Trophic Dynamics and Feeding Ecology of Skipjack Tuna (Katsuwonus pelamis) off Eastern and Western Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description, Data Collection, and Sampling
2.2. Stomach Content Analysis
2.3. Stable Isotope Analysis
2.4. Data Analysis
3. Results
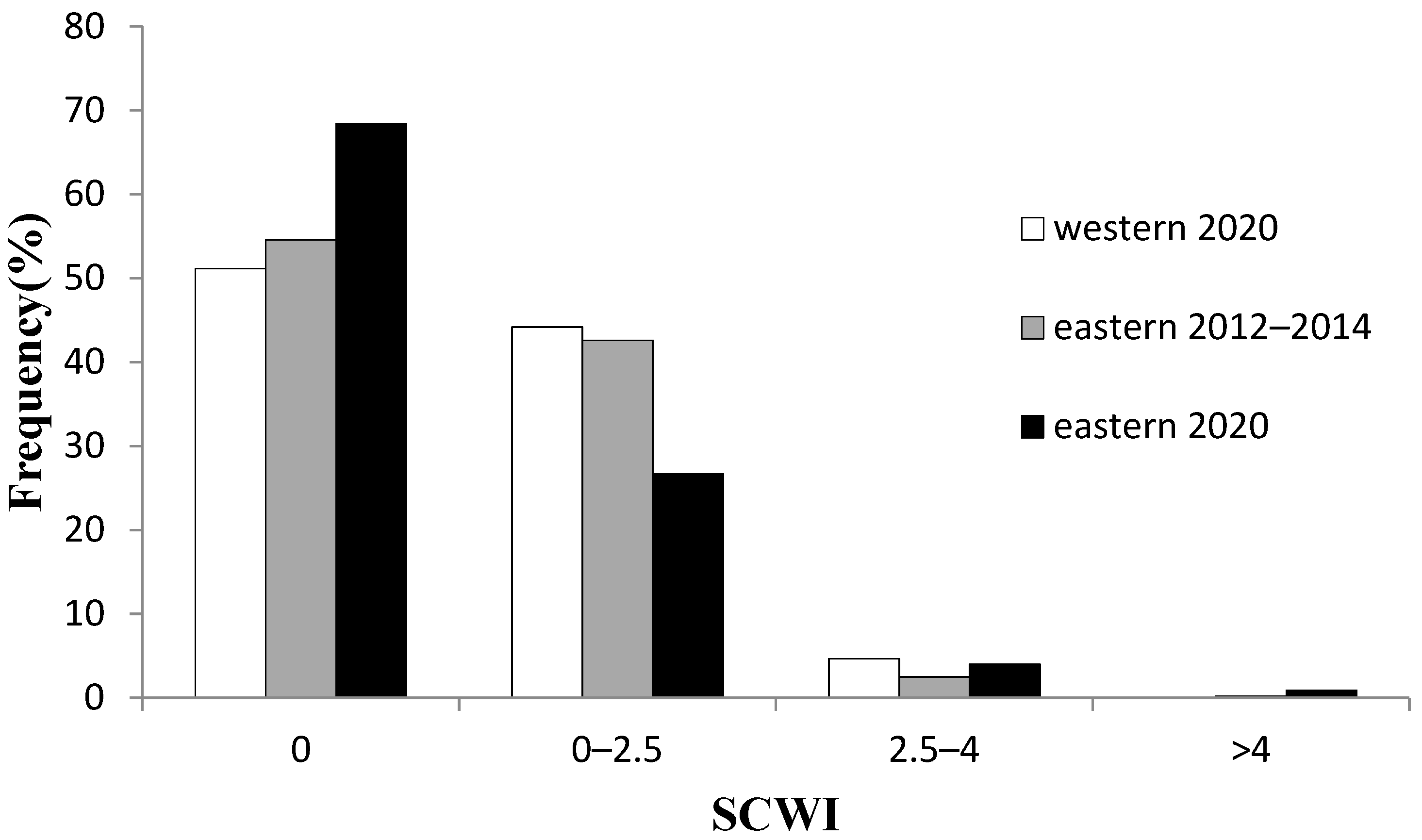
3.1. Size Class
3.2. Stomach Content Analysis
3.2.1. Regional Comparisons
3.2.2. Temporal Comparisons in Eastern Taiwan
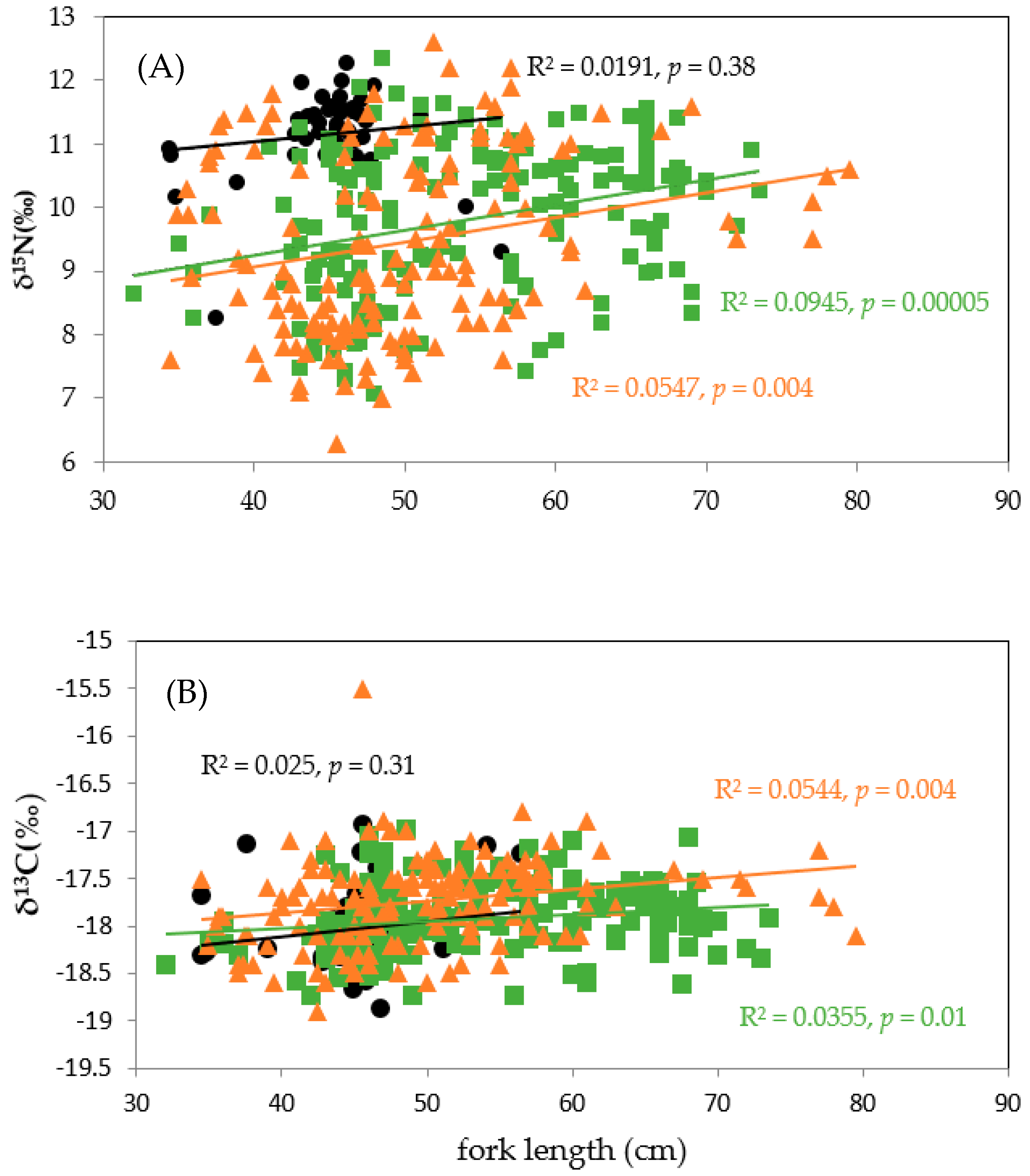
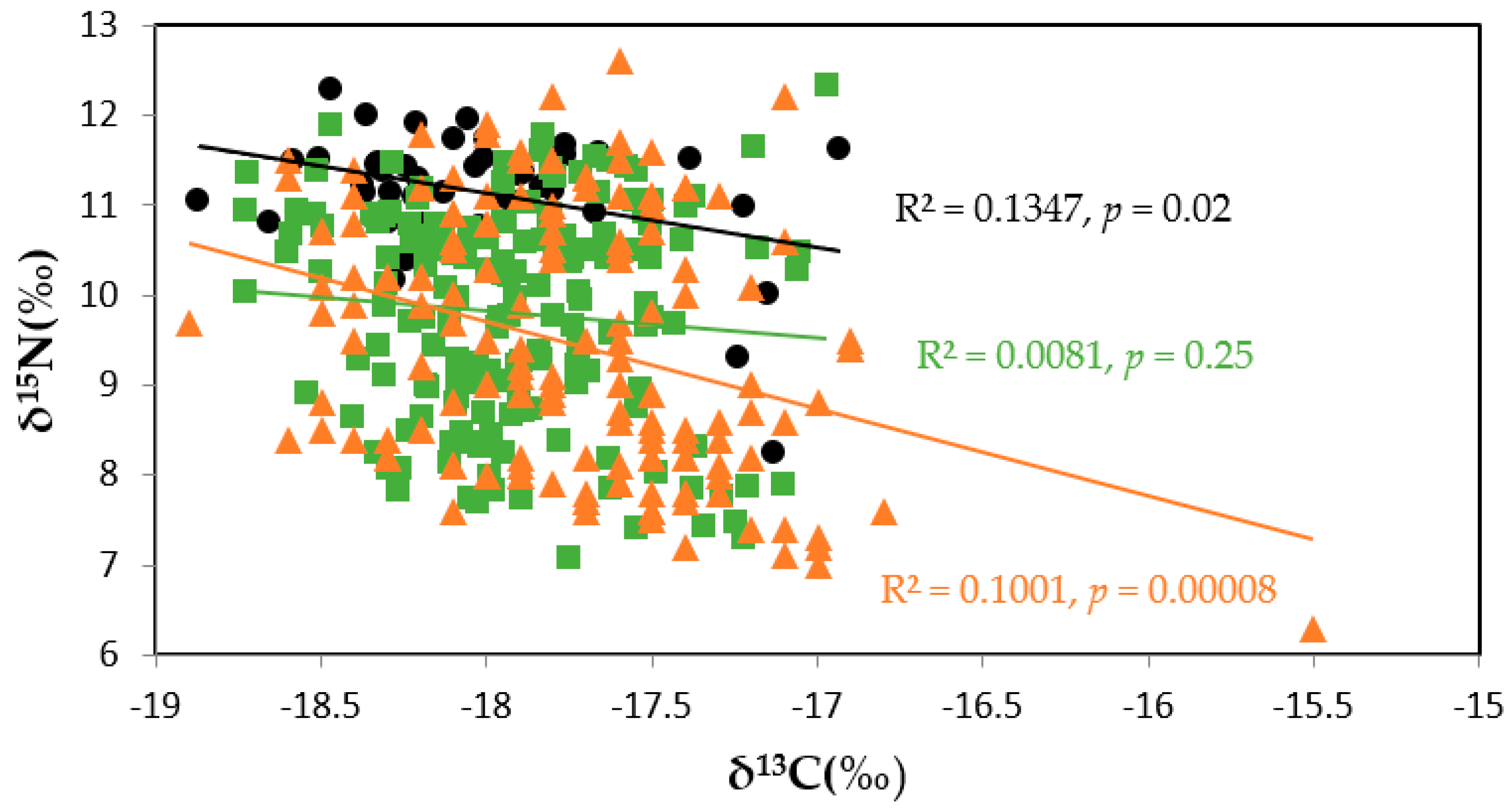
3.3. Stable Isotope Analysis
3.3.1. Regional Comparisons
3.3.2. Temporal Comparisons in Eastern Taiwan
3.4. Bayesian Mixing Models
Regional Comparisons
4. Discussion
4.1. Diet Reconstructions from SCA and SIA
4.2. Regional Effects on Diet
4.3. SI Dynamics across Skipjack Size
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dueri, S.; Guillotreau, P.; Jiménez-Toribio, R.; Oliveros-Ramos, R.; Bopp, L.; Maury, O. Food Security or Economic Profitability? Projecting the Effects of Climate and Socioeconomic Changes on Global Skipjack Tuna Fisheries under Three Management Strategies. Glob. Environ. Chang. 2016, 41, 1–12. [Google Scholar] [CrossRef]
- Hampton, J.; Williams, P.G. The Western and Central Pacific Tuna Fishery, 2001: Overview and Status of Stocks; Tuna Fisheries Assessment Report; Secretariat of the Pacific Community: Noumea, New Caledonia, 2003; p. 42. [Google Scholar]
- FAO (Ed.) The State of World Fisheries and Aquaculture. Sustainability in Action; FAO: Rome, Italy, 2020; 206p. [Google Scholar]
- Collette, B.B.; Nauen, C.E. FAO Species Catalogue. 2: Scombrids of the World: An Annotated and Illustrated Catalogue of Tuna, Mackerels, Bonitos and Related Species Known to Data; FAO Species Catalogue: Rome, Italy, 1983; pp. 42–90. [Google Scholar]
- Chiang, W.C.; Matsumoto, T.; Lin, S.J.; Chang, Q.X.; Musyl, M.K.; Ho, Y.S.; Ohta, F. Fine-Scale Vertical Movements and Behavior of Immature Skipjack Tuna (Katsuwonus Pelamis) off Eastern Taiwan. J. Mar. Sci. Technol. 2021, 29, 207–219. [Google Scholar] [CrossRef]
- Dambacher, J.M.; Young, J.W.; Olson, R.J.; Allain, V.; Galván-Magaña, F.; Lansdell, M.J.; Bocanegra-Castillo, N.; Alatorre-Ramírez, V.; Cooper, S.P.; Duffy, L.M. Analyzing Pelagic Food Webs Leading to Top Predators in the Pacific Ocean: A Graph-Theoretic Approach. Prog. Oceanogr. 2010, 86, 152–165. [Google Scholar] [CrossRef]
- Tsai, C.N.; Chiang, W.C.; Sun, C.L.; Shao, K.T.; Chen, S.Y.; Yeh, S.Z. Trophic Size-Structure of Sailfish Istiophorus Platypterus in Eastern Taiwan Estimated by Stable Isotope Analysis. J. Fish Biol. 2014, 84, 354–371. [Google Scholar] [CrossRef]
- Chang, C.T.; Chiang, W.C.; Chang, Y.C.; Musyl, M.K.; Sun, C.L.; Madigan, D.J.; Carlisle, A.B.; Hsu, H.H.; Chang, Q.X.; Su, N.J.; et al. Stable Isotope Analysis Reveals Ontogenetic Feeding Shifts in Pacific Blue Marlin (Makaira Nigricans) off Eastern Taiwan. J. Fish Biol. 2019, 94, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.C.; Chang, C.T.; Madigan, D.J.; Carlisle, A.B.; Musyl, M.K.; Chang, Y.C.; Hsu, H.H.; Su, N.J.; Sun, C.L.; Ho, Y.S.; et al. Stable Isotope Analysis Reveals Feeding Ecology and Trophic Position of Black Marlin off Eastern Taiwan. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 175, 104821. [Google Scholar] [CrossRef]
- Tanabe, T. Feeding Habits of Skipjack Tuna Katsuwonus Pelamis and Other Tuna Thunnus Spp. Juveniles in the Tropical Western Pacific. Fish. Sci. 2001, 67, 563–570. [Google Scholar] [CrossRef]
- Nakamura, E.L. Food and Feeding Habits of Skipjack Tuna (Katsuwonus Pelamis) from the Marquesas and Tuamotu Islands. Trans. Am. Fish. Soc. 1965, 94, 236–242. [Google Scholar] [CrossRef]
- Varela, J.L.; Cañavate, J.P.; Medina, A.; Mourente, G. Inter-regional variation in feeding patterns of skipjack tuna (Katsuwonus pelamis) inferred from stomach content, stable isotope and fatty acid analyses. Mar. Environ. Res. 2019, 152, 104821. [Google Scholar] [CrossRef]
- Coletto, J.L.; Botta, S.; Fischer, L.G.; Newsome, S.D.; Madureira, L.S.P. Isotope-based inferences of skipjack tuna feeding ecology and movement in the southwestern Atlantic Ocean. Mar. Environ. Res. 2021, 165, 105246. [Google Scholar] [CrossRef] [PubMed]
- Hyslop, E.J. Stomach Contents Analysis-a Review of Methods and Their Application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, J.S.; Waiwood, K.G.; Green, R.H. Rates of Digestion of Different Prey in Atlantic Cod (Gadus Morhua), Ocean Pout (Macrozoarces Americanus), Winter Flounder (Pseudopleuronectes Americanus), and American Plaice (Hippoglossoides Platessoides). Can. J. Fish. Aquat. Sci. 1982, 39, 651–659. [Google Scholar] [CrossRef]
- Galuardi, B.; Royer, F.; Golet, W.; Logan, J.; Neilson, J.; Lutcavage, M. Complex Migration Routes of Atlantic Bluefin Tuna (Thunnus Thynnus) Question Current Population Structure Paradigm. Can. J. Fish. Aquat. Sci. 2010, 67, 966–976. [Google Scholar] [CrossRef]
- Legler, N.D.; Johnson, T.B.; Heath, D.D.; Ludsin, S.A. Water Temperature and Prey Size Effects on the Rate of Digestion of Larval and Early Juvenile Fish. Trans. Am. Fish. Soc. 2010, 139, 868–875. [Google Scholar] [CrossRef]
- Suzuki, K.; Kasai, A.; Nakayama, K.; Tanaka, M. Differential Isotopic Enrichment and Half-Life among Tissues in Japanese Temperate Bass (Lateolabrax Japonicus) Juveniles: Implications for Analyzing Migration. Can. J. Fish. Aquat. Sci. 2011, 62, 671–678. [Google Scholar] [CrossRef]
- Madigan, D.J.; Litvin, S.Y.; Popp, B.N.; Carlisle, A.B.; Farwell, C.J.; Block, B.A. Tissue Turnover Rates and Isotopic Trophic Discrimination Factors in the Endothermic Teleost, Pacific Bluefin Tuna (Thunnus Orientalis). PLoS ONE 2012, 7, e49220. [Google Scholar] [CrossRef] [Green Version]
- Harvey, C.J.; Hanson, P.C.; Essington, T.E.; Brown, P.B.; Kitchell, J.F. Using Bioenergetics Models to Predict Stable Isotope Ratios in Fishes. Can. J. Fish. Aquat. Sci. 2002, 59, 115–124. [Google Scholar] [CrossRef] [Green Version]
- McClure, M.M.; McIntyre, P.B.; McCune, A.R. Notes on the Natural Diet and Habitat of Eight Danionin Fishes, Including the Zebrafish Danio Rerio. J. Fish Biol. 2006, 69, 553–570. [Google Scholar] [CrossRef]
- Goering, J.; Alexander, V.; Haubenstock, N. Seasonal variability of stable carbon and nitrogen isotope ratios of organisms in a North Pacific Bay. Estuar. Coast. Shelf Sci. 1990, 30, 239–260. [Google Scholar] [CrossRef]
- Post, D.M. Using Stable Isotopes to Estimate Trophic Position: Models, Methods, and Assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Olson, R.J.; Popp, B.N.; Graham, B.S.; López-Ibarra, G.A.; Galván-Magaña, F.; Lennert-Cody, C.E.; Bocanegra-Castillo, N.; Wallsgrove, N.J.; Gier, E.; Alatorre-Ramírez, V.; et al. Food-Web Inferences of Stable Isotope Spatial Patterns in Copepods and Yellowfin Tuna in the Pelagic Eastern Pacific Ocean. Prog. Oceanogr. 2010, 86, 124–138. [Google Scholar] [CrossRef]
- Ruiz-Cooley, R.I.; Gerrodette, T.; Fiedler, P.C.; Chivers, S.J.; Danil, K.; Ballance, L.T. Temporal Variation in Pelagic Food Chain Length in Response to Environmental Change. Sci. Adv. 2017, 3, e1701140. [Google Scholar] [CrossRef] [Green Version]
- Lorrain, A.; Pethybridge, H.; Cassar, N.; Receveur, A.; Allain, V.; Bodin, N.; Bopp, L.; Choy, C.A.; Duffy, L.; Fry, B.; et al. Trends in Tuna Carbon Isotopes Suggest Global Changes in Pelagic Phytoplankton Communities. Glob. Chang. Biol. 2019, 26, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Popp, B.N.; Graham, B.S.; Olson, R.J.; Hannides, C.C.S.; Lott, M.J.; López-Ibarra, G.A.; Galván-Magaña, F.; Fry, B. Insight into the Trophic Ecology of Yellowfin Tuna, Thunnus albacares, from Compound-Specific Nitrogen Isotope Analysis of Proteinaceous Amino Acids. In Terrestrial Ecology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 1, pp. 173–190. [Google Scholar]
- Frédérich, B.; Fabri, G.; Lepoint, G.; Vandewalle, P.; Parmentier, E. Trophic Niches of Thirteen Damselfishes (Pomacentridae) at the Grand Récif of Toliara, Madagascar. Ichthyol. Res. 2009, 56, 10–17. [Google Scholar] [CrossRef]
- Dempson, J.B.; Braithwaite, V.A.; Doherty, D.; Power, M. Stable Isotope Analysis of Marine Feeding Signatures of Atlantic Salmon in the North Atlantic. ICES J. Mar. Sci. 2010, 67, 52–61. [Google Scholar] [CrossRef]
- Friedland, K.D.; Reddin, D.G.; Castonguay, M. Ocean Thermal Conditions in the Post-Smolt Nursery of North American Atlantic Salmon. ICES J. Mar. Sci. 2003, 60, 343–355. [Google Scholar] [CrossRef] [Green Version]
- Friedland, K.D.; Reddin, D.G.; McMenemy, J.R.; Drinkwater, K.F. Multidecadal Trends in North American Atlantic Salmon (Salmo Salar) Stocks and Climate Trends Relevant to Juvenile Survival. Can. J. Fish. Aquat. Sci. 2003, 60, 563–583. [Google Scholar] [CrossRef] [Green Version]
- Friedland, K.D.; Chaput, G.; MacLean, J.C. The Emerging Role of Climate in Post-Smolt Growth of Atlantic Salmon. ICES J. Mar. Sci. 2005, 62, 1338–1349. [Google Scholar] [CrossRef]
- Baum, J.K.; Worm, B. Cascading Top-down Effects of Changing Oceanic Predator Abundances. J. Anim. Ecol. 2009, 78, 699–714. [Google Scholar] [CrossRef]
- Newsome, T.M.; Greenville, A.C.; Ćirović, D.; Dickman, C.R.; Johnson, C.N.; Krofel, M.; Letnic, M.; Ripple, W.J.; Ritchie, E.G.; Stoyanov, S.; et al. Top Predators Constrain Mesopredator Distributions. Nat. Commun. 2017, 8, 15469. [Google Scholar] [CrossRef]
- Jan, S.; Wang, J.; Chern, C.S.; Chao, S.Y. Seasonal Variation of the Circulation in the Taiwan Strait. J. Mar. Syst. 2002, 35, 249–268. [Google Scholar] [CrossRef]
- Pinkas, L.; Oliphant, M.S.; Iverson, I.L.K. Food Habits of Albacore, Bluefin Tuna, and Bonito in California Waters. Fish. Bull. 1971, 152, 1–105. [Google Scholar]
- Teece, M.A.; Fogel, M.L. Preparation of Ecological and Biochemical Samples for Isotope Analysis. In Handbook of Stable Isotope Analytical Techniques; Elsevier: Amsterdam, The Netherlands, 2004; pp. 177–202. [Google Scholar]
- Madigan, D.J.; Chiang, W.C.; Wallsgrove, N.J.; Popp, B.N.; Kitagawa, T.; Choy, C.A.; Tallmon, J.; Ahmed, N.; Fisher, N.S.; Sun, C.L. Intrinsic Tracers Reveal Recent Foraging Ecology of Giant Pacific Bluefin Tuna at Their Primary Spawning Grounds. Mar. Ecol. Prog. Ser. 2016, 553, 253–266. [Google Scholar] [CrossRef] [Green Version]
- Weng, J.S.; Lee, M.A.; Liu, K.M.; Hsu, M.S.; Hung, M.K.; Wu, L.J. Feeding Ecology of Juvenile Yellowfin Tuna from Waters Southwest of Taiwan Inferred from Stomach Contents and Stable Isotope Analysis. Mar. Coast. Fish. 2015, 7, 537–548. [Google Scholar] [CrossRef]
- Sogawa, S.; Sugisaki, H.; Tadokoro, K.; Ono, T.; Sato, E.; Shimode, S.; Kikuchi, T. Feeding Habits of Six Species of Euphausiids (Decapoda: Euphausiacea) in the Northwestern Pacific Ocean Determined by Carbon and Nitrogen Stable Isotope Ratios. J. Crust. Biol. 2017, 37, 29–36. [Google Scholar] [CrossRef]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montaña, C.G. Getting to the Fat of the Matter: Models, Methods and Assumptions for Dealing with Lipids in Stable Isotope Analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.M.; Martin, A.A.; Moore, B.C. Is Lipid Correction Necessary in the Stable Isotope Analysis of Fish Tissues? Rapid Commun. Mass Spectrom. 2016, 30, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Stock, B.C.; Jackson, A.L.; Ward, E.J.; Parnell, A.C.; Phillips, D.L.; Semmens, B.X. Analyzing Mixing Systems Using a New Generation of Bayesian Tracer Mixing Models. PeerJ 2018, 6, e5096. [Google Scholar] [CrossRef]
- Bond, A.L.; Diamond, A.W. Recent Bayesian Stable-Isotope Mixing Models Are Highly Sensitive to Variation in Discrimination Factors. Ecol. Appl. 2011, 21, 1017–1023. [Google Scholar] [CrossRef] [Green Version]
- Caut, S.; Angulo, E.; Courchamp, F. Variation in Discrimination Factors (Δ 15 N and Δ 13 C): The Effect of Diet Isotopic Values and Applications for Diet Reconstruction. J. Appl. Ecol. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Madigan, D.J.; Snodgrass, O.E.; Hyde, J.R.; Dewar, H. Stable Isotope Turnover Rates and Fractionation in Captive California Yellowtail (Seriola Dorsalis): Insights for Application to Field Studies. Sci. Rep. 2021, 11, 4466. [Google Scholar] [CrossRef]
- Batts, B.S. Food Habits of the Skipjack Tuna, Katsuwonus Pelamis, in North Carolina Waters. Chesap. Sci. 1972, 13, 193. [Google Scholar] [CrossRef]
- Baker, R.; Buckland, A.; Sheaves, M. Fish Gut Content Analysis: Robust Measures of Diet Composition. Fish Fish. 2014, 15, 170–177. [Google Scholar] [CrossRef]
- Magnuson, J.J. Digestion and Food Consumption by Skipjack Tuna (Katsuwonus Pelamis). Trans. Am. Fish. Soc. 1969, 98, 379–392. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, W.X. Large-Scale Spatial and Interspecies Differences in Trace Elements and Stable Isotopes in Marine Wild Fish from Chinese Waters. J. Hazard. Mater. 2012, 215–216, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.A.; Rice, A.N.; Lutcavage, M.E.; Skomal, G.B. Predicting Trophic Position in Sharks of the North-West Atlantic Ocean Using Stable Isotope Analysis. J. Mar. Biol. Ass. 2003, 83, 1347–1350. [Google Scholar] [CrossRef]
- Johnson, S.P.; Schindler, D.E. Trophic Ecology of Pacific Salmon (Oncorhynchus spp.) in the Ocean: A Synthesis of Stable Isotope Research. Ecol. Res. 2009, 24, 855–863. [Google Scholar] [CrossRef]
- Revill, A.T.; Young, J.W.; Lansdell, M. Stable Isotopic Evidence for Trophic Groupings and Bio-Regionalization of Predators and Their Prey in Oceanic Waters off Eastern Australia. Mar. Biol. 2009, 156, 1241–1253. [Google Scholar] [CrossRef]
- Hansen, J.; Hedeholm, R.; Sünksen, K.; Christensen, J.; Grønkjær, P. Spatial Variability of Carbon (δ13C) and Nitrogen (δ15N) Stable Isotope Ratios in an Arctic Marine Food Web. Mar. Ecol. Prog. Ser. 2012, 467, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Matić-Skoko, S.; Vrdoljak, D.; Uvanović, H.; Peharda, M.; Grønkjær, P. Spatial Variations in Feeding Ecology of Three Sparidae Species—A Stable Isotope Analysis. In Proceedings of the XVI European Congress of Ichthyology, Lausanne, Switzerland, 2–6 September 2019. [Google Scholar] [CrossRef]
- Hinz, H.; Moranta, J.; Balestrini, S.; Sciberras, M.; Pantin, J.R.; Monnington, J.; Zalewski, A.; Kaiser, M.J.; Sköld, M.; Jonsson, P.; et al. Stable Isotopes Reveal the Effect of Trawl Fisheries on the Diet of Commercially Exploited Species. Sci. Rep. 2017, 7, 6334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkley, R. The Kuroshio current. Sci. J. 1970, 6, 54–60. [Google Scholar]
- Kitchell, J.F.; Essington, T.E.; Boggs, C.H.; Schindler, D.E.; Walters, C.J. The Role of Sharks and Longline Fisheries in a Pelagic Ecosystem of the Central Pacific. Ecosystems 2002, 5, 202–216. [Google Scholar] [CrossRef]
- Allain, V.; Nicol, S.; Essington, T.; Okey, T.; Olson, R.; Kirby, D. An Ecopath with Ecosim model of the Western and Central Pacific Ocean warm pool pelagic ecosystem. In Proceedings of the Western and Central Pacific Fisheries Commission Scientific Committee Third Regular Session, Honolulu, HI, USA, 13–24 April 2007. [Google Scholar]
- Madigan, D.J.; Carlisle, A.B.; Dewar, H.; Snodgrass, O.E.; Litvin, S.Y.; Micheli, F.; Block, B.A. Stable Isotope Analysis Challenges Wasp-Waist Food Web Assumptions in an Upwelling Pelagic Ecosystem. Sci. Rep. 2012, 2, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukoschek, V.; McCormick, M. Ontogeny of Diet Changes in a Tropical Benthic Carnivorous Fish, Parupeneus Barberinus (Mullidae): Relationship between Foraging Behaviour, Habitat Use, Jaw Size, and Prey Selection. Mar. Biol. 2001, 138, 1099–1113. [Google Scholar] [CrossRef]
- Grossman, G.D. Ecological Aspects of Ontogenetic Shifts in Prey Size Utilization in the Bay Goby (Pisces: Gobiidae). Oecologia 1980, 47, 233–238. [Google Scholar] [CrossRef]
- Young, J.W.; Lansdell, M.J.; Campbell, R.A.; Cooper, S.P.; Juanes, F.; Guest, M.A. Feeding Ecology and Niche Segregation in Oceanic Top Predators off Eastern Australia. Mar. Biol. 2010, 157, 2347–2368. [Google Scholar] [CrossRef]
- Lin, H.J.; Kao, W.Y.; Wang, Y.T. Analyses of Stomach Contents and Stable Isotopes Reveal Food Sources of Estuarine Detritivorous Fish in Tropical/Subtropical Taiwan. Estuar. Coast. Shelf Sci. 2007, 73, 527–537. [Google Scholar] [CrossRef]

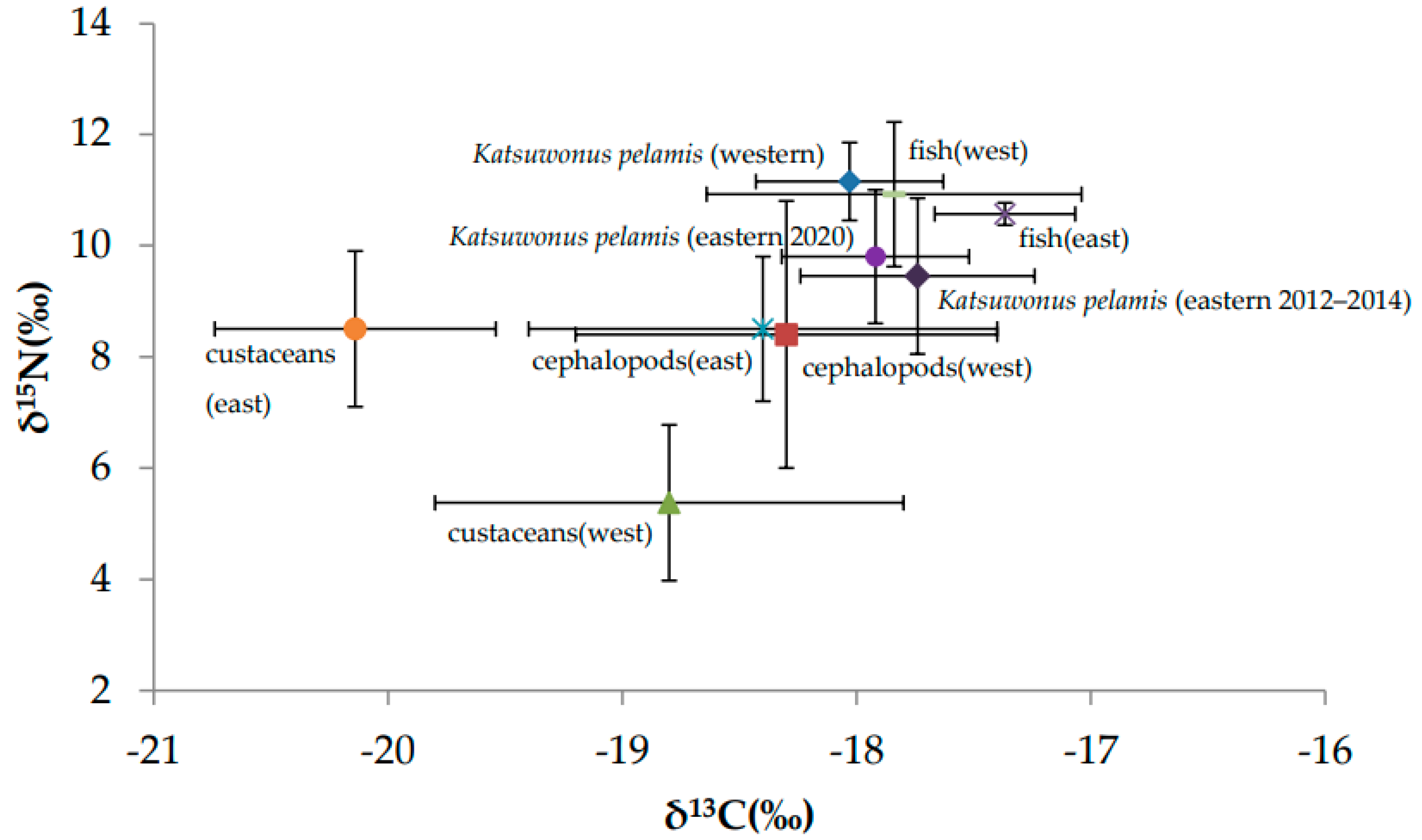
| Prey Item | n | Mean δ15N | Mean δ13C | Reference |
|---|---|---|---|---|
| Fish (west) | 48 | 10.9 (±1.3) | −17.8 (±0.8) | This study, [39] |
| Cephalopods (west) | 4 | 8.4 (±2.4) | −18.3 (±0.5) | [39] |
| Crustaceans (west) | 17 | 5.4 (±1.4) | −18.8 (±1.0) | [39] |
| Fish (east) | 14 | 10.6 (±0.2) | −17.4 (±0.3) | [8,39] |
| Cephalopods (east) | 11 | 8.5 (±1.3) | −18.4 (±0.9) | [8] |
| Crustaceans (east) | 198 | 8.5 (±1.4) | −20.1 (±0.6) | [40] |
| Prey Item | FO% | N% | W% | IRI |
|---|---|---|---|---|
| Teleostei | ||||
| Clupeidae | ||||
| Spratelloides gracilis | 4.7 | 29.9 | 29.8 | 277.6 |
| Myctophidae | ||||
| Benthosema pterotum | 2.3 | 34.6 | 7.0 | 96.8 |
| Leiognathidae | ||||
| Leiognathus bindus | 39.5 | 29.9 | 53.1 | 3282.9 |
| Leiognathus rivulatus | 2.3 | 1.9 | 2.0 | 8.9 |
| Caesionidae | ||||
| Pterocaesio digramma | 4.7 | 2.8 | 5.8 | 39.9 |
| Pomacentridae | ||||
| Chromis fumea | 2.3 | 0.9 | 2.3 | 7.6 |
| Prey Item | FO% | N% | W% | IRI |
|---|---|---|---|---|
| Crustaceans | ||||
| Sergestidae spp. | 2.5 | 1.2 | 0.1 | 3.4 |
| Euphausiidae spp. | 8.9 | 14.3 | 0.6 | 132.2 |
| Cymothoidae spp. | 8.2 | 1.7 | 0.4 | 16.9 |
| Cephalopoda | ||||
| Myopsida spp. | 25.3 | 12.5 | 37.5 | 1265.9 |
| Zooplankton | ||||
| Stomatopoda | 13.9 | 5.6 | 0.7 | 86.9 |
| Amphipoda | 1.3 | 2.2 | 0.1 | 2.8 |
| Megalopa | 5.1 | 1.9 | 0.1 | 10.0 |
| Teleostei | ||||
| Exocoetidae | ||||
| Exocoetidae spp. | 10.8 | 3.0 | 12.7 | 169.1 |
| Carangidae | ||||
| Decapterus macrosoma | 16.5 | 5.3 | 19.8 | 412.3 |
| Selar crumenophthalmus | 3.2 | 0.8 | 2.9 | 11.9 |
| Carangoides spp. | 1.9 | 0.5 | 0.2 | 1.4 |
| Decapterus kurroides | 1.9 | 0.6 | 2.9 | 6.7 |
| Chaetodontidae | ||||
| Chaetodontidae spp. | 1.3 | 0.3 | 0.1 | 0.5 |
| Ammodytidae | ||||
| Ammodytidae spp. | 10.1 | 26.5 | 7.8 | 347.7 |
| Trichiuridae | ||||
| Trichiurus lepturus | 5.7 | 1.2 | 0.9 | 12.2 |
| Acanthuridae | ||||
| Acanthuridae spp. | 1.3 | 0.2 | 0.1 | 0.4 |
| Balistoidei | ||||
| Ostraciidae spp. | 0.6 | 0.1 | 0.01 | 0.1 |
| Arothron stellatus | 0.6 | 0.5 | 0.1 | 0.4 |
| Monacanthidae spp. | 10.8 | 0.5 | 0.3 | 9.0 |
| Balistidae spp. | 3.2 | 1.1 | 2.6 | 11.8 |
| Dactylopteridae | ||||
| Dactylopteridae spp. | 1.3 | 0.4 | 0.1 | 0.7 |
| Pterois | ||||
| Pterois spp. | 9.5 | 0.2 | 0.03 | 2.3 |
| Monacanthidae | ||||
| Thamnaconus spp. | 0.6 | 0.1 | 0.1 | 0.1 |
| Engraulidae | ||||
| Engraulidae spp. | 5.7 | 19.2 | 9.8 | 165.1 |
| Prey Item | FO% | N% | W% | IRI |
|---|---|---|---|---|
| Crustaceans | ||||
| Squillidae | 6.7 | 14.2 | 1.8 | 111.1 |
| Sergestidae spp. | 1.8 | 1.5 | 0.1 | 2.9 |
| Cephalopoda | ||||
| Myopsida spp. | 17.6 | 10.2 | 23.1 | 442.7 |
| Teleostei | ||||
| Clupeidae | 2.4 | 13.7 | 0.6 | 34.5 |
| Exocoetidae | ||||
| Cheilopogon spp. | 16.4 | 14.9 | 26.3 | 609.0 |
| Polymixiidae | 1.2 | 0.6 | 0.04 | 0.3 |
| Fistulariidae | ||||
| Fistularia commersonii | 0.6 | 0.2 | 0.01 | 0.03 |
| Carangidae | ||||
| Decapterus spp. | 31.5 | 23.1 | 42.1 | 2054.3 |
| Bramidae | 4.2 | 1.5 | 0.1 | 2.9 |
| Emmelichthyidae | 0.6 | 0.2 | 0.02 | 0.1 |
| Chaetodontidae | ||||
| Chaetodon spp. | 0.6 | 0.2 | 0.2 | 0.2 |
| Ammodytidae | ||||
| Ariomma spp. | 1.8 | 9.5 | 1.1 | 19.2 |
| Trichiuridae | 6.1 | 4.4 | 1.8 | 37.5 |
| Scombridae | 6.1 | 2.3 | 15.3 | 106.7 |
| Balistidae | 1.8 | 1.1 | 0.1 | 2.0 |
| Tetraodontidae | ||||
| Lagocephalus spp. | 0.6 | 0.2 | 0.03 | 0.1 |
| Location/Years | n | Fork Length (FL, cm) | δ15N Value (Mean ± SD) | δ13C Value (Mean ± SD) | Trophic Position |
|---|---|---|---|---|---|
| Western/2020 | 43 | 45.0 ± 4.2 | 11.2 (±0.7) | −18.0 (±0.4) | 4.5 |
| Eastern/2012–2014 | 149 | 49.7 ± 8.7 | 9.5 (±1.4) | −17.7 (±0.5) | 3.8 |
| Eastern/2020 | 167 | 54.1 ± 9.2 | 9.8 (±1.2) | −17.9 (±0.4) | 3.9 |
| Class I (<39.9 cm, n = 4) | Class II (40–49.9 cm, n = 38) | Class III (>50 cm, n = 1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Prey Sample | Mean | 5% | 95% | Mean | 5% | 95% | Mean | 5% | 95% |
| Fish | 13 | 0 | 58 | 18 | 0 | 75 | 11 | 0 | 73 |
| Cephalopods | 63 | 1 | 89 | 75 | 0 | 100 | 84 | 6 | 100 |
| Crustaceans | 23 | 11 | 44 | 7 | 0 | 29 | 5 | 0 | 24 |
| Class I (<49.9 cm, n = 72) | Class II (50–59.9 cm, n = 41) | Class III (>60 cm, n = 54) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Prey Sample | Mean | 5% | 95% | Mean | 5% | 95% | Mean | 5% | 95% |
| Fish | 1 | 0 | 3 | 4 | 0 | 12 | 5 | 0 | 15 |
| Cephalopods | 47 | 8 | 100 | 54 | 18 | 99 | 38 | 0 | 84 |
| Crustaceans | 52 | 0 | 91 | 42 | 0 | 77 | 57 | 13 | 85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Chiang, W.-C.; Madigan, D.J.; Tsai, F.-Y.; Chiang, C.-L.; Hsu, H.-H.; Lin, S.-M.; Zhuang, M.-Y.; Sun, C.-T.; Chen, L.-C.; et al. Trophic Dynamics and Feeding Ecology of Skipjack Tuna (Katsuwonus pelamis) off Eastern and Western Taiwan. Molecules 2022, 27, 1073. https://doi.org/10.3390/molecules27031073
Chang Y-C, Chiang W-C, Madigan DJ, Tsai F-Y, Chiang C-L, Hsu H-H, Lin S-M, Zhuang M-Y, Sun C-T, Chen L-C, et al. Trophic Dynamics and Feeding Ecology of Skipjack Tuna (Katsuwonus pelamis) off Eastern and Western Taiwan. Molecules. 2022; 27(3):1073. https://doi.org/10.3390/molecules27031073
Chicago/Turabian StyleChang, Yun-Chen, Wei-Chuan Chiang, Daniel J. Madigan, Fu-Yuan Tsai, Ching-Lung Chiang, Hung-Hung Hsu, Shiow-Mei Lin, Mei-Ying Zhuang, Ching-Ting Sun, Lu-Chi Chen, and et al. 2022. "Trophic Dynamics and Feeding Ecology of Skipjack Tuna (Katsuwonus pelamis) off Eastern and Western Taiwan" Molecules 27, no. 3: 1073. https://doi.org/10.3390/molecules27031073
APA StyleChang, Y. -C., Chiang, W. -C., Madigan, D. J., Tsai, F. -Y., Chiang, C. -L., Hsu, H. -H., Lin, S. -M., Zhuang, M. -Y., Sun, C. -T., Chen, L. -C., & Wang, S. -P. (2022). Trophic Dynamics and Feeding Ecology of Skipjack Tuna (Katsuwonus pelamis) off Eastern and Western Taiwan. Molecules, 27(3), 1073. https://doi.org/10.3390/molecules27031073






