Screening of the Active Compounds against Neural Oxidative Damage from Ginseng Phloem Using UPLC-Q-Exactive-MS/MS Coupled with the Content-Effect Weighted Method
Abstract
:1. Introduction
2. Results
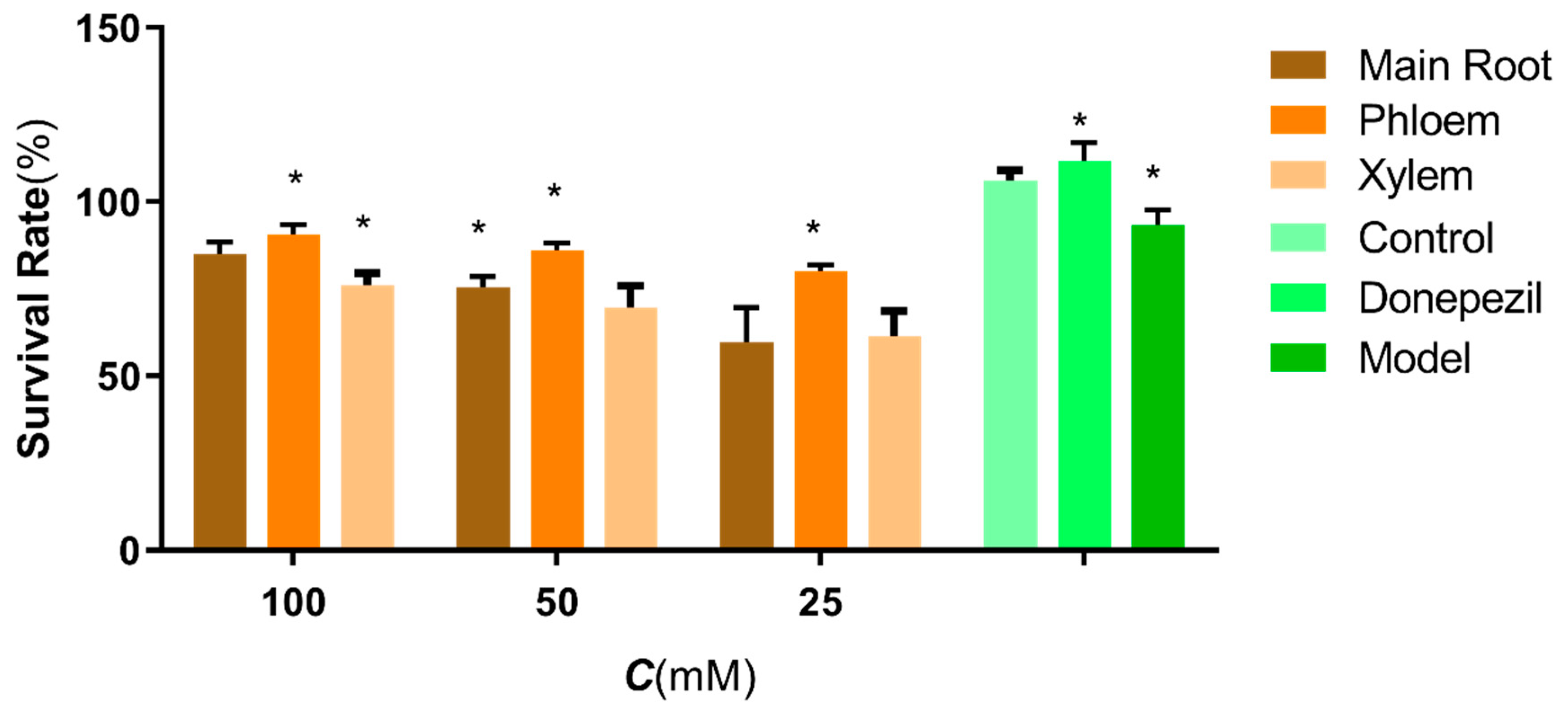
2.1. The Protective Effects of Different Parts of Ginseng on H2O2 Damage to SH-SY5Y Cells
2.2. Identification of Compounds in Phloem of the Ginseng by UPLC-Q-Exactive-MS/MS
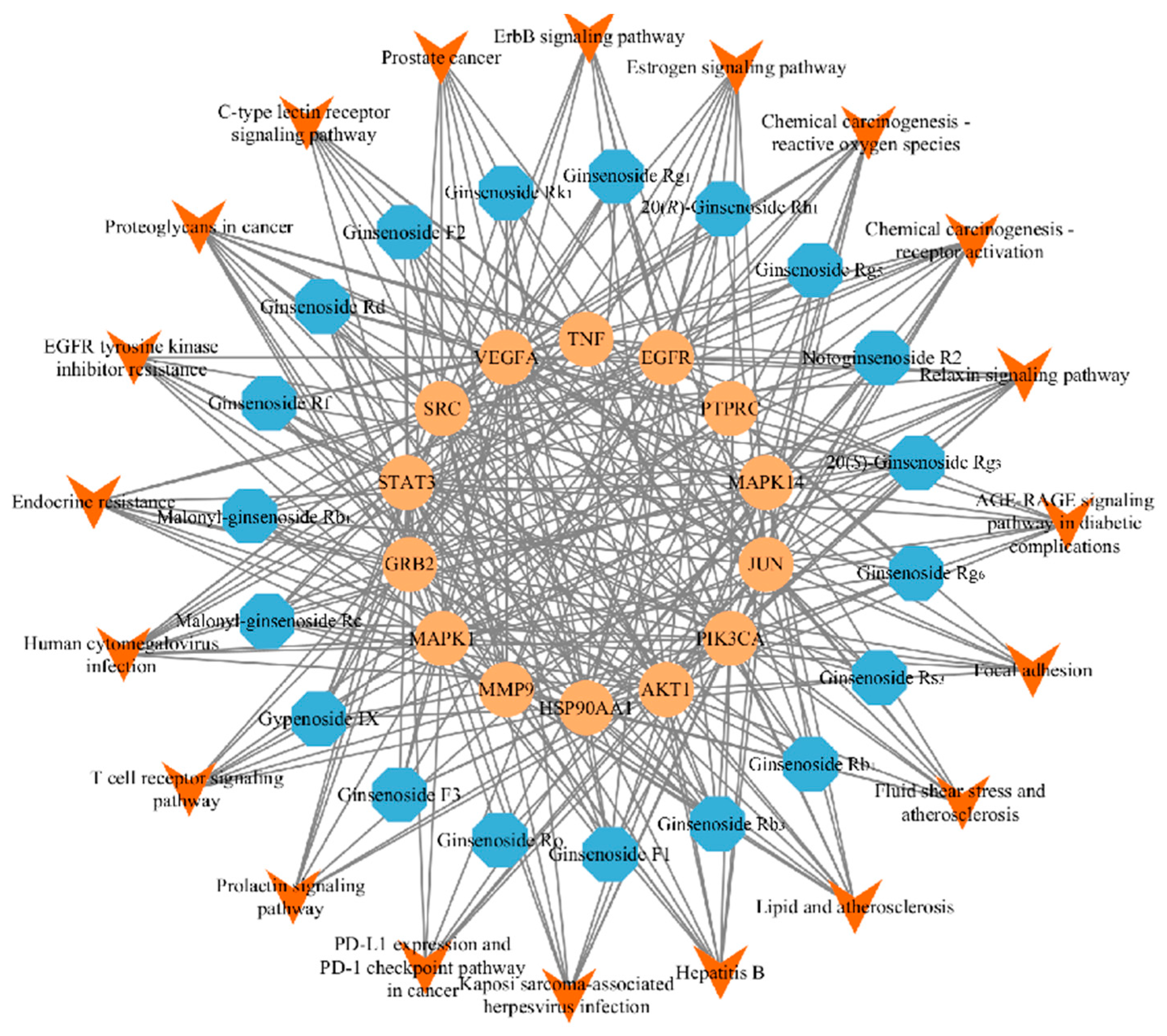
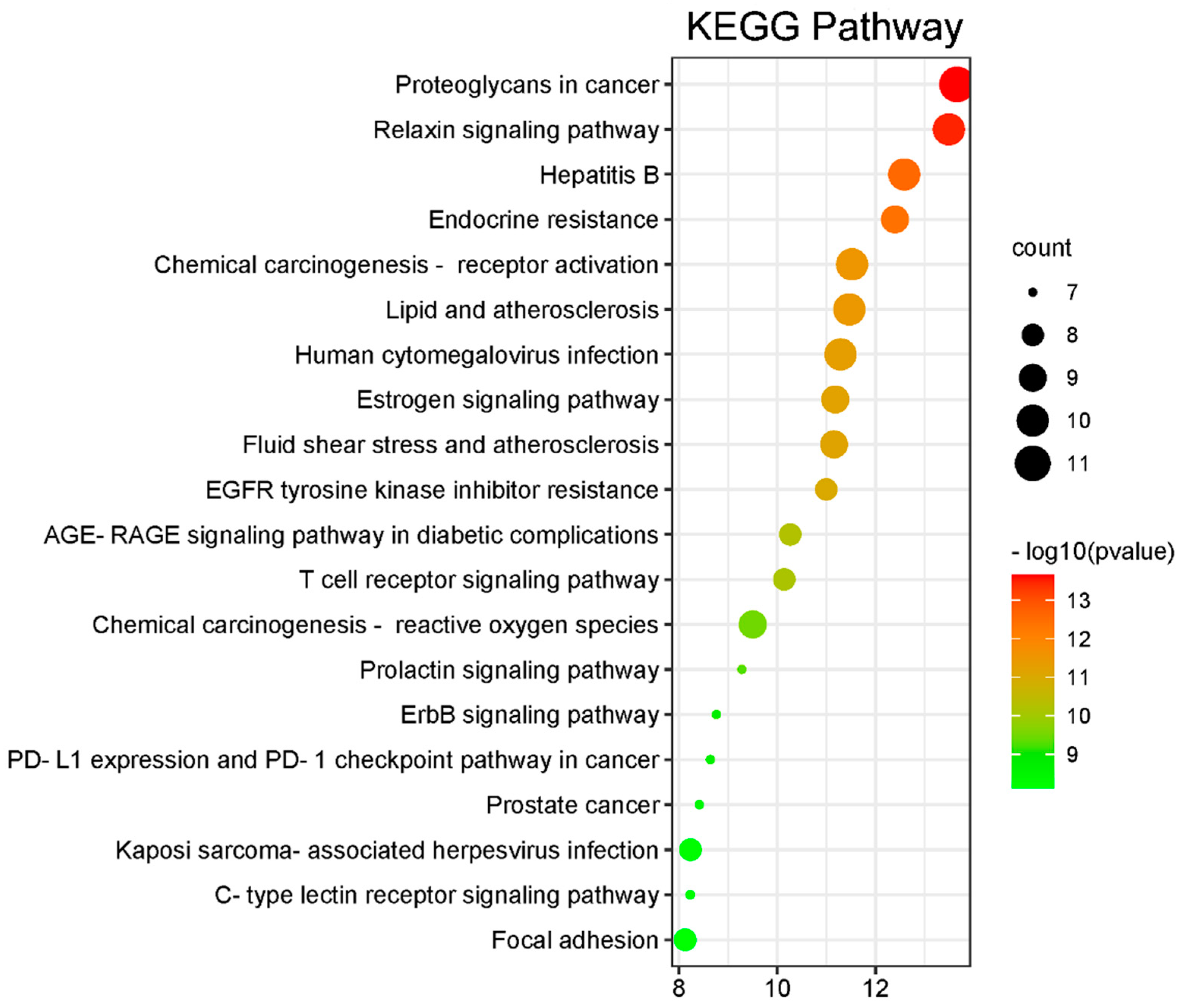
2.3. Analysis of the Network Pharmacology
2.4. Analysis of the Molecular Docking
2.5. Analysis of Content-Effect Weighted Method
2.6. The Protective Effect of Active Compounds on H2O2-Induced SH-SY5Y Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Sample Preparation
4.3. Cell Culture
4.4. MTT Assay
4.5. UPLC-Q-Exactive-MS/MS Conditions
4.6. Network Pharmacology Analysis
4.6.1. Construction of the Compound-Target and Disease-Target Networks
4.6.2. Protein–Protein Interaction and Pathway-Enrichment Analysis
4.6.3. Correlation Analysis
4.7. Molecular Docking
4.8. Establishment of the Weighted Value
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Miranda-Díaz, A.G.; García-Sánchez, A.; Cardona-Muñoz, E.G. Foods with potential prooxidant and antioxidant effects involved in Parkinson’s disease. Oxidative Med. Cell. Longev. 2020, 2020, 6281454. [Google Scholar] [CrossRef]
- Ryan, D.A.; Narrow, W.C.; Federoff, H.J.; Bowers, W.J. An improved method for generating consistent soluble amyloid-beta oligomer preparations for in vitro neurotoxicity studies. J. Neurosci. Methods 2010, 190, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Khobta, A.; Anderhub, S.; Kitsera, N.; Epe, B. Gene Silencing Induced by oxidative DNA base damage: Association with local decrease of histone H4 acetylation in the promoter region. Nucleic Acids Res. 2010, 38, 4285–4295. [Google Scholar] [CrossRef]
- Sánchez-Morán, I.; Rodríguez, C.; Lapresa, R.; Agulla, J.; Sobrino, T.; Castillo, J.; Bolaños, J.P.; Almeida, A. Nuclear WRAP53 promotes neuronal survival and functional recovery after stroke. Sci. Adv. 2020, 6, eabc5702. [Google Scholar] [CrossRef]
- Motohashi, H.; Kimura, M.; Fujita, R.; Inoue, A.; Pan, X.; Takayama, M.; Katsuoka, F.; Aburatani, H.; Bresnick, E.H.; Yamamoto, M. NF-E2 domination over Nrf2 promotes ROS accumulation and megakaryocytic maturation. Blood 2010, 115, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Botting, K.J.; Skeffington, K.L.; Niu, Y.; Allison, B.J.; Brain, K.L.; Itani, N.; Beck, C.; Logan, A.; Murray, A.J.; Murphy, M.P.; et al. Translatable mitochondria-targeted protection against programmed cardiovascular dysfunction. Sci. Adv. 2020, 6, eabb1929. [Google Scholar] [CrossRef]
- Gupta, R.; Karpatkin, S.; Basch, R.S. Hematopoiesis and stem cell renewal in long-term bone marrow cultures containing catalase. Blood 2006, 107, 1837–1846. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Guo, J.; Wang, Y.; Xiong, X.; Tao, H.; Li, J.; Jia, Y.; Hu, H.; Zhang, J. A broad-spectrum ROS-eliminating material for prevention of inflammation and drug-induced organ toxicity. Adv. Sci. 2018, 5, 1800781. [Google Scholar] [CrossRef] [Green Version]
- Imaida, K.; Fukushima, S.; Shirai, T.; Ohtani, M.; Nakanishi, K.; Ito, N. Promoting activities of butylated hydroxyanisole and butylated hydroxytoluene on 2-Stage urinary bladder carcinogenesis and inhibition of γ-glutamyl transpeptidase-positive foci development in the liver of rats. Carcinogenesis 1983, 4, 895–899. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.; Li, W.; Zhou, L.; Li, Q.; Wang, X.; He, P. A UPLC/MS-based metabolomics investigation of the protective effect of Ginsenosides Rg1 and Rg2 in mice with Alzheimer’s disease. J. Ginseng Res. 2016, 40, 9–17. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kang, S.M.; Vassy, J.L.; Shin, H.; Lee, Y.H.; Ahn, H.Y.; Choi, S.H.; Park, K.S.; Jang, H.C.; Lim, S. Efficacy and safety of ginsam, a vinegar extract from Panax ginseng, in type 2 diabetic patients: Results of a double-blind, placebo-controlled study: Antidiabetic effect and safety of ginsam. J. Diabetes Investig. 2012, 3, 309–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, W.; Zhao, F.; Zhang, C.; Ju, H.; Lu, W. Promotion of compound K production in Saccharomyces cerevisiae by glycerol. Microb. Cell Factories 2020, 19, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, K.; Wong, A. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Kim, H.J.; Park, M.S.; Ji, G.E. Effects of fermented ginseng root and ginseng berry on obesity and lipid metabolism in mice fed a high-fat diet. J. Ginseng Res. 2018, 42, 312–319. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Shim, J.S.; Song, M.-Y.; Yim, S.-V.; Lee, S.E.; Park, K.-S. Proteomic analysis reveals that the protective effects of ginsenoside Rb1 are associated with the actin cytoskeleton in β-amyloid-treated neuronal cells. J. Ginseng Res. 2016, 40, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Ghaeminia, M.; Rajkumar, R.; Koh, H.-L.; Dawe, G.S.; Tan, C.H. Ginsenoside Rg1 modulates medial prefrontal cortical firing and suppresses the hippocampo-medial prefrontal cortical long-term potentiation. J. Ginseng Res. 2018, 42, 298–303. [Google Scholar] [CrossRef]
- Feng, H.; Xue, M.; Deng, H.; Cheng, S.; Hu, Y.; Zhou, C. Ginsenoside and its therapeutic potential for cognitive impairment. Biomolecules 2022, 12, 1310. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.-S. Ginseng pharmacology. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Shin, E.-J.; Jeong, J.H.; Nguyen, B.-T.; Sharma, N.; Nah, S.-Y.; Chung, Y.H.; Lee, Y.; Byun, J.K.; Nabeshima, T.; Ko, S.K.; et al. Ginsenoside Re protects against serotonergic behaviors evoked by 2,5-dimethoxy-4-iodo-amphetamine in mice via inhibition of PKCδ-mediated mitochondrial dysfunction. Int. J. Mol. Sci. IJMS 2021, 22, 7219. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, Y.; Xu, L.; Qin, M.; Yi, T.; Chen, H.; Zhao, Z. Localization of ginsenosides in the rhizome and root of Panax ginseng by laser microdissection and liquid chromatography–quadrupole/time of flight-mass spectrometry. J. Pharm. Biomed. Anal. 2015, 105, 121–133. [Google Scholar] [CrossRef]
- Kubo, M.; Tani, T.; Katsuki, T.; Ishizaki, K.; Arichi, S. Histochemistry. I. Ginsenosides in ginseng (Panax ginseng C. A. Meyer, root). J. Nat. Prod. 1980, 43, 278–284. [Google Scholar] [CrossRef]
- Bai, H.; Wang, S.; Liu, J.; Gao, D.; Jiang, Y.; Liu, H.; Cai, Z. Localization of ginsenosides in Panax ginseng with different age by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry imaging. J. Chromatogr. B 2016, 1026, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Lucas, W.J.; Groover, A.; Lichtenberger, R.; Furuta, K.; Yadav, S.-R.; Helariutta, Y.; He, X.-Q.; Fukuda, H.; Kang, J.; Brady, S.M.; et al. The plant vascular system: Evolution, development and functionsF. J. Integr. Plant Biol. 2013, 55, 294–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Su, H.; Zhang, L.; Liao, B.-S.; Xiao, S.-M.; Dong, L.-L.; Hu, Z.-G.; Wang, P.; Li, X.-W.; Huang, Z.-H.; et al. Comprehensive characterization for ginsenosides biosynthesis in ginseng root by integration analysis of chemical and transcriptome. Molecules 2017, 22, 889. [Google Scholar] [CrossRef] [Green Version]
- Osbourn, A.E. Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell 1996, 8, 1821–1831. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, Y.; Wang, Y.; Xiao, W. Polypharmacology in drug discovery: A review from systems pharmacology perspective. Curr. Pharm. Des. 2016, 22, 3171–3181. [Google Scholar] [CrossRef] [Green Version]
- Lauria, A.; Bonsignore, R.; Bartolotta, R.; Perricone, U.; Martorana, A.; Gentile, C. Drugs polypharmacology by in Silico methods: New opportunities in drug discovery. Curr. Pharm. Des. 2016, 22, 3073–3081. [Google Scholar] [CrossRef]
- Boehr, D.D.; Nussinov, R.; Wright, P.E. The role of dynamic conformational ensembles in biomolecular recognition. Nat. Chem. Biol. 2009, 5, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zou, J.; Jia, Y.; Zhang, X.; Wang, C.; Shi, Y.; Guo, D.; Wu, Z.; Wang, F. The mechanism of lavender essential oil in the treatment of acute colitis based on “quantity–effect” weight coefficient network pharmacology. Front. Pharmacol. 2021, 12, 644140. [Google Scholar] [CrossRef]
- von Mering, C. String: Known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2004, 33, D433–D437. [Google Scholar] [CrossRef]
- Xie, J.; Yang, P.; Wei, H.; Mai, P.; Yu, X. Development of a prognostic nomogram based on an eight-gene signature for esophageal squamous cell carcinoma by weighted gene co-expression network analysis (WGCNA). Ann. Transl. Med. 2022, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Li, Y.; Liu, Q.; Shu, M. Identification and validation of an immune-associated RNA-binding proteins signature to predict clinical outcomes and therapeutic responses in glioma patients. Cancers 2021, 13, 1730. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Di Costanzo, L.; Christie, C.; Dalenberg, K.; Duarte, J.M.; Dutta, S.; et al. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019, 47, D464–D474. [Google Scholar] [CrossRef] [PubMed]
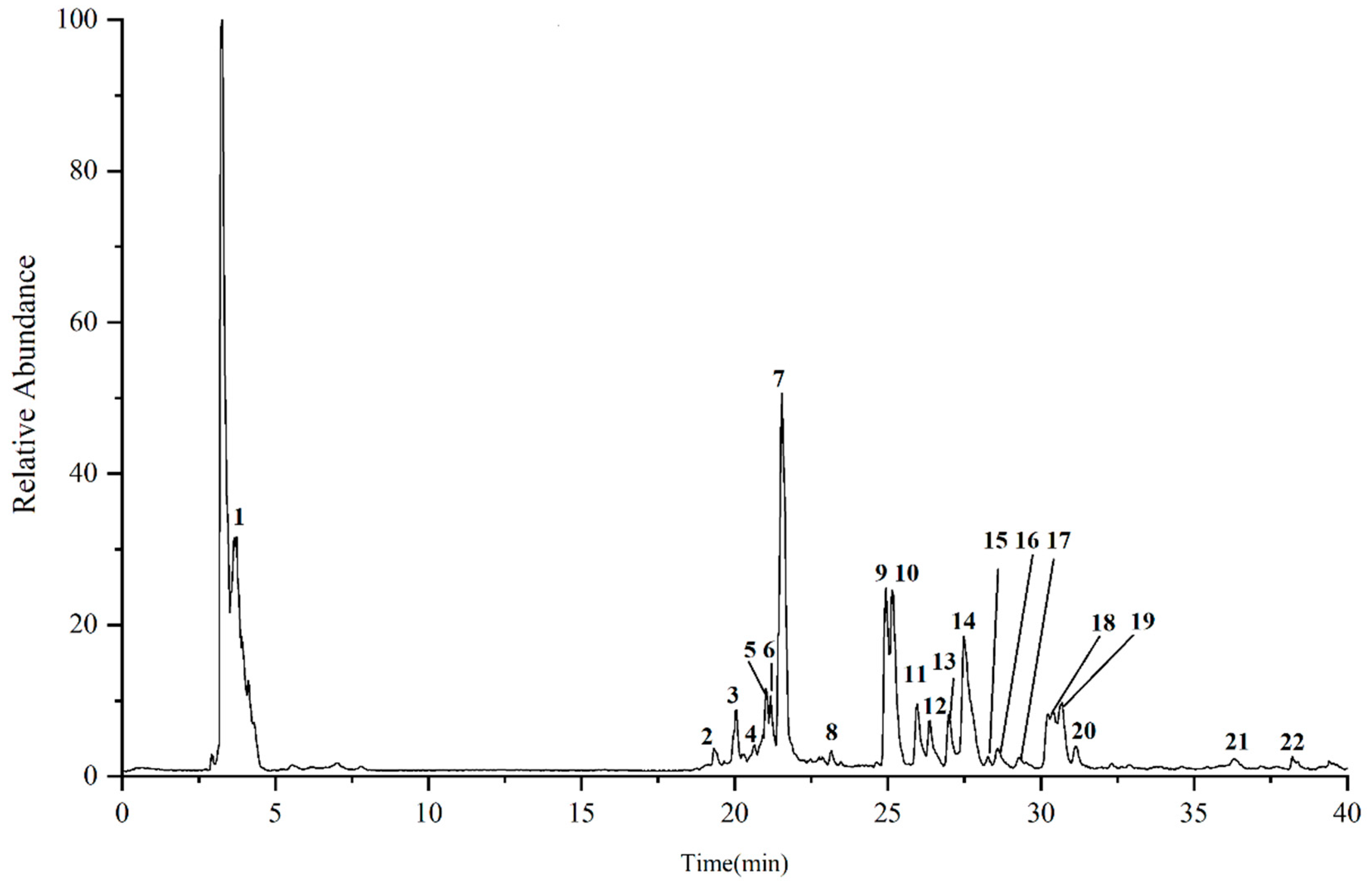




| No. | RT/min | [M-H]- | [M+HCOO]- | MS/MS Fragment Ions | Peak Area | Compound |
|---|---|---|---|---|---|---|
| 1 | 4.85 | - | 683.5000 | 475.3746; 161.0438 | 7,483,087.36 | Ginsenoside F1 |
| 2 | 19.32 | 851.4600 | - | - | 382,261.81 | Unknown |
| 3 | 20.06 | 799.0000 | - | 637.4302; 475.3775; 161.0445 | 2,462,891.31 | Ginsenoside Rg1 |
| 4 | 20.19 | 769.4744 | - | 637.4314; 475.3788 | 399,896.95 | Notoginsenoside R2 |
| 5 | 21.01 | 1163.5860 | - | 1077.5839; 945.5390; 783.4875 | 521,391.11 | Malonyl-ginsenoside Rc |
| 6 | 21.18 | 1193.5960 | - | 945.5067; 783.4598; 621.4125 | 417,224.70 | Malonyl-ginsenoside Rb1 |
| 7 | 21.54 | - | 991.9580 | 783.4615; 621.4111 | 3,865,902.28 | Ginsenoside Rd |
| 8 | 23.15 | 1077.5851 | - | 945.5088; 621.4125 | 151,726.43 | Ginsenoside Rb3 |
| 9 | 24.91 | 955.0521 | - | 631.3852; 455.3498; 119.0336 | 2,640,554.75 | Ginsenoside Ro |
| 10 | 25.17 | 799.4849 | - | 475.3657; 221.0609; 101.0247 | 3,432,721.17 | Ginsenoside Rf |
| 11 | 25.96 | 716.3397 | - | 783.4935; 621.4398; 459.3845 | 1,542,993.88 | Gypenoside IX |
| 12 | 26.35 | - | 769.3937 | 769.4711; 637.4299; 475.3777 | 1,580,772.42 | Ginsenoside F3 |
| 13 | 26.98 | - | 829.7129 | 621.4223; 459.3791; 161.0381 | 1,869,503.76 | Ginsenoside F2 |
| 14 | 27.48 | 1107.1880 | 945.5067; 783.4598; 621.4125 | 3,839,623.34 | Ginsenoside Rb1 | |
| 15 | 28.27 | 597.3012 | - | - | 149,999.06 | Unknown |
| 16 | 28.57 | - | 683.3636 | 475.3784; 161.0442 | 275,862.57 | 20(R)-Ginsenoside Rh1 |
| 17 | 29.29 | - | 811.6000 | 221.0579; 161.0381; 101.0186 | 146,976.50 | Ginsenoside Rk1 |
| 18 | 30.23 | 991.5514 | - | - | 998,851.03 | Unknown |
| 19 | 30.69 | - | 811.1000 | 603.4211; 441.3664; 221.0579 | 1,155,965.76 | Ginsenoside Rg5 |
| 20 | 31.12 | 783.4900 | - | 621.4309; 459.3768; 221.0598 | 296,078.61 | 20(S)-Ginsenoside Rg3 |
| 21 | 29.79 | - | 825.4038 | 783.4897; 621.4352; 459.3832 | 157,781.60 | Ginsenoside Rs3 |
| 22 | 35.46 | - | 811.2000 | 619.4207; 457.3665 | 169,659.90 | Ginsenoside Rg6 |
| NO. | Compound | Original Degree | Original Binding Energy | Content Coefficient | Effect Coefficient | Weighted Value | Original * Order | Order after Weighted | Order Changing after Weighted |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ginsenoside Rd | 10 | 90.8 | 26.30 | 3.25 | 856.02 | 1 | 2 | ↓1 |
| 2 | Ginsenoside Rk1 | 9 | 100.8 | 1.00 | 3.61 | 32.52 | 2 | 13 | ↓11 |
| 3 | Ginsenoside Rg1 | 9 | 89.2 | 16.76 | 3.20 | 482.69 | 3 | 4 | ↓1 |
| 4 | 20(R)-Ginsenoside Rh1 | 8 | 90.7 | 2.01 | 3.24 | 52.35 | 4 | 11 | ↓7 |
| 5 | Ginsenoside Rg5 | 8 | 85.6 | 7.86 | 3.07 | 193.04 | 5 | 7 | ↓2 |
| 6 | 20(S)-Ginsenoside Rg3 | 8 | 84.3 | 1.03 | 3.02 | 24.95 | 6 | 15 | ↓9 |
| 7 | Ginsenoside Rf | 8 | 79.4 | 23.36 | 2.85 | 531.74 | 7 | 3 | ↑4 |
| 8 | Ginsenoside F1 | 7 | 75.7 | 50.91 | 2.71 | 966.99 | 8 | 1 | ↑7 |
| 9 | Ginsenoside F2 | 7 | 71.1 | 12.72 | 2.55 | 226.90 | 9 | 6 | ↑3 |
| 10 | Notoginsenoside R2 | 7 | 70.6 | 2.72 | 2.53 | 48.19 | 10 | 12 | ↓2 |
| 11 | Gypenoside IX | 7 | 64.1 | 10.50 | 2.30 | 169.02 | 11 | 8 | ↑3 |
| 12 | Ginsenoside Rg6 | 6 | 65.1 | 1.15 | 2.33 | 16.16 | 12 | 18 | ↓6 |
| 13 | Ginsenoside F3 | 6 | 63.7 | 10.76 | 2.28 | 147.34 | 13 | 9 | ↑4 |
| 14 | Ginsenoside Rs3 | 5 | 52.6 | 1.07 | 1.89 | 10.12 | 14 | 19 | ↓5 |
| 15 | Ginsenoside Rb1 | 5 | 50.5 | 26.12 | 1.81 | 236.43 | 15 | 5 | ↑10 |
| 16 | Ginsenoside Rb3 | 5 | 45.8 | 2.01 | 2.46 | 24.78 | 16 | 16 | - |
| 17 | Malonyl-ginsenoside Rb1 | 5 | 43.4 | 2.84 | 1.56 | 22.08 | 17 | 17 | - |
| 18 | Malonyl-ginsenoside Rc | 5 | 43.3 | 3.55 | 1.55 | 27.49 | 18 | 14 | ↑4 |
| 19 | Ginsenoside Ro | 3 | 27.9 | 17.97 | 1.00 | 53.90 | 19 | 10 | ↑9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.-C.; Zhang, N.-X.; Shen, J.-M.; Lv, J.-W.; Zhang, K.-Y.; Sun, Y.; Li, H.; Wang, Y.-L.; Cheng, D.-D.; Zhao, M.-Y.; et al. Screening of the Active Compounds against Neural Oxidative Damage from Ginseng Phloem Using UPLC-Q-Exactive-MS/MS Coupled with the Content-Effect Weighted Method. Molecules 2022, 27, 9061. https://doi.org/10.3390/molecules27249061
Gao X-C, Zhang N-X, Shen J-M, Lv J-W, Zhang K-Y, Sun Y, Li H, Wang Y-L, Cheng D-D, Zhao M-Y, et al. Screening of the Active Compounds against Neural Oxidative Damage from Ginseng Phloem Using UPLC-Q-Exactive-MS/MS Coupled with the Content-Effect Weighted Method. Molecules. 2022; 27(24):9061. https://doi.org/10.3390/molecules27249061
Chicago/Turabian StyleGao, Xiao-Chen, Nan-Xi Zhang, Jia-Ming Shen, Jing-Wei Lv, Kai-Yue Zhang, Yao Sun, Hang Li, Yue-Long Wang, Duan-Duan Cheng, Meng-Ya Zhao, and et al. 2022. "Screening of the Active Compounds against Neural Oxidative Damage from Ginseng Phloem Using UPLC-Q-Exactive-MS/MS Coupled with the Content-Effect Weighted Method" Molecules 27, no. 24: 9061. https://doi.org/10.3390/molecules27249061
APA StyleGao, X.-C., Zhang, N.-X., Shen, J.-M., Lv, J.-W., Zhang, K.-Y., Sun, Y., Li, H., Wang, Y.-L., Cheng, D.-D., Zhao, M.-Y., Zhang, H., Li, C.-N., & Sun, J.-M. (2022). Screening of the Active Compounds against Neural Oxidative Damage from Ginseng Phloem Using UPLC-Q-Exactive-MS/MS Coupled with the Content-Effect Weighted Method. Molecules, 27(24), 9061. https://doi.org/10.3390/molecules27249061





