Development of Optogenetic Dual-Switch System for Rewiring Metabolic Flux for Polyhydroxybutyrate Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, Primers, and Culture Media
2.2. Plasmid and Strain Construction
2.3. Characterization of Light-Sensing Systems
2.4. PHB Fermentation
2.5. Analytical Method
2.6. Statistical Analysis
3. Results and Discussion
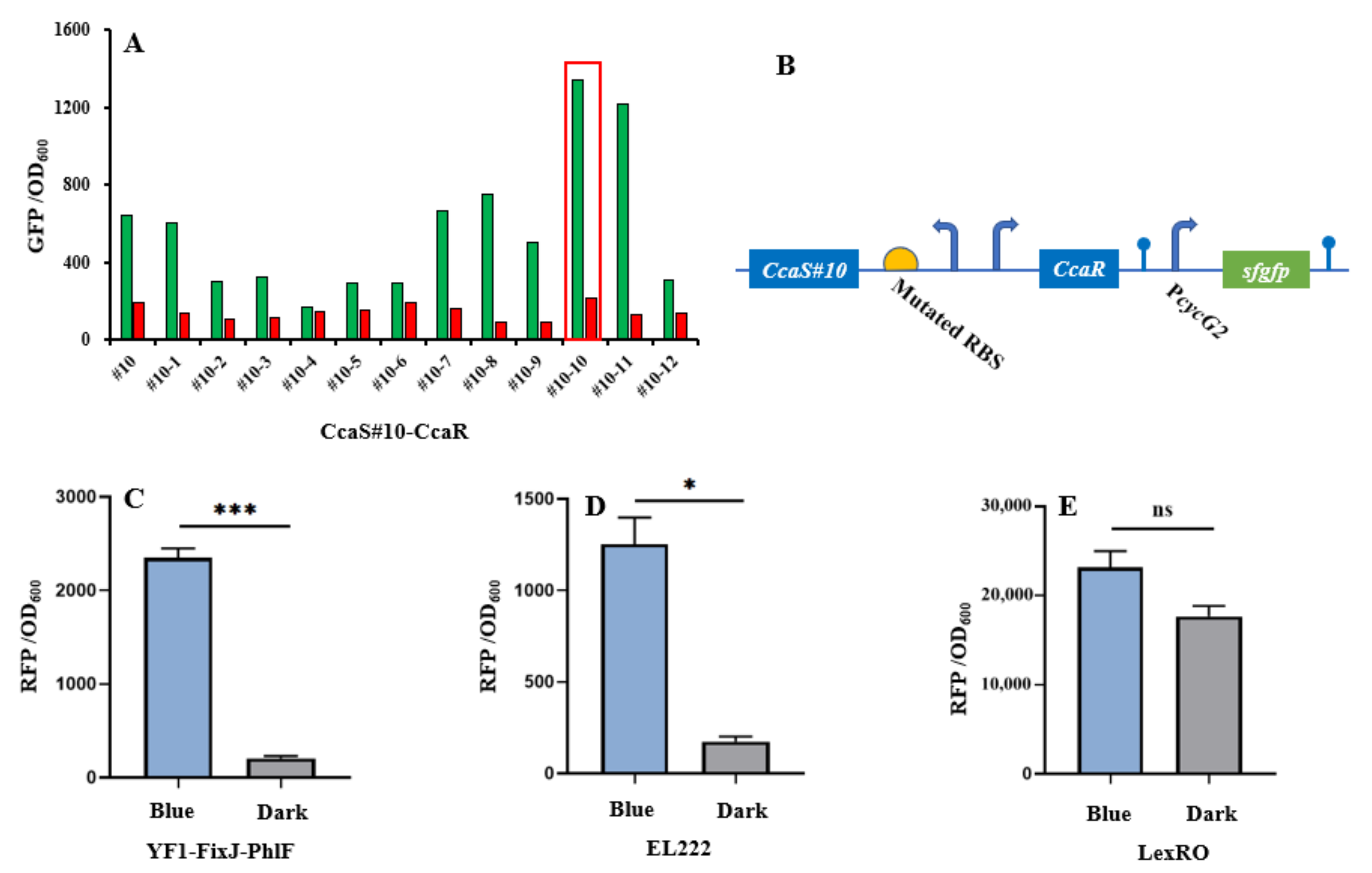
3.1. Characterization and Screening of Optogenetic Elements
3.2. Analysis of Crosstalk of Optogenetic Parts
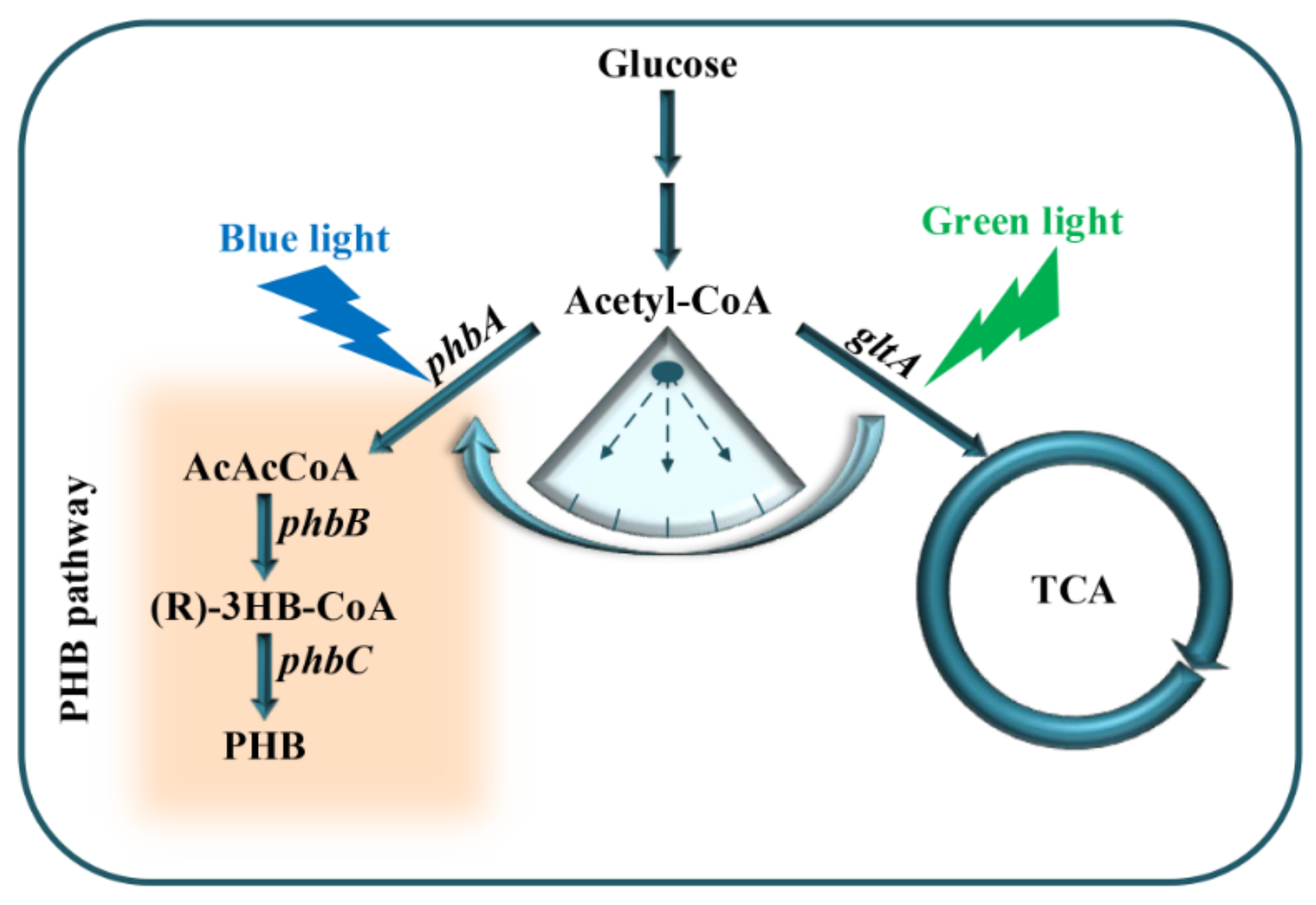
3.3. Construction of Optogenetic Dual-Switch System
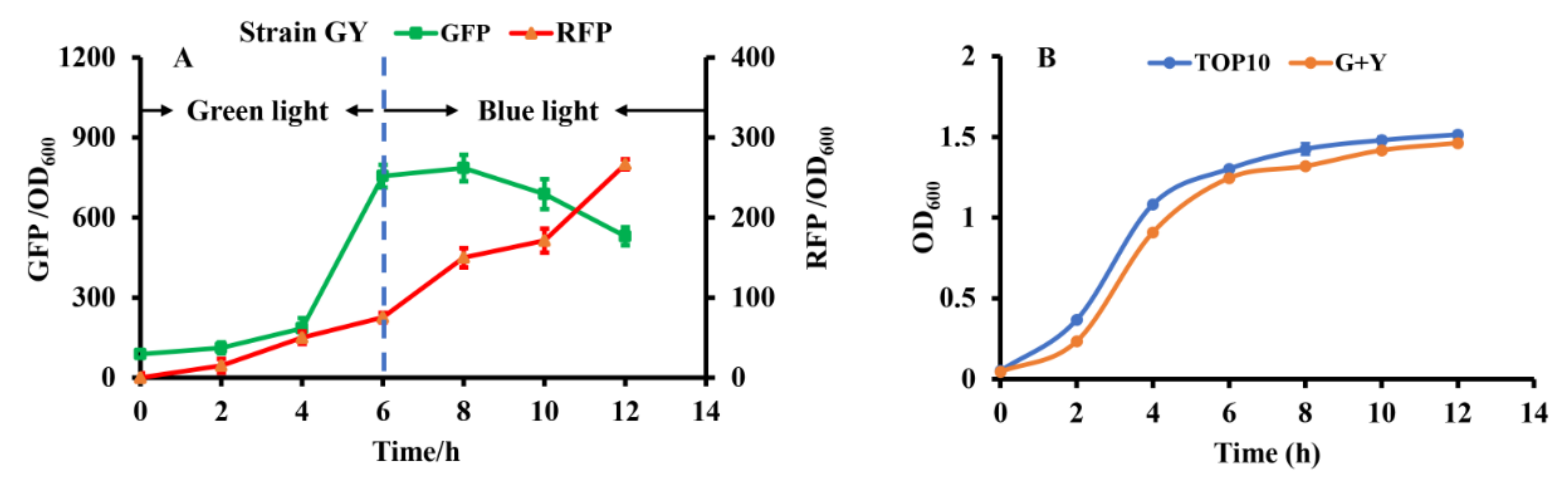
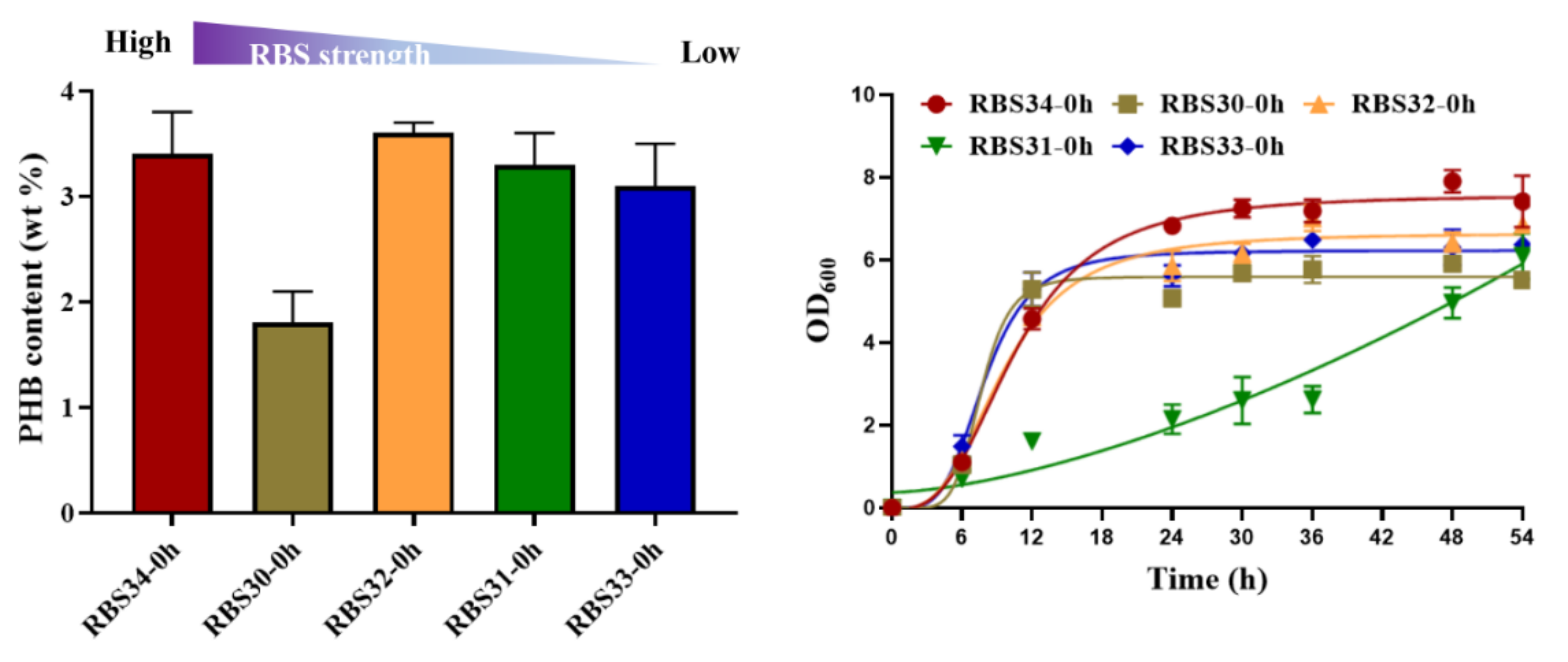
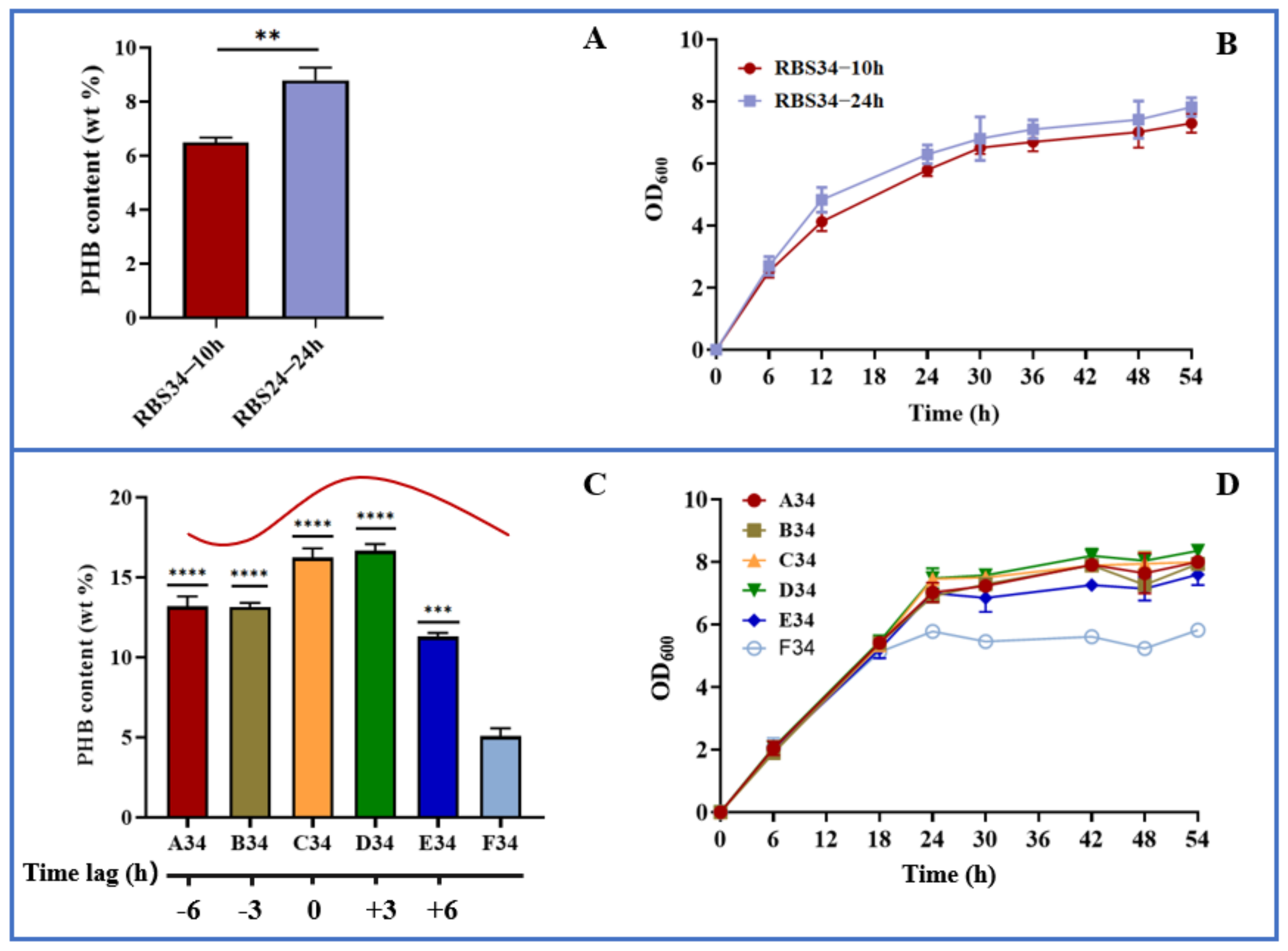
3.4. Dynamically Regulating Engineered E. coli for Enhancing PHB Production with Optogenetic Dual-Switch System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Purcell, E.B.; Crosson, S. Photoregulation in prokaryotes. Curr. Opin. Microbiol. 2008, 11, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Repina, N.A.; Rosenbloom, A.; Mukherjee, A.; Schaffer, D.V.; Kane, R.S. At Light Speed: Advances in Optogenetic Systems for Regulating Cell Signaling and Behavior. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 13–39. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, D.; Cho, Y.K. Natural photoreceptors and their application to synthetic biology. Trends Biotechnol. 2015, 33, 80–91. [Google Scholar] [CrossRef]
- Nash, A.I.; McNulty, R.; Shillito, M.E.; Swartz, T.E.; Bogomolni, R.A.; Luecke, H.; Gardner, K.H. Structural basis of photosensitivity in a bacterial light-oxygen-voltage/helix-turn-helix (LOV-HTH) DNA-binding protein. Proc. Natl. Acad. Sci. USA 2011, 108, 9449–9454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila-Pérez, M.; Hellingwerf, K.J.; Kort, R. Blue light activates the sigmaB-dependent stress response of Bacillus subtilis via YtvA. J. Bacteriol. 2006, 188, 6411–6414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Chen, X.; Yang, Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat. Methods 2012, 9, 266–269. [Google Scholar] [CrossRef]
- Hirose, Y.; Shimada, T.; Narikawa, R.; Katayama, M.; Ikeuchi, M. Cyanobacteriochrome CcaS is the green light receptor that induces the expression of phycobilisome linker protein. Proc. Natl. Acad. Sci. USA 2008, 105, 9528–9533. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, M.; Ferri, S.; Rögner, M.; Sode, K. Construction of a Miniaturized Chromatic Acclimation Sensor from Cyanobacteria with Reversed Response to a Light Signal. Sci. Rep. 2016, 6, 37595. [Google Scholar] [CrossRef] [Green Version]
- Ong, N.T.; Tabor, J.J. A Miniaturized Escherichia coli Green Light Sensor with High Dynamic Range. Chembiochem Eur. J. Chem. Biol. 2018, 19, 1255–1258. [Google Scholar] [CrossRef]
- Möglich, A.; Ayers, R.A.; Moffat, K. Design and signaling mechanism of light-regulated histidine kinases. J. Mol. Biol. 2009, 385, 1433–1444. [Google Scholar] [CrossRef] [Green Version]
- Tabor, J.J.; Levskaya, A.; Voigt, C.A. Multichromatic control of gene expression in Escherichia coli. J. Mol. Biol. 2011, 405, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zhang, J.; Jin, J.; Geng, Z.; Qi, Q.; Liang, Q. Programming Bacteria With Light-Sensors and Applications in Synthetic Biology. Front. Microbiol. 2018, 9, 2692. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Xu, X.; Miao, J.; Yao, J.; Liu, R.; Zhao, Y.; Chen, X.; Yang, Y. A single-component light sensor system allows highly tunable and direct activation of gene expression in bacterial cells. Nucleic Acids Res. 2020, 48, e33. [Google Scholar] [CrossRef] [PubMed]
- De Mena, L.; Rizk, P.; Rincon-Limas, D.E. Bringing Light to Transcription: The Optogenetics Repertoire. Front. Genet. 2018, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Sheets, M.B.; Wong, W.W.; Dunlop, M.J. Light-Inducible Recombinases for Bacterial Optogenetics. ACS Synth. Biol. 2020, 9, 227–235. [Google Scholar] [CrossRef]
- Yüz, S.G.; Ricken, J.; Wegner, S.V. Independent Control over Multiple Cell Types in Space and Time Using Orthogonal Blue and Red Light Switchable Cell Interactions. Adv. Sci. (Weinh. Baden-Wurtt. Ger.) 2018, 5, 1800446. [Google Scholar] [CrossRef]
- Abe, K.; Miyake, K.; Nakamura, M.; Kojima, K.; Ferri, S.; Ikebukuro, K.; Sode, K. Engineering of a green-light inducible gene expression system in Synechocystis sp. PCC6803. Microb. Biotechnol. 2014, 7, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Abe, K.; Ferri, S.; Nakajima, M.; Nakamura, M.; Yoshida, W.; Kojima, K.; Ikebukuro, K.; Sode, K. A green-light inducible lytic system for cyanobacterial cells. Biotechnol. Biofuels 2014, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandar, S.T.; Senoo, S.; Toya, Y.; Shimizu, H. Optogenetic switch for controlling the central metabolic flux of Escherichia coli. Metab. Eng. 2019, 55, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.M.; Zhang, Y.; Mehl, J.; Park, H.; Lalwani, M.A.; Toettcher, J.E.; Avalos, J.L. Optogenetic regulation of engineered cellular metabolism for microbial chemical production. Nature 2018, 555, 683–687. [Google Scholar] [CrossRef]
- Ding, Q.; Ma, D.; Liu, G.Q.; Li, Y.; Guo, L.; Gao, C.; Hu, G.; Ye, C.; Liu, J.; Liu, L.; et al. Light-powered Escherichia coli cell division for chemical production. Nat. Commun. 2020, 11, 2262. [Google Scholar] [CrossRef]
- Savenkova, L.; Gercberga, Z.; Nikolaeva, V.J.P.B. Mechanical properties and biodegradation characteristics of PHB-based films. Process Biochem. 2000, 35, 573–579. [Google Scholar] [CrossRef]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.Q. Grand Challenges for Industrializing Polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef]
- De Souza Pinto Lemgruber, R.; Valgepea, K.; Tappel, R.; Behrendorff, J.B.; Palfreyman, R.W.; Plan, M.; Hodson, M.P.; Simpson, S.D.; Nielsen, L.K.; Köpke, M.; et al. Systems-level engineering and characterisation of Clostridium autoethanogenum through heterologous production of poly-3-hydroxybutyrate (PHB). Metab. Eng. 2019, 53, 14–23. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.J.; Moon, M.; Lee, J.S.; Min, K. Recent progress and challenges in microbial polyhydroxybutyrate (PHB) production from CO(2) as a sustainable feedstock: A state-of-the-art review. Bioresour. Technol. 2021, 339, 125616. [Google Scholar] [CrossRef]
- Chang, Y.; Su, T.; Qi, Q.; Liang, Q. Easy regulation of metabolic flux in Escherichia coli using an endogenous type I-E CRISPR-Cas system. Microb. Cell Factories 2016, 15, 195. [Google Scholar] [CrossRef] [Green Version]
- Gu, F.; Jiang, W.; Mu, Y.; Huang, H.; Su, T.; Luo, Y.; Liang, Q.; Qi, Q. Quorum Sensing-Based Dual-Function Switch and Its Application in Solving Two Key Metabolic Engineering Problems. ACS Synth. Biol. 2020, 9, 209–217. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, Y.; Liang, Q.; Qi, Q. Autoinduced AND Gate Controls Metabolic Pathway Dynamically in Response to Microbial Communities and Cell Physiological State. ACS Synth. Biol. 2017, 6, 463–470. [Google Scholar] [CrossRef]
- Zhao, E.M.; Lalwani, M.A.; Chen, J.M.; Orillac, P.; Toettcher, J.E.; Avalos, J.L. Optogenetic Amplification Circuits for Light-Induced Metabolic Control. ACS Synth. Biol. 2021, 10, 1143–1154. [Google Scholar] [CrossRef]
- Schmidl, S.R.; Sheth, R.U.; Wu, A.; Tabor, J.J. Refactoring and optimization of light-switchable Escherichia coli two-component systems. ACS Synth. Biol. 2014, 3, 820–831. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; He, X.; Luo, Y.; Mu, Y.; Gu, F.; Liang, Q.; Qi, Q. Two Completely Orthogonal Quorum Sensing Systems with Self-Produced Autoinducers Enable Automatic Delayed Cascade Control. ACS Synth. Biol. 2020, 9, 2588–2599. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rodriguez, J.; Moser, F.; Song, M.; Voigt, C.A. Engineering RGB color vision into Escherichia coli. Nat. Chem. Biol. 2017, 13, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, J.; Sun, Z.; Luan, Y.; Li, Y.; Wang, J.; Liang, Q.; Qi, Q. Engineering an in vivo EP-bifido pathway in Escherichia coli for high-yield acetyl-CoA generation with low CO(2) emission. Metab. Eng. 2019, 51, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, P.; Devarajan, K.; Chua, T.K.; Zhang, H.; Gunawan, E.; Poh, C.L. Blue light-mediated transcriptional activation and repression of gene expression in bacteria. Nucleic Acids Res. 2016, 44, 6994–7005. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.F.; Ling, H.; Foo, J.L.; Chang, M.W. Synthetic genetic circuits for programmable biological functionalities. Biotechnol. Adv. 2019, 37, 107393. [Google Scholar] [CrossRef]
- Brophy, J.A.; Voigt, C.A. Principles of genetic circuit design. Nat. Methods 2014, 11, 508–520. [Google Scholar] [CrossRef] [Green Version]
- Davis, B.D.; Gilvarg, C. The role of the tricarboxylic acid cycle in acetate oxidation in Escherichia coli. J. Biol. Chem. 1956, 222, 307–319. [Google Scholar] [PubMed]
- Shen, R.; Ning, Z.Y.; Lan, Y.X.; Chen, J.C.; Chen, G.Q. Manipulation of polyhydroxyalkanoate granular sizes in Halomonas bluephagenesis. Metab. Eng. 2019, 54, 117–126. [Google Scholar] [CrossRef]
- Zhao, P.; Li, Q.; Tian, P.; Tan, T. Switching metabolic flux by engineering tryptophan operon-assisted CRISPR interference system in Klebsiella pneumoniae. Metab. Eng. 2021, 65, 30–41. [Google Scholar] [CrossRef]
- Mu, Q.; Zhang, S.; Mao, X.; Tao, Y.; Yu, B. Highly efficient production of L-homoserine in Escherichia coli by engineering a redox balance route. Metab. Eng. 2021, 67, 321–329. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, J.; Ma, W.; Yang, J.; Zhang, H.; Zhao, L.; Chen, S.; Zhang, S.; Hu, X.; Li, Y.; et al. Rebalancing microbial carbon distribution for L-threonine maximization using a thermal switch system. Metab. Eng. 2020, 61, 33–46. [Google Scholar] [CrossRef] [PubMed]

| Property | Photosensory System | |||
|---|---|---|---|---|
| RBS10–CcaS#10–CcaR | EL222 | YF1–FixJ–PhlF | LexRO | |
| Sensory light | Activation in green light Repression in red light | Activation in blue light Repression in darkness | Activation in blue light Repression in darkness | Activation in blue light Repression in darkness |
| Photosensory range | 10-fold | 7-fold | 10-fold | 1.3-fold |
| Leakage | Negligible | Fewer leakage in green light | No leakage | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Luo, Y.; Jiang, W.; Li, X.; Qi, Q.; Liang, Q. Development of Optogenetic Dual-Switch System for Rewiring Metabolic Flux for Polyhydroxybutyrate Production. Molecules 2022, 27, 617. https://doi.org/10.3390/molecules27030617
Wang S, Luo Y, Jiang W, Li X, Qi Q, Liang Q. Development of Optogenetic Dual-Switch System for Rewiring Metabolic Flux for Polyhydroxybutyrate Production. Molecules. 2022; 27(3):617. https://doi.org/10.3390/molecules27030617
Chicago/Turabian StyleWang, Sumeng, Yue Luo, Wei Jiang, Xiaomeng Li, Qingsheng Qi, and Quanfeng Liang. 2022. "Development of Optogenetic Dual-Switch System for Rewiring Metabolic Flux for Polyhydroxybutyrate Production" Molecules 27, no. 3: 617. https://doi.org/10.3390/molecules27030617
APA StyleWang, S., Luo, Y., Jiang, W., Li, X., Qi, Q., & Liang, Q. (2022). Development of Optogenetic Dual-Switch System for Rewiring Metabolic Flux for Polyhydroxybutyrate Production. Molecules, 27(3), 617. https://doi.org/10.3390/molecules27030617






