Physicochemical and Functional Properties of 2S, 7S, and 11S Enriched Hemp Seed Protein Fractions
Abstract
:1. Introduction
2. Results
2.1. Proximate Composition
2.2. Yield, Digestibility, Sulfhydryl Group, and Bound Carbohydrate
2.3. Amino Acid Composition
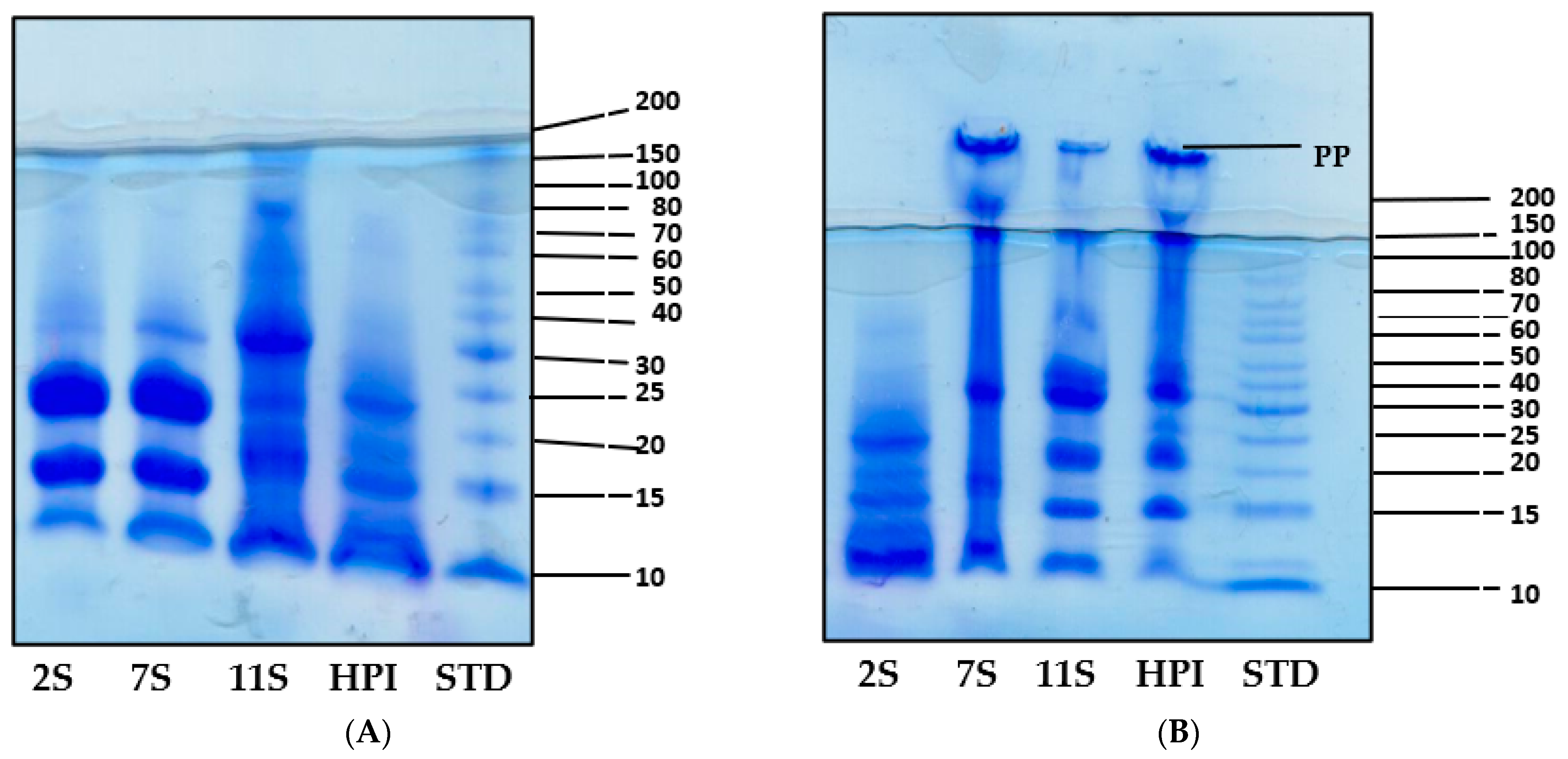
2.4. Gel Electrophoresis (SDS-PAGE)
2.5. Intrinsic Fluorescence Emission
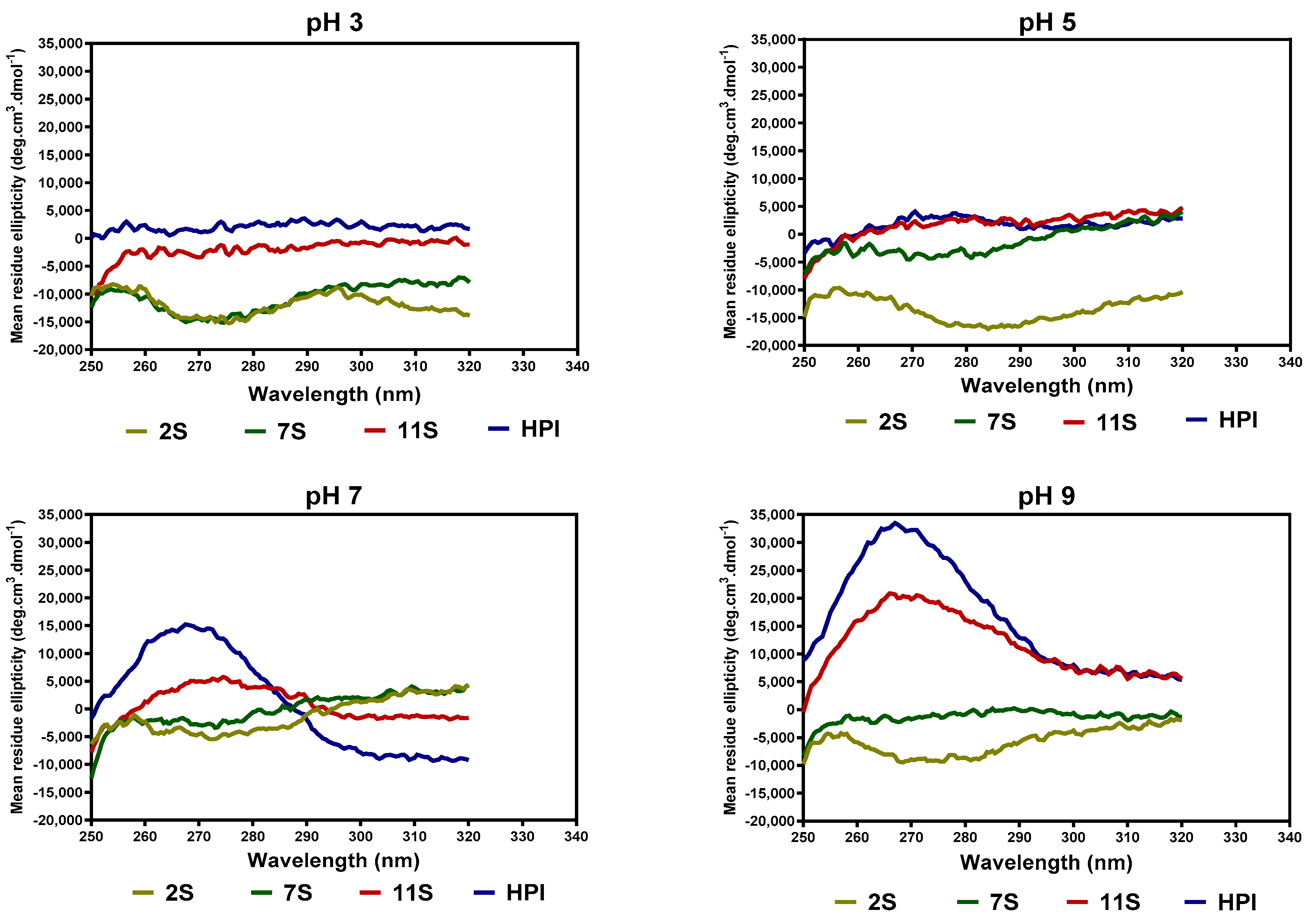
2.6. Secondary and Tertiary Structure Conformations
2.7. Protein Solubility Profiles
2.8. Water Holding Capacity (WHC), Oil Holding Capacity (OHC), and Least Gelation Concentration
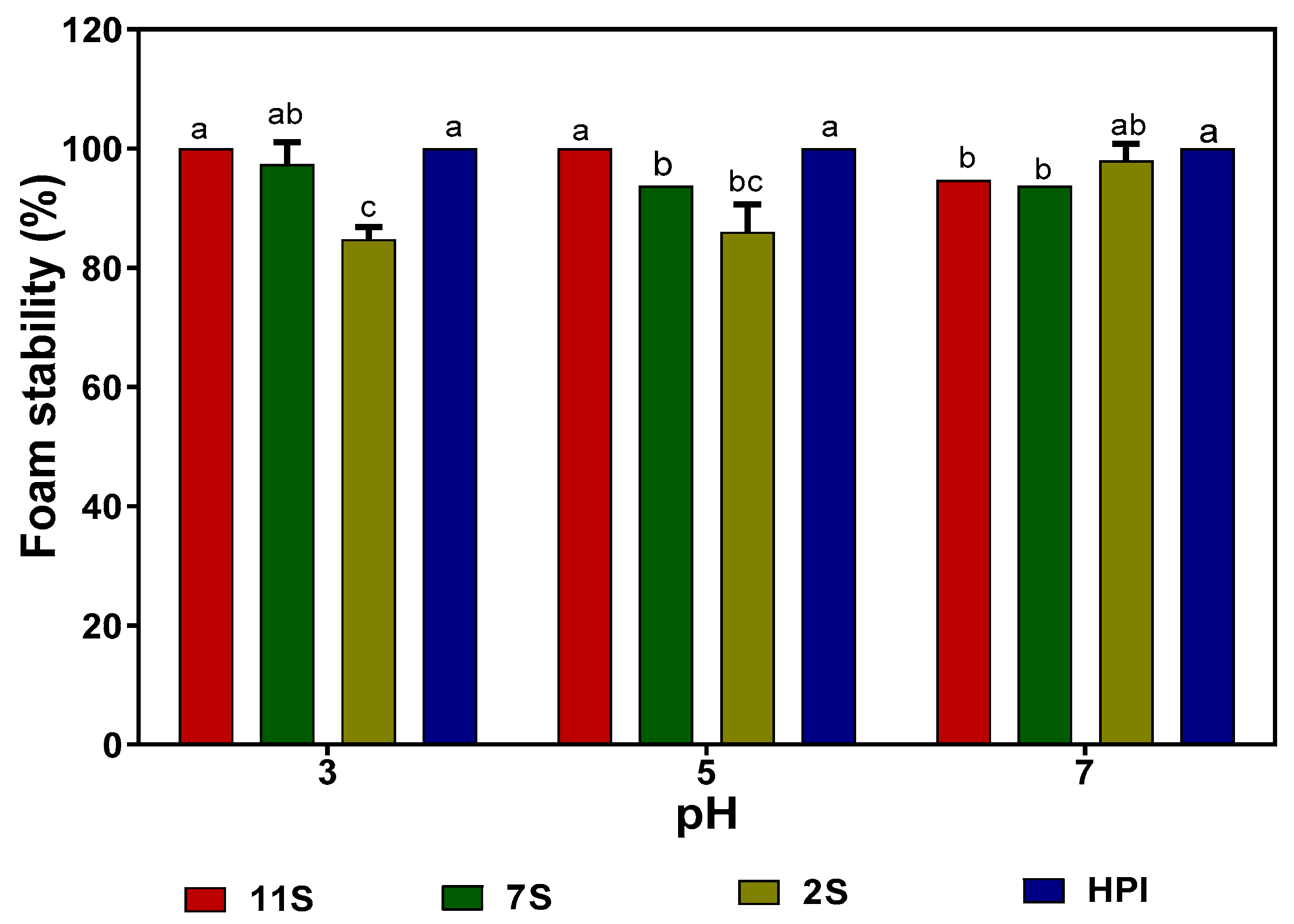
2.9. Foaming Capacity (FC) and Foam Stability (FS) of Hemp Seed Proteins
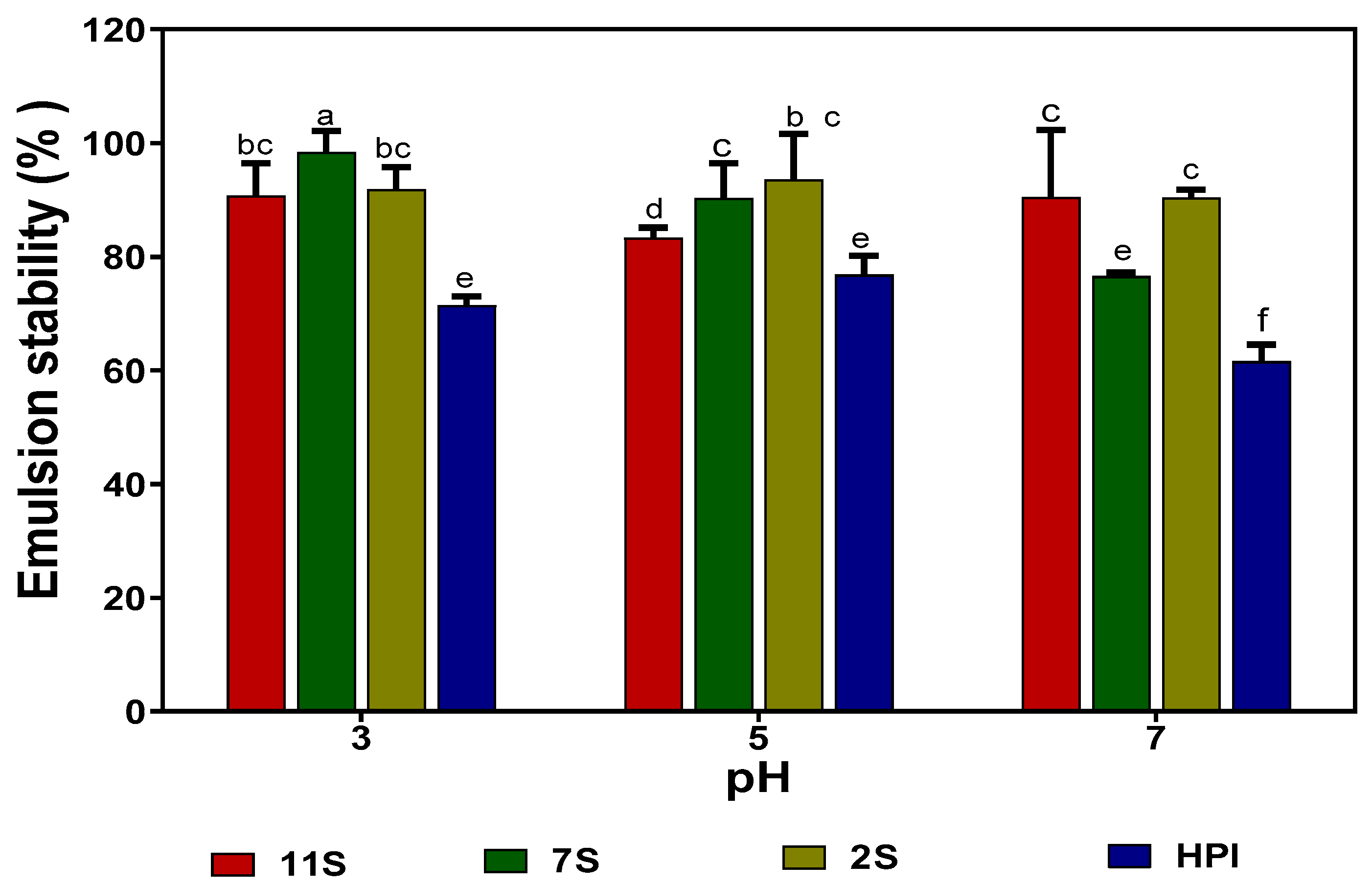
2.10. Emulsion Formation (Oil Droplet Size) and Stability
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Defatted Hemp Seed Flour (DHF)
4.3. Preparation of Hemp Seed Protein Isolate (HPI)
4.4. Preparation of 11S, 7S, and 2S Protein-Enriched Fractions
4.5. Proximate and Amino Acid Composition Analysis
4.6. Determination of In Vitro Protein Digestibility
4.7. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.8. Total and Exposed Sulfhydryl Contents
4.9. Intrinsic Fluorescence Emission
4.10. Measurements of Circular Dichroism (CD) Spectra
4.11. Protein Solubility (PS)
4.12. Water (WHC) and Oil (OHC) Holding Capacity
4.13. Least Gelation Concentration
4.14. Foaming Capacity (FC)
4.15. Emulsion Formation and Oil Droplet Size Measurement
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BCAA | Branched-chain amino acids |
| DHF | Defatted hemp seed flour |
| EAA | Essential amino acids |
| HPI | Hemp seed protein isolate |
| IVPD | In vitro protein digestibility |
| Sulfhydryl groups | SH |
| SCAA | Sulfur-containing amino acids |
| AAA | Aromatic amino acids |
| NCAA | Negatively charged amino acids |
| PCAA | Positively charged amino acids |
| HAA | Hydrophobic amino acids |
| Carbohydrates | CHO |
| FC | Foaming capacity |
| FS | Foam stability |
| ES | Emulsion stability |
| LGC | Least gelation concentration |
| WHC | Water holding capacity |
| OHC | Oil holding capacity |
| PS | Protein solubility |
| CD | Circular dichroism |
| FI | Fluorescence intensity |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
References
- Grand View Research. Protein Ingredients Market Size, Share & Trends Analysis Report by Product (Plant Proteins, Animal/Dairy Proteins, Microbe-Based Proteins, Insect Proteins), by Application, by Region and Segment Forecasts. 2020, pp. 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/protein-ingredients-market (accessed on 10 December 2021).
- Naghshi, S.; Sadeghi, O.; Willett, W.C.; Esmaillzadeh, A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2020, 370, m2412. [Google Scholar] [CrossRef] [PubMed]
- Dapcevic-Hadnadev, T.; Dizdar, M.; Pojić, M.; Krstonošić, V.; Zychowski, L.M.; Hadnadev, M. Emulsifying properties of hemp proteins: Effect of isolation technique. Food Hydrocoll. 2019, 89, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Hadnadev, M.; Dapcevic-Hadnadev, T.; Lazaridou, A.; Moschakis, T.; Michaelidou, A.M.; Popovic, S.; Biliaderis, C.G. Hempseed meal protein isolates prepared by different isolation techniques. Part I. physicochemical properties. Food Hydrocoll. 2018, 79, 526–533. [Google Scholar] [CrossRef]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the quality of protein from hemp seed (Cannabis sativa L.) products through the use of the protein digestibility-corrected amino acid score method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Gao, Z.; Xu, M.; Ohm, J.-B.; Rao, J.; Chen, B. The impact of hempseed dehulling on chemical composition, structure properties and aromatic profile of hemp protein isolate. Food Hydrocoll. 2020, 106, 105889. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Malomo, S.A.; He, R.; Aluko, R.E. Structural and functional properties of hemp seed protein products. J. Food Sci. 2014, 79, C1512. [Google Scholar] [CrossRef]
- Tang, C.-H.; Ten, Z.; Wang, X.-S.; Yang, X.-Q. Physicochemical and functional properties of hemp (Cannabis sativa L.) protein isolate. J. Agric. Food Chem. 2006, 54, 8945–8950. [Google Scholar] [CrossRef]
- Yin, S.W.; Tang, C.H.; Cao, J.S.; Hu, E.K.; Wen, Q.B.; Yang, X.Q. Effects of limited enzymatic hydrolysis with trypsin on the functional properties of hemp (Cannabis sativa L.) protein isolate. Food Chem. 2008, 106, 1004–1013. [Google Scholar] [CrossRef]
- Malomo, S.A.; Aluko, R.E. Conversion of a low protein hemp seed meal into a functional protein concentrate through enzymatic digestion of fibre coupled with membrane ultrafiltration. Innov. Food Sci. Emerg. Technol. 2015, 31, 151–159. [Google Scholar] [CrossRef]
- Malomo, S.A.; Aluko, R.E. A comparative study of the structural and functional properties of isolated hemp seed (Cannabis sativa L.) albumin and globulin fractions. Food Hydrocoll. 2015, 43, 743–752. [Google Scholar] [CrossRef]
- Wang, X.S.; Tang, C.H.; Yang, X.Q.; Gao, W.R. Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chem. 2008, 107, 11–18. [Google Scholar] [CrossRef]
- Ajibola, C.F.; Malomo, S.A.; Fagbemi, T.N.; Aluko, R.E. Polypeptide composition and functional properties of African yam bean seed (Sphenostylis stenocarpa) albumin, globulin and protein concentrate. Food Hydrocoll. 2016, 56, 189–200. [Google Scholar] [CrossRef]
- Mundi, S.; Aluko, R.E. Physicochemical and functional properties of kidney bean albumin and globulin protein fractions. Food Res. Int. 2012, 48, 299–306. [Google Scholar] [CrossRef]
- Kimura, A.; Fukuda, T.; Zhang, M.; Motoyama, S.; Maruyama, N.; Utsumi, S. Comparison of physicochemical properties of 7S and 11S globulins from pea, fava bean, cowpea, and French bean with those of soybean. J. Agric. Food Chem. 2008, 56, 10273–10279. [Google Scholar] [CrossRef]
- De Santis, M.A.; Rinaldi, M.; Menga, V.; Codianni, P.; Giuzio, L.; Fares, C.; Flagella, Z. Influence of Organic and Conventional Farming on Grain Yield and Protein Composition of Chickpea Genotypes. Agronomy 2021, 11, 191. [Google Scholar] [CrossRef]
- Yang, J.; Zamani, S.; Liang, L.; Chen, L. Extraction methods significantly impact pea protein composition, structure and gelling properties. Food Hydrocoll. 2021, 117, 106678. [Google Scholar] [CrossRef]
- Moreno, F.J.; Mellon, F.A.; Wickham, M.S.; Bottrill, A.R.; Mills, E.C. Stability of the major allergen Brazil nut 2S albumin (Ber e 1) to physiologically relevant in vitro gastrointestinal digestion. FEBS J. 2005, 272, 341–352. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148–3161. [Google Scholar] [CrossRef] [Green Version]
- Giroux, I.; Kurowska, E.M.; Freeman, D.J.; Carroll, K.K. Addition of arginine but not glycine to lysine plus methionine-enriched diets modulates serum cholesterol and liver phospholipids in rabbits. J. Nutr. 1999, 129, 1807–1813. [Google Scholar] [CrossRef] [Green Version]
- Tamanna, N.; Mahmood, N. Emerging roles of branched-chain amino acid supplementation in human diseases. Int. Sch. Res. Not. 2014, 2014, 235619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, T.; Izumi, N.; Charlton, M.R.; Sata, M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 2011, 54, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F.X. Spectra Methods of Characterizing Protein Conformation and Conformational Changes. In Protein Structure: A Practical Approach; Creighton, T.E., Ed.; Springer Publishing Co.: New York, NY, USA, 1989; pp. 251–285. [Google Scholar]
- Clara Sze, K.W.; Kshirsagar, H.H.; Venkatachalam, M.; Sathe, S.K. A circular dichroism and fluorescence spectrometric assessment of effects of selected chemical denaturants on soybean (Glycine max L.) storage proteins glycinin (11S) and β-conglycinin (7S). J. Agric. Food Chem. 2007, 55, 8745–8753. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Ma, C.Y. Structural characterization of globulin from common buckwheat (Fagopyrum esculentum Moench) using circular dichroism and Raman spectroscopy. Food Chem. 2007, 102, 150–160. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Z.; Kong, Y.; Ma, Z.; Wu, C.; Regenstein, J.M.; Teng, F.; Li, Y. Different commercial soy protein isolates and the characteristics of Chiba tofu. Food Hydrocoll. 2021, 110, 106115. [Google Scholar] [CrossRef]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta 2005, 175, 119–139. [Google Scholar] [CrossRef]
- Gonzalez-Perez, S.; Vereijken, J.M. Sunflower protein: Overview of their physicochemical, structural and functional properties. J. Sci. Food Agric. 2007, 87, 2173–2191. [Google Scholar] [CrossRef]
- Yildiz, G.; Ding, J.; Andrade, J.; Engeseth, N.J.; Feng, H. Effect of plant protein-polysaccharide complexes produced by mano-thermo-sonication and pH-shifting on the structure and stability of oil-in-water emulsions. Innov. Food Sci. Emerg. Technol. 2018, 47, 317–325. [Google Scholar] [CrossRef]
- Wang, X.; He, Z.; Zeng, M.; Qin, F.; Adhikari, B.; Chen, J. Effects of the size and content of protein aggregates on the rheological and structural properties of soy protein isolate emulsion gels induced by CaSO4. Food Chem. 2017, 221, 130–138. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, L.; Wei, F.; Xie, B.; Huang, F.; Huang, W.; Shi, J.; Huang, Q.; Tian, B.; Xue, S. Functional properties of protein isolates, globulin and albumin extracted from Ginkgo biloba seeds. Food Chem. 2011, 124, 1458–1465. [Google Scholar] [CrossRef]
- Moure, A.; Sineiro, J.; Domínguez, H.; Parajó, J.C. Functionality of oilseed protein products: A review. Food Res. Int. 2006, 39, 945–963. [Google Scholar] [CrossRef]
- Ragab, D.D.M.; Babiker, E.E.; Eltinay, A.H. Fractionation, solubility and functional properties of cowpea (Vigna unguiculata) proteins as affected by pH and/or salt concentration. Food Chem. 2004, 84, 207–212. [Google Scholar] [CrossRef]
- Tay, S.L.; Kasapis, S.; Perera, C.O.; Barlow, P.J. Functional and structural properties of 2S soy protein in relation to other molecular protein fractions. J. Agric. Food Chem. 2006, 54, 6046–6053. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhao, Y.; Li, T.; Li, D.; Chen, S.; Wu, N.; Jiang, L.; Wang, L. Effect of electrochemical modification on the structural characteristics and emulsion storage stability of soy protein isolate. Process Biochem. 2018, 75, 166–172. [Google Scholar] [CrossRef]
- Wang, J.-S.; Wang, A.-B.; Zang, X.-P.; Tan, L.; Xu, B.-Y.; Chen, H.-H.; Jin, Z.-Q.; Ma, W.-H. Physicochemical, functional and emulsion properties of edible protein from avocado (Persea americana Mill.) oil processing by-products. Food Chem. 2019, 288, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Peng, B.; Wang, M.; Zou, X.-G.; Yin, Y.-L.; Deng, Z.-Y. Characteristics and emulsifying properties of two protein fractions derived from the emulsion formed during aqueous extraction of Camellia oil. Food Hydrocoll. 2019, 87, 644–652. [Google Scholar] [CrossRef]
- Osemwota, E.C.; Alashi, A.M.; Aluko, R.E. Comparative study of the structural and functional properties of membrane-isolated and isoelectric pH precipitated green lentil seed protein isolates. Membranes 2021, 11, 694. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists Inc.: Washington, DC, USA, 1990. [Google Scholar]
- AOCS. American Oil Chemists’ Society Official Methods; AOCS: Chicago, IL, USA, 2006. [Google Scholar]
- Ijarotimi, S.O.; Malomo, S.A.; Fagbemi, T.N.; Osundahunsi, O.F.; Aluko, R.E. Structural and functional properties of Buchholzia coriacea seed flour and protein concentrate at different pH and protein concentrations. Food Hydrocoll. 2018, 74, 275–288. [Google Scholar] [CrossRef]
- Hsu, H.; Vavak, D.; Satterlee, L. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Aluko, R.E.; Mofolasayo, O.A.; Watts, B.M. Emulsifying and foaming properties of commercial yellow pea (Pisum sativum L.) seed flours. J. Agric. Food Chem. 2009, 57, 9793–9800. [Google Scholar] [CrossRef] [PubMed]



| Sample | Moisture (%) | Protein (%) | Fat (%) | Ash (%) | Fibre (%) |
|---|---|---|---|---|---|
| HPI | 4.11 ± 0.01 d | 87.14 ± 0.08 a | 2.14 ± 0.01 c | 8.63 ± 0.01 a | 0.11 ± 0.13 b |
| 11S | 4.84 ± 0.03 c | 87.23 ± 0.04 a | 6.46 ± 0.06 a | 1.50 ± 0.17 c | 1.12 ± 0.47 a |
| 7S | 5.18 ± 0.06 b | 57.70 ± 0.19 c | 5.33 ± 0.42 b | 8.66 ± 0.04 a | 1.04 ± 0.12 a |
| 2S | 8.45 ± 0.03 a | 66.34 ± 0.01 b | 0.67 ± 0.09 d | 6.31 ± 0.06 b | 0.01 ± 0.01 c |
| Sample | Protein Yield (%) | IVPD (%) | Exposed SH (µmol/g) | Total SH (µmol/g) | CHO (%) |
|---|---|---|---|---|---|
| HPI | 82.72 ± 4.36 a | 88.10 ± 0.26 a | 1.16 ± 0.02 c | 1.97 ± 0.07 b | 5.16 ± 0.95 b |
| 11S | 72.70 ± 2.30 b | 88.28 ± 0.17 a | 0.57 ± 0.04 d | 1.55 ± 0.22 c | 2.07 ± 0.09 c |
| 7S | 1.29 ± 0.11 d | 84.48 ± 0.30 b | 1.32 ± 0.07 b | 1.51 ± 0.12 c | 10.36 ± 0.53 a |
| 2S | 3.92 ± 0.15 c | 72.54 ± 0.52 c | 2.39 ± 0.14 a | 3.69 ± 0.05 a | 10.05 ± 0.49 c |
| Amino Acids | 2S | 7S | 11S | HPI | FAO-/WHO-Suggested Requirements (2–5 Years) |
|---|---|---|---|---|---|
| Asx | 7.50 | 9.15 | 11.04 | 11.60 | |
| Thr | 4.14 | 3.79 | 3.44 | 3.49 | 3.4 |
| Ser | 5.01 | 5.04 | 5.61 | 5.36 | |
| Glx | 25.63 | 20.95 | 18.44 | 18.13 | |
| Pro | 3.93 | 3.87 | 3.74 | 3.64 | |
| Gly | 5.75 | 4.17 | 4.06 | 4.18 | |
| Ala | 5.86 | 5.46 | 5.18 | 5.19 | |
| Cys | 4.88 | 2.24 | 1.56 | 1.22 | |
| Val | 3.07 | 4.87 | 4.74 | 5.32 | 3.5 |
| Met | 2.17 | 2.53 | 2.48 | 1.71 | |
| Ile | 1.99 | 3.70 | 3.81 | 4.36 | 2.8 |
| Leu | 4.02 | 6.34 | 6.61 | 6.90 | 6.6 |
| Tyr | 2.46 | 3.14 | 3.70 | 3.53 | |
| Phe | 1.43 | 3.67 | 4.49 | 4.78 | |
| His | 3.20 | 3.14 | 2.93 | 2.91 | 1.9 |
| Lys | 6.36 | 6.45 | 3.44 | 3.28 | 5.8 |
| Arg | 12.45 | 10.79 | 13.55 | 13.24 | |
| Trp | 0.18 | 0.70 | 1.19 | 1.14 | 1.1 |
| HAA | 30.86 | 38.45 | 40.00 | 40.80 | |
| AAA | 4.07 | 7.51 | 9.38 | 9.45 | 6.3 |
| NCAA | 33.13 | 30.1 | 29.04 | 29.73 | |
| PCAA | 22.01 | 20.38 | 19.92 | 19.43 | |
| SCAA | 7.05 | 4.77 | 4.04 | 2.93 | 2.5 |
| EAA | 26.56 | 35.19 | 33.13 | 33.89 | 32.8 |
| BCAA | 9.08 | 14.91 | 15.16 | 16.58 | |
| Arg/Lys ratio | 1.96 | 1.67 | 3.94 | 4.04 |
| pH | Samples | α-Helix (%) | β-Sheet (%) | β-Turns (%) | Unordered (%) |
|---|---|---|---|---|---|
| pH 3 | 2S | 80.40 ± 0.00 | 0.70 ±0.00 | 5.95 ± 0.01 | 1.53 ± 0.03 |
| 7S | 4.40 ± 0.00 | 31.18 ± 0.00 | 17.30 ± 0.00 | 46.66 ± 0.00 | |
| 11S | 3.10 ± 0.01 | 37.45 ± 0.02 | 17.45 ± 0.01 | 43.65 ± 0.00 | |
| HPI | 2.20 ± 0.01 | 43.45 ± 0.03 | 19.45 ± 0.01 | 34.95 ± 0.00 | |
| pH 5 | 2S | 18.60 ± 0.00 | 3.90 ± 0.01 | 12.50 ± 0.03 | 65.05 ± 0.04 |
| 7S | 1.40 ± 0.00 | 39.50 ± 0.00 | 20.35 ± 0.00 | 39.05 ± 0.00 | |
| 11S | 1.55 ± 0.00 | 43.25 ± 0.02 | 21.30 ± 0.01 | 35.50 ± 0.00 | |
| HPI | 1.85 ± 0.00 | 41.50 ± 0.01 | 20.30 ± 0.00 | 36.35 ± 0.01 | |
| pH 7 | 2S | 1.35 ± 0.01 | 37.55 ± 0.02 | 17.55 ± 0.00 | 47.05 ± 0.01 |
| 7S | 2.20 ± 0.00 | 40.80 ± 0.02 | 19.05 ± 0.03 | 38.85 ± 0.03 | |
| 11S | 3.45 ± 0.00 | 44.25 ± 0.00 | 20.30 ± 0.00 | 32.20 ± 0.00 | |
| HPI | 17.40 ± 0.01 | 26.05 ± 0.01 | 20.20 ± 0.04 | 36.15 ± 0.04 | |
| pH 9 | 2S | 4.55 ± 0.02 | 34.30 ± 0.00 | 17.30 ± 0.00 | 46.90 ± 0.00 |
| 7S | 3.30 ± 0.00 | 37.10 ± 0.00 | 17.05 ± 0.00 | 46.60 ± 0.00 | |
| 11S | 0.00 ± 0.00 | 55.45 ± 0.02 | 23.60 ± 0.01 | 24.15 ± 0.00 | |
| HPI | 0.00 ± 0.00 | 45.95 ± 0.01 | 13.70 ± 0.00 | 39.99 ± 0.03 |
| Sample | WHC (g/g) | OHC (g/g) | LGC (%) |
|---|---|---|---|
| HPI | 5.81 ± 0.01 a | 10.32 ± 0.01 a | 22.00 ± 0.00 c |
| 11S | 3.63 ± 0.03 c | 5.97 ± 0.08 b | 30.00 ± 0.00 d |
| 7S | 4.09 ± 0.21 b | 4.93 ± 0.51 b | 10.00 ± 0.00 a |
| 2S | 11.04 ± 0.01 a | 14.00 ± 0.00 b |
| Sample | pH 3.0 (%) | pH 5.0 (%) | pH 7.0 (%) |
|---|---|---|---|
| HPI | 75.00 ± 7.07 c | 55.00 ± 7.07 c | 75.00 ± 7.07 c |
| 11S | 60.00 ± 0.00 d | 60.00 ± 0.00 b | 90.00 ± 0.00 b |
| 7S | 95.00 ± 7.07 b | 60.00 ± 0.00 b | 60.00 ± 0.00 d |
| 2S | 195.00 ± 7.07 a | 185.00 ± 7.07 a | 150.00 ± 0.00 a |
| Sample | pH 3.0 (µm) | pH 5.0 (µm) | pH 7.0 (µm) |
|---|---|---|---|
| HPI | 12.65 ± 0.88 d | 6.23 ± 0.36 a | 4.90 ± 0.07 c |
| 11S | 5.50 ± 0.13 b | 6.06 ± 0.43 a | 5.66 ± 0.27 d |
| 7S | 7.62 ± 0.14 c | 6.79 ± 0.22 a | 4.45 ± 0.23 b |
| 2S | 4.19 ± 0.17 a | 4.29 ± 0.20 b | 2.25 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajibola, C.F.; Aluko, R.E. Physicochemical and Functional Properties of 2S, 7S, and 11S Enriched Hemp Seed Protein Fractions. Molecules 2022, 27, 1059. https://doi.org/10.3390/molecules27031059
Ajibola CF, Aluko RE. Physicochemical and Functional Properties of 2S, 7S, and 11S Enriched Hemp Seed Protein Fractions. Molecules. 2022; 27(3):1059. https://doi.org/10.3390/molecules27031059
Chicago/Turabian StyleAjibola, Comfort F., and Rotimi E. Aluko. 2022. "Physicochemical and Functional Properties of 2S, 7S, and 11S Enriched Hemp Seed Protein Fractions" Molecules 27, no. 3: 1059. https://doi.org/10.3390/molecules27031059
APA StyleAjibola, C. F., & Aluko, R. E. (2022). Physicochemical and Functional Properties of 2S, 7S, and 11S Enriched Hemp Seed Protein Fractions. Molecules, 27(3), 1059. https://doi.org/10.3390/molecules27031059







