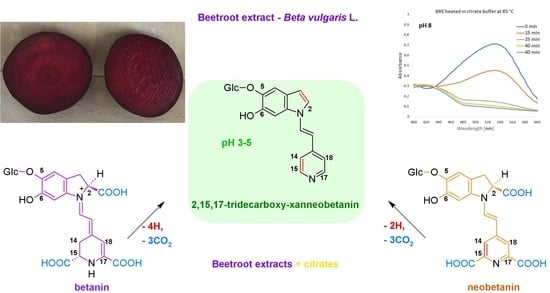
Influence of Citrates and EDTA on Oxidation and Decarboxylation of Betacyanins in Red Beet (Beta vulgaris L.) Betalain-Rich Extract
Abstract
1. Introduction
2. Results and Discussion
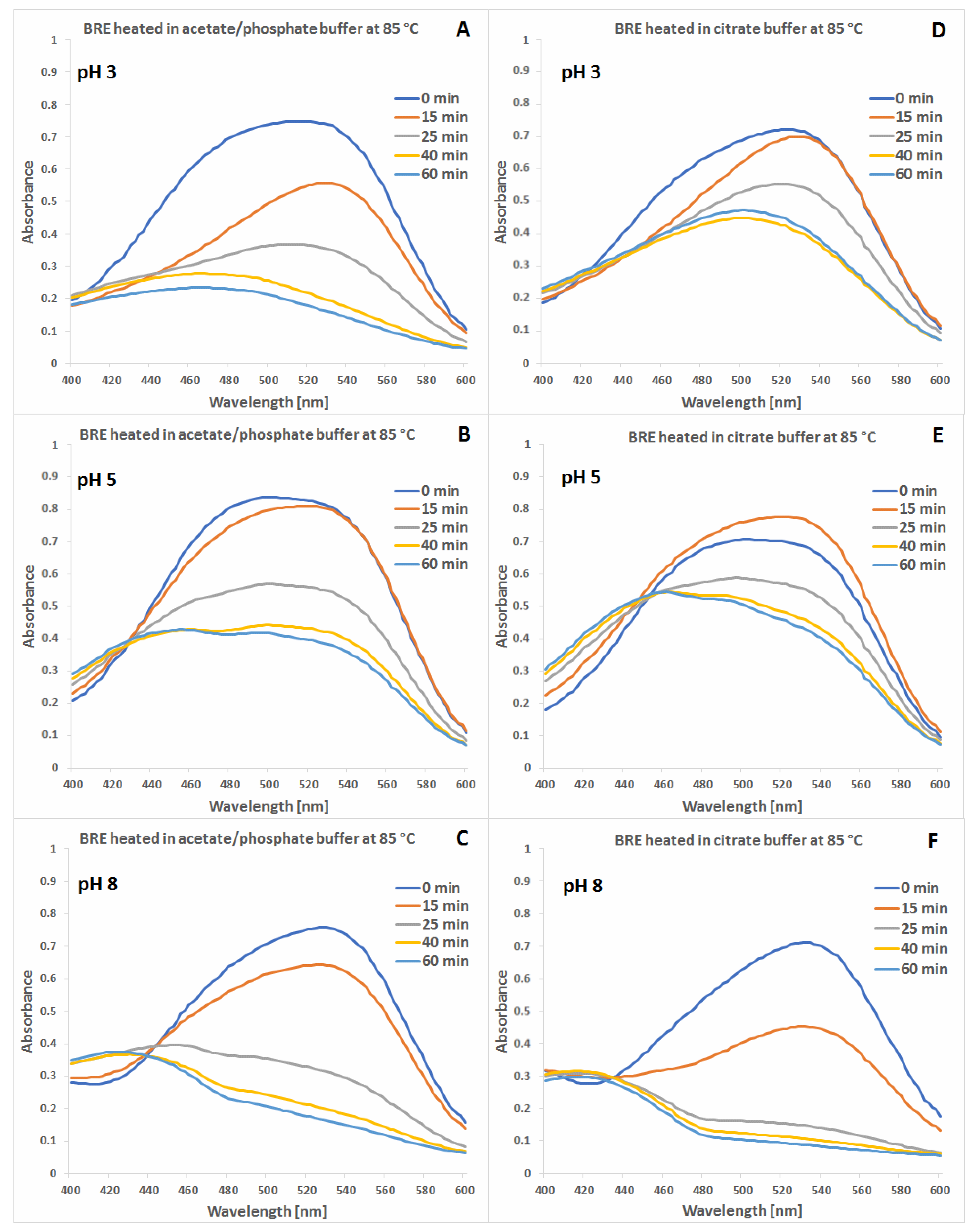
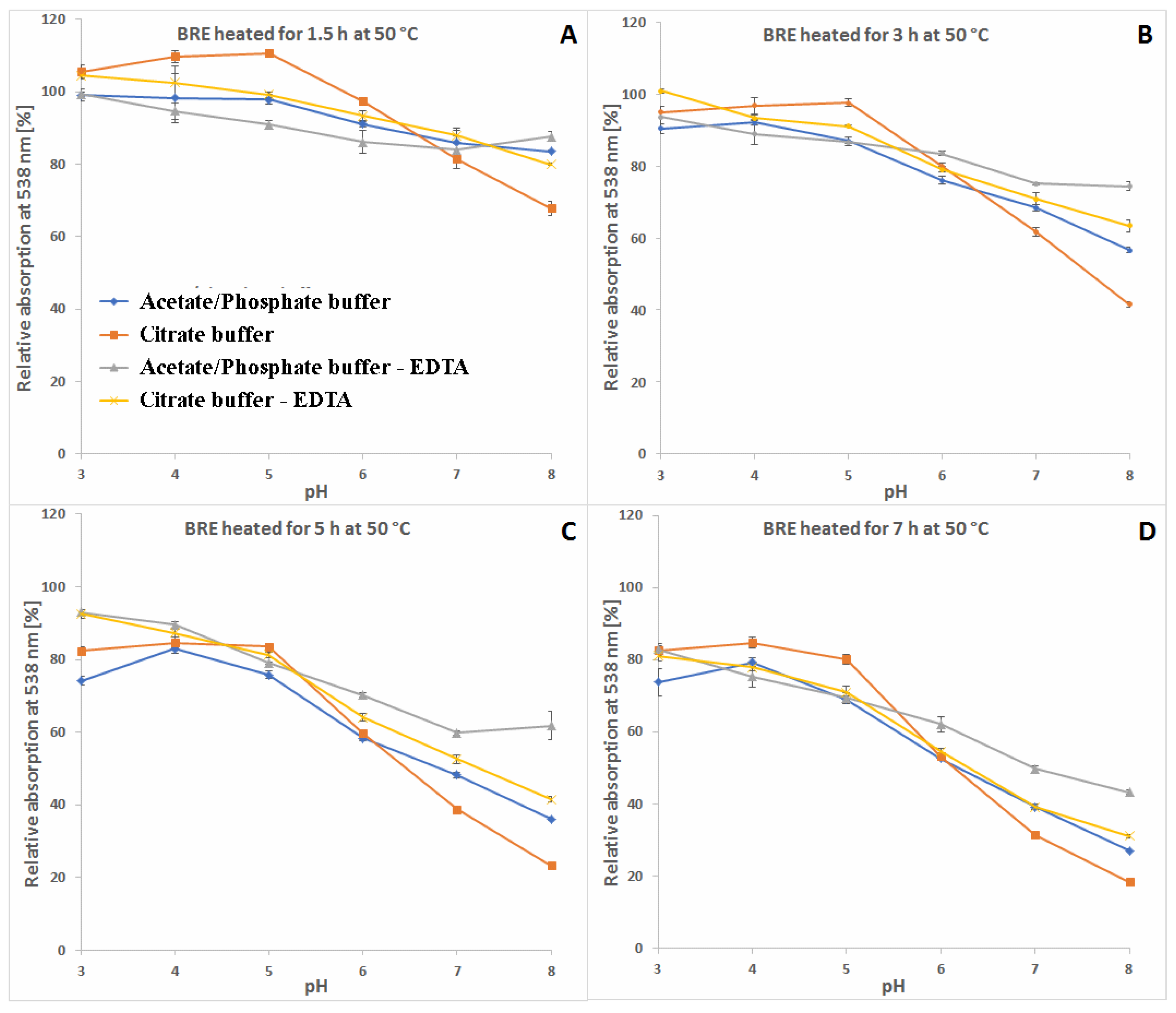
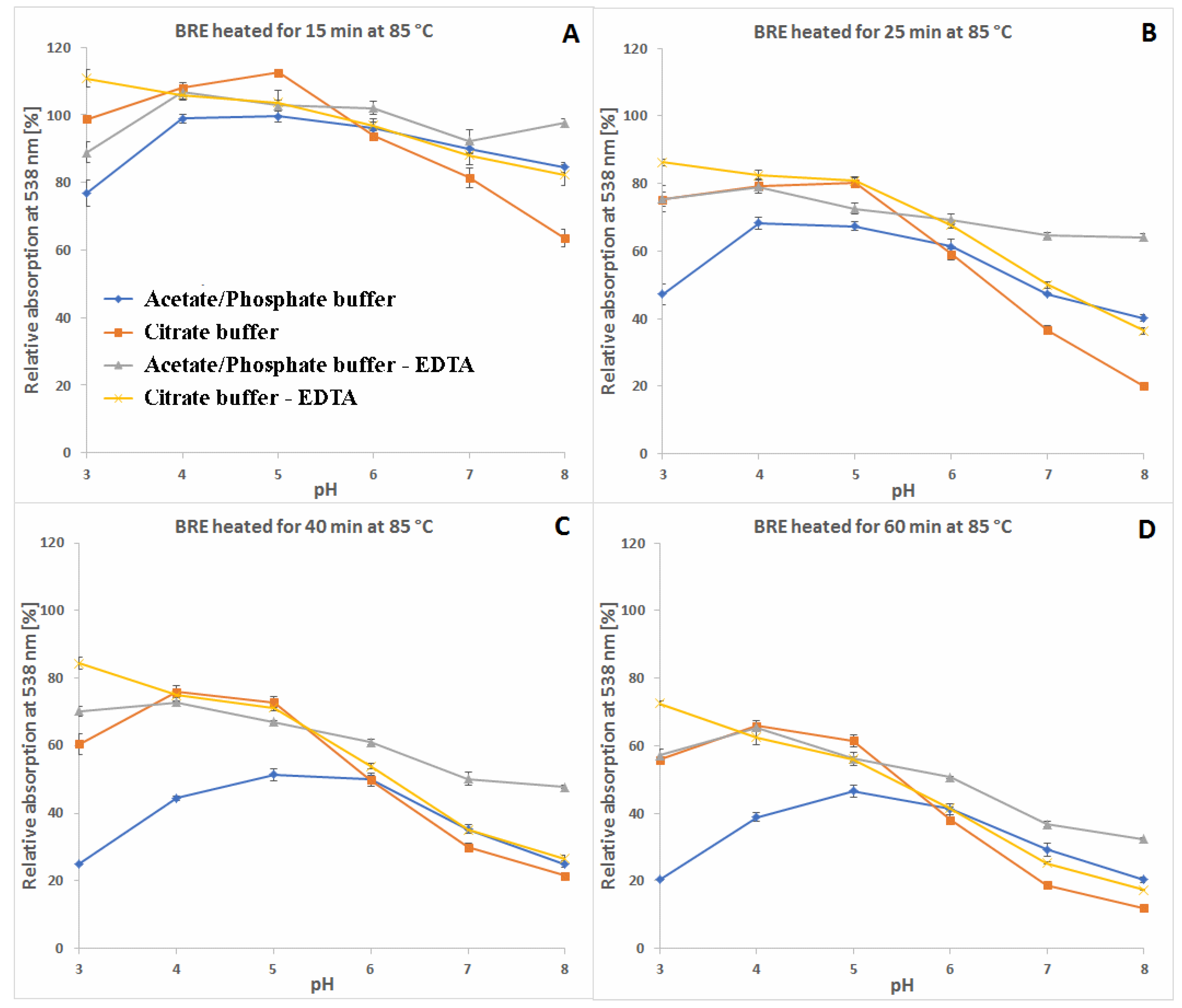
2.1. Spectrophotometric Monitoring of the BRE Heating Reaction Progress
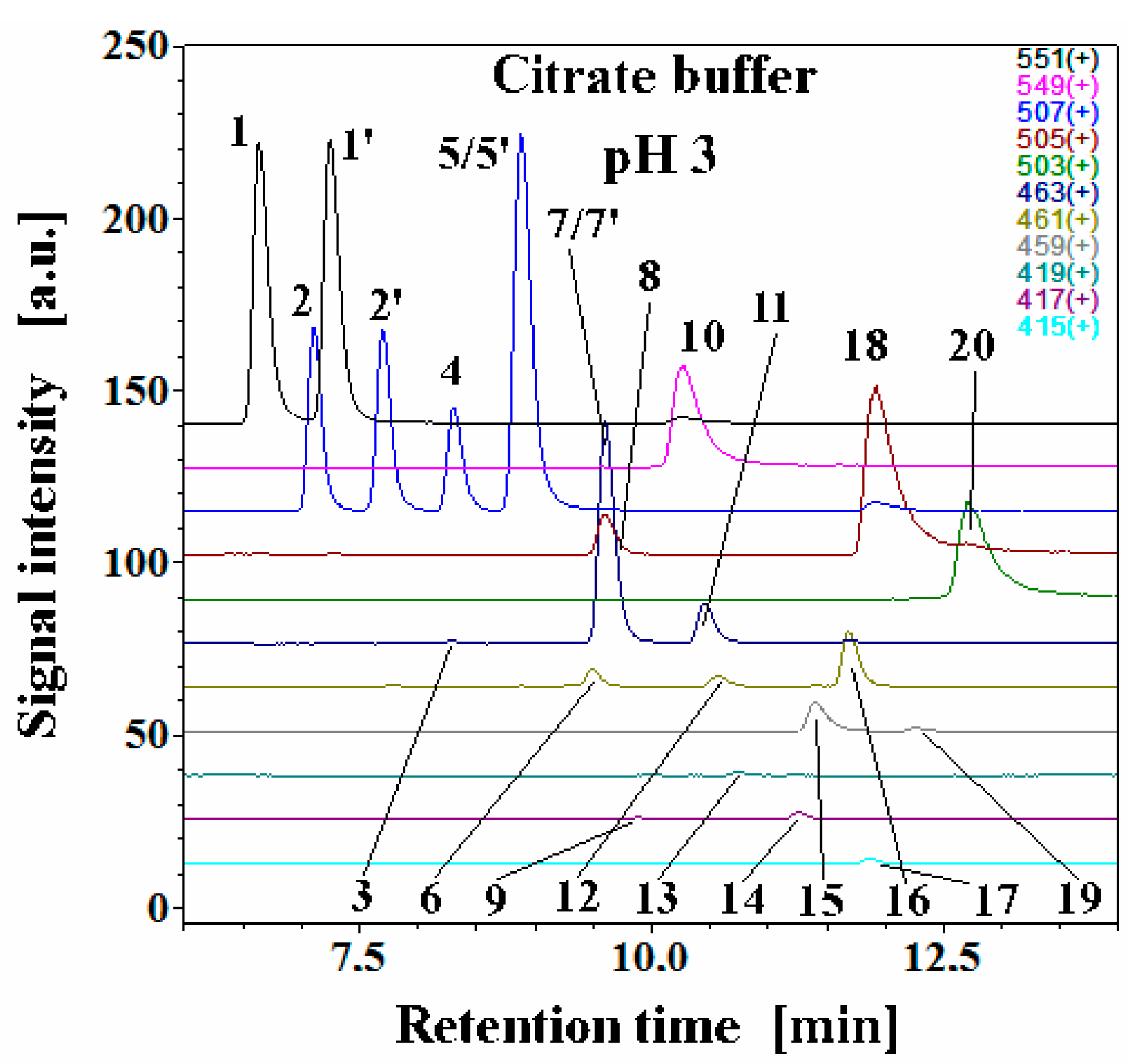
2.2. Chromatographic and Mass Spectrometric Monitoring of the Products Generated during the BRE Heating Experiments under the Influence of the Citric acid Concentration
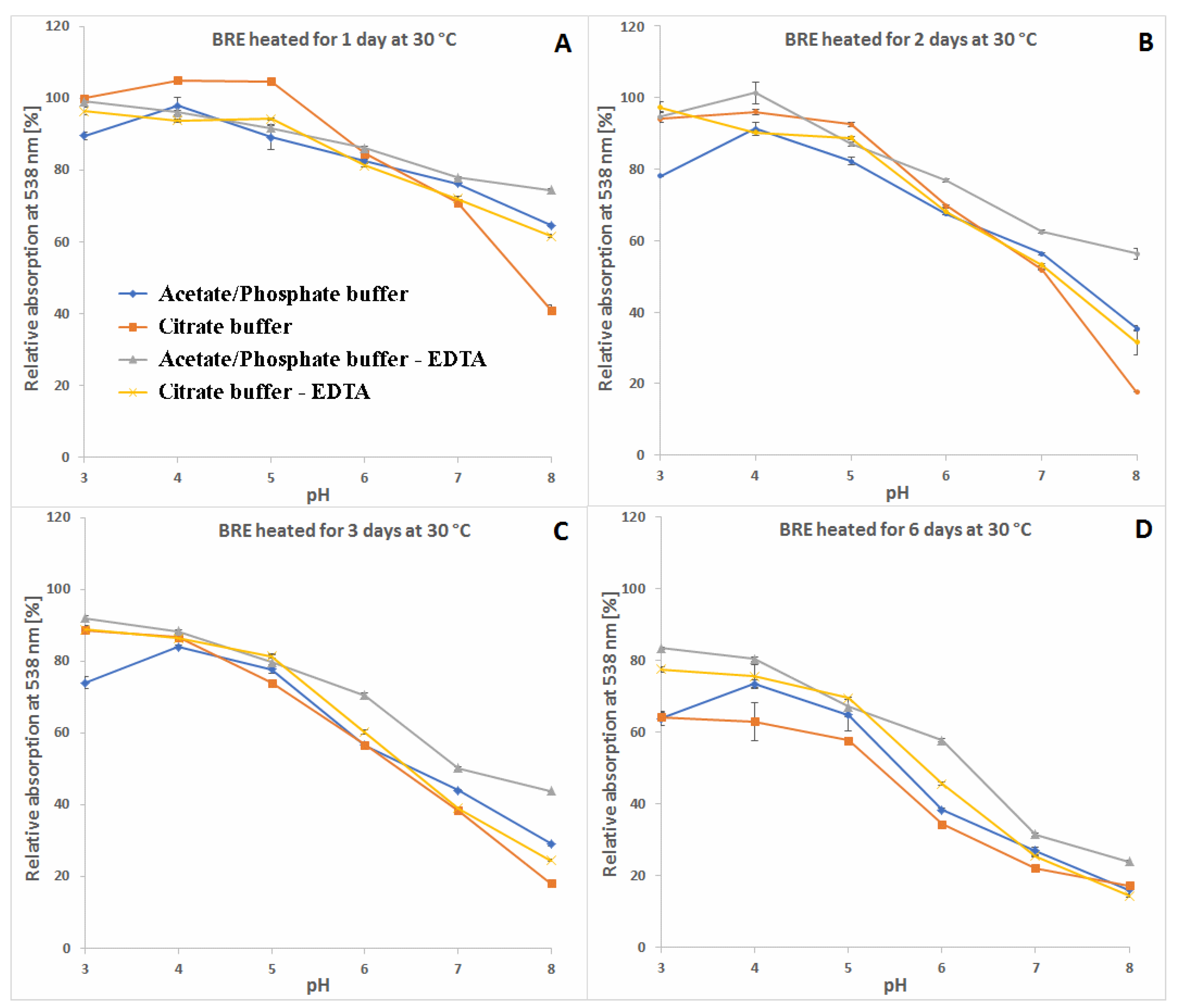
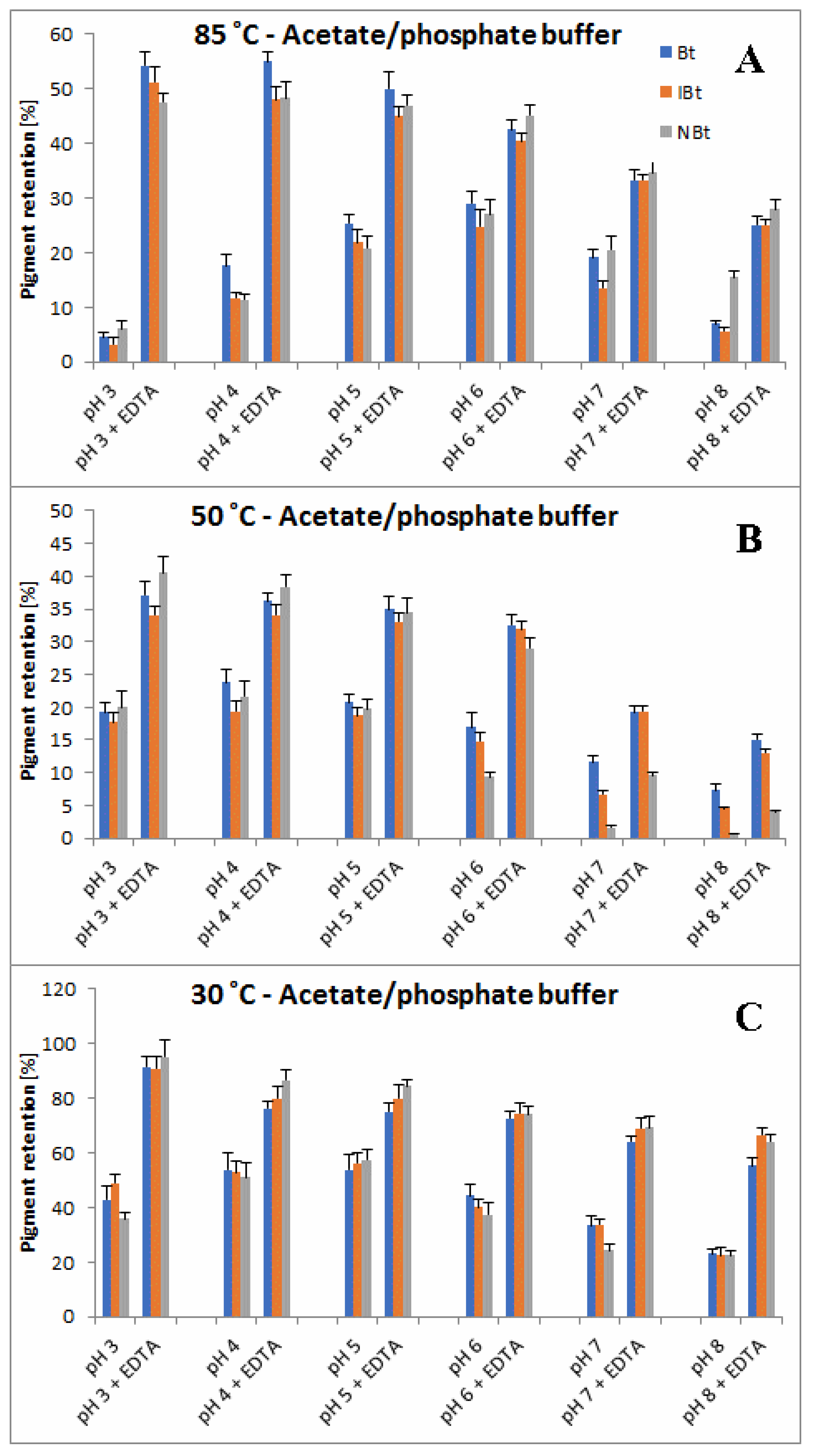
2.3. Determination of Citric Acid and EDTA Influence on Betanin/Isobetanin 1/1′ and Neobetanin 10 Substrate Retention Levels during the Heating of the BRE Extract
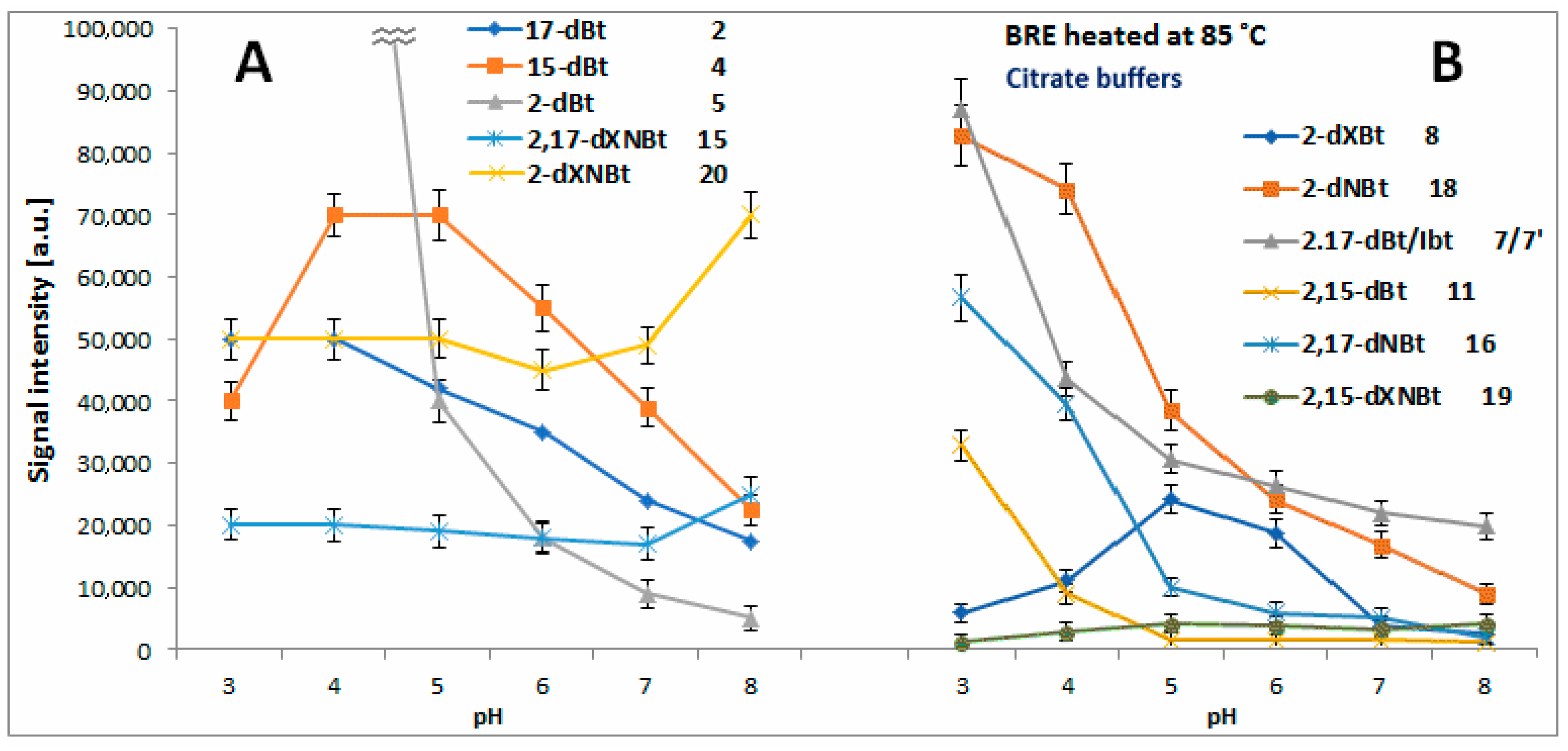
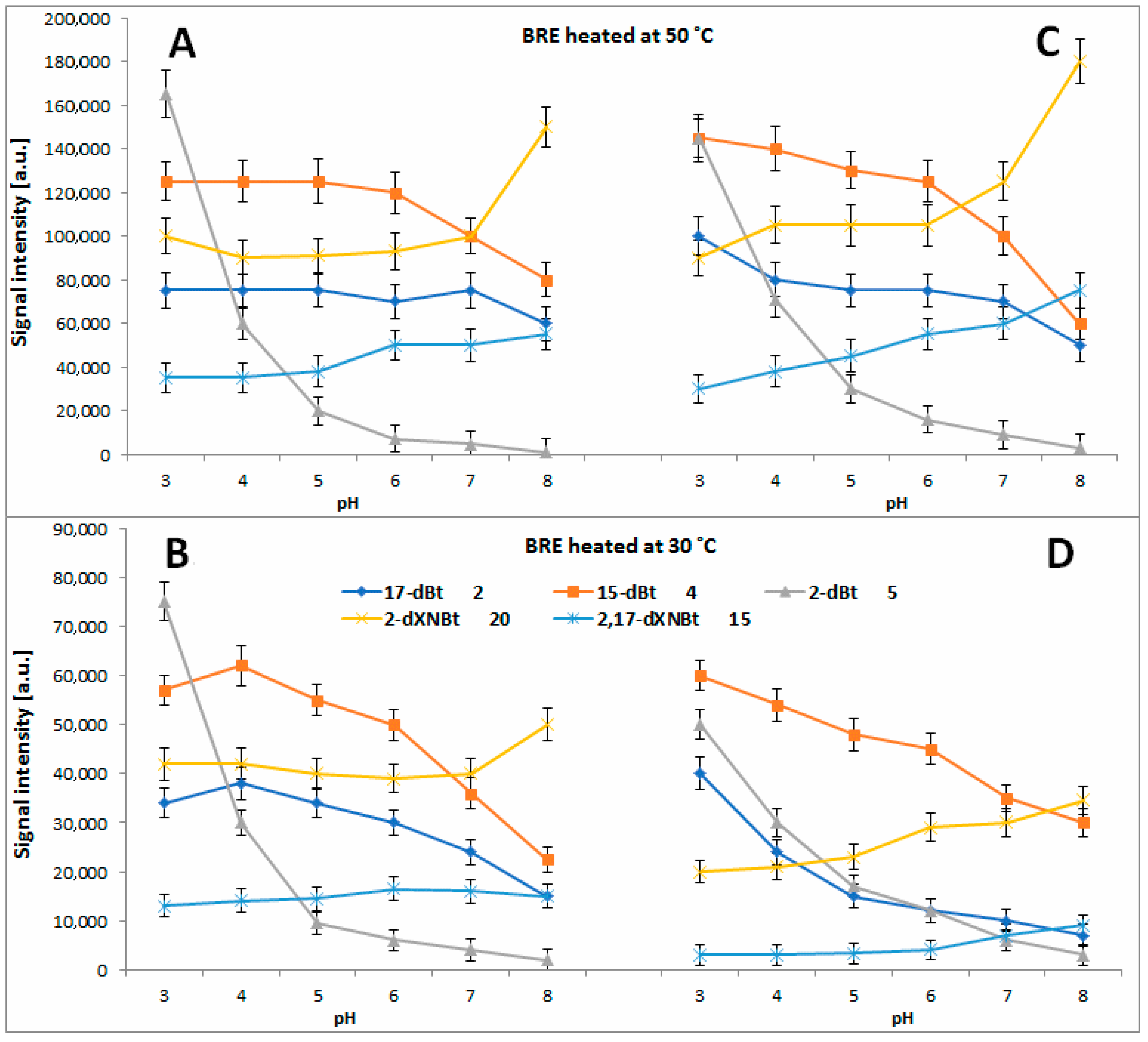
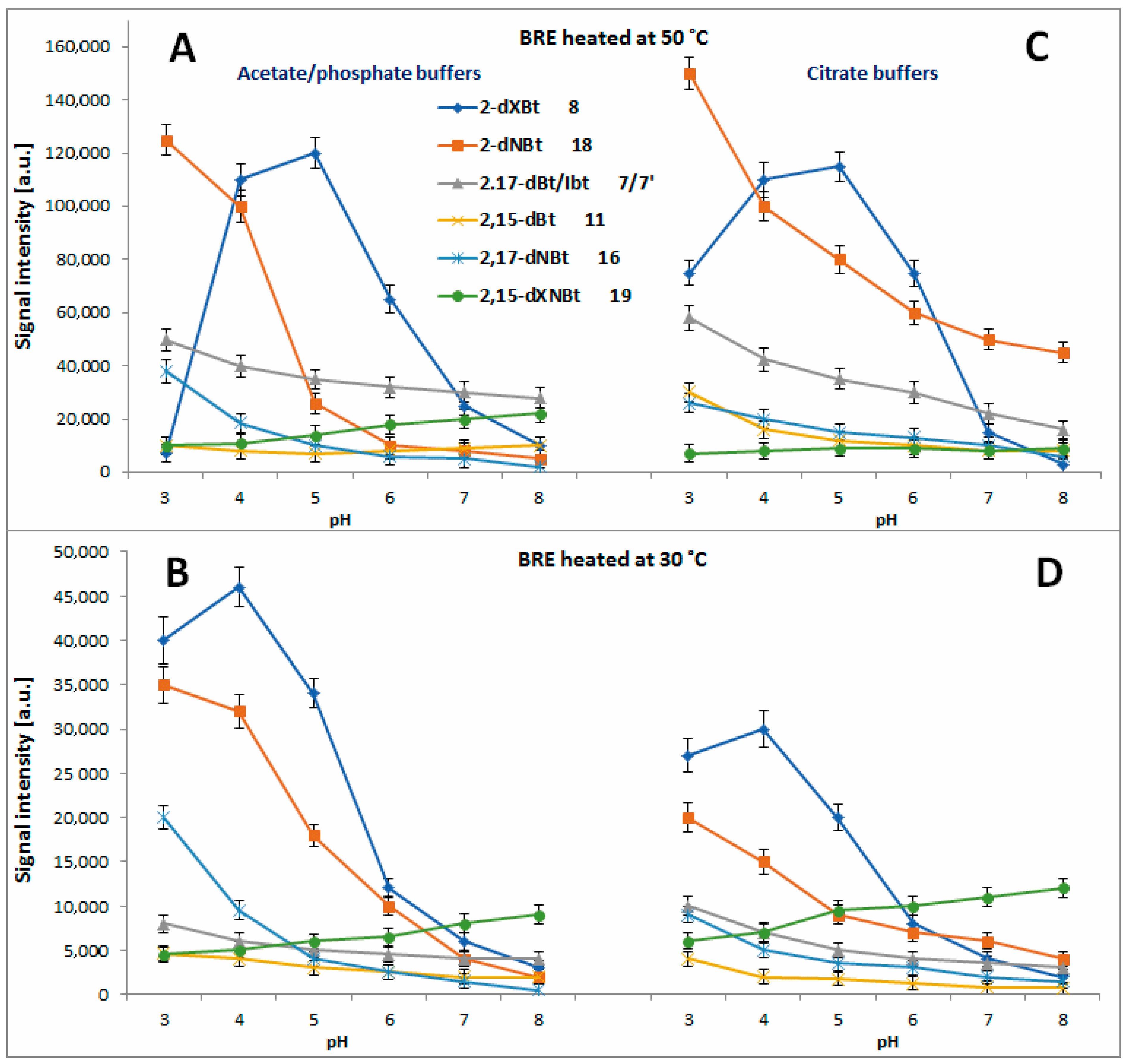
2.4. Determination of Citric Acid Influence on Signal Profiles of Chromophoric Betanin Derivatives Formed during BRE Heating
3. Materials and Methods
3.1. Reagents
3.2. Experiments on the Influence of Citrates and EDTA on the BRE Extract
3.3. LC-DAD-ESI-MS/MS Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kanner, J.; Harel, S.; Granit, R. Betalains—A new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef]
- Mabry, T.; Dreiding, A.S. The Betalains. In Recent Advances in Phytochemistry; Appleton: New York, NY, USA, 1968. [Google Scholar]
- Mabry, T.J. Selected topics from forty years of natural products research: Betalains to flavonoids, antiviral proteins, and neurotoxic nonprotein amino acids. J. Nat. Prod. 2001, 64, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Zryd, J.P.; Christinet, L. Plant Pigments and Their Manipulation, Annual Plant Review, Vol 14; RC Press/Blackwel: Oxford, UK, 2004. [Google Scholar]
- Georgiev, V.; Ilieva, M.; Bley, T.; Pavlov, A. Betalain production in plant in vitro systems. Acta Physiol. Plant. 2008, 30, 581–593. [Google Scholar] [CrossRef]
- Kumorkiewicz-Jamro, A.; Świergosz, T.; Sutor, K.; Spórna-Kucab, A.; Wybraniec, S. Multi-colored shades of betalains: Recent advances in betacyanin chemistry. Nat. Prod. Rep. 2021, 38, 2315–2346. [Google Scholar] [CrossRef]
- Cabanes, J.; Gandía-Herrero, F.; Escribano, J.; García-Carmona, F.; Jiménez-Atiénzar, M. One-step synthesis of betalains using a novel betalamic acid derivatized support. J. Agric. Food Chem. 2014, 62, 3776–3782. [Google Scholar] [CrossRef]
- Khan, M.I.; Kumar, A.; Giridhar, P. Betalains and expression of antioxidant enzymes during development and abiotic stress in Rivina humilis L. berries. Turk. J. Bot. 2016, 40, 28–36. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Apak, R. Current Issues in Antioxidant Measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Rao, G.S. Anticancer Effects of Red Beet Pigments. In Red Beet Biotechnology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 125–154. [Google Scholar]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef]
- Ito, N.; Fukushima, S.; Tsuda, H.; Shirai, T.; Tatematsu, M.; Imaida, K. Modification of chemical carcinogenesis by antioxidants. Princess Takamatsu Symp. 1983, 14, 381–389. [Google Scholar] [PubMed]
- Cunha, L.C.M.; Monteiro, M.L.G.; Costa-Lima, B.R.C.; Guedes-Oliveira, J.M.; Alves, V.H.M.; Almeida, A.L.; Tonon, R.V.; Rosenthal, A.; Conte-Junior, C.A. Effect of microencapsulated extract of pitaya (Hylocereus costaricensis) peel on color, texture and oxidative stability of refrigerated ground pork patties submitted to high pressure processing. Innov. Food Sci. Emerg. Technol. 2018, 49, 136–145. [Google Scholar] [CrossRef]
- Muíño, I.; Fuente, J.; Pérez, C.; Apeleo, E.; Pérez-Santaescolástica, C.; Cañeque, V.; Lauzurica, S.; Bermejo-Poza, R.; Díaz, A.M.T. Use of red wine polyphenols as a natural preservative in health-promoting omega-3 fatty acids-enriched lamb patties. Molecules 2018, 23, 3080. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.M.; Wang, C.X.; Iatropoulos, M.J. Toxicity studies of butylated hydroxanisole and butylated hydroxytoluene. II. Chronic feeding studies. Food Chem. Toxicol. 1990, 28, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Lorenzo, J.M. Effect of packaging conditions on shelf-life of foal fresh meat. Meat Sci. 2012, 91, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Islam, S.; Rather, L.J.; Mohammad, F. Phytochemistry, biological activities and potential of annatto in natural colorant production for industrial applications—A review. J. Adv. Res. 2016, 7, 499–514. [Google Scholar] [CrossRef]
- Shahid, M.; Islam, S.; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Vulić, J.J.; Cebović, T.N.; Canadanović, V.M.; Cetković, G.S.; Djilas, S.M.; Canadanović-Brunet, J.M.; Velićanski, A.S.; Cvetković, D.D.; Tumbas, V.T. Antiradical, antimicrobial and cytotoxic activities of commercial beetroot pomace. Food Funct. 2013, 4, 713–721. [Google Scholar] [CrossRef]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Castellar, R.; Obón, J.M.; Alacid, M.; Fernández-López, J.A. Color properties and stability of betacyanins from Opuntia fruits. J. Agric. Food Chem. 2003, 51, 2772–2776. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Przyjemnska, M.; Olejnik, A.; Dobrowolska-Zachwieja, A.; Grajek, W. In vitro effects of beetroot juice and chipson oxidative metabolism and apoptosis in neutrophils from obese individuals. Phytother. Res. 2009, 23, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Vidal, P.J.; López-Nicolás, J.M.; Gandía-Herrero, F.; García-Carmona, F. Inactivation of lipoxygenase and cyclooxygenase by natural betalains and semi-synthetic analogues. Food Chem. 2014, 154, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Bastos, E.L.; Schliemann, W. Betalains as Antioxidants. In Plant Antioxidants and Health; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Reference Series in Phytochemistry; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–44. [Google Scholar] [CrossRef]
- Pietrzkowski, Z.; Nemzer, B.; Spórna, A.; Stalica, P.; Tresher, W.; Keller, R.; Jimenez, R.; Michałowski, T.; Wybraniec, S. Influence of betalain rich extract on reduction of discomfort associated with osteoarthritis. New Med. 2010, 1, 12–17. [Google Scholar]
- Das, S.; Filippone, S.M.; Williams, D.S.; Das, A.; Kukreja, R.C. Beet root juice protects against doxorubicin toxicity in cardiomyocytes while enhancing apoptosis in breast cancer cells. Mol. Cell. Biochem. 2016, 421, 89–101. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Azuine, M.A.; Rao, G.S.; Arai, T.; Iida, A.; Tokuda, H. Cytotoxic effect of the red beetroot (Beta vulgaris L.) extract compared to doxorubicin (adriamycin) in the human prostate (PC-3) and breast (MCF-7) cancer cell lines. Anticancer Agents Med. Chem. 2012, 11, 280–284. [Google Scholar] [CrossRef]
- Czapski, J.; Mikołajczyk, K.; Kaczmarek, M. Relationship between antioxidant capacity of red beet juice and contents of its betalain pigments. Polish. J. Food Nutr. Sci. 2009, 59, 119–122. [Google Scholar]
- Wybraniec, S.; Stalica, P.; Spórna, A.; Nemzer, B.; Pietrzkowski, Z.; Michałowski, T. Antioxidant activity of betanidin: Electrochemical study in aqueous media. J. Agric. Food Chem. 2011, 59, 12163–12170. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Szymusiak, H.; Malinowska, P. Betanin, the main pigment of red beet-molecular origin of its exceptionally high free radical scavenging activity. Food Addit. Contam. 2006, 23, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. The role of phenolic hydroxy groups in the free radical scavenging activity of betalains. J. Nat. Prod. 2009, 72, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Valentão, P.; Faria, J.; Ferreres, F.; Sousa, C.; Gil-Izquierdo, A.; Pinho, B.R.; Andrade, P.B. Phytochemical investigations and biological potential screening with cellular and non-cellular models of globe amaranth (Gomphrena globosa L.) inflorescences. Food Chem. 2012, 135, 756–763. [Google Scholar] [CrossRef]
- Escribano, J.; Pedreño, M.A.; García-Carmona, F.; Muñoz, R. Characterization of the antiradical activity of betalains from Beta vulgaris L. roots. Phytochem. Anal. 1998, 9, 124–127. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. HPLC characterization of betalains from plants in the Amaranthaceae. J. Chromatogr. Sci. 2005, 43, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant activities of sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Wagner, A.E.; Motafakkerazad, R.; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem. Toxicol. 2014, 73, 119–126. [Google Scholar] [CrossRef]
- Gentile, C.; Tesoriere, L.; Allegra, M.; Livrea, M.A.; D’Alessio, P. Antioxidant betalains from cactus pear (Opuntia ficus-indica) inhibit endothelial ICAM-1 expression. Ann. N. Y. Acad. Sci. 2004, 1028, 481–486. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A.; Čanadanović-Brunet, J.; Ćetković, G.; Tumbas Šaponjac, V. Microencapsulates and extracts from red beetroot pomace modify antioxidant capacity, heat damage and colour of pseudocereals-enriched einkorn water biscuits. Food Chem. 2018, 268, 40–48. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation-structural and chromatic aspects. J. Food Sci. 2006, 71, 41–50. [Google Scholar] [CrossRef]
- Kaimainen, M. Stability of Natural Colorants of Plant Origin. Ph.D. Thesis, University of Turku, Turku, Finland, 2014. [Google Scholar]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Havlíková, L.; Miková, K.; Kyzlink, V. Heat stability of betacyanins. Z. Lebensm. Unters. Forsch. 1983, 177, 247–250. [Google Scholar] [CrossRef]
- Kidoń, M.; Czapski, J. Wpływ obróbki termicznej na zawartość barwników betalainowych i zdolność przeciwutleniającą buraka ćwikłowego. Żywność Nauk. Technol. Jakość 2007, 1, 124–131. [Google Scholar]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Betaxanthins as substrates for tyrosinase. an approach to the role of tyrosinase in the biosynthetic pathway of betalains. Plant Physiol. 2005, 138, 421–432. [Google Scholar] [CrossRef]
- Wybraniec, S.; Nowak-Wydra, B.; Mizrahi, Y. 1H and 13C NMR spectroscopic structural elucidation of new decarboxylated betacyanins. Tetrahedron Lett. 2006, 47, 1725–1728. [Google Scholar] [CrossRef]
- Attoe, E.L.; von Elbe, J.H. Degradation kinetics of betanin in solutions as influenced by oxygen. J. Agric. Food Chem. 1982, 30, 708–712. [Google Scholar] [CrossRef]
- Pasch, J.H. Stability containing organic in buffered solutions acids, metal cations, or sequestrants materials&methods. J. Food Sci. 1979. [Google Scholar] [CrossRef]
- Savolainen, K.; Kuusi, T. The stability properties of golden beet and red beet pigments: Influence of pH, temperature, and some stabilizers. Z. Lebensm. Unters. Forsch. 1978, 166, 19–22. [Google Scholar] [CrossRef]
- Herbach, K.M.; Rohe, M.; Stintzing, F.C.; Carle, R. Structural and chromatic stability of purple pitaya (Hylocereus polyrhizus [Weber] Britton&Rose) betacyanins as affected by the juice matrix and selected additives. Food Res. Int. 2006, 39, 667–677. [Google Scholar]
- Pasch, J.H.; von Elbe, J.H. Betanine stability in buffered solutions containing organic acids, metal cations, antioxidants, or sequestrants. J. Food Sci. 1979, 44, 72–75. [Google Scholar]
- Barrera, F.A.; Reynoso, C.R.; Gonzales de Mejia, E. Estabilidad de las betalainas extraidas del garambullo (Myrtillocactus geometrizans). Food Sci. Technol. Int. 1998, 4, 115–120. [Google Scholar] [CrossRef]
- Bilyk, A.; Kolodij, M.A.; Sapers, G.M. Stabilization of red beet pigments with isoascorbic acid. J. Food Sci. 1981, 46, 1616–1617. [Google Scholar] [CrossRef]
- Attoe, E.L.; von Elbe, J.H. Oxygen involvement in betanine degradation: Effect of antioxidants. J. Agric. Food Chem. 1985, 50, 106–110. [Google Scholar] [CrossRef]
- Bilyk, A.; Howard, M. Reversibility of thermal degradation of betacyanins under the influence of isoascorbic acid. J. Agric. Food Chem. 1982, 30, 906–908. [Google Scholar] [CrossRef]
- Wybraniec, S. Formation of decarboxylated betacyanins in heated purified betacyanin fractions from red beet root (Beta vulgaris L.) monitored by LC-MS/MS. J. Agric. Food Chem. 2005, 53, 3483–3487. [Google Scholar] [CrossRef]
- Wybraniec, S.; Michalowski, T. New pathways of betanidin and betanin enzymatic oxidation. J. Agric. Food Chem. 2011, 59, 9612–9622. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, S.; Starzak, K.; Skopińska, A.; Nemzer, B.; Pietrzkowski, Z.; Michałowski, T. Studies on nonenzymatic oxidation mechanisms in neobetanin, betanin, and decarboxylated betanins. J. Agric. Food Chem. 2013, 61, 6465–6476. [Google Scholar] [CrossRef]
- Nemzer, B.; Pietrzkowski, Z.; Spórna, A.; Stalica, P.; Thresher, W.; Michałowski, T.; Wybraniec, S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Kumorkiewicz, A.; Szmyr, N.; Popenda, Ł.; Pietrzkowski, Z.; Wybraniec, S. Alternative mechanisms of betacyanin oxidation by complexation and radical generation. J. Agric. Food Chem. 2019, 67, 7455–7465. [Google Scholar] [CrossRef] [PubMed]
- Kumorkiewicz, A.; Sutor, K.; Nemzer, B.; Pietrzkowski, Z.; Wybraniec, S. Thermal decarboxylation of betacyanins in red beet betalain-rich extract. Pol. J. Food Nutr. Sci. 2020, 70, 7–14. [Google Scholar] [CrossRef]
- Wybraniec, S.; Starzak, K.; Skopińska, A.; Szaleniec, M.; Słupski, J.; Mitka, K.; Kowalski, P.; Michałowski, T. Effects of metal cations on betanin stability in aqueous-organic solutions. Food Sci. Biotechnol. 2013, 22, 353–363. [Google Scholar] [CrossRef]
- Han, D.; Kim, S.J.; Kim, S.H.; Kim, D.M. Repeated regeneration of degraded red beet juice pigments in the presence of antioxidants. J. Food Sci. 1998, 63, 69–72. [Google Scholar] [CrossRef]
- Santos, R.P.; Souza, L.M.; Balieiro, A.L.; Soares, C.M.F.; Lima, A.S.; Souza, R.L. Integrated process of extraction and purification of betanin from Opuntia ficus-indica using aqueous two-phase systems based on THF and sodium salts. Sep. Sci. Technol. 2018, 53, 2–11. [Google Scholar] [CrossRef]
- Sutor-Świeży, K.; Antonik, M.; Proszek, J.; Nemzer, B.; Pietrzkowski, Z.; Popenda, Ł.; Świergosz, T.; Wybraniec, S. Dehydrogenation of Betacyanins in Heated Betalain-Rich Extracts of Red Beet (Beta vulgaris L.). Int. J. Mol. Sci. 2022, 23, 1245. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Stability and color changes of thermally treated betanin, phyllocactin, and hylocerenin solutions. J. Agric. Food Chem. 2006, 54, 390–398. [Google Scholar] [CrossRef]
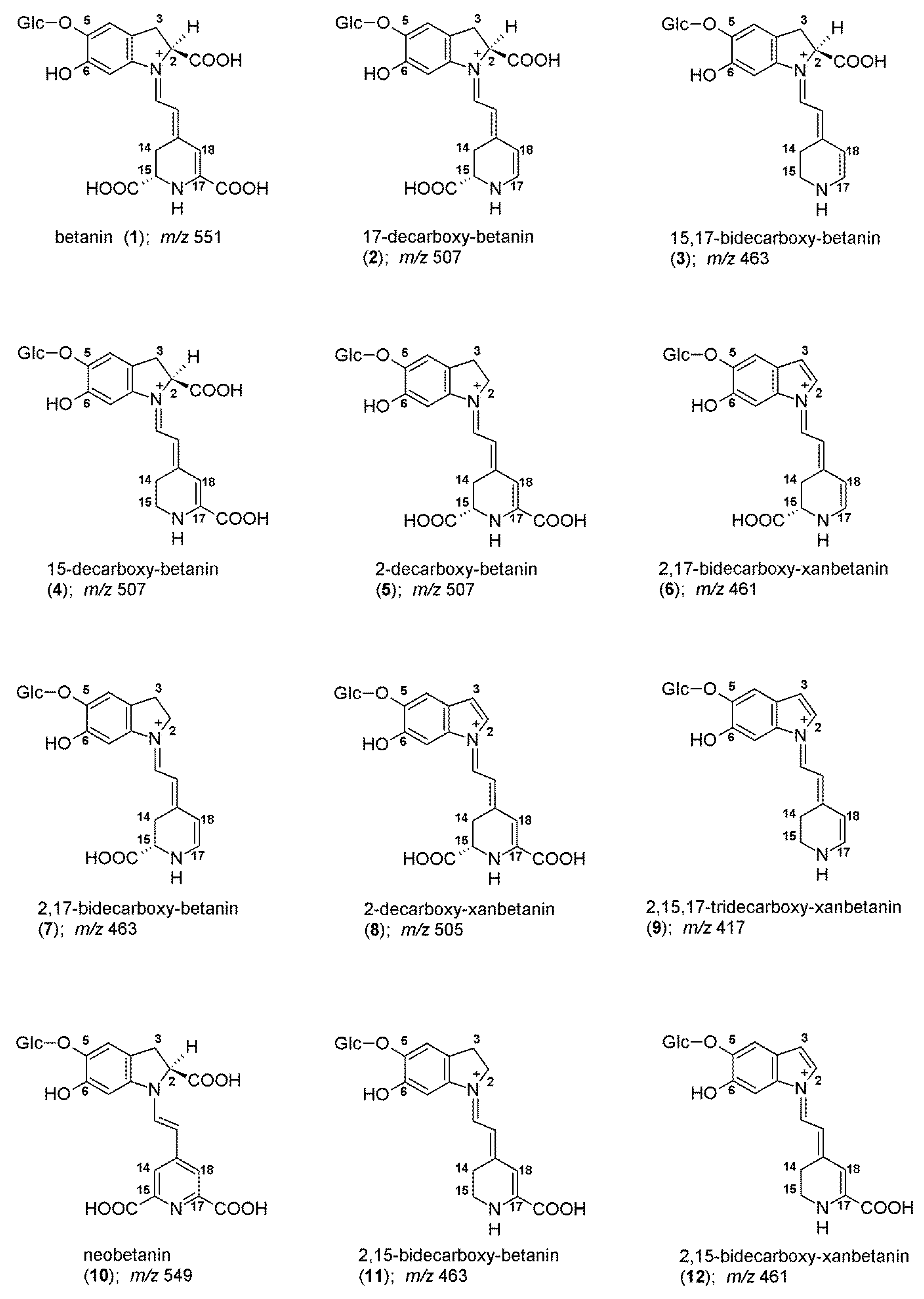





| tR | λmax | m/z | |||
|---|---|---|---|---|---|
| No. | Pigment | Abbreviation | [min] | [nm] | [M + H]+ |
| non-decarboxylated betacyanins | |||||
| 1/1′ | betanin/isobetanin | Bt/IBt | 6.6/7.3 | 536 | 551 |
| 10 | neobetanin | NBt | 10.3 | 468 | 549 |
| mono-decarboxylated betacyanins | |||||
| 2/2′ | 17-decarboxy-betanin/-isobetanin | 17-dBt/IBt | 7.1/7.7 | 505 | 507 |
| 4 | 15-decarboxy-betanin | 15-dBt | 8.3 | 527 | 507 |
| 5/5′ | 2-decarboxy-betanin/-isobetanin | 2-dBt/IBt | 8.9 | 533 | 507 |
| bi- and tri-decarboxylated betacyanins | |||||
| 3 | 15,17-bidecarboxy-betanin a | 15,17-dBt | 8.3 | 494 | 463 |
| 7/7′ | 2,17-bidecarboxy-betanin/-isobetanin | 2,17-dBt/IBt | 9.6 | 507 | 463 |
| 11 | 2,15-bidecarboxy-betanin | 2,15-dBt | 10.4 | 532 | 463 |
| 13 | 2,15,17-tridecarboxy-betanin a | 2,15,17-dBt | 10.7 | 503 | 419 |
| mono-decarboxylated dehydro-betacyanins | |||||
| 8 | 2-decarboxy-xanbetanin a | 2-dXBt | 9.6 | 446 | 505 |
| 18 | 2-decarboxy-neobetanin | 2-dNBt | 12.0 | 480 | 505 |
| 20 | 2-decarboxy-xanneobetanin | 2-dXNBt | 12.7 | 422 | 503 |
| bi-decarboxylated dehydro-betacyanins | |||||
| 6 | 2,17-decarboxy-xanbetanin a | 2,17-dXBt | 9.5 | 460 | 461 |
| 12 | 2,15-bidecarboxy-xanbetanin a | 2,15-dXBt | 10.6 | 478 | 461 |
| 16 | 2,17-bidecarboxy-neobetanin a | 2,17-dNBt | 11.7 | 459 | 461 |
| bi-decarboxylated xanneobetacyanins | |||||
| 15 | 2,17-bidecarboxy-xanneobetanin | 2,17-dXNBt | 11.4 | 407 | 459 |
| 19 | 2,15-bidecarboxy-xanneobetanin a | 2,15-dXNBt | 12.3 | 427 | 459 |
| tri-decarboxylated dehydro-betacyanins | |||||
| 9 | 2,15,17-tridecarboxy-xanbetanin a | 2,15,17-dXBt | 9.9 | - | 417 |
| 14 | 2,15,17-tridecarboxy-neobetanin a | 2,15,17-dNBt | 11.3 | 442 | 417 |
| 17 | 2,15,17-tridecarboxy-xanneobetanin | 2,15,17-dXNBt | 11.9 | 394 | 415 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutor-Świeży, K.; Proszek, J.; Popenda, Ł.; Wybraniec, S. Influence of Citrates and EDTA on Oxidation and Decarboxylation of Betacyanins in Red Beet (Beta vulgaris L.) Betalain-Rich Extract. Molecules 2022, 27, 9054. https://doi.org/10.3390/molecules27249054
Sutor-Świeży K, Proszek J, Popenda Ł, Wybraniec S. Influence of Citrates and EDTA on Oxidation and Decarboxylation of Betacyanins in Red Beet (Beta vulgaris L.) Betalain-Rich Extract. Molecules. 2022; 27(24):9054. https://doi.org/10.3390/molecules27249054
Chicago/Turabian StyleSutor-Świeży, Katarzyna, Justyna Proszek, Łukasz Popenda, and Sławomir Wybraniec. 2022. "Influence of Citrates and EDTA on Oxidation and Decarboxylation of Betacyanins in Red Beet (Beta vulgaris L.) Betalain-Rich Extract" Molecules 27, no. 24: 9054. https://doi.org/10.3390/molecules27249054
APA StyleSutor-Świeży, K., Proszek, J., Popenda, Ł., & Wybraniec, S. (2022). Influence of Citrates and EDTA on Oxidation and Decarboxylation of Betacyanins in Red Beet (Beta vulgaris L.) Betalain-Rich Extract. Molecules, 27(24), 9054. https://doi.org/10.3390/molecules27249054