Nucleophilic Radiofluorination Using Tri-tert-Butanol Ammonium as a Bifunctional Organocatalyst: Mechanism and Energetics
Abstract
1. Introduction
2. Results
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, E.; Hooker, J.M.; Ritter, T. Nickel-mediated oxidative fluorination for PET with aqueous [18F] fluoride. J. Am. Chem. Soc. 2012, 134, 17456–17458. [Google Scholar] [CrossRef] [PubMed]
- Tredwell, M.; Preshlock, S.M.; Taylor, N.J.; Gruber, S.; Huiban, M.; Passchier, J.; Mercier, J.; Génicot, C.; Gouverneur, V. A general copper-mediated nucleophilic 18F fluorination of arenes. Angew. Chemie 2014, 126, 7885–7889. [Google Scholar] [CrossRef]
- Edwards, R.; Westwell, A.D.; Daniels, S.; Wirth, T. Convenient synthesis of diaryliodonium salts for the production of [18F]F-DOPA. European J. Org. Chem. 2015, 2015, 625–630. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Neumann, K.D.; Uppaluri, S.; Cerny, R.L.; DiMagno, S.G. Improved arene fluorination methodology for I(III) salts. Org. Lett. 2010, 12, 3352–3355. [Google Scholar] [CrossRef][Green Version]
- Edwards, R.; Wirth, T. [18F] 6-fluoro-3,4-dihydroxy-l-phenylalanine–recent modern syntheses for an elusive radiotracer. J. Label. Compd. Radiopharm. 2015, 58, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Pretze, M.; Wängler, C.; Wängler, B. 6-[18F] fluoro-L-DOPA: A well-established neurotracer with expanding application spectrum and strongly improved radiosyntheses. Biomed Res. Int. 2014, 2014, 674063. [Google Scholar] [CrossRef]
- Oh, Y.H.; Choi, H.; Lee, S.S.; Lee, S. Toward the Robust Synthesis of [18F]F-DOPA: Quantum Chemical Analysis of SNAr Cold Fluorination of Diaryl Iodonium Salt by 19F−. Bull. Korean Chem. Soc. 2020, 41, 200–405. [Google Scholar] [CrossRef]
- Lee, J.W.; Oliveira, M.T.; Jang, H.B.; Lee, S.; Chi, D.Y.; Kim, D.W.; Song, C.E. Hydrogen-bond promoted nucleophilic fluorination: Concept, mechanism and applications in positron emission tomography. Chem. Soc. Rev. 2016, 45, 4638–4650. [Google Scholar] [CrossRef]
- Krasikova, R.N. Nucleophilic Synthesis of 6-l-[18F] F-DOPA. Is Copper-Mediated Radiofluorination the Answer? Molecules 2020, 25, 4365. [Google Scholar] [CrossRef]
- Maisonial-Besset, A.; Serre, A.; Ouadi, A.; Schmitt, S.; Canitrot, D.; Léal, F.; Miot-Noirault, E.; Brasse, D.; Marchand, P.; Chezal, J.M. Base/Cryptand/Metal-Free Automated Nucleophilic Radiofluorination of [18F]FDOPA from Iodonium Salts: Importance of Hydrogen Carbonate Counterion. European J. Org. Chem. 2018, 2018, 7058–7065. [Google Scholar] [CrossRef]
- Zlatopolskiy, B.D.; Zischler, J.; Urusova, E.A.; Endepols, H.; Kordys, E.; Frauendorf, H.; Mottaghy, F.M.; Neumaier, B. A Practical One-Pot Synthesis of Positron Emission Tomography (PET) Tracers via Nickel-Mediated Radiofluorination. ChemistryOpen 2015, 4, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.S.; Lee, S.S.; Oh, Y.H.; Lee, S.H.; Kim, S.E.; Kim, D.W.; Lee, B.C.; Lee, S.; Raffel, D.M. Control of reactivity and selectivity of guanidinyliodonium salts toward 18F-Labeling by monitoring of protecting groups: Experiment and theory. J. Fluor. Chem. 2019, 227, 109387. [Google Scholar] [CrossRef]
- Shinde, S.S.; Bolik, K.-V.; Maschauer, S.; Prante, O. 18F-Fluorination Using Tri-Tert-Butanol Ammonium Iodide as Phase-Transfer Catalyst: An Alternative Minimalist Approach. Pharmaceuticals 2021, 14, 833. [Google Scholar] [CrossRef] [PubMed]
- Ichiishi, N.; Brooks, A.F.; Topczewski, J.J.; Rodnick, M.E.; Sanford, M.S.; Scott, P.J.H. Copper-catalyzed [18F]fluorination of (Mesityl)(aryl)iodonium salts. Org. Lett. 2014, 16, 3224–3227. [Google Scholar] [CrossRef]
- Zischler, J.; Kolks, N.; Modemann, D.; Neumaier, B.; Zlatopolskiy, B.D. Alcohol-enhanced Cu-mediated radiofluorination. Chem. Eur. J. 2017, 23, 3251–3256. [Google Scholar] [CrossRef] [PubMed]
- Stenhagen, I.S.R.; Kirjavainen, A.K.; Forsback, S.J.; Jørgensen, C.G.; Robins, E.G.; Luthra, S.K.; Solin, O.; Gouverneur, V. [18F] Fluorination of an arylboronic ester using [18F] selectfluor bis (triflate): Application to 6-[18F] fluoro-l-DOPA. Chem. Commun. 2013, 49, 1386–1388. [Google Scholar] [CrossRef]
- Makaravage, K.J.; Brooks, A.F.; Mossine, A.V.; Sanford, M.S.; Scott, P.J.H. Copper-mediated radiofluorination of arylstannanes with [18F] KF. Org. Lett. 2016, 18, 5440–5443. [Google Scholar] [CrossRef]
- Kuik, W.-J.; Kema, I.P.; Brouwers, A.H.; Zijlma, R.; Neumann, K.D.; Dierckx, R.A.J.O.; DiMagno, S.G.; Elsinga, P.H. In vivo biodistribution of no-carrier-added 6-18F-fluoro-3, 4-dihydroxy-L-phenylalanine (18F-DOPA), produced by a new nucleophilic substitution approach, compared with carrier-added 18F-DOPA, prepared by conventional electrophilic substitution. J. Nucl. Med. 2015, 56, 106–112. [Google Scholar] [CrossRef]
- Inkster, J.A.H.; Akurathi, V.; Sromek, A.W.; Chen, Y.; Neumeyer, J.L.; Packard, A.B. A non-anhydrous, minimally basic protocol for the simplification of nucleophilic 18F-fluorination chemistry. Sci. Rep. 2020, 10, 6818. [Google Scholar] [CrossRef]
- Richarz, R.; Krapf, P.; Zarrad, F.; Urusova, E.A.; Neumaier, B.; Zlatopolskiy, B.D. Neither azeotropic drying, nor base nor other additives: A minimalist approach to 18F-labeling. Org. Biomol. Chem. 2014, 12, 8094–8099. [Google Scholar] [CrossRef]
- Shinde, S.S.; Maschauer, S.; Prante, O. Sweetening Pharmaceutical Radiochemistry by 18F-Fluoroglycosylation: Recent Progress and Future Prospects. Pharmaceuticals 2021, 14, 1175. [Google Scholar] [CrossRef] [PubMed]
- Minn, H.; Kauhanen, S.; Seppänen, M.; Nuutila, P. 18F-FDOPA: A multiple-target molecule. J. Nucl. Med. 2009, 50, 1915–1918. [Google Scholar] [CrossRef]
- Morrish, P.K.; Sawle, G.V.; Brooks, D.J. Regional changes in [18F] dopa metabolism in the striatum in Parkinson’s disease. Brain 1996, 119, 2097–2103. [Google Scholar] [CrossRef]
- Zhu, L.; Ploessl, K.; Kung, H.F. Expanding the scope of fluorine tags for PET imaging. Science 2013, 342, 429–430. [Google Scholar] [CrossRef]
- Preshlock, S.; Tredwell, M.; Gouverneur, V. 18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem. Rev. 2016, 116, 719–766. [Google Scholar] [CrossRef]
- Lee, E.; Kamlet, A.S.; Powers, D.C.; Neumann, C.N.; Boursalian, G.B.; Furuya, T.; Choi, D.C.; Hooker, J.M.; Ritter, T. A fluoride-derived electrophilic late-stage fluorination reagent for PET imaging. Science 2011, 334, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Tredwell, M.; Gouverneur, V. 18F labeling of arenes. Angew. Chemie-Int. Ed. 2012, 51, 11426–11437. [Google Scholar] [CrossRef]
- Wang, B.; Graskemper, J.W.; Qin, L.; DiMagno, S.G. Regiospecific reductive elimination from diaryliodonium salts. Angew. Chemie Int. Ed. 2010, 49, 4079–4083. [Google Scholar] [CrossRef]
- Kim, D.W.; Jeong, H.; Lim, S.T.; Sohn, M. Tetrabutylammonium Tetra (tert-Butyl Alcohol)-Coordinated Fluoride as a Facile Fluoride Source. Angew. Chemie 2008, 120, 8532–8534. [Google Scholar] [CrossRef]
- Boer, S.A.; Foyle, E.M.; Thomas, C.M.; White, N.G. Anion coordination chemistry using O–H groups. Chem. Soc. Rev. 2019, 48, 2596–2614. [Google Scholar] [CrossRef]
- Lee, J.W.; Yan, H.; Jang, H.B.; Kim, H.K.; Park, S.W.; Lee, S.; Chi, D.Y.; Song, C.E. Bis-terminal hydroxy polyethers as all-purpose, multifunctional organic promoters: A mechanistic investigation and applications. Angew. Chemie-Int. Ed. 2009, 48, 7683–7686. [Google Scholar] [CrossRef]
- Kim, D.W.; Ahn, D.S.; Oh, Y.H.; Lee, S.; Kil, H.S.; Oh, S.J.; Lee, S.J.; Kim, J.S.; Ryu, J.S.; Moon, D.H.; et al. A new class of SN2 reactions catalyzed by protic solvents: Facile fluorination for isotopic labeling of diagnostic molecules. J. Am. Chem. Soc. 2006, 128, 16394–16397. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Song, C.E.; Chi, D.Y. New method of fluorination using potassium fluoride in ionic liquid: Significantly enhanced reactivity of fluoride and improved selectivity. J. Am. Chem. Soc. 2002, 124, 10278–10279. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.L.; Valle, M.S.; Pliego Jr, J.R. Nucleophilic Fluorination with KF Catalyzed by 18-Crown-6 and Bulky Diols: A Theoretical and Experimental Study. J. Org. Chem. 2020, 85, 15457–15465. [Google Scholar] [CrossRef]
- Oh, Y.-H.; Yun, W.; Kim, C.-H.; Jang, S.-W.; Lee, S.-S.; Lee, S.; Kim, D.-W. Inter- and intramolecular organo-catalysis of SN2 fluorination by crown ether: Kinetics and quantum chemical analysis. Molecules 2021, 26, 2947. [Google Scholar] [CrossRef]
- Shinde, S.S.; Khonde, N.S.; Kumar, P. Tri–tert-Butanolamine as an Organic Promoter in Nucleophilic Fluorination. ChemistrySelect 2017, 2, 118–122. [Google Scholar] [CrossRef]
- Lee, S.-S.; Jung, H.-K.; Shinde, S.S.; Lee, S. Mechanistic study of nucleophilic fluorination promoted by tri-tert-butanolamine. J. Fluor. Chem. 2017, 197, 80–86. [Google Scholar] [CrossRef]
- Seeman, J.I. The Curtin-Hammett principle and the Winstein-Holness equation: New definition and recent extensions to classical concepts. J. Chem. Educ. 1986, 63, 42. [Google Scholar] [CrossRef]
- Choi, H.; Oh, Y.-H. Mechanism of promotion of SN2 fluorination by [Bmim]F in solvent-free environment: Quantum chemical analysis. Chem. Phys. Lett. 2020, 756, 137857. [Google Scholar] [CrossRef]
- Bouvet, S.; Pegot, B.; Marrot, J.; Magnier, E. Solvent free nucleophilic introduction of fluorine with [bmim][F]. Tetrahedron Lett. 2014, 55, 826–829. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.; Guan, Q.; Wang, F. Ab initio kinetics predictions for the role of pre-reaction complexes in hydrogen abstraction from 2-butanone by OH radicals. RSC Adv. 2020, 10, 33205–33212. [Google Scholar] [CrossRef]
- Dalessandro, E.V.; Pliego, J.R. Theoretical design of new macrocycles for nucleophilic fluorination with KF: Thiourea-crown-ether is predicted to overcome [2.2.2]-cryptand. Mol. Syst. Des. Eng. 2020, 5, 1513–1523. [Google Scholar] [CrossRef]
- Dékány, A.Á.; Kovács, G.Z.; Czakó, G. High-Level Systematic Ab Initio Comparison of Carbon-and Silicon-Centered SN2 Reactions. J. Phys. Chem. A 2021, 125, 9645–9657. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Yang, L.; Hase, W.L. How a Solvent Molecule Affects Competing Elimination and Substitution Dynamics. Insight into Mechanism Evolution with Increased Solvation. J. Am. Chem. Soc. 2018, 140, 10995–11005. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, N.; et al. Gaussian 16; Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hohenstein, E.G.; Chill, S.T.; Sherrill, C.D. Assessment of the performance of the M05− 2X and M06− 2X exchange-correlation functionals for noncovalent interactions in biomolecules. J. Chem. Theory Comput. 2008, 4, 1996–2000. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]

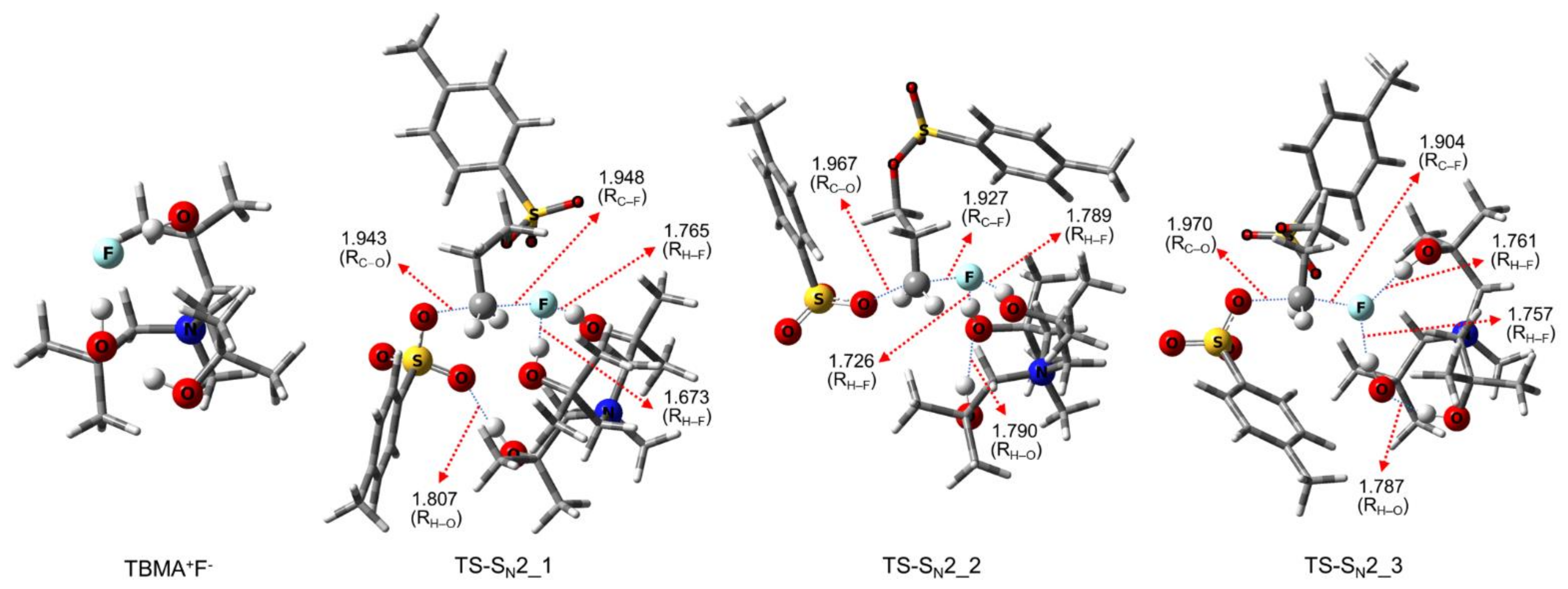
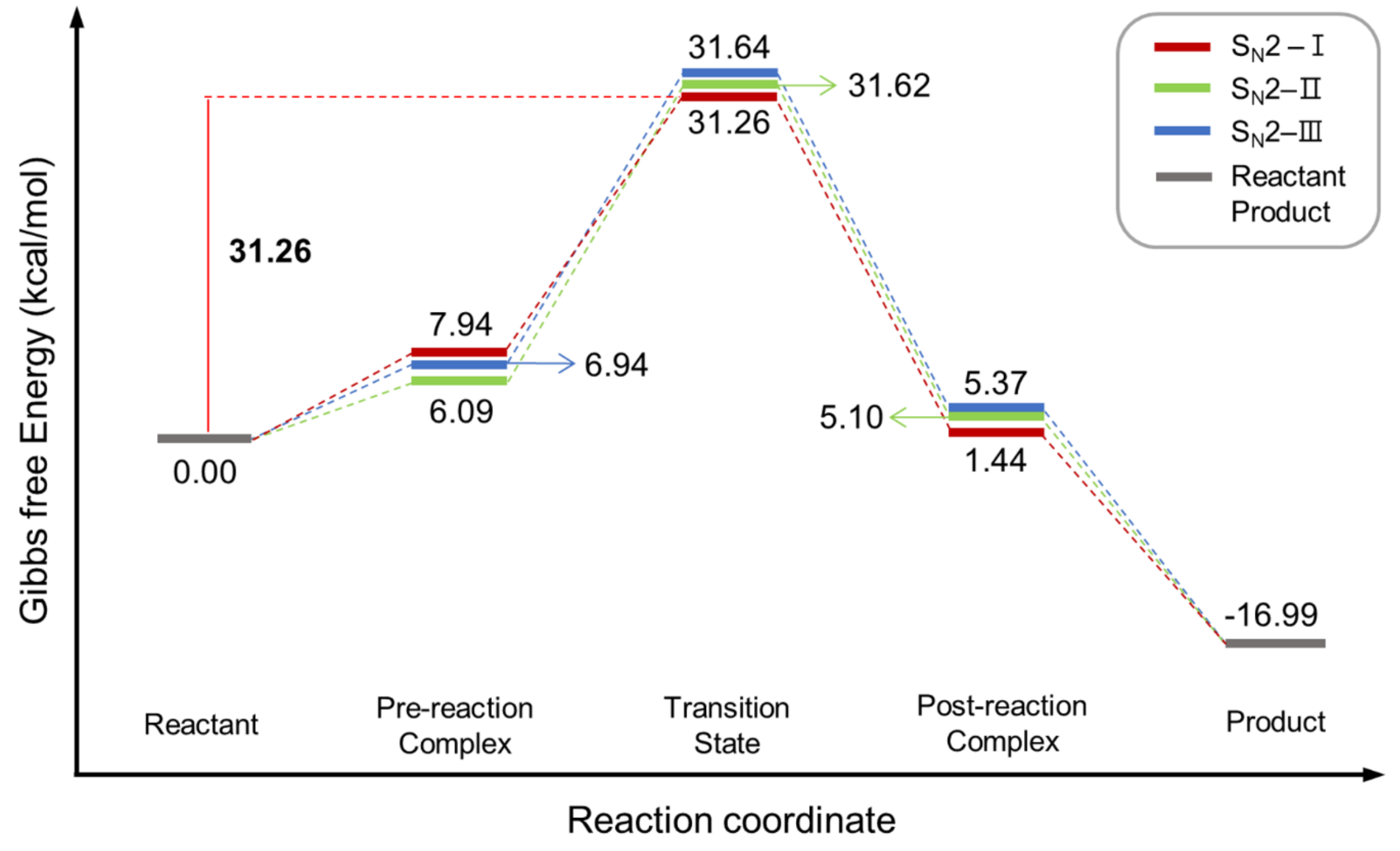
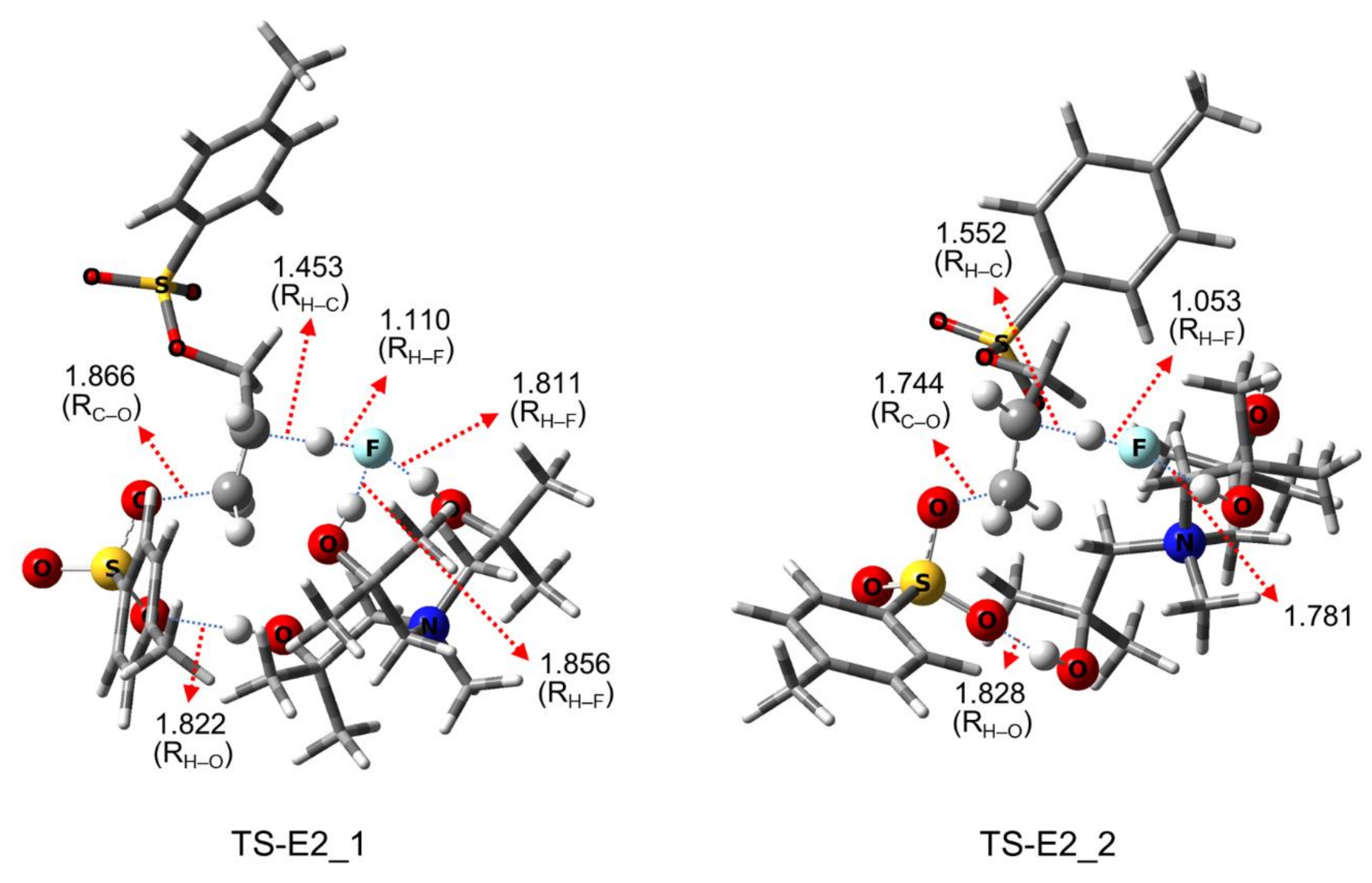
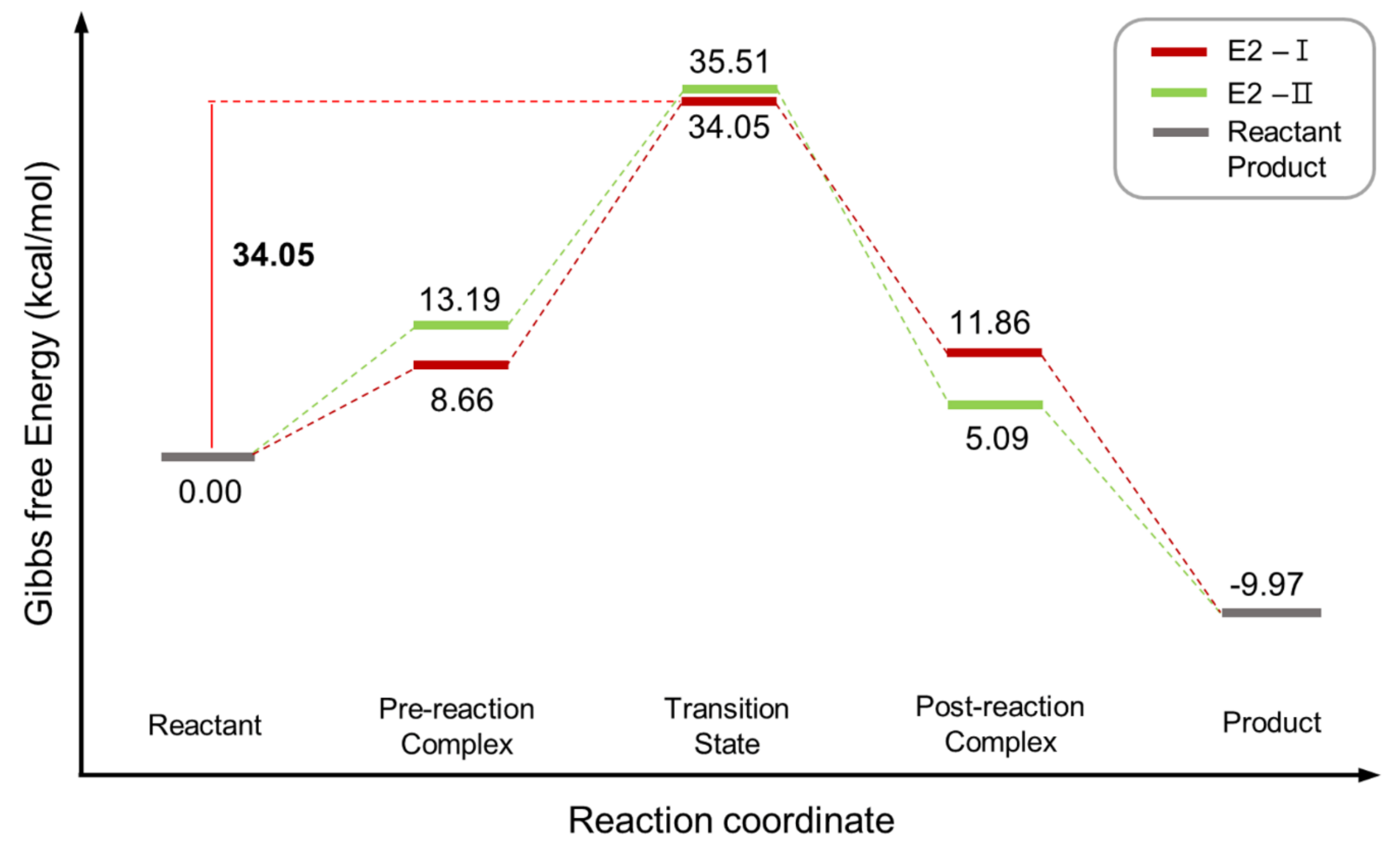
| Entry | Promoter | Recovery of [18F]fluoride [%] | Solvent (0.5 mL) | RCY[%] (Radio-TLC) | |
|---|---|---|---|---|---|
| [18F]2 | [18F]3 | ||||
| 1 | TBMA-I | 98.8 | CH3CN | 21 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, Y.-H.; Shinde, S.S.; Lee, S. Nucleophilic Radiofluorination Using Tri-tert-Butanol Ammonium as a Bifunctional Organocatalyst: Mechanism and Energetics. Molecules 2022, 27, 1044. https://doi.org/10.3390/molecules27031044
Oh Y-H, Shinde SS, Lee S. Nucleophilic Radiofluorination Using Tri-tert-Butanol Ammonium as a Bifunctional Organocatalyst: Mechanism and Energetics. Molecules. 2022; 27(3):1044. https://doi.org/10.3390/molecules27031044
Chicago/Turabian StyleOh, Young-Ho, Sandip S. Shinde, and Sungyul Lee. 2022. "Nucleophilic Radiofluorination Using Tri-tert-Butanol Ammonium as a Bifunctional Organocatalyst: Mechanism and Energetics" Molecules 27, no. 3: 1044. https://doi.org/10.3390/molecules27031044
APA StyleOh, Y.-H., Shinde, S. S., & Lee, S. (2022). Nucleophilic Radiofluorination Using Tri-tert-Butanol Ammonium as a Bifunctional Organocatalyst: Mechanism and Energetics. Molecules, 27(3), 1044. https://doi.org/10.3390/molecules27031044







