The Anti-Adiposity Mechanisms of Ampelopsin and Vine Tea Extract in High Fat Diet and Alcohol-Induced Fatty Liver Mouse Models
Abstract
1. Introduction
2. Results
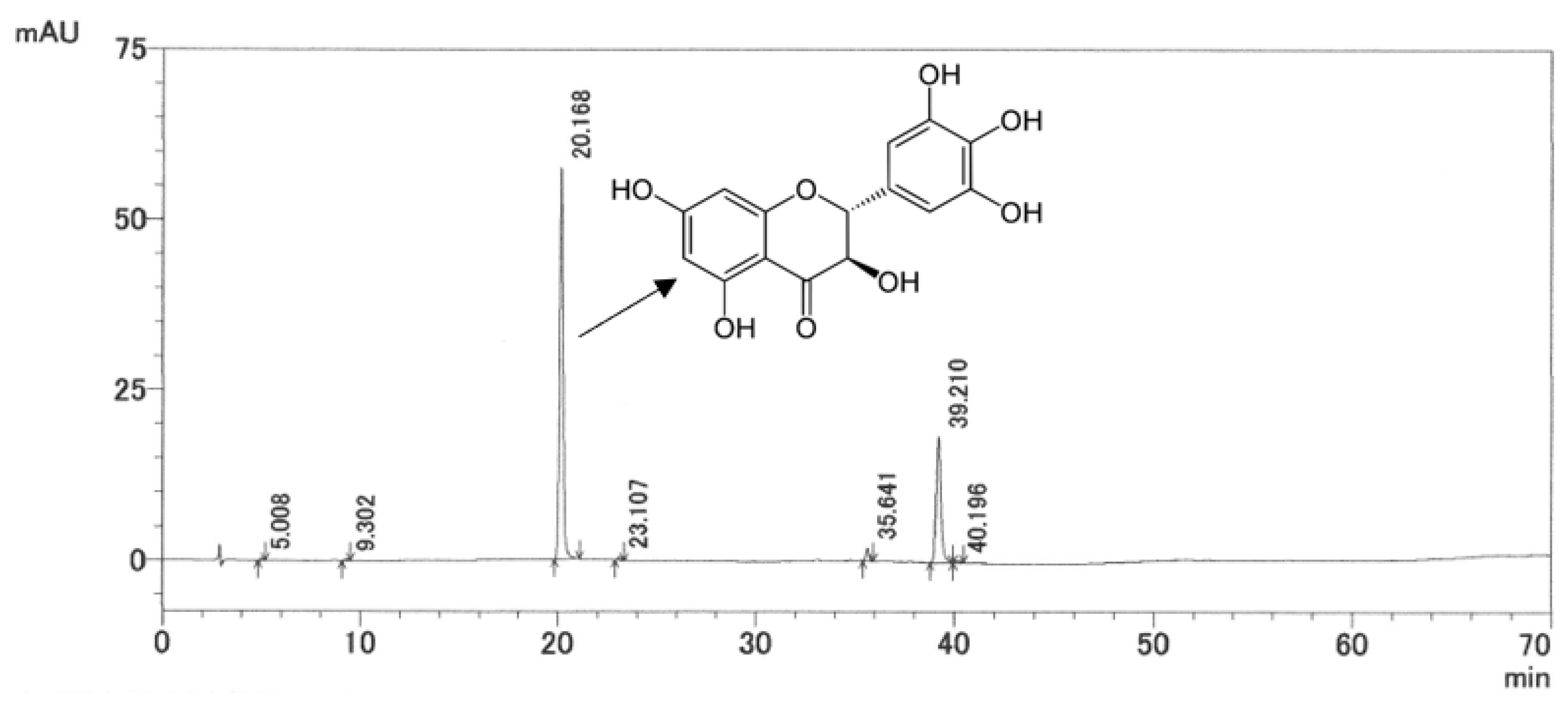
2.1. HPLC Analysis of AG Extract
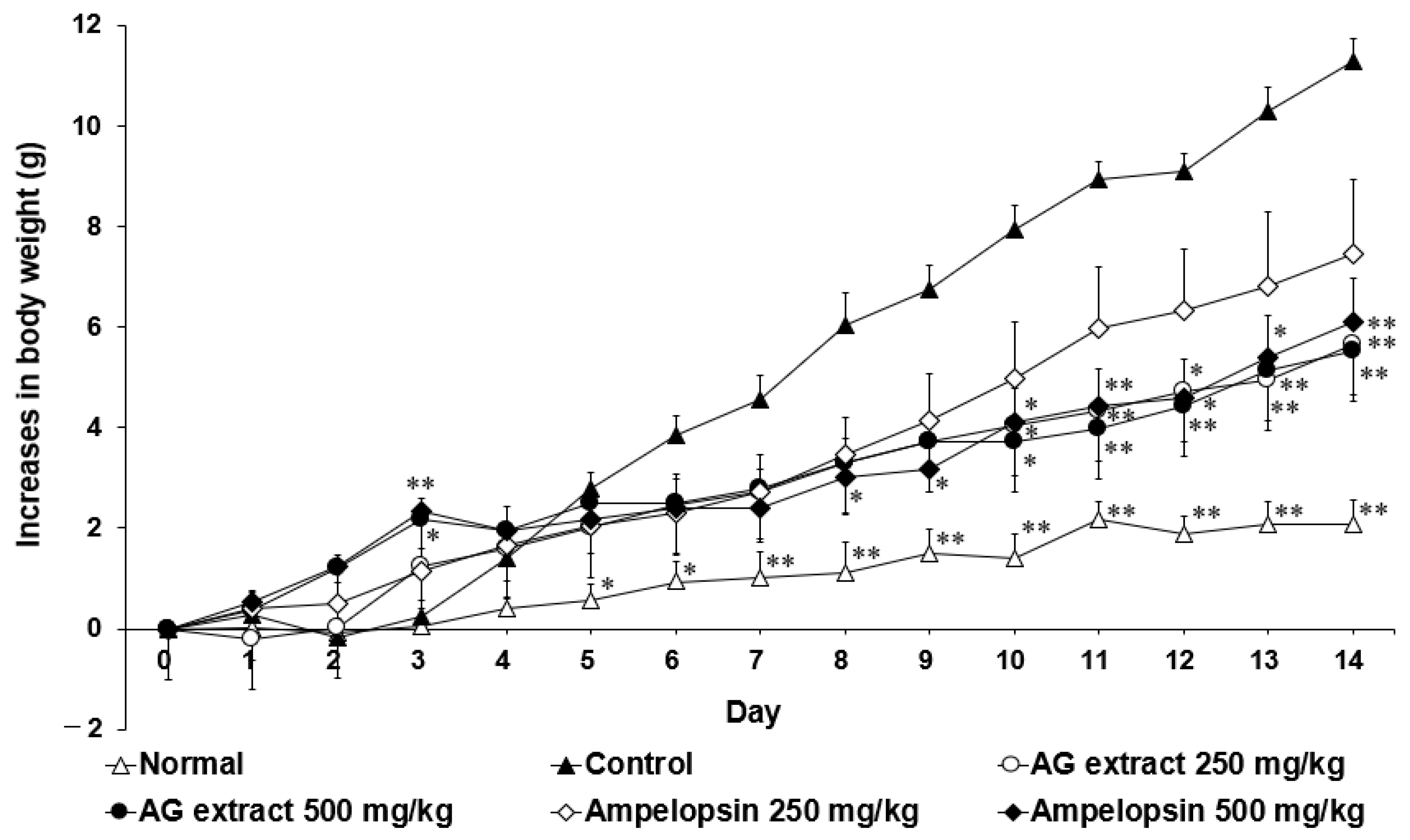
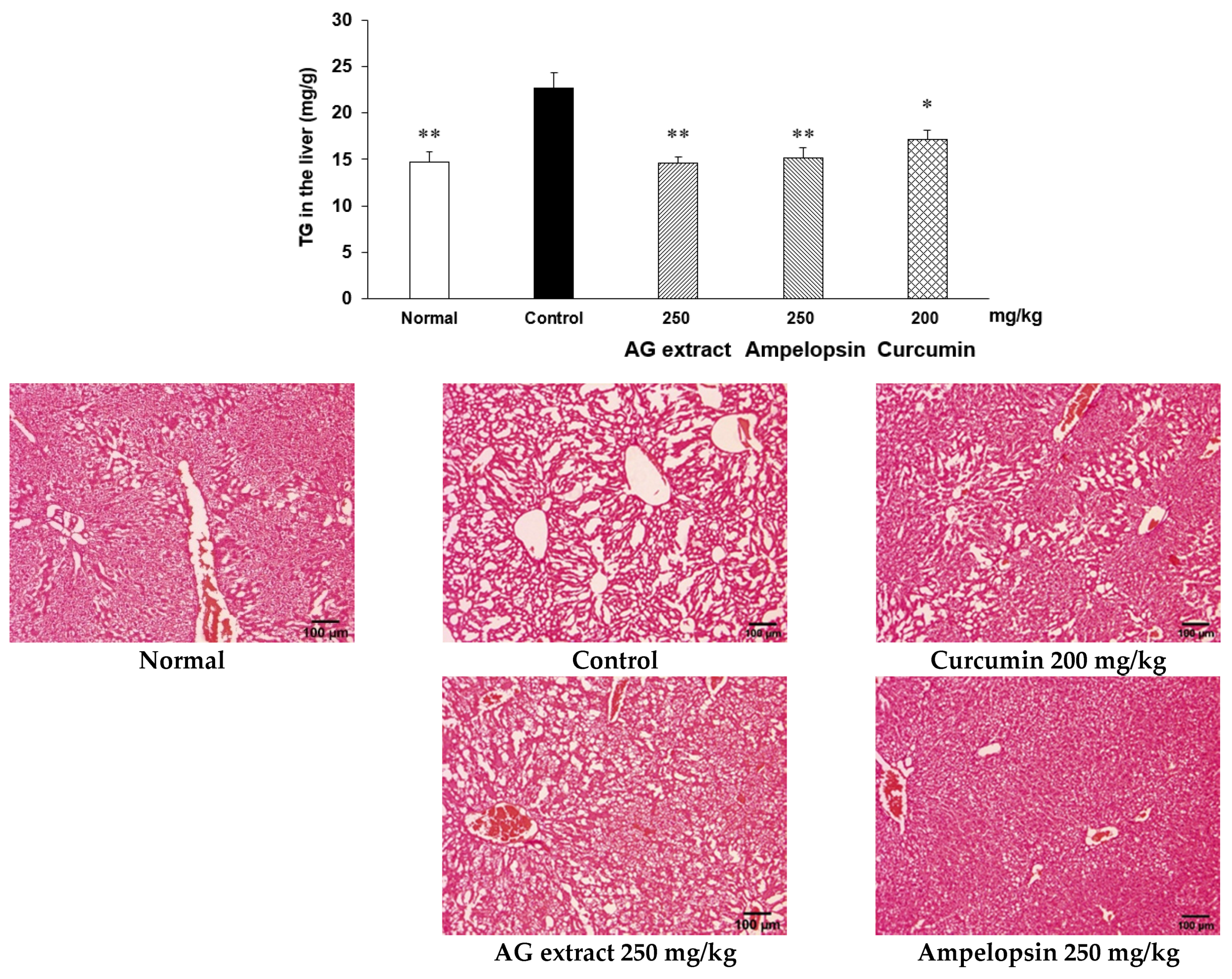
2.2. Effects of AG Extract and Ampelopsin on HFD-Induced NAFLD in Mice
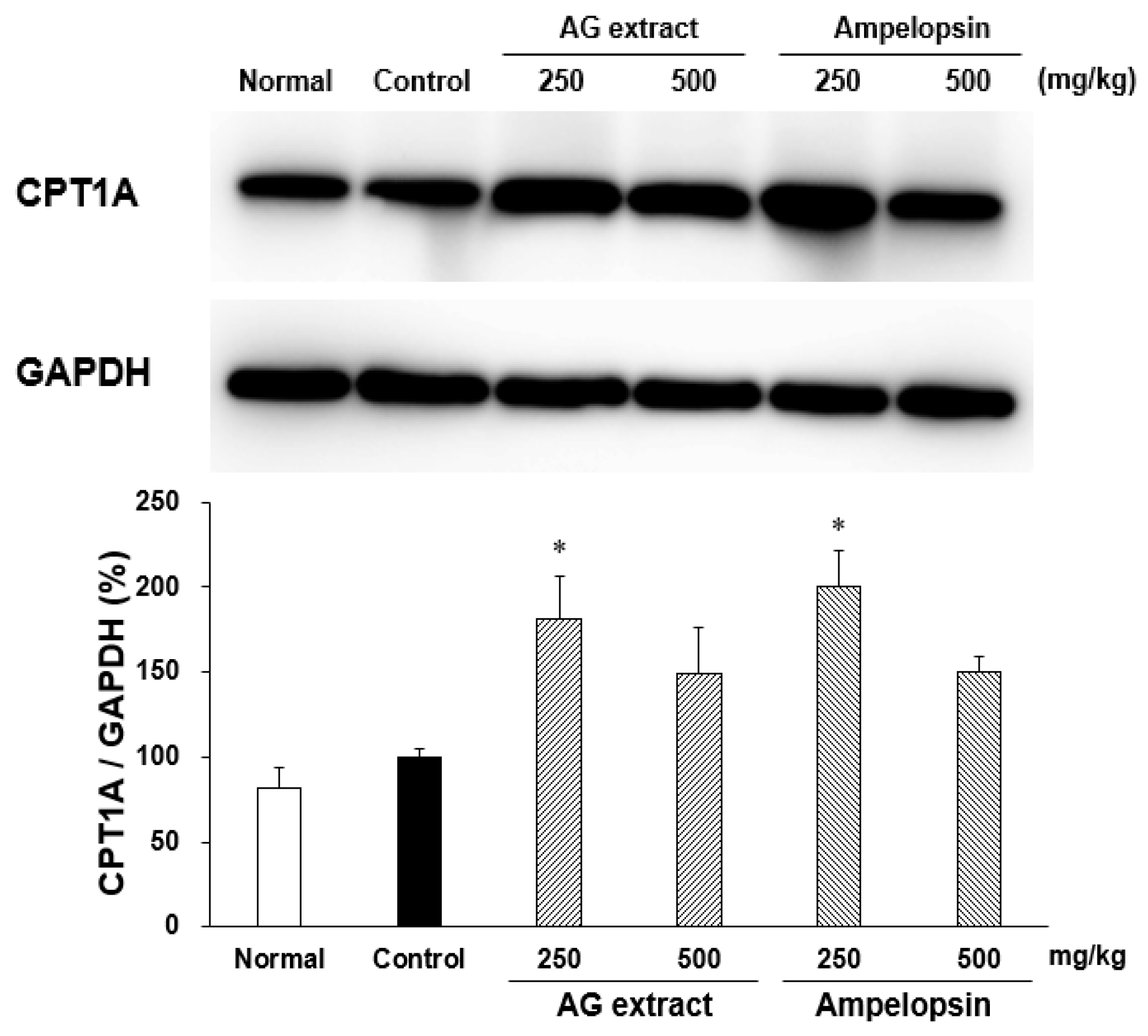
2.3. Effects of AG Extract and Ampelopsin on CPT1A Expression in the Liver of HFD-Fed Mice
2.4. Effects of AG Extract and Ampelopsin on Lipid Absorption in Olive Oil-Loaded Mice
2.5. Effects of AG Extract and Ampelopsin on Lipolysis in Differentiated 3T3-L1 Adipocytes
2.6. Effects of AG Extract and Ampelopsin on Free Fatty Acid (FFA) and Glycerol Release from the Rat Epididymal Fat Pad
2.7. Effects of AG Extract and Ampelopsin on Pancreatic Lipase Activity
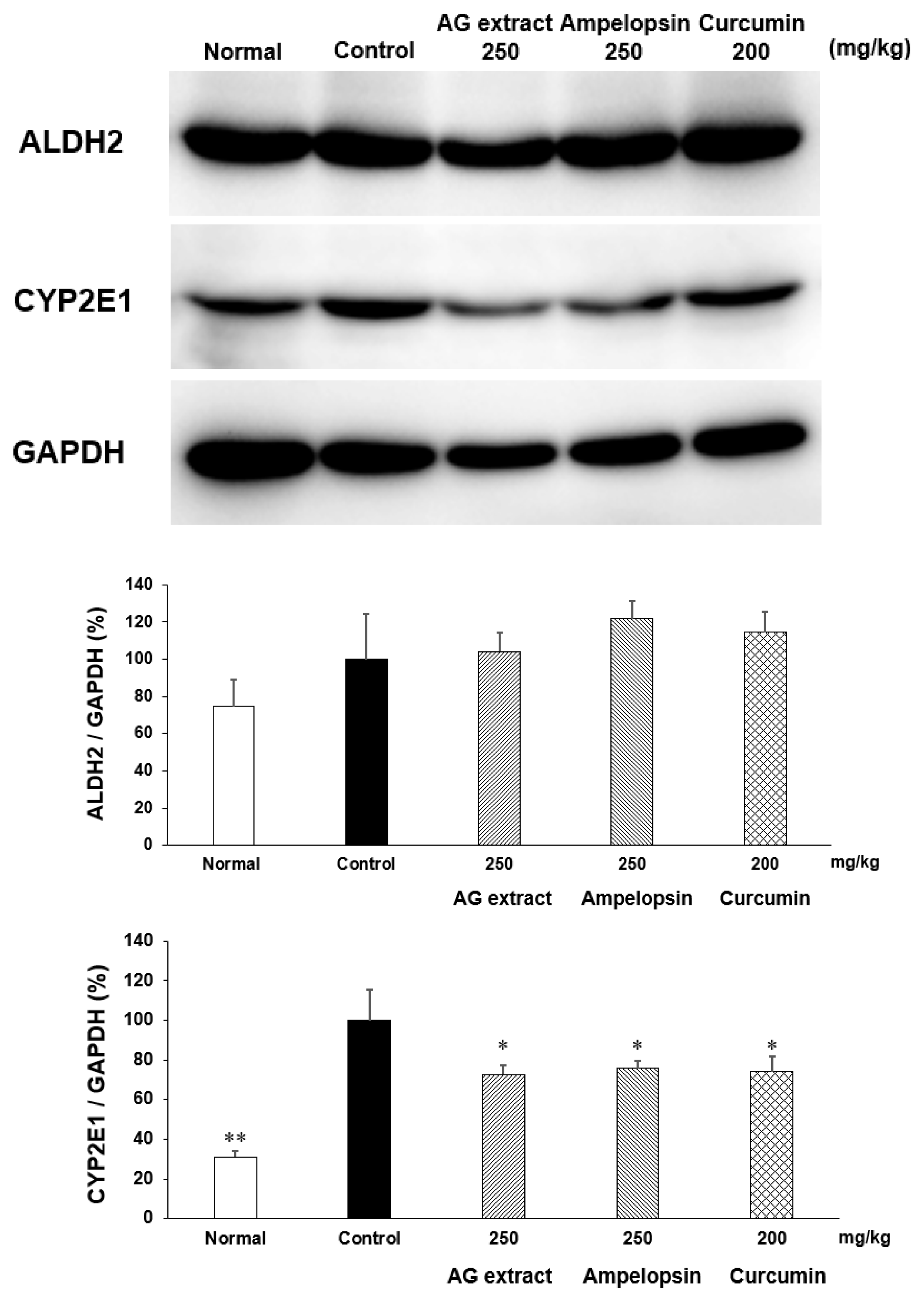
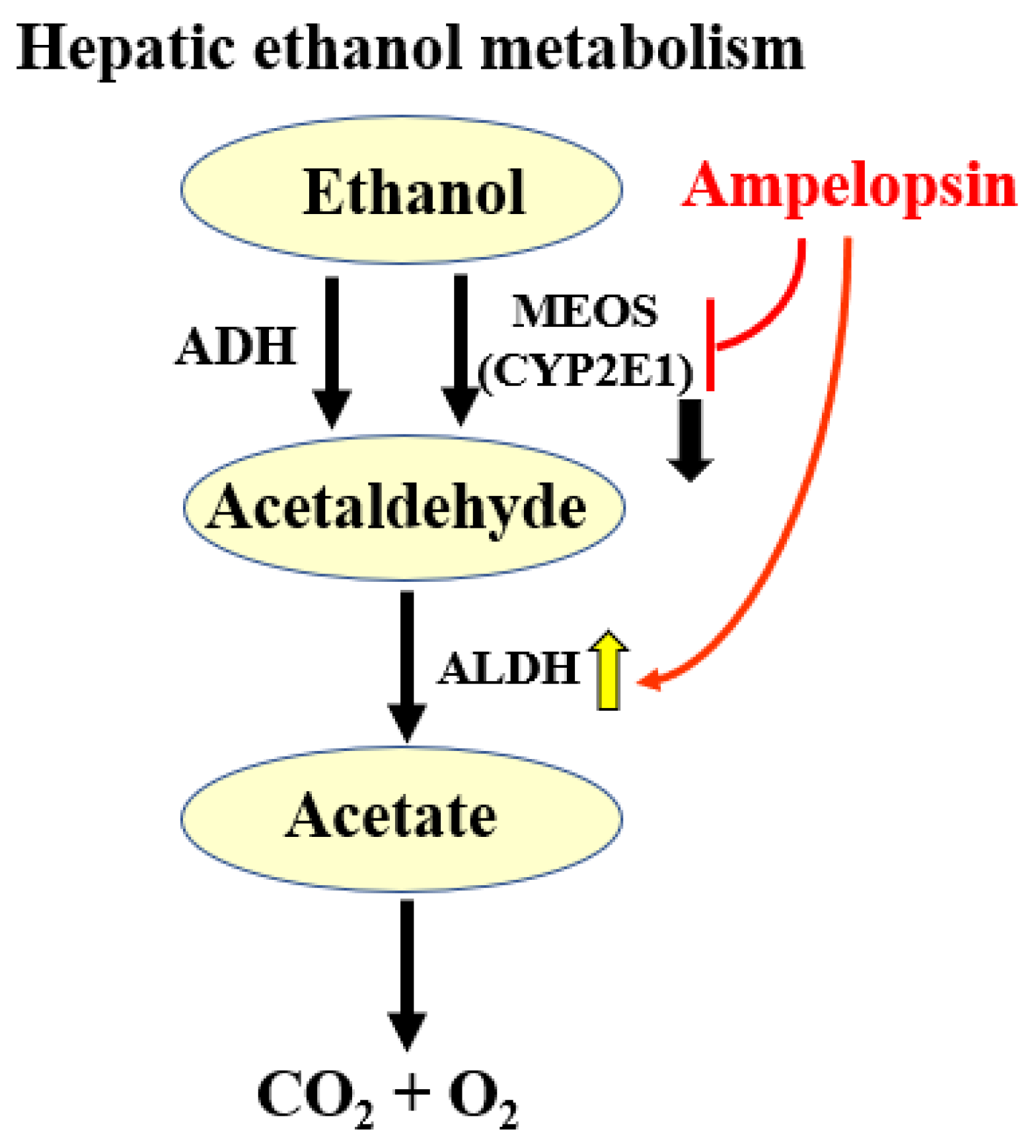
2.8. Effects of AG Extract and Ampelopsin on Alcohol Diet-Fed Mice
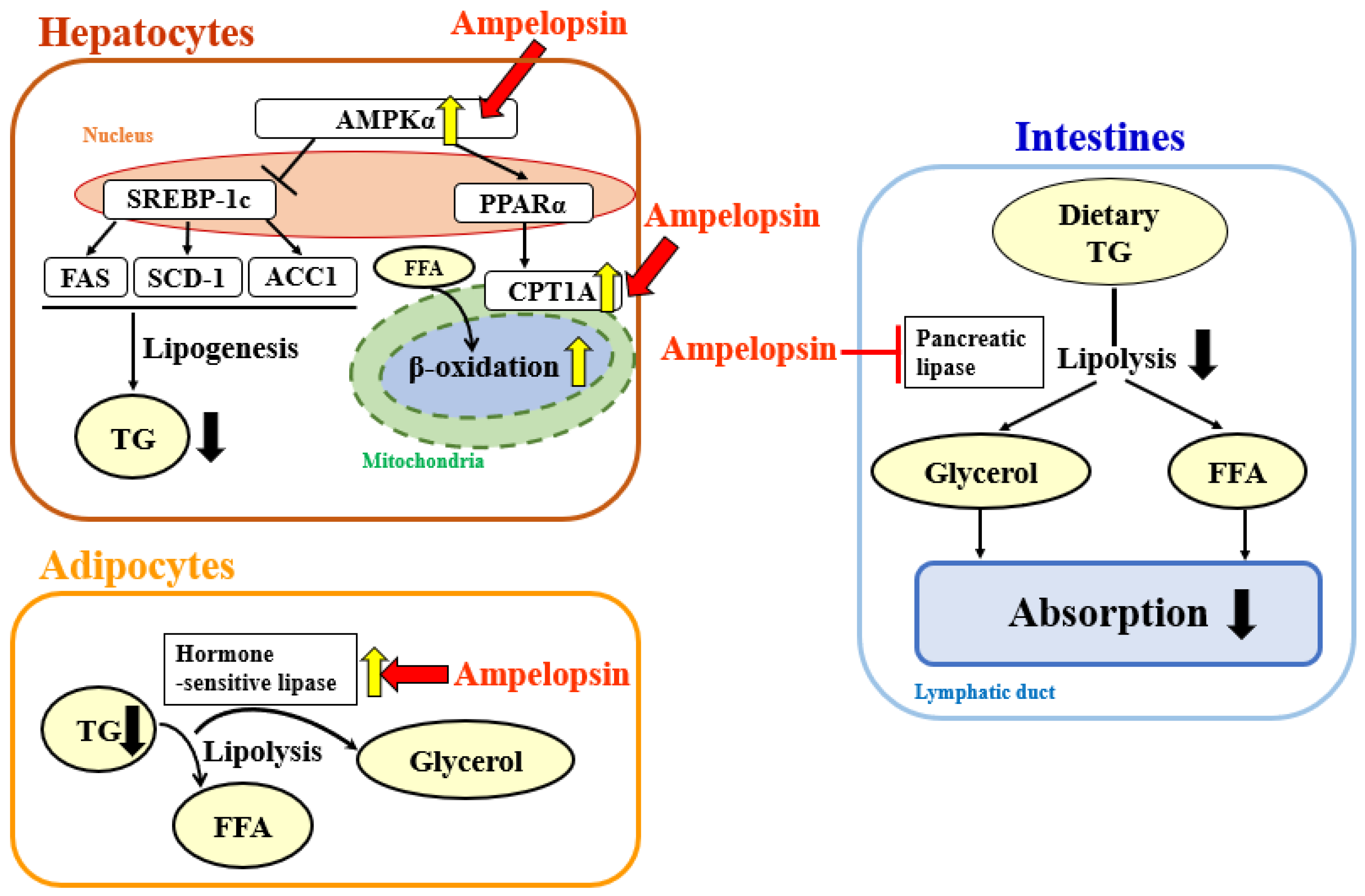
3. Discussion
4. Materials and Methods
4.1. Materials and Preparation
4.2. Animals and Cells
4.3. Chemicals and Reagents
4.4. Detection of Ampelopsin in AG Extract
4.5. HFD-Induced NAFLD in Mice
4.6. Serum TG Changes in Olive Oil-Loaded Mice
4.7. TG Decomposition in Differentiated 3T3-L1 Adipocytes
4.8. FFA and Glycerol Release from Rat Epididymal Fat Pads
4.9. Pancreatic Lipase Inhibition Test
4.10. Alcohol Diet-Induced Alcoholic Fatty Liver Disease in Mice
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| AG | Ampelopsis grossedentata |
| NAFLD | non-alcoholic fatty liver disease |
| HFD | high-fat diet |
| CPT | carnitine palmitoyltransferase |
| GOT | glutamic oxaloacetic transaminase |
| GPT | glutamic pyruvic transaminase |
| TG | triglyceride |
| ALDH | aldehyde dehydrogenase |
| CYP2E1 | cytochrome P450, family 2, subfamily e1 |
| ACC | acetyl CoA carboxylase |
| FFA | free fatty acid |
| ADH | alcohol dehydrogenase |
| AMPK | adenosine 5′-monophosphate-activated protein kinase |
| SREBP-1c | sterol regulatory element binding protein 1c |
| PPAR | peroxisome proliferation-activated receptor |
| FAS | fatty acid synthase |
| ACOX1 | acyl-coenzyme A oxidase 1 |
| Ucp1 | uncoupling protein 1 |
| Adrb3 | adrenoreceptor β3 |
| MEK/ERK | mitogen-activated protein kinase /extracellular signal-regulated kinase |
| MEOS | microsomal ethanol-oxidizing system |
| ROS | reactive oxygen species |
| DMSO | dimethyl sulfoxide |
| T-Cho | total cholesterol |
| DMEM | Dulbecco’s modified Eagle medium |
| FBS | fetal bovine serum |
| PBS | phosphate-buffered saline |
| PS | penicillin-streptomycin solution |
| BSA | bovine serum albumin |
| IBMX | 1-methyl-3-isobutylxanthine |
| NA | norepinephrine |
| DEX | dexamethasone |
| RIPA | radioimmunoprecipitation |
| HRP | horse radish peroxidase |
| PVDF | polyvinylidene fluoride |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| HPLC | high performance liquid chromatography |
References
- Dent, R.; McPherson, R.; Harper, M.-E. Factors affecting weight loss variability in obesity. Metabolism 2020, 113, 154388. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2018, 92, 82–97. [Google Scholar] [CrossRef]
- Keith, J.N. Pharmacotherapy in Treatment of Obesity. Gastroenterol. Clin. N. Am. 2016, 45, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Yoon, M. Regulation of Obesity by Antiangiogenic Herbal Medicines. Molecules 2020, 25, 4549. [Google Scholar] [CrossRef]
- Son, J.W.; Kim, S. Comprehensive Review of Current and Upcoming Anti-Obesity Drugs. Diabetes Metab. J. 2020, 44, 802–818. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; Fornasini, G.F. Pharmacokinetics of L-Carnitine. Clin. Pharmacokinet. 2003, 42, 941–967. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial Effects of Green Tea—A Review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Zhou, C.B.; Zhang, M.X.; Miu, R.L.; Chen, W.P. Research progress on active components and efficacy of vine tea. Tea Fujian 2014, 3, 2–4. [Google Scholar]
- Zhang, Q.; Zhao, Y.; Zhang, M.; Zhang, Y.; Ji, H.; Shen, L. Recent advances in research on vine tea, a potential and functional herbal tea with dihydromyricetin and myricetin as major bioactive compounds. J. Pharm. Anal. 2020, 11, 555–563. [Google Scholar] [CrossRef]
- Wan, W.; Jiang, B.; Sun, L.; Xu, L.; Xiao, P. Metabolomics reveals that vine tea (Ampelopsis grossedentata) prevents high-fat-diet-induced metabolism disorder by improving glucose homeostasis in rats. PLoS ONE 2017, 12, e0182830. [Google Scholar] [CrossRef]
- Xie, K.; He, X.; Chen, K.; Sakao, K.; Hou, D.-X. Ameliorative effects and molecular mechanisms of vine tea on western diet-induced NAFLD. Food Funct. 2020, 11, 5976–5991. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Zhao, X.L.; Wan, J.; Ran, L.; Qin, Y.; Wang, X.F.; Gao, Y.X.; Shu, F.R.; Zhang, Y.; Liu, P.; et al. Dihy-dromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial. Pharmacol. Res. 2015, 99, 74–81. [Google Scholar] [CrossRef]
- Silva, J.; Yu, X.; Moradian, R.; Folk, C.; Spatz, M.H.; Kim, P.; Bhatti, A.A.; Davies, D.L.; Liang, J. Dihydromyricetin Protects the Liver via Changes in Lipid Metabolism and Enhanced Ethanol Metabolism. Alcohol. Clin. Exp. Res. 2020, 44, 1046–1060. [Google Scholar] [CrossRef]
- Rajendram, R.; Rajendram, R.; Preedy, V.R. Chapter 35. Ethanol Metabolism and Implications for Disease. In Neuropathology of Drug Addictions and Substance Misuse; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, pp. 377–388. [Google Scholar] [CrossRef]
- Fang, J.; Wang, F.; Song, H.; Wang, Z.; Zuo, Z.; Cui, H.; Jia, Y.; Deng, J.; Yu, S.; Hu, Y.; et al. AMPKα pathway involved in hepatic triglyceride metabolism disorder in diet-induced obesity mice following Escherichia coli Infection. Aging 2018, 10, 3161–3172. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Hitoe, S.; Nakamura, S.; Matsuda, H. Purple Tea and Its Extract Suppress Diet-induced Fat Accumulation in Mice and Human Subjects by Inhibiting Fat Absorption and Enhancing Hepatic Carnitine Palmitoyltransferase Expression. Int. J. Biomed. Sci. 2015, 11, 67–75. [Google Scholar]
- Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Effect of Spirulina lipids on high-fat and high-sucrose diet induced obesity and hepatic lipid accumulation in C57BL/6J mice. J. Funct. Foods 2020, 65, 103741. [Google Scholar] [CrossRef]
- Zeng, Y.; Hua, Y.Q.; Wang, W.; Zhang, H.; Xu, X.L. Modulation of SIRT1-mediated signaling cascades in the liver contributes to the amelioration of nonalcoholic steatohepatitis in high fat fed middle-aged LDL receptor knockout mice by dihydromyricetin. Biochem. Pharmacol. 2020, 175, 113927. [Google Scholar] [CrossRef] [PubMed]
- Hinds, T.D.J.; Creeden, J.F.; Gordon, D.M.; Stec, D.F.; Donald, M.C.; Stec, D.E. Bilirubin Nanoparticles Reduce Diet-Induced Hepatic Steatosis, Improve Fat Utilization, and Increase Plasma β-Hydroxybutyrate. Front. Pharmacol. 2020, 11, 594574. [Google Scholar] [CrossRef]
- Green, H.; Kehinde, O. Sublines of mouse 3T3 cells that accumulate lipid. Cell 1974, 1, 113–116. [Google Scholar] [CrossRef]
- Poulos, S.P.; Dodson, M.V.; Hausman, G.J. Cell line models for differentiation: Preadipocytes and adipocytes. Exp. Biol. Med. 2010, 235, 1185–1193. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, M.; Lang, H.; Zhou, Y.; Mi, M. Dihydromyricetin enhances glucose uptake by inhibition of MEK/ERK pathway and consequent down-regulation of phosphorylation of PPARγ in 3T3-L1 cells. J. Cell. Mol. Med. 2018, 22, 1247–1256. [Google Scholar] [CrossRef]
- Michalik, L.; Auwerx, J.; Berger, J.P.; Chatterjee, V.K.; Glass, C.K.; Gonzalez, F.J.; Grimaldi, P.A.; Kadowaki, T.; Lazar, M.A.; O’Rahilly, S.; et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006, 58, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.R.; Anastasi, M.R.; Zhang, V.J.; Russell, D.L.; Robker, R.L. Regulation of Fatty Acid Oxidation in Mouse Cumulus-Oocyte Complexes during Maturation and Modulation by PPAR Agonists. PLoS ONE 2014, 9, e87327. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Moller, D.E. The Mechanisms of Action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Wagner, K.-D. The Role of PPARs in Disease. Cells 2020, 9, 2367. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.H.; Shen, C.Y. Peroxisome proliferator-activated receptor γ in white and brown adipocyte regulation and differentiation. Physiol. Res. 2020, 69, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Guerrero-Juarez, C.F.; Chen, S.X.; Zhang, X.; Yin, M.; Li, F.; Wu, S.; Chen, J.; Li, M.; Liu, Y.; et al. Diet-induced obesity promotes infection by impairment of the innate antimicrobial defense function of dermal adipocyte progenitors. Sci. Transl. Med. 2021, 13, eabb5280. [Google Scholar] [CrossRef]
- Charrier, A.; Xu, X.; Guan, B.-J.; Ngo, J.; Wynshaw-Boris, A.; Hatzoglou, M.; Buchner, D.A. Adipocyte-specific deletion of zinc finger protein 407 results in lipodystrophy and insulin resistance in mice. Mol. Cell. Endocrinol. 2020, 521, 111109. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, L.; Kim, S.M. Vanadium-protein complex inhibits human adipocyte differentiation through the activation of β-catenin and LKB1/AMPK signaling pathway. PLoS ONE 2020, 15, e0239547. [Google Scholar] [CrossRef]
- Regnier, M.; Polizzi, A.; Smati, S.; Lukowicz, C.; Fougerat, A.; Lippi, Y.; Fouché, E.; Lasserre, F.; Naylies, C.; Bétoulières, C.; et al. Hepatocyte-specific deletion of Pparα promotes NAFLD in the context of obesity. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef]
- Gordon, D.M.; Neifer, K.L.; Hamoud, A.R.A.; Hawk, C.F.; Nestor-Kalinoski, A.L.; Miruzzi, S.A.; Morran, M.P.; Adeosun, S.O.; Sarver, J.G.; Erhardt, P.W.; et al. Bilirubin remodels murine white adipose tissue by reshaping mito-chondrial activity and the coregulator profile of peroxisome proliferator–activated receptor α. J. Biol. Chem. 2020, 29, 9804–9822. [Google Scholar] [CrossRef] [PubMed]
- Traversy, G.; Chaput, J.-P. Alcohol Consumption and Obesity: An Update. Curr. Obes. Rep. 2015, 4, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Bendsen, N.T.; Christensen, R.; Bartels, E.M.; Kok, F.J.; Sierksma, A.; Raben, A.; Astrup, A. Is beer consumption related to measures of abdominal and general obesity? A systematic review and meta-analysis. Nutr. Rev. 2012, 71, 67–87. [Google Scholar] [CrossRef]
- Sayon-Orea, C.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Alcohol consumption and body weight: A systematic review. Nutr. Rev. 2011, 69, 419–431. [Google Scholar] [CrossRef]
- Yeomans, M.R. Alcohol, appetite and energy balance: Is alcohol intake a risk factor for obesity? Physiol. Behav. 2010, 100, 82–89. [Google Scholar] [CrossRef]
- National Institute on Alcohol Abuse and Alcoholism. Alcohol Metabolism: An Update. 2007. Available online: https://pubs.niaaa.nih.gov/publications/aa72/aa72.htm (accessed on 1 April 2007).
- Wang, J.-H.; Batey, R.G.; George, J. Role of ethanol in the regulation of hepatic stellate cell Function. World J. Gastroenterol. 2006, 12, 6926–6932. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Zhang, Q.Y.; Li, L.Y.; Wang, B.; Zhao, Y.Y.; Guo, D.A. Simultaneous determination and pharmacokinetic studies of dihy-dromyricetin and myricetin in rat plasma by HPLC-DAD after oral administration of Ampelopsis grossedentata decoction. J. Chromatogr. B 2007, 860, 4–9. [Google Scholar] [CrossRef]
- Fernández, P.L.; Martín, M.J.; González, A.G.; Pablos, F. HPLC determination of catechins and caffeine in tea. Differentiation of green, black and instant teas. Analyst 2000, 125, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Tanaka, J.; Kikuchi, M.; Fukuda, T.; Ito, H.; Hatano, T.; Yoshida, T. Effect of Polyphenol-Rich Extract from Walnut on Diet-Induced Hypertriglyceridemia in Mice via Enhancement of Fatty Acid Oxidation in the Liver. J. Agric. Food Chem. 2009, 57, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Seki, E.; Aitani, M. Inhibitory effect of green coffee bean extract on fat accumulation and body weight gain in mice. BMC Complement. Altern. Med. 2006, 6, 9. [Google Scholar] [CrossRef]
- Motoyashiki, T.; Miyaki, M.; Yoshida, A.; Morita, T.; Ueki, H. A vanadyl sulfate-bovine serum albumin complex stimulates the release of lipoprotein lipase activity from isolated rat fat pads through an increase in the cellular content of cAMP and myo-inositol 1, 4, 5-trisphosphate. Biol. Pharm. Bull. 1999, 22, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Okano, G.; Shimomura, Y. Fatty Acid Release Rate of Adipose Tissue from Rats on a High Carbohydrate or a High Fat Diet and its Response to PTU Administration. J. Jpn. Soc. Food Nutr. 1981, 34, 335–340. [Google Scholar] [CrossRef][Green Version]
- Baskaran, P.; Thyagarajan, B. Measurement of Basal and Forskolin-stimulated Lipolysis in Inguinal Adipose Fat Pads. J. Vis. Exp. 2017, 125, e55625. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Shimoda, H.; Nishida, N.; Takada, M.; Matsuda, H. Salacia reticulata and Its Polyphenolic Constituents with Lipase Inhibitory and Lipolytic Activities Have Mild Antiobesity Effects in Rats. J. Nutr. 2002, 132, 1819–1824. [Google Scholar] [CrossRef]
- Ninomiya, K.; Matsuda, H.; Shimoda, H.; Nishida, N.; Kasajima, N.; Yoshino, T.; Morikawa, T.; Yoshikawa, M. Carnosic acid, a new class of lipid absorption inhibitor from sage. Bioorg. Med. Chem. Lett. 2004, 14, 1943–1946. [Google Scholar] [CrossRef]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef] [PubMed]



| Dose (mg/kg) | Body Weight (g) | Liver (g) | Epididymal Fat (g) | Perirenal Fat (g) | |
|---|---|---|---|---|---|
| Normal | - | 45.24 ± 0.62 ** | 1.65 ± 0.04 ** | 0.98 ± 0.09 ** | 0.53 ± 0.07 ** |
| Control | - | 53.76 ± 0.81 | 2.15 ± 0.08 | 2.65 ± 0.18 | 1.05 ± 0.06 |
| AG extract | 250 | 47.85 ± 0.82 * | 1.83 ± 0.04 * | 1.88 ± 0.15 ** | 0.71 ± 0.05 * |
| 500 | 48.82 ± 1.97 | 1.85 ± 0.09 * | 1.86 ± 0.16 ** | 0.85 ± 0.07 | |
| Ampelopsin | 250 | 49.12 ± 1.94 | 1.80 ± 0.08 ** | 1.87 ± 0.16 ** | 0.84 ± 0.09 |
| 500 | 48.54 ± 1.28 | 1.90 ± 0.06 | 1.87 ± 0.12 ** | 1.01 ± 0.11 |
| Dose (mg/kg) | TG (mg/dL) | T-Cho (mg/dL) | |
|---|---|---|---|
| Normal | - | 97.5 ± 7.3 * | 148.7 ± 19.3 * |
| Control | - | 144.9 ± 13.2 | 235.4 ± 36.2 |
| AG extract | 250 | 98.5 ± 12.1 * | 199.1 ± 19.0 |
| 500 | 106.0 ± 14.2 | 197.6 ± 11.6 | |
| Ampelopsin | 250 | 87.8 ± 9.3 ** | 213.7 ± 10.5 |
| 500 | 109.0 ± 9.8 | 214.7 ± 13.5 |
| Concentration (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Control | 1 | 3 | 10 | 30 | 100 | ||
| TG (%) | AG extract | 100.0 ± 1.1 | 90.1 ± 2.5 | 89.9 ± 2.2 ** | 85.6 ± 1.2 ** | 92.7 ± 2.1 * | 85.2 ± 1.2 ** |
| Ampelopsin | 100.0 ± 3.2 | 99.7 ± 2.1 | 90.0 ± 4.2 | 99.2 ± 6.0 | 91.7 ± 4.3 | 83.2 ± 1.7 * | |
| Viability (%) | AG extract | 100.0 ± 1.6 | 103.0 ± 1.0 | 100.0 ± 1.1 | 99.0 ± 1.5 | 99.4 ± 1.7 | 94.0 ± 1.4 * |
| Ampelopsin | 100.0 ± 2.4 | 99.7 ± 1.8 | 99.8 ± 2.8 | 101.8 ± 2.4 | 102.5 ± 2.4 | 89.5 ± 1.6 * | |
| Concentration (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | NA (10) | 1 | 3 | 10 | 30 | 100 | ||
| FFA (%) | AG extract | 100.0 ± 0.3 | 478.4 ± 7.5 ** | 133.4 ± 1.4 ** | 137.4 ± 0.7 ** | 138.0 ± 0.3 ** | 136.4 ± 1.2 ** | 124.3 ± 0.3 ** |
| Ampelopsin | 100.0 ± 1.4 | 559.1 ± 1.0 ** | 119.7 ± 1.0 ** | 117.0 ± 1.3 ** | 118.1 ± 1.8 ** | 123.6 ± 3.2 ** | 116.6 ± 0.4 ** | |
| Glycerol (%) | AG extract | 100.0 ± 3.3 | 217.5 ± 2.8 ** | 117.1 ± 1.1 ** | 112.3 ± 0.4 ** | 119.3 ± 0.6 ** | 110.0 ± 0.9 * | 102.2 ± 3.0 |
| Ampelopsin | 100.0 ± 1.0 | 348.8 ± 3.0 ** | 108.3 ± 0.8 ** | 112.2 ± 1.8 ** | 106.8 ± 0.8 ** | 124.9 ± 2.0 ** | 95.6 ± 0.5 | |
| Inhibition (%) | |||||||
|---|---|---|---|---|---|---|---|
| Control | 1 (µg/mL) | 3 | 10 | 30 | 100 | IC50 (µg/mL) | |
| AG extract | 0.0 ± 2.0 | −0.5 ± 2.8 | 8.4 ± 0.7 | 22.8 ± 11.5 | 36.7 ± 2.4 ** | 58.8 ± 0.9 ** | 97.7 |
| Ampelopsin | 0.0 ± 3.9 | 17.3 ± 3.0 | 11.6 ± 3.8 | 28.8 ± 2.5 ** | 44.0 ± 2.3 ** | 66.7 ± 5.0 ** | 45.9 |
| Orlistat | 0.0 ± 3.8 | 77.4 ± 5.0 ** | 81.6 ± 1.5 ** | 90.2 ± 0.4 ** | 95.6 ± 0.1 ** | 97.4 ± 0.2 ** | <0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Miyasaka, K.; Yamada, W.; Takeda, S.; Shimizu, N.; Shimoda, H. The Anti-Adiposity Mechanisms of Ampelopsin and Vine Tea Extract in High Fat Diet and Alcohol-Induced Fatty Liver Mouse Models. Molecules 2022, 27, 607. https://doi.org/10.3390/molecules27030607
Wu J, Miyasaka K, Yamada W, Takeda S, Shimizu N, Shimoda H. The Anti-Adiposity Mechanisms of Ampelopsin and Vine Tea Extract in High Fat Diet and Alcohol-Induced Fatty Liver Mouse Models. Molecules. 2022; 27(3):607. https://doi.org/10.3390/molecules27030607
Chicago/Turabian StyleWu, Jianbo, Kenchi Miyasaka, Wakana Yamada, Shogo Takeda, Norihito Shimizu, and Hiroshi Shimoda. 2022. "The Anti-Adiposity Mechanisms of Ampelopsin and Vine Tea Extract in High Fat Diet and Alcohol-Induced Fatty Liver Mouse Models" Molecules 27, no. 3: 607. https://doi.org/10.3390/molecules27030607
APA StyleWu, J., Miyasaka, K., Yamada, W., Takeda, S., Shimizu, N., & Shimoda, H. (2022). The Anti-Adiposity Mechanisms of Ampelopsin and Vine Tea Extract in High Fat Diet and Alcohol-Induced Fatty Liver Mouse Models. Molecules, 27(3), 607. https://doi.org/10.3390/molecules27030607





