Preliminary Studies of Antimicrobial Activity of New Synthesized Hybrids of 2-Thiohydantoin and 2-Quinolone Derivatives Activated with Blue Light
Abstract
:1. Introduction
2. Results and Discussion
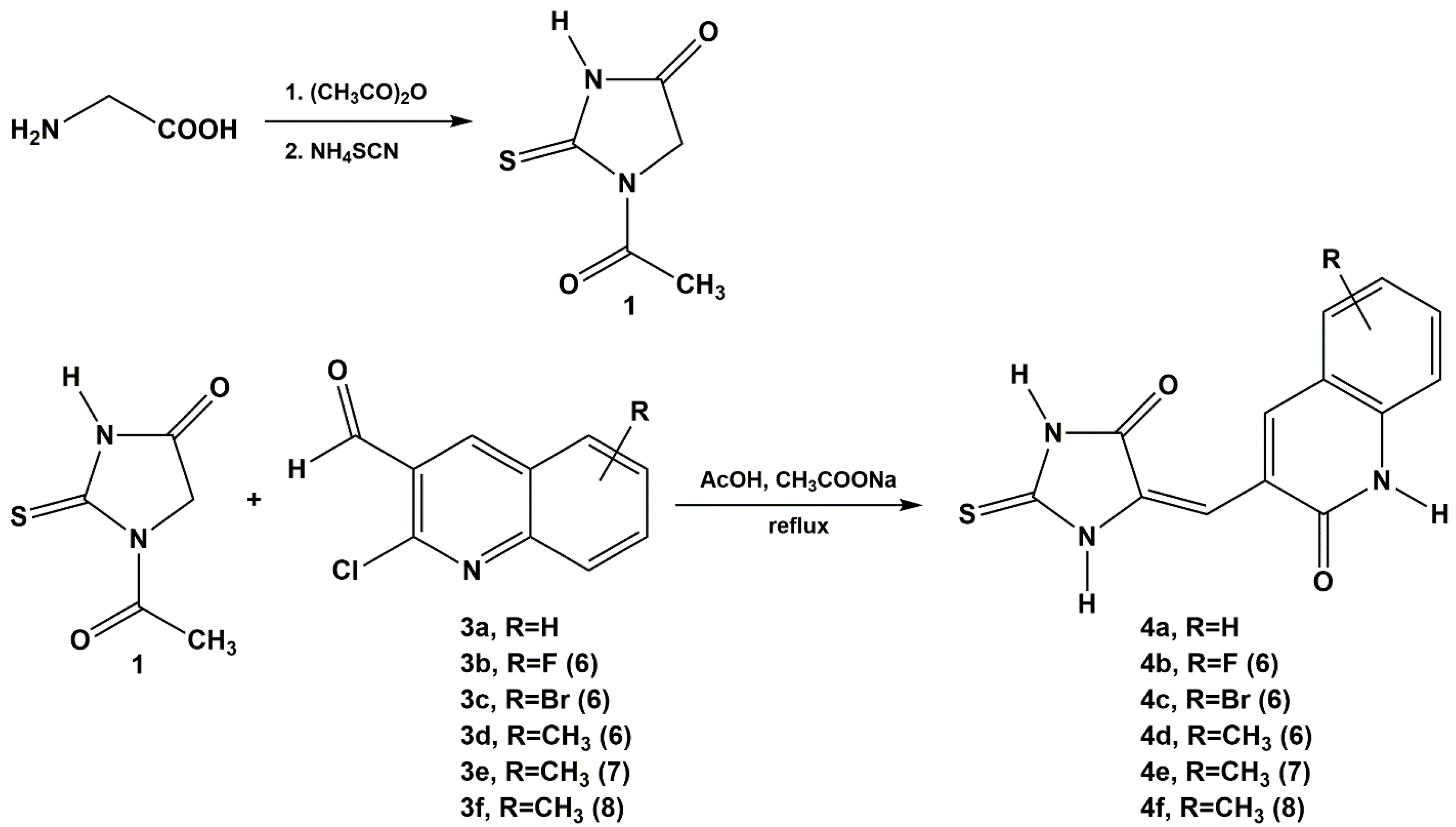
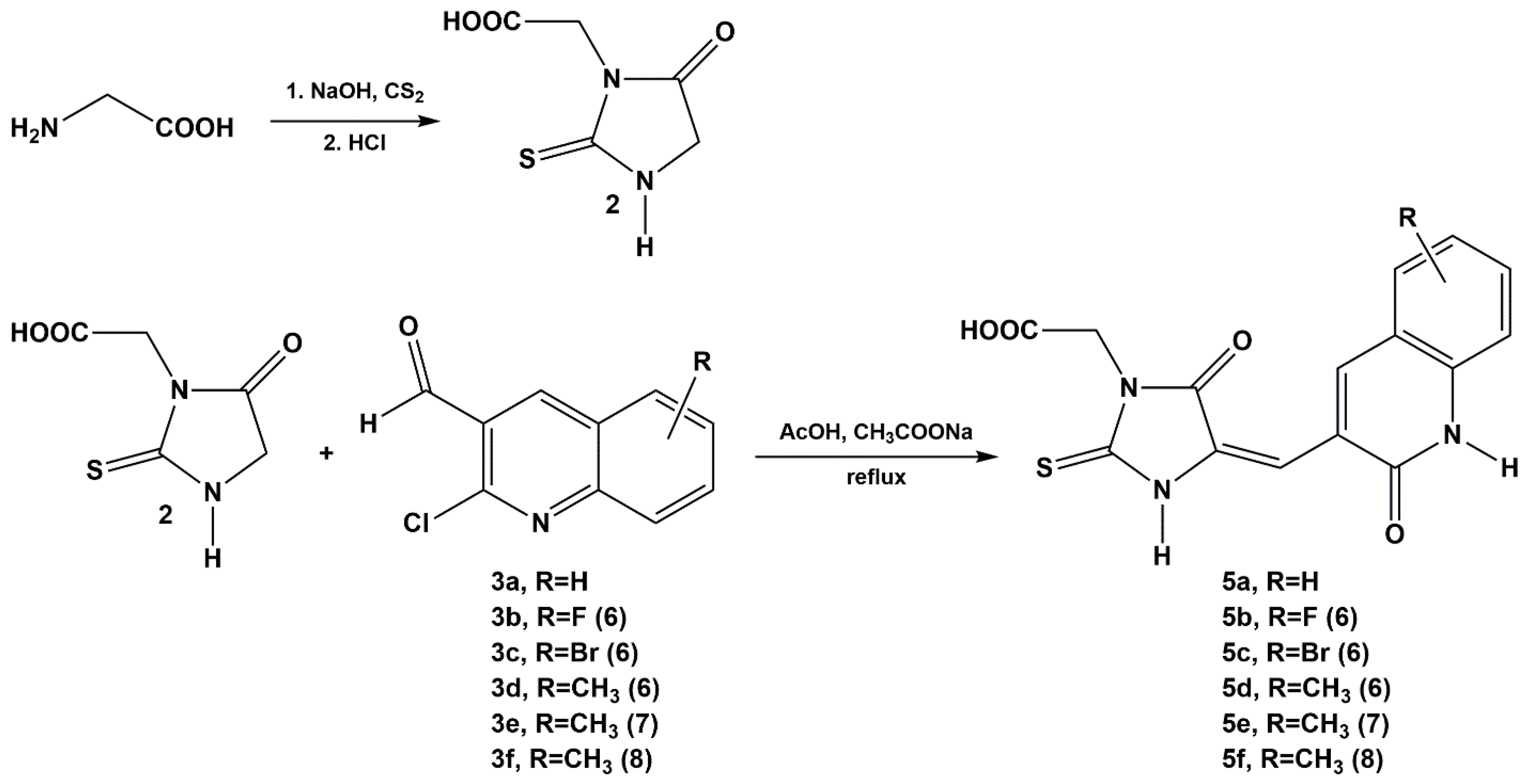
2.1. Chemistry
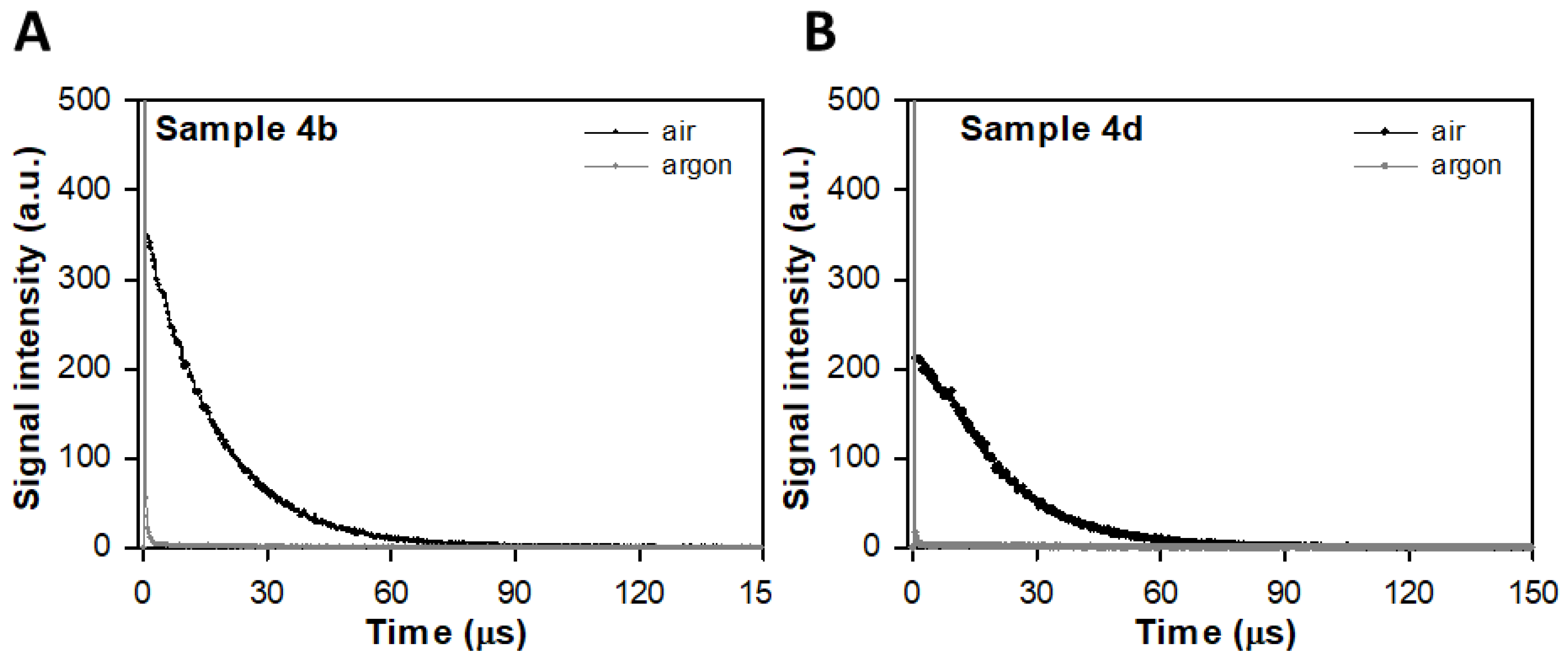
2.2. Direct Detection of Singlet Oxygen (1Δg, 1O2*) Phosphorescence at 1270 nm
2.3. Antibacterial Activity
2.3.1. Antibacterial Activity under Dark Conditions
2.3.2. Antibacterial Activity under Blue Light Irradiation
2.3.3. Structure and Microbiological Activity Relationship
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Condensation of 1-acetyl-2-thiohydantoin and 2-thiohydantoin-3-acetic Acid with 2-chloro-3-quinolinecarboxaldehyde Derivatives
3.1.2. Spectrometry and Spectroscopy
3.2. Direct Detection of Singlet Oxygen (1Δg, 1O2*) Phosphorescence at 1270 nm
3.3. Antibacterial Activity Assay In Vitro
3.3.1. Bacterial Strains
3.3.2. Antibacterial Assay Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- El-Hady, H.A.; Mohamed, S.M.; Al-Shareef, H.F.; El-Mekawy, R.E. Synthesis, reactions and aplications of 2-thiohydantoin derivatives. Acta Pol. Pharm. Drug Res. 2019, 76, 971–986. [Google Scholar] [CrossRef]
- Tejchman, W.; Orwat, B.; Korona-Głowniak, I.; Barbasz, A.; Kownacki, I.; Latacz, G.; Handzlik, J.; Żesławska, E.; Malm, A. Highly efficient microwave synthesis of rhodanine and 2-thiohydantoin derivatives and determination of relationships between their chemical structures and antibacterial activity. RSC Adv. 2019, 9, 39367–39380. [Google Scholar] [CrossRef] [Green Version]
- Camargo de Carvalho, P.G.; Ribeiro, J.M.; Garbin, R.P.B.; Nakazato, G.; Ogatta, S.F.Y.; de Fátima, A.; Bispo, M.d.L.F.; Macedo, F. Synthesis and Antimicrobial Activity of Thiohydantoins Obtained from L-Amino Acids. Lett. Drug Des. Discov. 2020, 17, 94–102. [Google Scholar] [CrossRef]
- Mutlaq, D.Z.; Al-Shawi, A.A.A.; AbdulJabar, L.A. Antioxidant and Antimicrobial Activities of Some Novel 2-Thiohydantoin Derivatives. Egypt. J. Chem. 2021, 64, 1315–1321. [Google Scholar] [CrossRef]
- Raghavan, S.; Manogaran, P.; Gadepalli Narasimha, K.K.; Kalpattu Kuppusami, B.; Mariyappan, P.; Gopalakrishnan, A.; Venkatraman, G. Synthesis and anticancer activity of novel curcumin-quinolone hybrids. Bioorg. Med. Chem. Lett. 2015, 25, 3601–3605. [Google Scholar] [CrossRef]
- Buchynskyy, A.; Gillespie, J.R.; Herbst, Z.M.; Ranade, R.M.; Buckner, F.S.; Gelb, M.H. 1-Benzyl-3-aryl-2-thiohydantoin Derivatives as New Anti-Trypanosoma brucei Agents: SAR and in Vivo Efficacy. ACS Med. Chem. Lett. 2017, 8, 886–891. [Google Scholar] [CrossRef] [Green Version]
- Nitsche, C.; Schreier, V.N.; Behnam, M.A.M.; Kumar, A.; Bartenschlager, R.; Klein, C.D. Thiazolidinone-Peptide Hybrids as Dengue Virus Protease Inhibitors with Antiviral Activity in Cell Culture. J. Med. Chem. 2013, 56, 8389–8403. [Google Scholar] [CrossRef]
- Horta, P.; Secrieru, A.; Coninckx, A.; Cristiano, M.L.S. Quinolones for applications in medical chemistry: Synthesis and Structure. In Targets in Heterocyclic Systems; Attanasi, O.A., Merino, P., Spinelli, D., Eds.; Societa Chimica Italiana: Rome, Italy, 2018; pp. 260–297. [Google Scholar]
- Senerovic, L.; Opsenica, D.; Moric, I.; Aleksic, I.; Spasić, M.; Vasiljevic, B. Quinolines and Quinolones as Antibacterial, Antifungal, Anti-virulence, Antiviral and Anti-parasitic Agents. In Advances in Microbiology, Infectious Diseases and Public Health; Donelli, G., Ed.; Springer International Publishing: Cham, Switzerland, 2020; Volume 14, pp. 37–69. [Google Scholar]
- Al-Amiery, A.A.; Al-Bayati, R.I.H.; Saour, K.Y.; Radi, M.F. Cytotoxicity, antioxidant, and antimicrobial activities of novel 2-quinolone derivatives derived from coumarin. Res. Chem. Intermed. 2012, 38, 559–569. [Google Scholar] [CrossRef]
- Joseph, B.; Darro, F.; Béhard, A.; Lesur, B.; Collignon, F.; Decaestecker, C.; Frydman, A.; Guillaumet, G.; Kiss, R. 3-Aryl-2-Quinolone Derivatives: Synthesis and Characterization of In Vitro and In Vivo Antitumor Effects with Emphasis on a New Therapeutical Target Connected with Cell Migration. J. Med. Chem. 2002, 45, 2543–2555. [Google Scholar] [CrossRef]
- Liu, X.; Deng, J.; Xu, Z.; Lv, Z.-S.; Wang, J.; Feng, L.; Liu, M. Recent Advances of 2-Quinolone-Based Derivatives as Anti-Tubercular Agents. Anti-Infect. Agents 2018, 16, 4–10. [Google Scholar] [CrossRef]
- Salman, G.A. Synthesis of Novel 3-Acetyl N-methyl-2- Quinolone Derivatives with Expected Antimicrobial Activity. Al-Mustansiriyah J. Sci. 2018, 28, 73–79. [Google Scholar] [CrossRef]
- Khamkhenshorngphanuch, T.; Kulkraisri, K.; Janjamratsaeng, A.; Plabutong, N.; Thammahong, A.; Manadee, K.; Na Pombejra, S.; Khotavivattana, T. Synthesis and Antimicrobial Activity of Novel 4-Hydroxy-2-quinolone Analogs. Molecules 2020, 25, 3059. [Google Scholar] [CrossRef]
- Insuasty, D.; Abonia, R.; Insuasty, B.; Quiroga, J.; Laali, K.K.; Nogueras, M.; Cobo, J. Microwave-Assisted Synthesis of Diversely Substituted Quinoline-Based Dihydropyridopyrimidine and Dihydropyrazolopyridine Hybrids. ACS Comb. Sci. 2017, 19, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Bolakatti, G.; Palkar, M.; Katagi, M.; Hampannavar, G.; Karpoormath, R.V.; Ninganagouda, S.; Badiger, A. Novel series of benzo[d]thiazolyl substituted-2-quinolone hybrids: Design, synthesis, biological evaluation and in-silico insights. J. Mol. Struct. 2021, 1227, 129413. [Google Scholar] [CrossRef]
- Bondock, S.; Gieman, H. Synthesis, antibacterial and anticancer evaluation of some new 2-chloro-3-hetarylquinolines. Res. Chem. Intermed. 2015, 41, 8381–8403. [Google Scholar] [CrossRef]
- Radini, I.A.M.; Elsheikh, T.M.Y.; El-Telbani, E.M.; Khidre, R.E. New Potential Antimalarial Agents: Design, Synthesis and Biological Evaluation of Some Novel Quinoline Derivatives as Antimalarial Agents. Molecules 2016, 21, 909. [Google Scholar] [CrossRef] [Green Version]
- Slonczewski, J.L.; Foster, J.W.; Zinser, E.R. Microbiology: An Evolving Science, 5th ed.; W.W. Norton&Company, Inc.: New York, NY, USA, 2020. [Google Scholar]
- Davis, S.L.; Neuhauser, M.M.; McKinnon, P.S. Quinolones. In Antimicrobial Chemotherapy and Vaccines, 2nd ed.; Yu, V.L., Edwards, G., McKinnon, P.S., Peloquin, C., Morse, G.D., Eds.; Antimicrobial Agents; Esun Technologies: Pittsburgh, PA, USA, 2005; Volume 2, pp. 337–366. [Google Scholar]
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.-M.; Bäumler, W. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 7223–7228. [Google Scholar] [CrossRef] [Green Version]
- Merchat, M.; Bertolini, G.; Giacomini, P.; Villaneuva, A.; Jori, G. Meso-substituted cationic porphyrins as efficient photosensitizers of Gram-positive and Gram-negative bacteria. J. Photochem. Photobiol. B Biol. 1996, 32, 153–157. [Google Scholar] [CrossRef]
- Merchat, M.; Spikes, J.D.; Bertoloni, G.; Jori, G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J. Photochem. Photobiol. B Biol. 1996, 35, 149–157. [Google Scholar] [CrossRef]
- Minnock, A.; Vernon, D.I.; Schofield, J.; Griffiths, J.; Howard Parish, J.; Brown, S.B. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both Gram-negative and Gram-positive bacteria. J. Photochem. Photobiol. B Biol. 1996, 32, 159–164. [Google Scholar] [CrossRef]
- Zanin, I.C.J.; Gonçalves, R.B.; Junior, A.B.; Hope, C.K.; Pratten, J. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: An in vitro study. J. Antimicrob. Chemother. 2005, 56, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S. Definition of Type I And Type II Photosensitized Oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, W.; Abels, C.; Karrer, S.; Weiß, T.; Messmann, H.; Landthaler, M.; Szeimies, R.M. Photo-oxidative killing of human colonic cancer cells using indocyanine green and infrared light. Br. J. Cancer 1999, 80, 360–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaeli, A.; Feitelson, J. Reactivity of Singlet Oxygen toward Amino Acids and Peptides. Photochem. Photobiol. 1994, 59, 284–289. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.-L. Oxidatively generated base damage to cellular DNA. Free Radic. Biol. Med. 2010, 49, 9–21. [Google Scholar] [CrossRef]
- Bumah, V.V.; Masson-Meyers, D.S.; Tong, W.; Castel, C.; Enwemeka, C.S. Optimizing the bactericidal effect of pulsed blue light on Propionibacterium acnes—A correlative fluorescence spectroscopy study. J. Photochem. Photobiol. B Biol. 2020, 202, 111701. [Google Scholar] [CrossRef]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. Updates 2012, 15, 223–236. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Kim, J.S.; Lim, W.B.; Jeon, S.M.; Kim, O.S.; Koh, J.-T.; Kim, C.-S.; Choi, H.R.; Kim, O.J. In Vitro Bactericidal Effects of 625, 525, and 425 nm Wavelength (Red, Green, and Blue) Light-Emitting Diode Irradiation. Photomed. Laser Surg. 2013, 31, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Morici, P.; Battisti, A.; Tortora, G.; Menciassi, A.; Checcucci, G.; Ghetti, F.; Sgarbossa, A. The in vitro Photoinactivation of Helicobacter pylori by a Novel LED-Based Device. Front. Microbiol. 2020, 11, 283. [Google Scholar] [CrossRef] [Green Version]
- Rapacka-Zdończyk, A.; Woźniak, A.; Michalska, K.; Pierański, M.; Ogonowska, P.; Grinholc, M.; Nakonieczna, J. Factors Determining the Susceptibility of Bacteria to Antibacterial Photodynamic Inactivation. Front. Med. 2021, 8, 642609. [Google Scholar] [CrossRef]
- Hassan, A.Y.; Sarg, M.T.; Said, M.M.; El-Sebaey, S.A. Utility of thieno[2,3-b] pyridine derivatives in the synthesis of some condensed heterocyclic compounds with expected biological activity. Univers. Org. Chem. 2013, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Reyes, S.; Burgess, K. On Formation of Thiohydantoins from Amino Acids under Acylation Conditions. J. Org. Chem. 2006, 71, 2507–2509. [Google Scholar] [CrossRef] [PubMed]
- Nadaraj, V.; Selvi, S.T. The effective reaction of 2-chloro-3-formylquinoline and acetic acid/sodium acetate under microwave irradiation. Int. J. Eng. Sci. Technol. 2011, 3, 297–302. [Google Scholar] [CrossRef]
- Nastasa, V.; Pascu, A.; Boni, M.; Smarandache, A.; Staicu, A.; Pascu, M.L. Insights into the photophysics of zinc phthalocyanine and photogenerated singlet oxygen in DMSO-water mixture. Colloids Surf. A Physicochem. Eng. Asp. 2016, 505, 197–203. [Google Scholar] [CrossRef]
- Entradas, T.; Waldron, S.; Volk, M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J. Photochem. Photobiol. B Biol. 2020, 204, 111787. [Google Scholar] [CrossRef] [PubMed]
- Arnbjerg, J.; Paterson, M.J.; Nielsen, C.B.; Jørgensen, M.; Christiansen, O.; Ogilby, P.R. One- and Two-Photon Photosensitized Singlet Oxygen Production: Characterization of Aromatic Ketones as Sensitizer Standards. J. Phys. Chem. A 2007, 111, 5756–5767. [Google Scholar] [CrossRef] [PubMed]
- Martí, C.; Jürgens, O.; Cuenca, O.; Casals, M.; Nonell, S. Aromatic ketones as standards for singlet molecular oxygen O2(1Δg) photosensitization. Time-resolved photoacoustic and near-IR emission studies. J. Photochem. Photobiol. A Chem. 1996, 97, 11–18. [Google Scholar] [CrossRef]
- Schmidt, R.; Tanielian, C.; Dunsbach, R.; Wolff, C. Phenalenone, a universal reference compound for the determination of quantum yields of singlet oxygen O2(1Δg) sensitization. J. Photochem. Photobiol. A Chem. 1994, 79, 11–17. [Google Scholar] [CrossRef]
- Martinez, L.J.; Sik, R.H.; Chignell, C.F. Fluoroquinolone Antimicrobials: Singlet Oxygen, Superoxide and Phototoxicity. Photochem. Photobiol. 1998, 67, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Zoltan, T.; Rivas, C.; Ramirez, A.; Cordero, T.; Díaz, Y.; Izzo, C.; Cárdenas, Y.M.; López, V.; Gómez, L.; et al. Synthesis, primary photophysical and antibacterial properties of naphthyl ester cinoxacin and nalidixic acid derivatives. J. Photochem. Photobiol. B Biol. 2008, 92, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zoltan, T.; Vargas, F.; Izzo, C. UV-Vis Spectrophotometrical and Analytical Methodology for the Determination of Singlet Oxygen in New Antibanterials Drugs. Anal. Chem. Insights 2007, 2, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. New York Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikaido, H. Prevention of drug access to bacterial targets: Permeability barriers and active efflux. Science 1994, 264, 382–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinstein, E.; Lagacé-Wiens, P. Quinolones. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1239–1248. [Google Scholar]
- Mortimer, P.G.S.; Piddok, L.J.V. The accumulation of five antibacterial agents in porin-deficient mutants of Escherichia coli. J. Antimicrob. Chemother. 1993, 32, 195–213. [Google Scholar] [CrossRef]
- Nikaido, H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob. Agents Chemother. 1989, 33, 1831–1836. [Google Scholar] [CrossRef] [Green Version]
- Walden, D.M.; Khotimchenko, M.; Hou, H.; Chakravarty, K.; Varshney, J. Effects of Magnesium, Calcium, and Aluminum Chelation on Fluoroquinolone Absorption Rate and Bioavailability: A Computational Study. Pharmaceutics 2021, 13, 594. [Google Scholar] [CrossRef]
- Trotsko, N.; Kosikowska, U.; Paneth, A.; Wujec, M.; Malm, A. Synthesis and antibacterial activity of new (2,4-dioxothiazolidin-5-yl/ylidene)acetic acid derivatives with thiazolidine-2,4-dione, rhodanine and 2-thiohydantoin moieties. Saudi Pharm. J. 2018, 26, 568–577. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.-H.; Shin, D. Recent applications of hydantoin and thiohydantoin in medicinal chemistry. Eur. J. Med. Chem. 2019, 164, 517–545. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Feix, J.B.; Sieber, F.; Thomas, J.P.; Girotti, A.W. Photodynamic action of merocyanine 540 on artificial and natural cell membranes: Involvement of singlet molecular oxygen. Proc. Natl. Acad. Sci. USA 1987, 84, 2999–3003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, T.; Gupta, A.; Huang, Y.-Y.; Yin, R.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Tegos, G.P.; Hamblin, M.R. Blue Light Rescues Mice from Potentially Fatal Pseudomonas aeruginosa Burn Infection: Efficacy, Safety, and Mechanism of Action. Antimicrob. Agents Chemother. 2013, 57, 1238–1245. [Google Scholar] [CrossRef] [Green Version]
- Fila, G.; Kawiak, A.; Grinholc, M.S. Blue light treatment of Pseudomonas aeruginosa: Strong bactericidal activity, synergism with antibiotics and inactivation of virulence factors. Virulence 2017, 8, 938–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronqui, M.R.; de Aguiar Coletti, T.M.S.F.; de Freitas, L.M.; Miranda, E.T.; Fontana, C.R. Synergistic antimicrobial effect of photodynamic therapy and ciprofloxacin. J. Photochem. Photobiol. B Biol. 2016, 158, 122–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, C.M.; Green, G.M. Quinolones: A comprehensive review. Am. Fam. Physician 2002, 65, 455–464. [Google Scholar]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
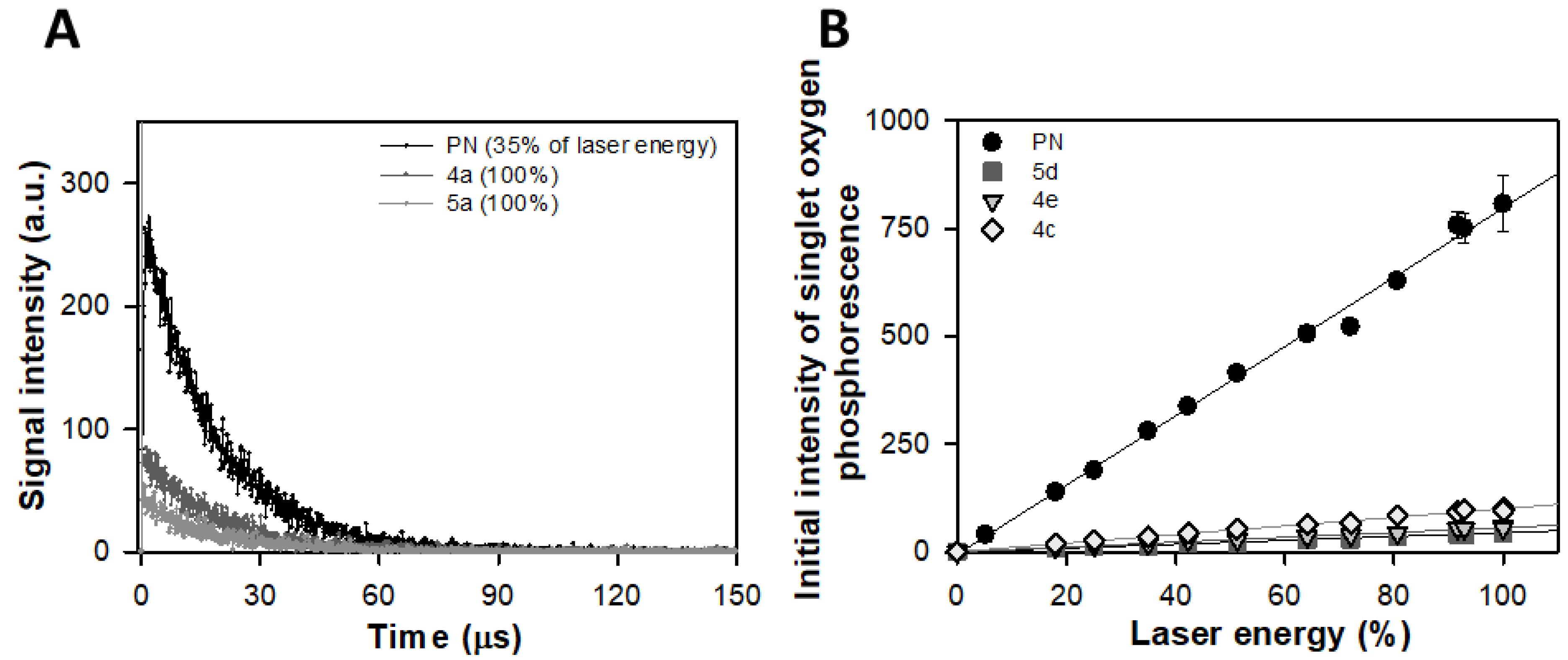

| Sample | Relative Efficiency of 1O2 (1Δg) Generation (%) |
|---|---|
| PN (standard) | 100 |
| 4c | 12.43 (±0.02) |
| 4a | 10.90 (±1.07) |
| 4b | 10.23 (±0.93) |
| 4f | 9.81 (±0.24) |
| 4d | 8.54 (±0.23) |
| 5c | 7.98 (±0.56) |
| 4e | 7.71 (±0.98) |
| 5a | 5.91 (±0.66) |
| 5f | 5.77 (±1.14) |
| 5d | 5.66 (±0.15) |
| 5b | 4.93 (±0.22) |
| 5e | 3.93 (±2.10) |
| Microorganism | 4a | 4b | 4c | 4d | 4e | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | |
| E. coli | nd | nd | nd | nd | nd | nd | nd | nd | 500 | nd |
| P. aeruginosa | nd | nd | nd | nd | nd | nd | nd | nd | 1000 | nd |
| K. pneumoniae | nd | nd | nd | nd | nd | nd | 250 | nd | 31.25 | 1000 |
| E. faecalis | nd | nd | 7.82 | 250 | nd | nd | 7.82 | 250 | 7.82 | 500 |
| S. aureus | nd | nd | 7.82 | 125 | nd | nd | 125 | nd | 31.25 | 1000 |
| B. subtilis | nd | nd | nd | nd | nd | nd | 7.82 | 250 | 62.5 | nd |
| Microorganism | 4f | 5b | 5c | 5e | 5f | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | |
| E. coli | 62.5 | 1000 | nd | nd | nd | nd | nd | nd | 500 | nd |
| P. aeruginosa | 31.25 | 500 | nd | nd | nd | nd | nd | nd | 250 | nd |
| K. pneumoniae | 62.5 | 1000 | nd | nd | nd | nd | nd | nd | 250 | nd |
| E. faecalis | 62.5 | 1000 | 250 | nd | 62.5 | 1000 | nd | nd | 250 | nd |
| S. aureus | 31.25 | 500 | 250 | nd | 62.5 | 1000 | nd | nd | 250 | nd |
| B. subtilis | 31.25 | 500 | 250 | nd | 125 | nd | nd | nd | 62.5 | 1000 |
| Microorganism | 4b | 4d | 4e | 4f | 5b | 5c | 5f |
|---|---|---|---|---|---|---|---|
| E. coli | - | - | - | 16 | - | - | - |
| P. aeruginosa | - | - | - | 16 | - | - | - |
| K. pneumoniae | - | - | 32 | 16 | - | - | - |
| E. faecalis | 32 | 32 | 64 | 16 | - | 16 | - |
| S. aureus | 16 | - | 32 | 16 | - | 16 | - |
| B. subtilis | - | 32 | - | 16 | - | - | 16 |
| Microorganism | Ciprofloxacin | ||
|---|---|---|---|
| MIC (μg/mL) | MBC (μg/mL) | MBC/MIC Ratio | |
| E. coli ATCC 25922 | 0.24 | 0.48 | 2 |
| P. aeruginosa ATCC 27853 | 0.26 | 0.31 | 1.19 |
| K. pneumoniae ATCC 700603 | 7.81 | 7.81 | 1 |
| E. faecalis ATCC 29212 | 1 | 1 | 1 |
| S. aureus ATCC 29213 | 7.81 | 7.81 | 1 |
| B. subtilis ATCC 6633 | 0.98 | 0.98 | 1 |
| Microorganism | 4a | 4b | 4c | 4d | 4e | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | |
| E. coli | nd | nd | 125 | 1000 | nd | nd | 7.82 | 250 | 500 | nd |
| P. aeruginosa | nd | nd | 7.82 | 250 | 3.91 | 125 | 3.91 | 125 | 3.91 | 3.91 |
| K. pneumoniae | nd | nd | 62.5 | 1000 | nd | nd | 62.5 | 1000 | 15.63 | 500 |
| E. faecalis | nd | nd | 7.82 | 250 | nd | nd | 7.82 | 250 | 15.63 | 500 |
| S. aureus | nd | nd | 7.82 | 125 | nd | nd | 3.91 | 125 | 31.25 | 1000 |
| B. subtilis | nd | nd | 7.82 | 125 | nd | nd | 3.91 | 125 | 3.91 | 250 |
| Microorganism | 4f | 5b | 5c | 5e | 5f | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | |
| E. coli | 62.5 | 1000 | nd | nd | nd | nd | nd | nd | 500 | nd |
| P. aeruginosa | 7.82 | 125 | nd | nd | 3.91 | 3.91 | nd | nd | 7.82 | 250 |
| K. pneumoniae | 62.5 | 1000 | nd | nd | 62.5 | 1000 | nd | nd | 250 | nd |
| E. faecalis | 62.5 | 1000 | 31.25 | 1000 | 62.5 | 1000 | nd | nd | 62.5 | nd |
| S. aureus | 3.91 | 250 | 125 | 1000 | 62.5 | 1000 | nd | nd | 15.63 | 500 |
| B. subtilis | 3.91 | 250 | 125 | 1000 | 3.91 | 250 | nd | nd | 3.91 | 250 |
| Microorganism | 4b | 4d | 4e | 4f | 5b | 5c | 5f |
|---|---|---|---|---|---|---|---|
| E. coli | 8 | 32 | - | 16 | - | - | - |
| P. aeruginosa | 32 | 32 | 1 | 16 | - | 1 | - |
| K. pneumoniae | 16 | 16 | 32 | 16 | - | 16 | - |
| E. faecalis | 32 | 32 | 32 | 16 | 32 | 16 | - |
| S. aureus | 16 | 32 | 32 | 64 | 8 | 16 | 32 |
| B. subtilis | 16 | 32 | 64 | 64 | 8 | 64 | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kania, A.; Tejchman, W.; Pawlak, A.M.; Mokrzyński, K.; Różanowski, B.; Musielak, B.M.; Greczek-Stachura, M. Preliminary Studies of Antimicrobial Activity of New Synthesized Hybrids of 2-Thiohydantoin and 2-Quinolone Derivatives Activated with Blue Light. Molecules 2022, 27, 1069. https://doi.org/10.3390/molecules27031069
Kania A, Tejchman W, Pawlak AM, Mokrzyński K, Różanowski B, Musielak BM, Greczek-Stachura M. Preliminary Studies of Antimicrobial Activity of New Synthesized Hybrids of 2-Thiohydantoin and 2-Quinolone Derivatives Activated with Blue Light. Molecules. 2022; 27(3):1069. https://doi.org/10.3390/molecules27031069
Chicago/Turabian StyleKania, Agnieszka, Waldemar Tejchman, Anna M. Pawlak, Krystian Mokrzyński, Bartosz Różanowski, Bogdan M. Musielak, and Magdalena Greczek-Stachura. 2022. "Preliminary Studies of Antimicrobial Activity of New Synthesized Hybrids of 2-Thiohydantoin and 2-Quinolone Derivatives Activated with Blue Light" Molecules 27, no. 3: 1069. https://doi.org/10.3390/molecules27031069






