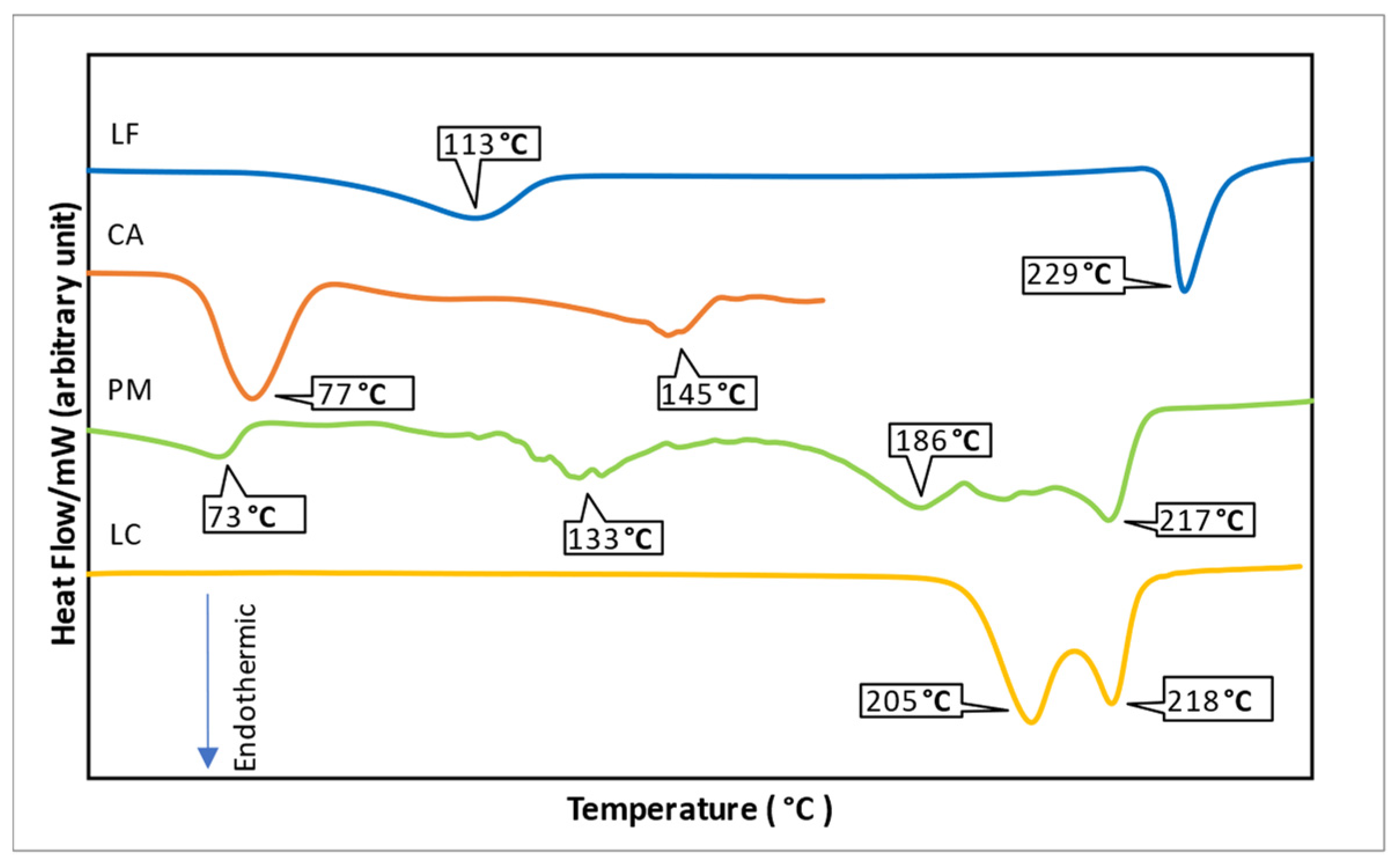

| No. | Mechanisms | Antiviral | Indication |
|---|---|---|---|
| 1. | Nucleoside Reverse Transcriptase Inhibitor (NRTI) Block the synthesis of viral nucleic acid | Acyclovir | - Oral First episode genital herpes treatment, recurrent genital herpes treatment, genital herpes suppression, herpes proctitis treatment, first episode orolabial herpes treatment, recurrent orolabial herpes treatment, orolabial herpes suppression, varicella treatment (age ≥ 2 years), zoster treatment - Intravenous Severe herpes simplex virus treatment, mucocutaneous herpes in the immunocompromised host treatment, herpes encephalitis treatment, neonatal herpes simplex virus infection treatment, varicella or zoster in the immunosuppressed host treatment - Topical (5% cream) Herpes labialis treatment |
| Ganciclovir | - Topical (0.15% gel) Keratitis - Intravenous cytomegalovirus retinitis treatment | ||
| Valganciclovir | Oral cytomegalovirus retinitis treatment, cytomegalovirus prophylaxis (transplant patients) | ||
| Penciclovir | Topical (1% cream) Herpes labialis or herpes genitalis | ||
| Valacyclovir | Oral First episode genital herpes treatment, recurrent genital herpes treatment, genital herpes suppression, first episode orolabial herpes treatment, recurrent orolabial herpes treatment, orolabial herpes treatment, varicella (age > 2 years), zoster | ||
| Famciclovir | Oral First episode genital herpes treatment, recurrent genital herpes treatment, genital herpes in the HIV-infected host treatment, genital herpes suppression, first episode orolabial herpes treatment, recurrent orolabial herpes treatment, orolabial herpes suppression, zoster | ||
| Cidofovir | Intravenous cytomegalovirus retinitis treatment | ||
| Trifluridine | Topical (1% solution) Acyclovir-resistant herpes simplex virus infection | ||
| Foscarnet | Intravenous Acyclovir-resistant herpes simplex virus and varicella-zoster virus infection, cytomegalovirus retinitis treatment | ||
| Lamivudine | Oral Chronic hepatitis B, antiretroviral (in pregnancy) | ||
| Zidovudine | First-line antiretroviral (in pregnancy), decrease the rate of clinical disease progression and prolong survival in HIV-infected individuals. | ||
| Abacavir | Oral Antiretroviral (in pregnancy) | ||
| Emtricitabine | Oral Antiretroviral (in pregnancy) | ||
| Tenofovir | Oral Chronic hepatitis B HBV infection; antiretroviral (in pregnancy); pre-exposure prophylaxis to reduce HIV acquisition in men who have sex with men, in heterosexually active men and women, and in injection drug users. | ||
| Stavudine | Oral Antiretroviral | ||
| Didanosine | Antiretroviral | ||
| adefovir dipivoxil | Oral Chronic hepatitis B | ||
| Entecavir | Oral Chronic hepatitis B | ||
| Telbivudine | Oral Chronic hepatitis B | ||
| 2. | Protease Inhibitor (PI) Block viral late protein synthesis and processing | Ritonavir | Antiretroviral (in pregnancy) |
| Saquinavir | Antiretroviral (in pregnancy) | ||
| Tipranavir | Antiretroviral | ||
| Atazanavir | Antiretroviral (in pregnancy) | ||
| Lopinavir | Antiretroviral (in pregnancy) | ||
| Darunavir | Antiretroviral (in pregnancy) | ||
| Nelfinavir | Antiretroviral | ||
| Indinavir | Antiretroviral | ||
| Fosamprenavir | Antiretroviral | ||
| Boceprevir | Oral Chronic hepatitis C | ||
| Telaprevir | Oral Chronic hepatitis C | ||
| Simeprevir | Oral Hepatitis C | ||
| 3. | Nonnucleoside Reverse Transcriptase Inhibitor (NNRTI) Block the synthesis of viral nucleic acid | Nevirapine | Antiretroviral (in pregnancy) |
| Rilpivirine | Antiretroviral | ||
| Etravirine | Antiretroviral | ||
| Efavirenz | Antiretroviral | ||
| Delavirdine | Antiretroviral | ||
| 4. | Integrase Strand Transfer Inhibitors (INSTI) Block viral nucleic acid integration into the genome | Raltegravir | Antiretroviral |
| Elvitegravir | Antiretroviral | ||
| Dolutegravir | Antiretroviral | ||
| 5. | Entry Inhibitors Block viral attachment and entry into the cell | Maraviroc | Oral Treatment of experienced adult patients infected with only CCR5-tropic HIV-1 detectable who are resistant to other antiretroviral agents |
| Enfuvirtide | Subcutaneous Antiretroviral (HIV) | ||
| Docosanol | Topical (10% cream) Keratitis | ||
| 6. | Interferons (speculated to have multiple sites of action) | interferon alfa 2b | Subcutaneous/intramuscular Chronic hepatitis B, acute hepatitis C |
| pegylated interferon alfa 2a | Subcutaneous Chronic hepatitis B, chronic hepatitis C | ||
| pegylated interferon alfa 2b | Subcutaneous Chronic hepatitis C | ||
| 7. | Neuraminidase Inhibitor Block viral release from the cell | Oseltamivir | Oral Anti-influenza A and B |
| Zanamivir | Inhalation Anti-influenza A and B | ||
| Peramivir | Intravenous Anti-influenza A and B | ||
| 8. | Inhibit viral uncoating process | Amantadine | Anti-influenza A |
| Rimantadine | Anti-influenza A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugrahani, I.; Susanti, E.; Adawiyah, T.; Santosa, S.; Laksana, A.N. Non-Covalent Reactions Supporting Antiviral Development. Molecules 2022, 27, 9051. https://doi.org/10.3390/molecules27249051
Nugrahani I, Susanti E, Adawiyah T, Santosa S, Laksana AN. Non-Covalent Reactions Supporting Antiviral Development. Molecules. 2022; 27(24):9051. https://doi.org/10.3390/molecules27249051
Chicago/Turabian StyleNugrahani, Ilma, Emy Susanti, Tazkia Adawiyah, Safira Santosa, and Agnesya Namira Laksana. 2022. "Non-Covalent Reactions Supporting Antiviral Development" Molecules 27, no. 24: 9051. https://doi.org/10.3390/molecules27249051
APA StyleNugrahani, I., Susanti, E., Adawiyah, T., Santosa, S., & Laksana, A. N. (2022). Non-Covalent Reactions Supporting Antiviral Development. Molecules, 27(24), 9051. https://doi.org/10.3390/molecules27249051