Cell Culture-Based Assessment of Toxicity and Therapeutics of Phytochemical Antioxidants
Abstract
1. Introduction
2. Medicinal Plants in Cancer Treatment and Management
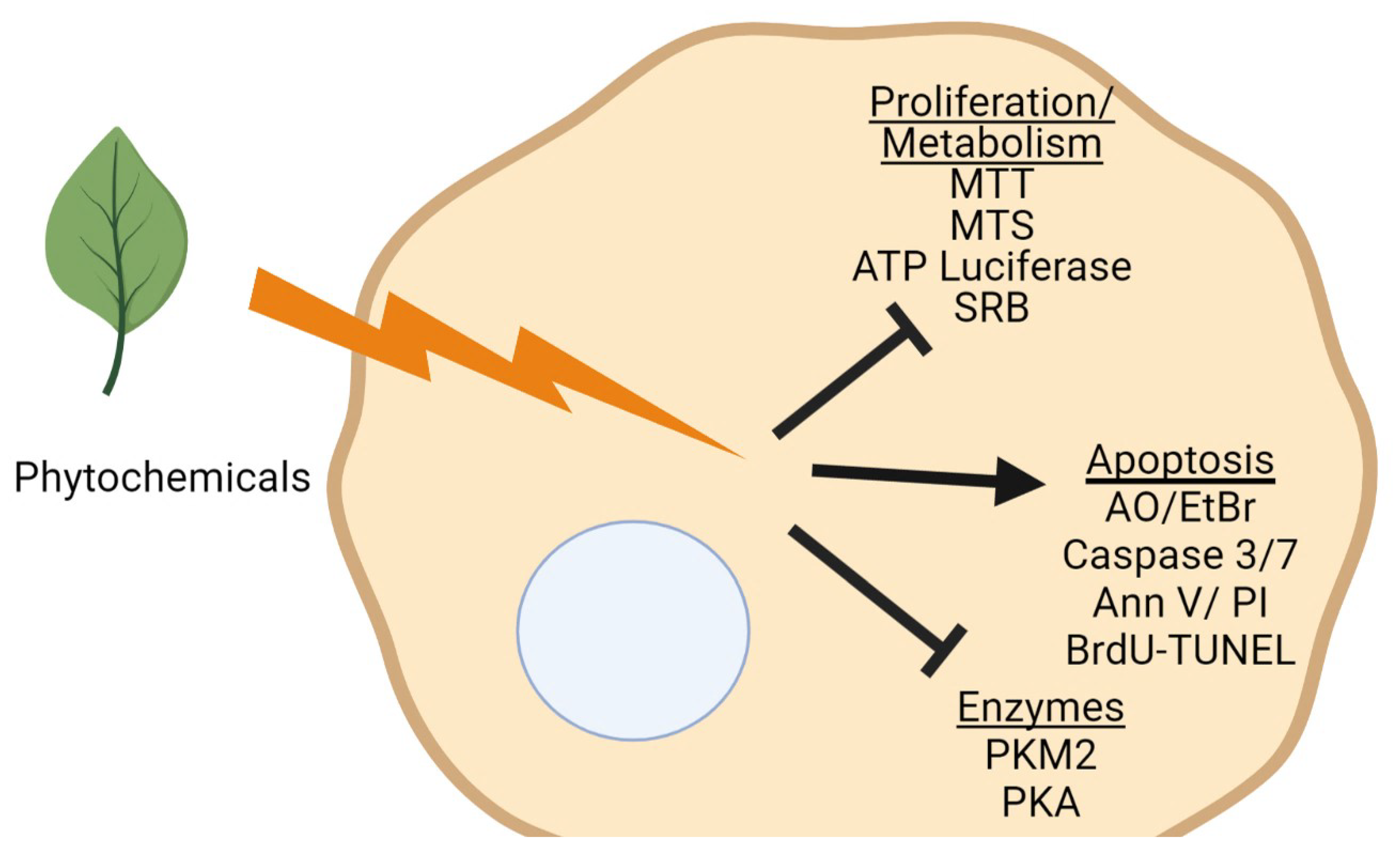
3. Determining Anti-Cancer Potential of Phytochemicals
3.1. Cancer Cell Lines
| Cell Line | Culture Medium | Supplementation | Medicinal Plant | Reference |
|---|---|---|---|---|
| Androgen-dependent growing human prostate cancer cell line, LNCaP (lymph node prostate cancer) | Roswell Park Memorial Institute (RPMI) 1640 medium | 10% fetal calf serum (FCS), penicillin (100 U/mL), streptomycin (100 U/mL), and 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) | Prunus africana | [51] |
| Human hepatoma Hep3B cells stablyexpressing green fluorescent protein (GFP)-AR or yellow fluorescent protein (YFP)-AR-cyan fluorescent protein (CFP) | Minimum Essential Medium Eagle, alpha modification (ɑ-MEM) | 5% FCS, 2 mM L-glutamine, penicillin (100 U/mL), streptomycin (100 U/mL), and Genticin (G418) | Prunus africana | [51] |
| Monkey kidney CV1 cells | Dulbecco’s modified Eagle’s medium (DMEM) | 10% (v/v) FCS, penicillin (100 IU/mL) and streptomycin (100 IU/mL) | Prunus africana | [37] |
| Mouse mammary breast cancer cell line, mouse colon cancer cell line and Vero cells (monkey kidney cells) | Earl’s Minimum Essential Media (EMEM) | Penicillin, streptomycin and 10% fetal bovine serum (FBS) | Prunus africana | [52] |
| Human embryonic kidney cells, HEK293 | EMEM | Glutamine, 10% FBS and antibiotics (100 μg/mL penicillin, 100 μg/mL streptomycin) | Moringa oleifera, Prunus africana | [53] |
| Colorectal adenocarcinoma cell line, Caco-2 | EMEM | Glutamine, 10% FBS and antibiotics | Moringa oleifera, Prunus africana | [53] |
| Hepatocellular carcinoma cell line, HepG2 | EMEM | Glutamine, 10% FBS and antibiotics | Moringa oleifera, Prunus africana | [53] |
| HepG-2, Caco-2 and the non-cancer cell line HEK293 | EMEM + glutamine | 10% FBS, 100 μg/mL penicillin and 100 μg/mL streptomycin | Prunus africana | [54] |
| Human prostate carcinoma LNCaP cells | RPMI-1640 medium | 10% (v/v) FCS, 1% (v/v) penicillin and streptomycin, 1% (v/v) L-glutamin and 1% (v/v) sodium pyruvate | Prunus africana | [55] |
| Human prostate carcinoma cell lines PC3, PC3-ARwt | DMEM | 10% (v/v) FCS, 1% (v/v) penicillin and streptomycin, 1% (v/v) L-glutamin (and 600 μg/mL geneticin for PC3- ARwt) | Prunus africana | [55] |
| Human prostate cancer C4-2 cells | DMEM | 10% (v/v) FCS, 20% F12, 5 μg/mL Insulin, 13.6 pg/mL T3 (3,3′,5-triiodo-L-thyronine sodium salt), 5 μg/mL apotransferrin, 0.25 μg/mL Biotin, 1% (v/v) penicillin and streptomycin | Prunus africana | [55] |
| Human prostatic myofibroblasts and fibroblasts (HPMF) | Endothelial basal medium MCDB 131 | 1 × L-glutamine, 5% FCS, 1 × MEM vitamins solution, 1× insulin-tranferrin-selenium liquid media supplement, and 1% (v/v) antimycotic/ antibiotic solution | Prunus africana | [56] |
| Madin-Darby canine kidney epithelial cell line (MDCK cells) | DMEM | 1 × L-glutamine, 5% FCS and 1% (v/v) antibiotic solution | Prunus africana | [56] |
| Vero E6, CT 26-CL 25 colon cancer cells and Hep2 throat cancer cells | MEM medium | 10% FBS, 1% L-glutamine and 1% antibiotic solution | Prunus africana | [57] |
| Human ileoceacal adenocarcinoma, HCT-8 cell line | RPMI-1640 | 10% heat inactivated FBS, 2 mM L-glutamine, 50 μg/mL of penicillin-G, and 50 μg/mL of streptomycin sulfate | Moringa oleifera | [18] |
| Human breast cancer, MDA-MB-231 cell line | DMEM | 10% heat inactivated FBS, 2 mM L-glutamine, 50 μg/mL of penicillin-G, and 50 μg/mL of streptomycin sulfate | Moringa oleifera | [18] |
| Human B-lymphoblastoid cells, Raji | RPMI-1640 | 10% fetal calf serum (FCS) containing n-butyric acid (3 mM) and teleocidin B-4 (50 nM) | Moringa oleifera | [58] |
3.2. Recent Advances in Cell Culture Models for Testing Anticancer Drugs
3.3. Real-Time Assessments of Cell Culture Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Vander Hoorn, S.; Lopez, A.D.; Murray, C.J.; Ezzati, M. Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005, 366, 1784–1793. [Google Scholar] [CrossRef]
- Herbst, R.S.; Bajorin, D.F.; Bleiberg, H.; Blum, D.; Hao, D.; Johnson, B.E.; Ozols, R.F.; Demetri, G.D.; Ganz, P.A.; Kris, M.G.; et al. Clinical Cancer Advances 2005: Major research advances in cancer treatment, prevention, and screening—A report from the American Society of Clinical Oncology. J. Clin. Oncol. 2006, 24, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the Evolution of Cancer Treatment Therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Bono, A. Phytochemical antioxidants for health and medicine—A move towards nature. Biotechnol. Mol. Biol. Rev. 2007, 1, 97–104. [Google Scholar]
- Kumari, P.; Kumari, C.; Singh, P.S. Phytochemical screening of selected medicinal plants for secondary metabolites. Int. J. Life-Sci. Sci. Res. 2017, 3, 1151–1157. [Google Scholar] [CrossRef]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern extraction methods for preparation of bioactive plant extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Ncube, A.; Afolayan, A.; Okoh, A. Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. Afr. J. Biotechnol. 2008, 7, 1797–1806. [Google Scholar] [CrossRef]
- Albuquerque Costa, R.; de Sousa, O.V.; Hofer, E.; Mafezoli, J.; Barbosa, F.G.; dos Fernandes Vieira, R.H.S. Thiocarbamates from Moringa oleifera seeds bioactive against virulent and multidrug-resistant Vibrio species. BioMed Res. Int. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Zamboni, M.; Lorenzoni, E.; Montanaro, L.; Sperti, S.; Stirpe, F. Inhibition of protein synthesis in vitro by proteins from the seeds of Momordica charantia (bitter pear melon). Biochem. J. 2015, 186, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Bijina, B.; Chellappan, S.; Basheer, S.M.; Elyas, K.K.; Bahkali, A.H.; Chandrasekaran, M. Protease inhibitor from Moringa oleifera leaves: Isolation, purification, and characterization. Process. Biochem. 2011, 46, 2291–2300. [Google Scholar] [CrossRef]
- Igwe, K.; Madubuike, A.; Ikenga, C.; Otuokere, I.; Amaku, F. Studies of the medicinal plant Pausinystalia yohimbe ethanol leaf extract phytocomponents by GCMS analysis. Int. J. Sci. Res. Manag. 2016, 4, 4116–4122. [Google Scholar] [CrossRef]
- Komakech, R.; Kang, Y.; Lee, J.; Omujal, F. A review of the potential of phytochemicals from Prunus africana (Hook f.) Kalkman stem bark for chemoprevention and chemotherapy of prostate cancer. Evid.-Based Complement. Altern. Med. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Shenouda, N.; Sakla, M.; Newton, L.; Besch-Williford, C.; Greenberg, N.; MacDonald, R.; Lubahn, D. Phytosterol Pygeum africanum regulates prostate cancer in vitro and in vivo. Endocrine 2007, 31, 72–81. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Albalawi, S.M.; Athar, M.T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines. PLoS ONE 2015, 10, e0135814. [Google Scholar] [CrossRef] [PubMed]
- Eweka, A.O.; Om’Iniabohs, F.A.E.; Momodu, O. The histological effects of mixed diet containing Pausinystalia yohimbe ground stem bark on the kidney of adult Wistar rats (Rattus norvegicus). Biol. Med. 2010, 2, 30–36. [Google Scholar]
- Lo, H.-Y.; Ho, T.-Y.; Lin, C.; Li, C.-C.; Hsiang, C.-Y. Momordica charantia and Its Novel Polypeptide Regulate Glucose Homeostasis in Mice via Binding to Insulin Receptor. J. Agric. Food Chem. 2013, 61, 2461–2468. [Google Scholar] [CrossRef]
- Karan, M.; Kumar Jena, A.; Sharma, N.; Vasisht, K.; Efferth, T. A systematic analysis of Prunus species with a focus on management plan of Prunus africana (Hook.f.) Kalkman: An autochthon plant of Africa. Eur. J. Med. Plants 2017, 20, 1–24. [Google Scholar] [CrossRef]
- Wavinya Nyamai, D.; Musyoki, M.A.; Wambua, F.; Matheri, F. Phytochemical Profile of Prunus africana Stem Bark from Kenya. J. Pharmacogn. Nat. Prod. 2015, 1, 8. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013, 1, 168–182. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef]
- Lee, J.H.; Khor, T.O.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Kong, A.-N.T. Dietary Phytochemicals and Cancer Prevention: Nrf2 signaling, Epigenetics, and Cell DeathMechanisms in Blocking Cancer Initiation and Progression. Pharmacol. Ther. 2013, 137, 153–171. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Niciforovic, N.; Topuzovic, M.; Solujic, S. Total phenolic content, flavonoid concentrations and antioxidant activity, of the whole plant and plant parts extracts from Teucrium montanum L. var. montanum, f. supinum (L.) reichenb. Biotechnol. Biotechnol. Equip. 2011, 25, 2222–2227. [Google Scholar] [CrossRef]
- Mitreski, I.; Petreska Stanoeva, J.; Stefova, M.; Stefkov, G.; Kulevanova, S. Polyphenols in Representative Teucrium Species in the Flora of R. Macedonia: LC/DAD/ESI-MS n Profile and Content. Nat. Prod. Comm. 2014, 9. [Google Scholar] [CrossRef]
- Asuzu, P.; Aryee, A.; Trompeter, N.; Mann, Y.; Besong, S.; Duncan, R.; Cooper, C. In Vitro Assessment of Efficacy and Cytotoxicity of Prunus africana Extracts on Prostate Cancer C4-2 Cells. bioRxiv 2021. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25 (Suppl. S2), 41–59. [Google Scholar] [CrossRef]
- Singh, M.; Patra, S.; Singh, R.K. Common techniques and methods for screening of natural products for developing of anticancer drugs. In Evolutionary Diversity as a Source for Anticancer Molecules; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Das, S. Antibacterial activity of Prunus africana stem bark extract against Shigella spp. World J. Pharm. Pharm. Sci. 2017, 6, 1155–1160. [Google Scholar] [CrossRef][Green Version]
- Jeruto, P.; Mutai, C.; Catherine, L.; Ouma, G. Phytochemical constituents of some medicinal plants. J. Anim. Plant Sci. 2011, 9, 1201–1210. [Google Scholar]
- Madivoli, E.S.; Maina, E.G.; Kairigo, P.K.; Murigi, M.K.; Ogilo, J.K.; Nyangau, J.O.; Kimani, P.K.; Kipyegon, C.; Madivoli, E. In vitro antioxidant and antimicrobial activity of Prunus africana (Hook. f.) Kalkman (bark extracts) and Harrisonia abyssinica Oliv. extracts (bark extracts): A comparative study. J. Med. Plants Econ. Dev. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Ngule, M.C.; Ndiku, M.H.; Ramesh, F. Chemical constituents screening and in vitro antibacterial assessment of Prunus africana bark hydromethanolic extract. J. Nat. Sci. Res. 2014, 4, 85–90. [Google Scholar]
- Kadu, C.; Parich, A.; Schueler, S.; Konrad, H.; Muluvi, G.M.; Eyog-Matig, O.; Muchugi, A.; Williams, V.L.; Ramamonjisoa, L.; Kapinga, C.; et al. Bioactive constituents in Prunus africana: Geographical variation throughout Africa and associations with environmental and genetic parameters. Phytochemistry 2012, 83, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Schleich, S.; Papaioannou, M.; Baniahmad, A.; Matusch, R. Activity-guided isolation of an antiandrogenic compound of Pygeum africanum. Planta Med. 2006, 72, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sharma, B.; Kanwar, S.S.; Kumar, A. Lead Phytochemicals for Anticancer Drug Development. Front. Plant Sci. 2016, 7, 1667. [Google Scholar] [CrossRef]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Lee, J.-H.; Kong, A.-N.T. Plants vs. Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability. Anticancer. Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Ferreira, D.; Adega, F.; Chaves, R. The Importance of Cancer Cell Lines as in vitro Models in Cancer Methylome Analysis and Anticancer Drugs Testing. In Oncogenomics and Cancer Proteomics—Novel Approaches in Biomarkers Discovery and Therapeutic Targets in Cancer; IntechOpen: London, UK, 2013; pp. 139–166. [Google Scholar]
- Cunningham, D.; You, Z. In vitro and in vivo model systems used in prostate cancer research. J. Biol. Methods 2015, 2, e17. [Google Scholar] [CrossRef]
- Vela, I.; Chen, Y. Prostate cancer organoids: A potential new tool for testing drug sensitivity. Expert Rev. Anticancer Ther. 2015, 15, 261–263. [Google Scholar] [CrossRef]
- Goodspeed, A.; Heiser, L.M.; Gray, J.W.; Costello, J.C. Tumor-derived cell lines as molecular models of cancer pharmacogenomics. Mol. Cancer Res. 2016, 14, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, S.-J.; Langley, R.R.; Fidler, I.J. Modulation of the cancer cell transcriptome by culture media formulations and cell density. Int. J. Oncol. 2015, 46, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Arodin Selenius, L.; Wallenberg Lundgren, M.; Jawad, R.; Danielsson, O.; Björnstedt, M. The Cell Culture Medium Affects Growth, Phenotype Expression and the Response to Selenium Cytotoxicity in A549 and HepG2 Cells. Antioxidants 2019, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.J.; Kingsley, E.A. Human prostate cancer cell lines. In Prostate Cancer Methods and Protocols; Russell, P.J., Jackson, P., Kingsley, E., Eds.; Springer: Totowa, NJ, USA, 2003; Volume 81, pp. 21–40. [Google Scholar] [CrossRef]
- Chen, Q.; Watson, J.T.; Marengo, S.R.; Decker, K.S.; Coleman, I.; Nelson, P.S.; Sikes, R.A. Gene expression in the LNCaP human prostate cancer progression model: Progression associated expression in vitro corresponds to expression changes associated with prostate cancer progression in vivo. Cancer Lett. 2006, 244, 274–288. [Google Scholar] [CrossRef]
- Liu, A.Y.; Brubaker, K.D.; Goo, Y.A.; Quinn, J.E.; Kral, S.; Sorensen, C.M.; Vessella, R.L.; Belldegrun, A.S.; Hood, L.E. Lineage relationship between LNCaP and LNCaP-derived prostate cancer cell lines. Prostate 2004, 60, 98–108. [Google Scholar] [CrossRef]
- Lange, T.; Ullrich, S.; Müller, I.; Nentwich, M.F.; Stübke, K.; Feldhaus, S.; Knies, C.; Hellwinkel, O.J.C.; Vessella, R.L.; Abramjuk, C.; et al. Human Prostate Cancer in a Clinically Relevant Xenograft Mouse Model: Identification of β(1,6)-Branched Oligosaccharides as a Marker of Tumor Progression. Clin. Cancer Res. 2012, 18, 1364–1373. [Google Scholar] [CrossRef]
- Thalmann, G.N.; Sikes, R.A.; Wu, T.T.; Degeorges, A.; Chang, S.M.; Ozen, M.; Pathak, S.; Chung, L.W.K. LNCaP progression model of human prostate cancer: Androgen-independence and osseous metastasis. Prostate 2000, 44, 91–103. [Google Scholar] [CrossRef]
- Hessenkemper, W.; Roediger, J.; Bartsch, S.; Houtsmuller, A.B.; van Royen, M.E.; Petersen, I.; Grimm, M.-O.; Baniahmad, A. A natural androgen receptor antagonist induces cellular senescence in prostate cancer cells. Mol. Endocrinol. 2014, 28, 1831–1840. [Google Scholar] [CrossRef]
- Nabende, P.; Karanja, S.; Mwatha, J.; Wachira, S. Anti-proliferative activity of Prunus africana, Warburgia stuhlmannii and Maytenus senegalensis extracts in breast and colon cancer cell lines. Eur. J. Med. Plants 2015, 5, 366–376. [Google Scholar] [CrossRef]
- Chepkoech, M.F. Phytochemistry and anti-cancer potential of compounds isolated from Kenyan medicinal plants, Moringa oleifera and Prunus africana. Phytochemistry 2015, 1–199. [Google Scholar]
- Maiyo, F.; Moodley, R.; Singh, M. Phytochemistry, cytotoxicity and apoptosis studies of Β-sitosterol-3-oglucoside and Β -amyrin from prunus Africana. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, M.; Schleich, S.; Prade, I.; Degen, S.; Roell, D.; Schubert, U.; Tanner, T.; Claessens, F.; Matusch, R.; Baniahmad, A. The natural compound atraric acid is an antagonist of the human androgen receptor inhibiting cellular invasiveness and prostate cancer cell growth. J. Cell. Mol. Med. 2019, 13, 2210–2223. [Google Scholar] [CrossRef] [PubMed]
- Boulbès, D.; Soustelle, L.; Costa, P.; Haddoum, M.; Bali, J.P.; Hollande, F.; Magous, R. Pygeum africanum extract inhibits proliferation of human cultured prostatic fibroblasts and myofibroblasts. BJU Int. 2006, 98, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Yiaile, A.L. In Vitro Antiploriferative Activity, Phytochemical Composition and Toxicity Studies of Fagaropsis Angolensis and Prunus Africana Crude Extracts. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 2017. [Google Scholar]
- Murakami, A.; Kitazono, Y.; Jiwajinda, S.; Koshimizu, K.; Ohigashi, H. Niaziminin, a thiocarbamate from the leaves of Moringa oleifera, holds a strict structural requirement for inhibition of tumor-promoter-induced epstein- barr virus activation. Planta Med. 1998, 64, 319–323. [Google Scholar] [CrossRef]
- McCauley, J.; Zivanovic, A.; Skropeta, D. Bioassays for anticancer activities. Methods Mol. Biol. 2013, 1055, 191–205. [Google Scholar] [CrossRef]
- Aslantürk, Ö.S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages. In Genotoxicity—A Predictable Risk to Our Actual World; IntechOpen: London, UK, 2018. [Google Scholar]
- Chakrabarti, R.; Kundu, S.; Kumar, S.; Chakrabarti, R. Vitamin A as an enzyme that catalyzes the reduction of MTT to formazan by vitamin C. J. Cell. Biochem. 2000, 80, 133–138. [Google Scholar] [CrossRef]
- Lee, J.W.; Shin, K.D.; Kim, K.-H.; Kim, E.-J.; Han, S.-S.; Jeong, T.C.; Koh, W.S. A Three-step Method of Immunotoxicity Assessment. Toxicol. Res. 2000, 16, 317–323. [Google Scholar]
- Kumar, S.; Bajaj, S.; Bodla, R. Preclinical screening methods in cancer. Indian J. Pharmacol. 2016, 48, 481–486. [Google Scholar] [CrossRef]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Liu, K.; Liu, P.-c.; Liu, R.; Wu, X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res. 2015, 21, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mueller, H.; Kassack, M.U.; Wiese, M. Comparison of the usefulness of the MTT, ATP, and calcein assays to predict the potency of cytotoxic agents in various human cancer cell lines. J. Biomol. Screen. 2004, 9, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Moorthi, C.; Kathiresan, K.; Krishnan, K.; Manavalan, R. In-vitro Cell Based Assay: A Preferred Anticancer Drug Screening Techniques for The Academic Researchers. J. Pharm. Res. 2011, 4, 671–675. [Google Scholar]
- Salic, A.; Mitchison, T.J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 2415–2420. [Google Scholar] [CrossRef]
- Gurski, L.A.; Petrelli, N.J.; Jia, X.; Farach-Carson, M.C. 3D Matrices for Anti-Cancer Drug Testing and Development. Oncol. Issues 2017, 25, 20–25. [Google Scholar] [CrossRef]
- Lovitt, C.; Shelper, T.; Avery, V. Advanced cell culture techniques for cancer drug discovery. Biology 2014, 3, 345–367. [Google Scholar] [CrossRef]
- Kitaeva, K.V.; Rutland, C.S.; Rizvanov, A.A.; Solovyeva, V.V. Cell Culture Based in vitro Test Systems for Anticancer Drug Screening. Front. Bioeng. Biotechnol. 2020, 8, 322. [Google Scholar] [CrossRef]
- Meijer, T.G.; Naipal, K.A.; Jager, A.; Van Gent, D.C. Ex vivo tumor culture systems for functional drug testing and therapy response prediction. Future Sci. OA 2017, 3, FSO190. [Google Scholar] [CrossRef]
- Rathod, C.P.; Dhawale, S.C.; Kshirsagar, R.V. Recent Trends in Screening and Evaluation Methods of Anticancer Drugs. Indo Am. J. Pharm. Res. 2011, 111, 506–515. [Google Scholar]
- Blatt, N.L.; Mingaleeva, R.N.; Khaiboullina, S.F.; Lombardi, V.C.; Rizvanov, A.A. Application of cell and tissue culture systems for anticancer drug screening. World Appl. Sci. J. 2013, 23, 315–325. [Google Scholar] [CrossRef]
- Falasca, M.; Raimondi, C.; Maffucci, T. Boyden chamber. Methods Mol. Biol. 2011, 769, 87–95. [Google Scholar] [CrossRef]
- Wessely, A.; Waltera, A.; Reichert, T.E.; Stöckl, S.; Grässel, S.; Bauer, R.J. Induction of ALP and MMP9 activity facilitates invasive behavior in heterogeneous human BMSC and HNSCC 3D spheroids. FASEB J. 2019, 33, 11884–11893. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Sakamoto, R.; Rahman, M.M.; Shimomura, M.; Itoh, M.; Nakatsura, T. Time-lapse imaging assay using the BioStation CT: A sensitive drug-screening method for three-dimensional cell culture. Cancer Sci. 2015, 106, 757–765. [Google Scholar] [CrossRef][Green Version]
- Weaver, V.M.; Lelièvre, S.A.; Lakins, J.N.; Chrenek, M.A.; Jones, J.C.R.; Giancotti, F.G.; Werb, Z.; Bissell, M.J. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2002, 2, 205–216. [Google Scholar] [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, S.; Chen, Z.; Shin, H.K.; Lee, S.Y.; Moon, H.E.; Paek, S.H.; Park, S. 3D bioprinted vascularized tumour for drug testing. Int. J. Mol. Sci. 2020, 21, 2993. [Google Scholar] [CrossRef] [PubMed]
- Regnault, C.; Dheeman, D.S.; Hochstetter, A. Microfluidic devices for drug assays. High-Throughput 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Sameni, M.; Osuala, K.O.; Moin, K.; Mattingly, R.R.; Sloane, B.F. Spatio-temporal modeling and live-cell imaging of proteolysis in the 4D microenvironment of breast cancer. Cancer Metastasis Rev. 2019, 38, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Isherwood, B.J.; Timpson, P.; McGhee, E.J.; Anderson, K.I.; Canel, M.; Serrels, A.; Brunton, V.G.; Carragher, N.O. Live Cell in Vitro and in Vivo Imaging Applications: Accelerating Drug Discovery. Pharmaceutics 2011, 3, 141–170. [Google Scholar] [CrossRef]
- Lanigan, T.M.; Rasmussen, S.M.; Weber, D.P.; Athukorala, K.S.; Campbell, P.L.; Fox, D.A.; Ruth, J.H. Real time visualization of cancer cell death, survival and proliferation using fluorochrome-transfected cells in an IncuCyte® imaging system. J. Biol. Methods 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.; Ali, A.Y. Boswellia frereana suppresses HGF-mediated breast cancer cell invasion and migration through inhibition of c-Met signalling. J. Transl. Med. 2018, 16, 281. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asuzu, P.C.; Trompeter, N.S.; Cooper, C.R.; Besong, S.A.; Aryee, A.N.A. Cell Culture-Based Assessment of Toxicity and Therapeutics of Phytochemical Antioxidants. Molecules 2022, 27, 1087. https://doi.org/10.3390/molecules27031087
Asuzu PC, Trompeter NS, Cooper CR, Besong SA, Aryee ANA. Cell Culture-Based Assessment of Toxicity and Therapeutics of Phytochemical Antioxidants. Molecules. 2022; 27(3):1087. https://doi.org/10.3390/molecules27031087
Chicago/Turabian StyleAsuzu, Peace C., Nicholas S. Trompeter, Carlton R. Cooper, Samuel A. Besong, and Alberta N. A. Aryee. 2022. "Cell Culture-Based Assessment of Toxicity and Therapeutics of Phytochemical Antioxidants" Molecules 27, no. 3: 1087. https://doi.org/10.3390/molecules27031087
APA StyleAsuzu, P. C., Trompeter, N. S., Cooper, C. R., Besong, S. A., & Aryee, A. N. A. (2022). Cell Culture-Based Assessment of Toxicity and Therapeutics of Phytochemical Antioxidants. Molecules, 27(3), 1087. https://doi.org/10.3390/molecules27031087






