Gamma-Irradiation-Induced Degradation of the Water-Soluble Polysaccharide from Auricularia polytricha and Its Anti-Hypercholesterolemic Activity
Abstract
:1. Introduction
2. Results and Discussion
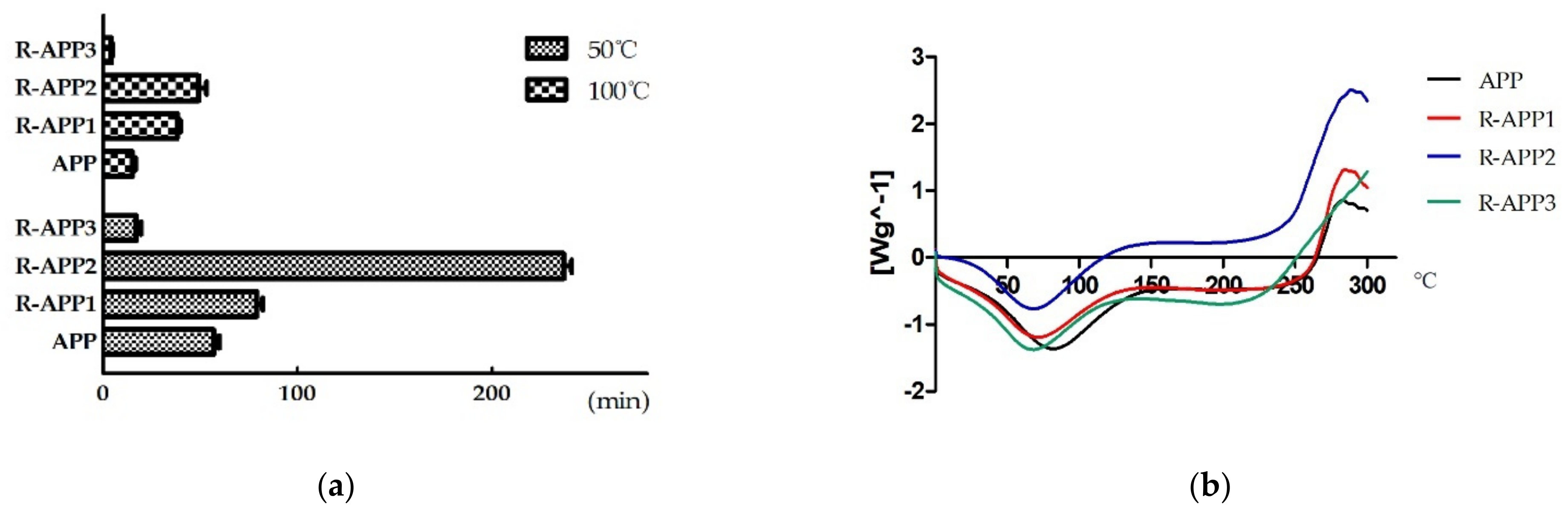
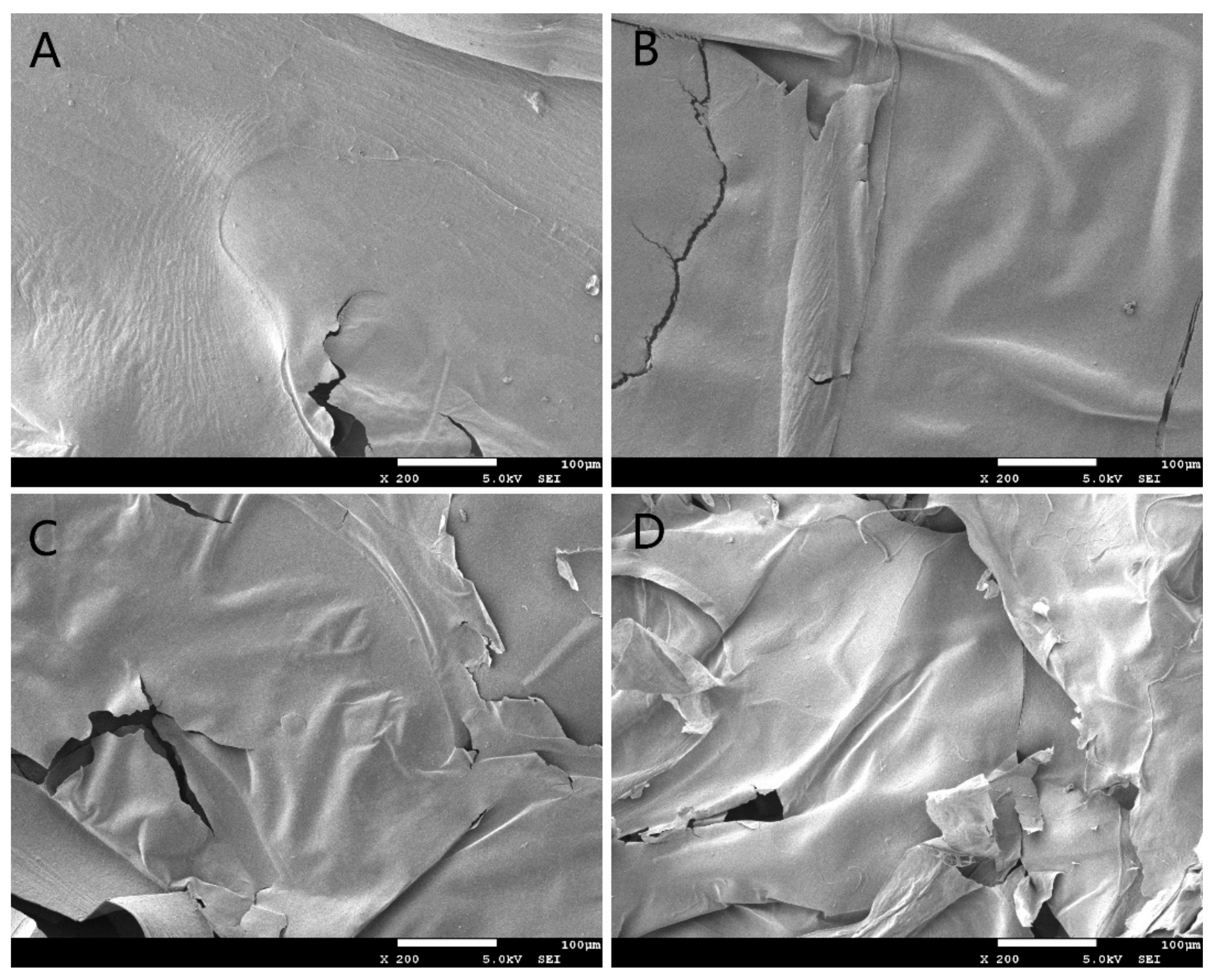
2.1. Effect of Gamma Irradiation on the Physicochemical Properties of APPs
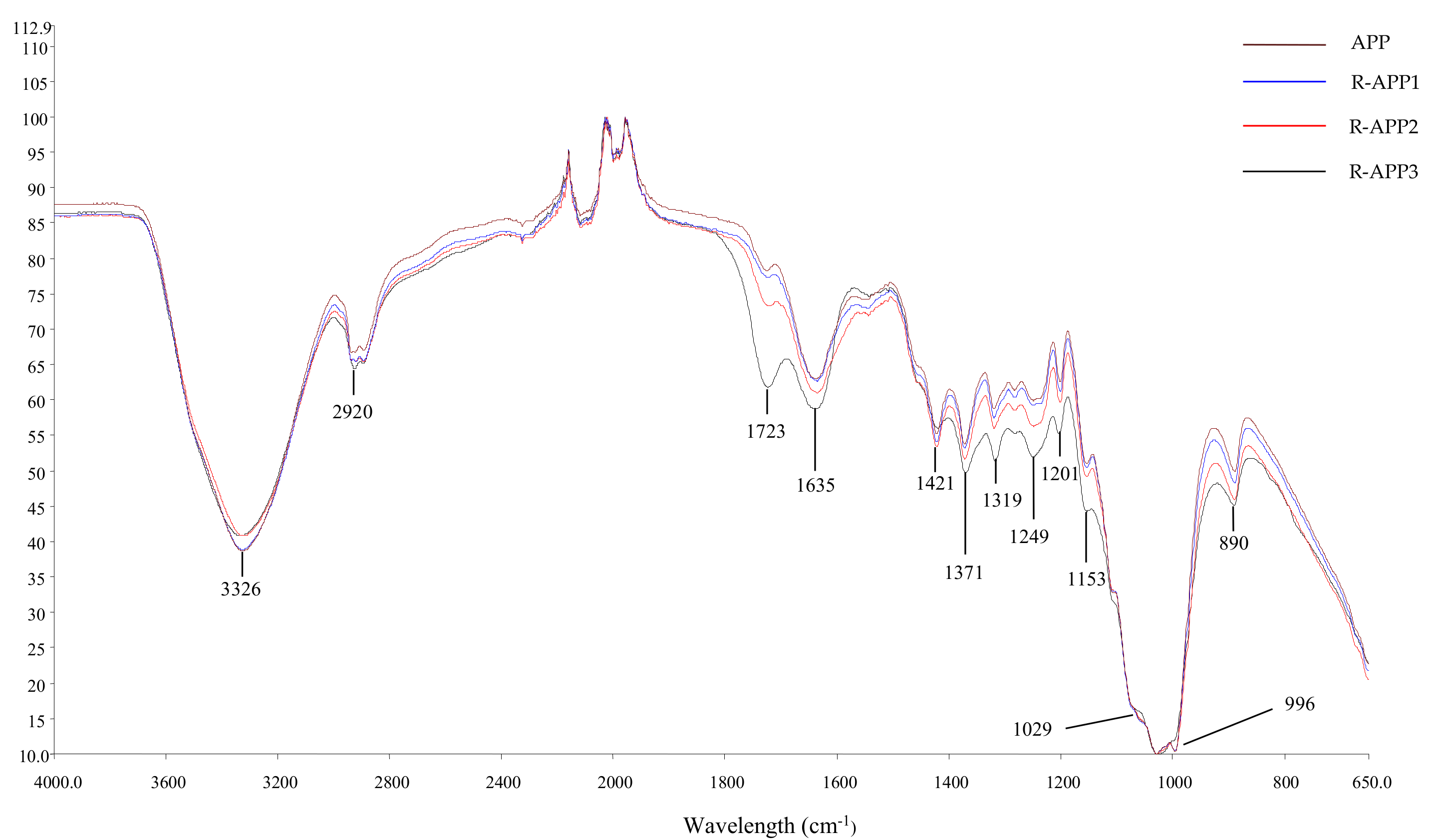
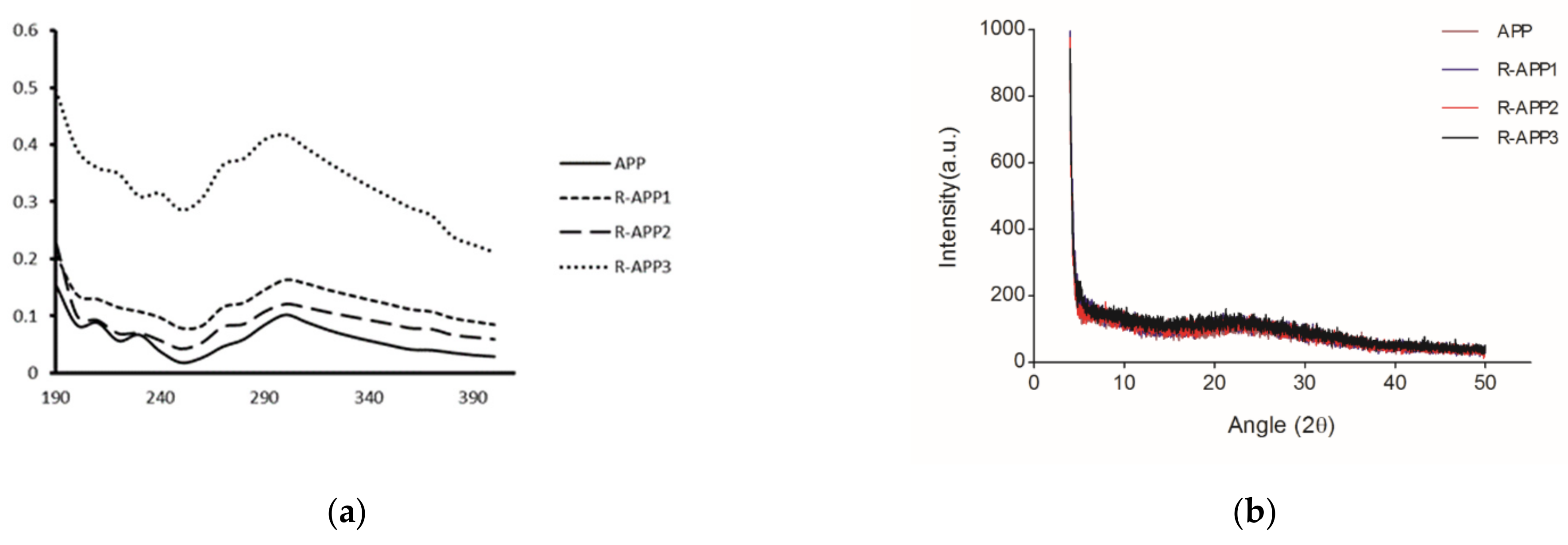
2.2. Spectroscopic Analysis of APPs
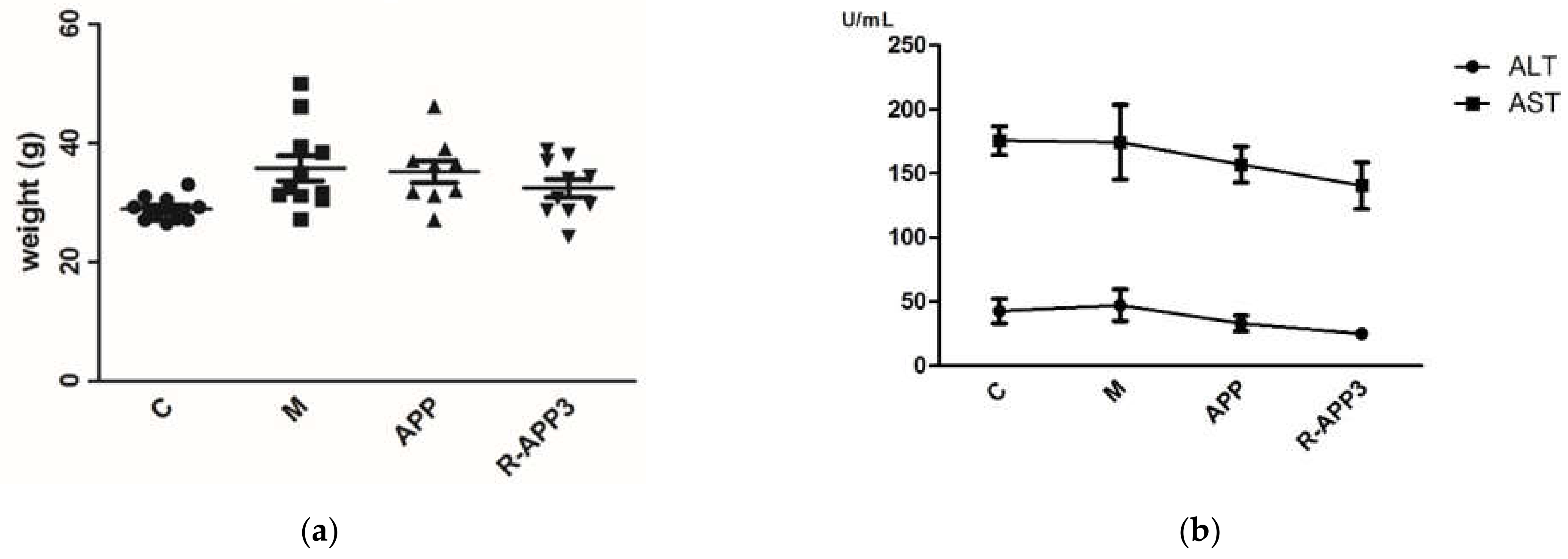
2.3. Anti-Hypercholesterolemic Activity of APPs after Gamma Irradiation
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Polysaccharide from Auricularia polytricha
3.3. Physicochemical Characteristics of Polysaccharide from Auricularia polytricha
3.3.1. Viscosity
3.3.2. Molecular Weight Determination
3.3.3. Solubility of APPs
3.3.4. Thermal Analysis
3.3.5. Scanning Electron Microscopy (SEM)
3.4. Spectroscopic Characterization
3.4.1. Fourier-Transform Infrared (FTIR) Analysis
3.4.2. X-ray Diffraction Patterns
3.4.3. UV Spectra
3.5. Anti-Hypercholesterolemic Activities of Polysaccharides from Auricularia polytricha
3.5.1. Animals and Treatment
3.5.2. Measurement of Biochemical Parameters
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Arora, S.; Tandon, C.; Tandon, S. Evaluation of the cytotoxic effects of CAM therapies: An in vitro study in normal kidney cell lines. Sci. World J. 2014, 2014, 452892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Chen, Y.; Xin, M.; Luo, Q.; Gu, J.; Zhao, M.; Xu, X.; Lu, X.; Song, G. Structure analysis and antimutagenic activity of a novel salt-soluble polysaccharide from Auricularia polytricha. J. Sci. Food Agric. 2013, 93, 3225–3230. [Google Scholar] [CrossRef]
- Yu, M.; Qing, Y.; Luo, X.; Yang, Z.; Zheng, L. Isolation of an anti-tumor polysaccharide from Auricularia polytricha (Jew’s ear) and its effects on macrophage activation. Eur. Food Res. Technol. 2009, 228, 477–485. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, Y. Purification, chemical characterization and antioxidant activities of a novel polysaccharide from Auricularia polytricha. Int. J. Biol. Macromol. 2018, 120, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Qian, L.; Zhou, Y. Hypolipidemic activity of the polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 62, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, J.; Zhou, Q.; Lu, H.; Wu, Y.; Wu, W. Polysaccharide from fuzi (FPS) prevents hypercholesterolemia in rats. Lipids Health Dis. 2010, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zhao, W.; Xie, B.; Liu, H. Effects of Auricularia auricula and its polysaccharide on diet-induced hyperlipidemia rats by modulating gut microbiota. J. Funct. Foods 2020, 72, 104038. [Google Scholar] [CrossRef]
- Zhao, S.; Rong, C.; Liu, Y.; Xu, F.; Wang, S.; Duan, C.; Chen, J.; Wu, X. Extraction of a soluble polysaccharide from Auricularia polytricha and evaluation of its anti-hypercholesterolemic effect in rats. Carbohydr. Polym. 2015, 122, 39–45. [Google Scholar] [CrossRef]
- Jiang, Y.; Qi, X.; Gao, K.; Liu, W.; Li, N.; Cheng, N.; Ding, G.; Huang, W.; Wang, Z.; Xiao, W. Relationship between molecular weight, monosaccharide composition and immunobiologic activity of Astragalus polysaccharides. Glycoconj. J. 2016, 33, 755–761. [Google Scholar] [CrossRef]
- Miyanishi, N.; Iwamoto, Y.; Watanabe, E.; Odaz, T. Induction of TNF-alpha production from human peripheral blood monocytes with beta-1,3-glucan oligomer prepared from laminarin with beta-1,3-glucanase from Bacillus clausii NM-1. J. Biosci. Bioeng. 2003, 95, 192–195. [Google Scholar] [CrossRef]
- Lee, K.H.; Cho, C.H.; Rhee, K.H. Synergic anti-tumor activity of gamma-irradiated exo-polysaccharide from submerged culture of Grifola frondosa. J. Med. Plants Res. 2011, 5, 2378–2386. [Google Scholar]
- Choi, J.-i.; Kim, H.-J.; Kim, J.-H.; Byun, M.-W.; Chun, B.S.; Ahn, D.H.; Hwang, Y.-J.; Kim, D.-J.; Kim, G.H.; Lee, J.-W. Application of gamma irradiation for the enhanced physiological properties of polysaccharides from seaweeds. Appl. Radiat. Isot. 2009, 67, 1277–1281. [Google Scholar] [CrossRef]
- Shah, A.; Masoodi, F.A.; Gani, A.; Ashwar, B.A. Effect of gamma-irradiation on antioxidant and antiproliferative properties of oat beta-glucan. Radiat. Phys. Chem. 2015, 117, 120–127. [Google Scholar] [CrossRef]
- Choi, J.I.; Kim, H.J. Preparation of low molecular weight fucoidan by gamma-irradiation and its anticancer activity. Carbohydr. Polym. 2013, 97, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Gani, A.; Shah, A.; Masoodi, F.A.; Hussain, P.R.; Wani, I.A.; Khanday, F.A. Effect of gamma-irradiation on structural, functional and antioxidant properties of beta-glucan extracted from button mushroom (Agaricus bisporus). Innov. Food Sci. Emerg. 2015, 31, 123–130. [Google Scholar] [CrossRef]
- Choi, J.I.; Kim, H.J.; Lee, J.W. Structural feature and antioxidant activity of low molecular weight laminarin degraded by gamma irradiation. Food Chem. 2011, 129, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.I.; Kim, H.J.; Kim, J.H.; Lee, J.W. Enhanced Biological Activities of Laminarin Degraded by Gamma-Ray Irradiation. J. Food Biochem. 2012, 36, 465–469. [Google Scholar] [CrossRef]
- Hussain, P.R.; Wani, I.A.; Suradkar, P.P.; Dar, M.A. Gamma irradiation induced modification of bean polysaccharides: Impact on physicochemical, morphological and antioxidant properties. Carbohydr. Polym. 2014, 110, 183–194. [Google Scholar] [CrossRef]
- Cheong, K.L.; Wang, L.Y.; Wu, D.T.; Hu, D.J.; Zhao, J.; Li, S.P. Microwave-Assisted Extraction, Chemical Structures, and Chain Conformation of Polysaccharides from a Novel Cordyceps Sinensis Fungus UM01. J. Food Sci. 2016, 81, C2167–C2174. [Google Scholar] [CrossRef]
- Relleve, L.; Abad, L. Characterization and antioxidant properties of alcoholic extracts from gamma irradiated kappa-carrageenan. Radiat. Phys. Chem. 2015, 112, 40–48. [Google Scholar] [CrossRef]
- Jin, W.P.; Xu, W.; Li, Z.S.; Li, J.; Zhou, B.; Zhang, C.L.; Li, B. Degraded konjac glucomannan by gamma-ray irradiation assisted with ethanol: Preparation and characterization. Food Hydrocolloid. 2014, 36, 85–92. [Google Scholar] [CrossRef]
- Li, Y.J.; Ha, Y.M.; Wang, F.; Li, Y.F. Effect of irradiation on the molecular weight, structure and apparent viscosity of xanthan gum in aqueous solution. Adv. Mater. Res. 2011, 239, 2632–2637. [Google Scholar] [CrossRef]
- Choi, J.I.; Lee, S.G.; Han, S.J.; Cho, M.; Lee, P.C. Effect of gamma irradiation on the structure of fucoidan. Radiat. Phys. Chem. 2014, 100, 54–58. [Google Scholar] [CrossRef]
- Charlesby, A. Crosslinking and degradation of polymers. Radiat. Phys. Chem. 1981, 18, 59–66. [Google Scholar] [CrossRef]
- Kong, L.; Yu, L.; Feng, T.; Yin, X.; Liu, T.; Dong, L. Physicochemical characterization of the polysaccharide from Bletilla striata: Effect of drying method. Carbohydr. Polym. 2015, 125, 1–8. [Google Scholar] [CrossRef]
- Akram, K.; Shahbaz, H.M.; Kim, G.R.; Farooq, U.; Kwon, J.H. Improved Extraction and Quality Characterization of Water-Soluble Polysaccharide from Gamma-Irradiated Lentinus edodes. J. Food Sci. 2017, 82, 296–303. [Google Scholar] [CrossRef]
- Alijani, S.; Balaghi, S.; Mohammadifar, M.A. Effect of gamma irradiation on rheological properties of polysaccharides exuded by A. fluccosus and A. gossypinus. Int. J. Biol. Macromol. 2011, 49, 471–479. [Google Scholar] [CrossRef]
- Emeje, M.; Isimi, C.; Byrn, S.; Fortunak, J.; Kunle, O.; Ofoefule, S. Extraction and physicochemical characterization of a new polysaccharide obtained from the fresh fruits of abelmoschus esculentus. Iran. J. Pharm. Res. 2011, 10, 237–246. [Google Scholar]
- Chan, K.W.; Ismail, M.; Mohd Esa, N.; Imam, M.U.; Ooi, J.; Khong, N.M.H. Dietary supplementation of defatted kenaf (Hibiscus cannabinus L.) seed meal and its phenolics-saponins rich extract effectively attenuates diet-induced hypercholesterolemia in rats. Food Funct. 2018, 9, 925–936. [Google Scholar] [CrossRef]
- Reyna-Villasmil, N.; Bermudez-Pirela, V.; Mengual-Moreno, E.; Arias, N.; Cano-Ponce, C.; Leal-Gonzalez, E.; Souki, A.; Inglett, G.E.; Israili, Z.H.; Hernández-Hernández, R.; et al. Oat derived beta-glucan significantly improves HDLC and diminishes LDLC and non-HDL cholesterol in overweight individuals with mild hypercholesterolemia. Am. J. Ther. 2007, 14, 203–212. [Google Scholar] [CrossRef]
- Chen, J.; Seviour, R. Medicinal importance of fungal beta-(1/3), (1/6)-glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Knodel, L.C.; Talbert, R.L. Adverse Effects of Hypolipidaemic Drugs. Med. Toxicol. Advers. Drug Exp. 1987, 2, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, D.; Song, G.; Zuo, C.; Qiao, X.; Qin, S. The effect of combination therapy of allicin and fenofibrate on high fat diet-induced vascular endothelium dysfunction and liver damage in rats. Lipids Health Dis. 2010, 9, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Chen, F.; Cheng, H.; Huang, G. Modification and application of polysaccharide from traditional Chinese medicine such as Dendrobium officinale. Int. J. Biol. Macromol. 2020, 157, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Pan, C.; Yang, X.; Chen, S.; Qi, B.; Huang, H. Degradation of Codium cylindricum polysaccharides by H2O2-Vc-ultrasonic and H2O2-Fe(2+)-ultrasonic treatment: Structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 182, 129–135. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Kim, D.; Nah, J.-W. Advances in functionalizing fucoidans and alginates (bio)polymers by structural modifications: A review. Chem Eng. J. 2019, 355, 33–48. [Google Scholar] [CrossRef]
- Du, B.; Zeng, H.; Yang, Y.; Bian, Z.; Xu, B. Anti-inflammatory activity of polysaccharide from Schizophyllum commune as affected by ultrasonication. Int. J. Biol. Macromol. 2016, 91, 100–105. [Google Scholar] [CrossRef]
- Hafsa, J.; Chaouch, M.A.; Charfeddine, B.; Rihouey, C.; Limem, K.; Le Cerf, D.; Rouatbi, S.; Majdoub, H. Effect of ultrasonic degradation of hyaluronic acid extracted from rooster comb on antioxidant and antiglycation activities. Pharm. Biol. 2017, 55, 156–163. [Google Scholar] [CrossRef]
- Silva, I.; Machado, F.; Moreno, M.J.; Nunes, C.; Coreta-Gomes, F. Polysaccharide Structures and Their Hypocholesterolemic Potential. Molecules 2021, 26, 4559. [Google Scholar] [CrossRef]
- Tungland, B.C.; Meyer, D. Nondigestible oligo-and polysaccharides (dietary fiber): Their physiology and role in human health and food. Compr. Rev. Food Sci. Food Saf. 2002, 1, 90–109. [Google Scholar] [CrossRef]
- Immerstrand, T.; Andersson, K.; Wange, C.; Rascon, A.; Christian, T.; Rickard, Ö. Effects of oat bran, processed to different molecular weights of β-glucan, on plasma lipids and caecal formation of SCFA in mice. Br. J. Nutr. 2010, 104, 364–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellegard, L.; Andersson, H. Oat bran rapidly increases bile acid excretion and bile acid synthesis: An ileostomy study. Eur. J. Clin. Nutr. 2007, 61, 938–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; White, P.J. Optimizing the molecular weight of oat β-glucan for in vitro bile acid binding and fermentation. J. Agric. Food Chem. 2011, 59, 10322–10328. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.J.; Lee, S.Y.; Yoo, M.A.; Lee, H.G. Structural andbiological characterization of sulfated-derivatized oat β-glucan. J. Agric. Food Chem. 2006, 54, 3815–3818. [Google Scholar] [CrossRef]

| Sample | Viscosity (cP) | Mw(kD) | Mn(kD) | Polydispersity (Mw/Mn) |
|---|---|---|---|---|
| APP | 132.65 ± 12.45 | 6820 | 1360 | 5.01 |
| R-APP1 | 86.54 ± 16.66 | 2590 | 306 | 8.49 |
| R-APP2 | 15.43 ± 2.75 | 325 | 102 | 3.19 |
| R-APP3 | 4.76 ± 1.48 | 34 | 8.59 | 3.97 |
| Group | Parameters (Units) | ||||
|---|---|---|---|---|---|
| Serum TC (mmol/L) | Serum TG (mmol/L) | Serum LDL –c (mmol/L) | Hepatic TG (mg/g Pro) | Hepatic TC (mg/g Pro) | |
| C | 4.22 ± 0.17 b | 1.23 ± 0.04 b | 1.22 ± 0.08 c | 84.32 ± 7.16 c | 33.76 ± 2.23 c |
| M | 5.77 ± 0.18 a | 1.78 ± 0.14 a | 2.04 ± 0.11 a | 147.77 ± 12.32 a | 86.32 ± 6.36 a |
| APP | 5.93 ± 0.14 a | 1.37 ± 0.09 ab | 1.93 ± 0.14 ab | 126.86 ± 7.48 ab | 76.87 ± 3.68 ab |
| R-APP3 | 4.37 ± 0.25 b | 1.39 ± 0.07 ab | 1.80 ± 0.19 b | 101.11 ± 8.02 bc | 68.82 ± 3.85 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Xiong, C.; Huang, W. Gamma-Irradiation-Induced Degradation of the Water-Soluble Polysaccharide from Auricularia polytricha and Its Anti-Hypercholesterolemic Activity. Molecules 2022, 27, 1110. https://doi.org/10.3390/molecules27031110
Li P, Xiong C, Huang W. Gamma-Irradiation-Induced Degradation of the Water-Soluble Polysaccharide from Auricularia polytricha and Its Anti-Hypercholesterolemic Activity. Molecules. 2022; 27(3):1110. https://doi.org/10.3390/molecules27031110
Chicago/Turabian StyleLi, Ping, Chuan Xiong, and Wenli Huang. 2022. "Gamma-Irradiation-Induced Degradation of the Water-Soluble Polysaccharide from Auricularia polytricha and Its Anti-Hypercholesterolemic Activity" Molecules 27, no. 3: 1110. https://doi.org/10.3390/molecules27031110





