Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases
Abstract
1. Introduction
2. Origin and Synthesis of Glucosinolates (GLs) and Isothiocyanates (ITCs)
3. Glucosinolates (GLs)
4. Isothiocyanates (ITCs)
5. Mechanism of Cardio- and Neuroprotective Effect
5.1. Cardioprotective Effect
5.1.1. Sulforaphane (SFN)
5.1.2. Phenethyl Isothiocyanate (PEITC)
5.1.3. Moringin (MG)
5.1.4. Erucin (ER)
5.1.5. Allyl Isothiocyanate (AITC)
5.1.6. Indole-3-Carbinol (I3C)
5.2. Neuroprotective Effect
5.2.1. Sulforaphane (SFN)
5.2.2. Phenethyl Isothiocyanate (PEITC)
5.2.3. Moringin (MG)
5.2.4. Erucin (ER)
5.2.5. Allyl Isothiocyanate (AITC)
5.2.6. Indole-3-Carbinol (I3C)
6. Adverse Effects of Glucosinolates (GLs) and Isothiocyanates (ITCs)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Wahbi, K. CNS-disease affecting the heart: Brain—Heart disorders. J. Neurol. Sci. 2014, 345, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Morales, I.; Farías, G.A.; Cortes, N.; Maccioni, R.B. Neuroinflammation and Neurodegeneration. Update Dement. IntechOpen 2016, 17–47. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Deuschl, G. Neuroinflammation—A common thread in neurological disorders. Nat. Rev. 2019, 15, 429–430. [Google Scholar] [CrossRef]
- Shah, S.M.A.; Akram, M.; Riaz, M.; Munir, N.; Rasool, G. Cardioprotective Potential of Plant-Derived Molecules: A Scientific and Medicinal Approach. Dose-Response Int. J. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Galuppo, M.; Montaut, S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. An overview on neuroprotective effects of isothiocyanates for the treatment of neurodegenerative diseases. Fitoterapia 2015, 106, 12–21. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kopeć, A.; Piątkowska, E.; Borczak, B.; Leszczyńska, T. The Beneficial Effects of Brassica Vegetables on Human Health. Rocz. Panstw. Zakl. Hig. 2012, 63, 389–395. [Google Scholar] [PubMed]
- Kala, C.; Ali, S.S.; Ahmad, N.; Gilani, S.J.; Khan, N.A. Isothiocyanates: A review. Res. J. Pharmacogn. 2018, 5, 71–89. [Google Scholar] [CrossRef]
- Ioannides, C.; Hanlon, N.; Konsue, N. Isothiocyanates: A Chemical Class of Potential Nutraceuticals. Open Nutraceuticals J. 2010, 3, 55–62. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Li, L.; Zhou, X.; Li, N.; Sun, M.; Lv, J.; Xu, Z. Herbal drugs against cardiovascular disease: Traditional medicine and modern development. Drug Discov. Today 2015, 20, 1074–1086. [Google Scholar] [CrossRef]
- Bernstein, N.; Akram, M.; Daniyal, M.; Koltai, H.; Fridlender, M.; Gorelick, J. Antiinflammatory Potential of Medicinal Plants: A Source for Therapeutic Secondary Metabolites. Adv. Agron. 2018, 150, 131–183. [Google Scholar]
- Palliyaguru, D.L.; Yuan, J.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the power of plants to people. Mol. Nutr. Food Res. 2019, 62, 1–23. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates From Cruciferous Vegetables and Their Potential Role in Chronic Disease: Investigating the Preclinical and Clinical Evidence. Front. Pharmacol. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Fimognari, C.; Turrini, E.; Ferruzzi, L.; Lenzi, M.; Hrelia, P. Natural isothiocyanates: Genotoxic potential versus chemoprotection. Mutat. Res. 2012, 750, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Igado, O.O.; Olopade, J.O. A Review on the Possible Neuroprotective Effects of Moringa Oleifera Leaf Extract. Niger. J. Physiol. Sci. 2016, 31, 183–187. [Google Scholar]
- Fenwick, G.R.; Heaney, R.K.; Mullin, W.J.; Vanetten, C.H. Glucosinolates and their breakdown products in food and food plants. CRC Crit. Rev. Food Sci. Nutr. 2009, 18, 123–201. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, L.; Heinrichs, H.; Hornbacher, J.; Papenbrock, J.; Nguyen-Thanh, B.; Selmar, D. Protein content and glucosinolates from Moringa oleifera Lam. – New insights into an auspicious commodity. J. Appl. Bot. Food Qual. 2020, 93, 257–265. [Google Scholar] [CrossRef]
- Brunelli, D.; Tavecchio, M.; Falcioni, C.; Frapolli, R.; Erba, E.; Iori, R.; Rollin, P.; Barillari, J.; Manzotti, C.; Morazzoni, P. The isothiocyanate produced from glucomoringin inhibits NF-κB and reduces myeloma growth in nude mice in vivo. Biochem. Pharmacol. 2010, 79, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Mellon, F.A.; Foidl, N.; Pratt, J.H.; Dupont, M.S.; Perkins, L.; Kroon, P.A. Profiling Glucosinolates and Phenolics in Vegetative and Reproductive Tissues of the Multi-Purpose Trees Moringa oleifera L. (Horseradish Tree) and Moringa stenopetala L. J. Agric. Food Chem 2003, 51, 3546–3553. [Google Scholar] [CrossRef]
- Di Gioia, F.; Pinela, J.; Bailón, A.D.H.; Fereira, I.C.F.R.; Petropoulos, S.A. The dilemma of “good ” and “ bad ” glucosinolates and the potential to regulate their content. In Glucosinolates: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–31. ISBN 9780128164938. [Google Scholar]
- Possenti, M.; Baima, S.; Raffo, A.; Durazzo, A.; Giusti, A.M.; Natella, F. Glucosinolates in Food. In Glucosinolates; Mérillon, M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–47. [Google Scholar]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Lafarga, T.; Bobo, G.; Vinas, I.; Collazo, C.; Aguilo-Aguayo, I. Effects of thermal and non-thermal processing of cruciferous vegetables on glucosinolates and its derived forms. J. Food Sci. Technol. Technol 2018, 55, 1973–1981. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 1–57. [Google Scholar] [CrossRef]
- Traka, M.; Mithen, R. Glucosinolates, isothiocyanates and human health. Phytochem Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Melrose, J. The Glucosinolates: A Sulphur Glucoside Family of Mustard Anti-Tumour and Antimicrobial Phytochemicals of Potential Therapeutic Application. Biomedicines 2019, 7, 62. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 9601691, Glucobrassicin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Glucobrassicin (accessed on 31 December 2021).
- National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 6602383, Glucoraphanin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Glucoraphanin (accessed on 31 December 2021).
- National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 656555, Gluconasturtiin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Gluconasturtiin (accessed on 31 December 2021).
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.-N.T. Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr. Pharmacol. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Jaafaru, M.S.; Karim, N.A.A.; Eliaser, E.M.; Waziri, P.M.; Ahmed, H.; Barau, M.M.; Kong, L.; Abdull Razis, A.F. Nontoxic Glucomoringin-Isothiocyanate (GMG-ITC) Rich Soluble Extract Induces Apoptosis and Inhibits Proliferation of Human Prostate Adenocarcinoma Cells (PC-3). Nutrients 2018, 10, 1174. [Google Scholar] [CrossRef] [PubMed]
- Jaafaru, M.S.; Abd Karim, N.A.; Enas, M.E.; Rollin, P.; Mazzon, E.; Abdull Razis, A.F. Protective Effect of Glucosinolates Hydrolytic Products in Neurodegenerative Diseases (NDDs). Nutrients 2018, 10, 580. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Burčul, F.; Mekinić, I.G.; Radan, M.; Rollin, P.; Blazevic, I. Isothiocyanates: Cholinesterase inhibiting, antioxidant, and anti-inflammatory activity. J. Enzym. Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef]
- Prabu, S.L.; Umamaheswari, A.; Puratchikody, A. Phytopharmacological potential of the natural gift Moringa oleifera Lam and its therapeutic application: An overview. Asian Pac. J. Trop. Med. 2019, 12, 485–498. [Google Scholar] [CrossRef]
- Munday, R.; Munday, C.M. Induction of Phase II Detoxification Enzymes in Rats by Plant-Derived Isothiocyanates: Comparison of Allyl Isothiocyanate with Sulforaphane and Related Compounds. J. Agric. Food Chem. 2004, 52, 1867–1871. [Google Scholar] [CrossRef]
- Clarke, J.D.; Hsu, A.; Riedl, K.; Bella, D.; Schwartz, S.J.; Stevens, J.F.; Ho, E. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol. Res. 2011, 64, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Hollands, W.; Teucher, B.; Needs, P.W.; Narbad, A.; Ortori, C.A.; Barrett, D.; Rossiter, J.T.; Mithen, R.; Kroon, P.A. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared. Mol. Nutr. Food Res. 2012, 56, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Piragine, E.; Pagnotta, E.; Ugolini, L.; Mannelli, L.D.C.; Testai, L.; Ghelardini, C.; Lazzeri, L.; Calderone, V.; Martelli, A. Anticancer properties of erucin, an H2S - releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC - 1). Phyther. Res. 2019, 33, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Bricker, G.V.; Riedl, K.M.; Ralston, R.A.; Tober, K.L.; Oberyszyn, T.M.; Schwartz, S.J. Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Mol. Nutr. Food Res. 2014, 58, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Rohn, S.; Mewis, I.; Schreiner, M.; Kroh, L.W. Influence of the chemical structure on the thermal degradation of the glucosinolates in broccoli sprouts. Food Chem. 2012, 130, 1–8. [Google Scholar] [CrossRef]
- Gupta, P.; Wright, S.E.; Kim, S.; Srivastava, S.K. Phenethyl isothiocyanate: A comprehensive review of anti-cancer mechanisms. Biochim. Biophys. Acta. 2014, 1846, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhauser, C.; Mithen, R.; et al. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, 219–265. [Google Scholar] [CrossRef] [PubMed]
- Platz, S.; Piberger, A.L.; Budnowski, J.; Herz, C.; Schreiner, M.; Blaut, M.; Hartwig, A.; Lamy, E.; Hanske, L.; Rohn, S. Bioavailability and biotransformation of sulforaphane and erucin metabolites in different biological matrices determined by LC – MS – MS. Anal. Bioanal. Chem. 2015, 407, 1819–1829. [Google Scholar] [CrossRef]
- Kolm, R.H.; Danielson, U.H.; Zhang, Y.; Talalay, P.; Mannervik, B. Isothiocyanates as substrates for human glutathione transferases: Structure-activity studies. Biochem. J. 1995, 311, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, N.; Poynton, C.L.; Coldham, N.; Sauer, M.J.; Ioannides, C. The aliphatic isothiocyanates erucin and sulforaphane do not effectively up-regulate NAD(P)H: Quinone oxidoreductase (NQO1) in human liver compared with rat. Mol. Nutr. Food Res. 2009, 53, 836–844. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 9548605, Glucotropaeolin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9548605 (accessed on 31 December 2021).
- National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 6911854, Sinigrin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sinigrin (accessed on 31 December 2021).
- National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 16741, Phenethyl isothiocyanate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phenethyl-isothiocyanate (accessed on 31 December 2021).
- National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 2346, Benzyl isothiocyanate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Benzyl-isothiocyanate (accessed on 31 December 2021).
- National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 5971, Allyl isothiocyanate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Allyl-isothiocyanate (accessed on 31 December 2021).
- National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 9577379, (R)-sulforaphane. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/R_-sulforaphane (accessed on 31 December 2021).
- Deshmukh, P.; Unni, S.; Krishnappa, G.; Padmanabhan, B. The Keap1 – Nrf2 pathway: Promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys. Rev. 2017, 9, 41–56. [Google Scholar] [CrossRef]
- Aekthammarat, D.; Tangsucharit, P.; Pannangpetch, P.; Sriwantanad, T.; Sibmooh, N. Moringa oleifera leaf extract enhances endothelial nitric oxide production leading to relaxation of resistance artery and lowering of arterial blood pressure. Biomed. Pharmacother. 2020, 130, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sita, G.; Hrelia, P.; Tarozzi, A.; Morroni, F. Isothiocyanates Are Promising Compounds against Oxidative Stress, Neuroinflammation and Cell Death that May Benefit Neurodegeneration in Parkinson’ s Disease. Int. J. Mol. Sci. 2016, 17, 1454. [Google Scholar] [CrossRef]
- Matsui, T.; Nakamura, N.; Ojima, A.; Nishino, Y.; Yamagishi, S. Sulforaphane reduces advanced glycation end products (AGEs) -induced inflammation in endothelial cells and rat aorta. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 797–807. [Google Scholar] [CrossRef]
- Silva-Palacios, A.; Ostolga-Chavarría, M.; Sánchez-Garibay, C.; Rojas-Morales, P.; Galván-Arzate, S.; Buelna-Chontal, M.; Pavón, N.; Pedraza-Chaverrí, J.; Königsberg, M.; Zazueta, C. Sulforaphane protects from myocardial ischemia-reperfusion damage through the balanced activation of Nrf2/AhR. Free Radic. Biol. Med. 2019, 143, 331–340. [Google Scholar] [CrossRef]
- Ma, T.; Zhu, D.; Chen, D.; Zhang, Q.; Dong, H.; Wu, W.; Lu, H.; Wu, G. Sulforaphane, a Natural Isothiocyanate Compound, Improves Cardiac Function and Remodeling by Inhibiting Oxidative Stress and Inflammation in a Rabbit Model of Chronic Heart Failure. Med. Sci. Monit. 2018, 24, 1473–1483. [Google Scholar] [CrossRef]
- Pereyra, K.V.; Andrade, D.C.; Toledo, C.; Schwarz, K.G.; Uribe-Ojeda, A.; Ríos-Gallardo, A.P.; Quintanilla, R.A.; Contreras, S.; Mahn, A.; Rio, R. Del Dietary supplementation of a sulforaphane-enriched broccoli extract protects the heart from acute cardiac stress. J. Funct. Foods 2020, 75, 1–7. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, G.; Huang, E.C.; Huang, J.; Cai, J.; Cai, L.; Wang, S.; Keller, B.B. Sulforaphane Prevents Right Ventricular Injury and Reduces Pulmonary Vascular Remodeling in Pulmonary Arterial Hypertension. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Bose, C.; Alves, I.; Singh, P.; Palade, P.T.; Carvalho, E.; Børsheim, E.; Cheema, S.J.A.; Boerma, M.; Awasthi, S.; Singh, S.P. Sulforaphane prevents age-associated cardiac and muscular dysfunction through Nrf2 signaling. Aging Cell 2020, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, R.; Zhan, Z.; Li, X.; Zhou, F.; Xing, A.; Jiang, C.; Chen, Y.; An, L. Beneficial Effects of Sulforaphane Treatment in Alzheimer’s Disease May Be Mediated through Reduced HDAC1/3 and Increased P75NTR Expression. Front. Aging Neurosci. 2017, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Miao, Q. Sulforaphane Ameliorates Neurobehavioral Deficits and Protects the Brain From Amyloid β Deposits and Peroxidation in Mice With Alzheimer-Like Lesions. Am. J. Alzheimer’s Dis. Other Demen. 2015, 30, 183–191. [Google Scholar] [CrossRef]
- Hou, T.-T.; Yang, H.-Y.; Wang, W.; Wu, Q.-Q.; Tian, Y.-R.; Jia, J.-P. Sulforaphane Inhibits the Generation of Amyloid-β Oligomer and Promotes Spatial Learning and Memory in Alzheimer’s Disease (PS1V97L) Transgenic Mice. J. Alzheimer’s Dis. 2018, 62, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Buonfiglio, M.; Corona, G.; Chirafisi, J.; Vafeiadou, K.; Angeloni, C.; Hrelia, S.; Hrelia, P.; Spencer, J.P.E. Sulforaphane protects cortical neurons against 5-S-cysteinyl-dopamine-induced toxicity through the activation of ERK1/2, Nrf-2 and the upregulation of detoxification enzymes. Mol. Nutr. Food Res. 2010, 54, 532–542. [Google Scholar] [CrossRef]
- Morroni, F.; Tarozzi, A.; Sita, G.; Bolondi, C.; Manuel, J.; Moraga, Z.; Cantelli-forti, G.; Hrelia, P. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson’s disease. Neurotoxicology 2013, 36, 63–71. [Google Scholar] [CrossRef]
- Zhao, J.; Kobori, N.; Aronowski, J.; Dash, P.K. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci. Lett. 2006, 393, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Ping, Z.; Liu, W.; Kang, Z.; Cai, J.; Wang, Q.; Cheng, N.; Wang, S.; Wang, S.; Zhang, J.H.; Sun, X. Sulforaphane protects brains against hypoxic – ischemic injury through induction of Nrf2-dependent phase 2 enzyme. Brain Res. 2010, 1343, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.; Kim, M.; Kim, J.; Sung, J.; Park, S.T.; Ahn, S.-W. The Anti-Inflammatory Effect of Sulforaphane in Mice with Experimental Autoimmune Encephalomyelitis. J. Korean Med. Sci. 2019, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Y.; Zhang, G.; Fang, H.; Wang, H.; Zang, H.; Xie, T.; Wang, W. Activation of Nrf2-ARE signal pathway protects the brain from damage induced by epileptic seizure. Brain Res. 2014, 1544, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Gao, Q.; Zhao, P.; Gao, Y.; Xi, Y.; Wang, X.; Liang, Y.; Shi, H.; Ma, Y. Sulforaphane produces antidepressant- and anxiolytic-like effects in adult mice. Behav. Brain Res. 2016, 301, 55–62. [Google Scholar] [CrossRef]
- Mas, S.; Gasso, P.; Triasa, G.; Bernardo, M.; Lafuente, A. Sulforaphane protects SK-N-SH cells against antipsychotic-induced oxidative stress. Fundam. Clin. Pharmacol. 2011, 26, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.; Kim, Y.; Nam, C.; Whiteman, M. β-Phenylethyl and 8-methylsulphinyloctyl isothiocyanates, constituents of watercress, suppress LPS induced production of nitric oxide and prostaglandin E2 in RAW 264. 7 macrophages. Nitric Oxide 2005, 12, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Nakatsuji, Y.; Maeda, A.; Ota, H.; Kamikubo, R.; Miyoshi, N.; Nakamura, Y.; Akagawa, M. Phenethyl isothiocyanate activates leptin signaling and decreases food intake. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gwon, M.-H.; Im, Y.-S.; Seo, A.-R.; Kim, K.Y.; Moon, H.-R.; Yun, J.-M. Phenethyl Isothiocyanate Protects against High Fat/Cholesterol Diet-Induced Obesity and Atherosclerosis in C57BL/6 Mice. Nutrients 2020, 12, 3657. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, M.; Wang, J.; Wang, S.; Zhang, J.; Guo, Y.; Tang, Y.; Gu, J. NRF2-Related Epigenetic Modifications in Cardiac and Vascular Complications of Diabetes Mellitus. Front. Endocrinol. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Ho, J.-N.; Yoon, H.-G.; Park, C.-S.; Kim, S.; Jun, W.; Choue, R.; Lee, J. Isothiocyanates Ameliorate the Symptom of Heart Dysfunction and Mortality in a Murine AIDS Model by Inhibiting Apoptosis in the Left Ventricle 1. J. Med. Food 2012, 15, 781–787. [Google Scholar] [CrossRef]
- Li, Y.; Ji, Q.; Wang, Z.; Shen, L.; He, B. Moringa oleifera seeds mitigate myocardial injury and prevent ventricular failure induced by myocardial infarction. Am. J. Transl. Res. 2020, 12, 4511–4521. [Google Scholar] [PubMed]
- Nandave, M.; Ojha, S.K.; Joshi, S.; Kumari, S.; Arya, D.S. Moringa oleifera Leaf Extract Prevents Isoproterenol-Induced Myocardial Damage in Rats: Evidence for an Antioxidant, Antiperoxidative, and Cardioprotective Intervention. J. Med. Food 2009, 12, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, T.; Joshi, U.; Desai, V.; Desai, T.; Tirgar, P. Investigation of Cardiotonic Activity of Moringa oleifera Roots in Doxorubicin-Induced Congestive Heart Failure. J. Pharm. Res. 2012, 5, 3687–3691. [Google Scholar]
- Jaja-chimedza, A.; Zhang, L.; Wol, K.; Graf, B.L.; Kuhn, P.; Moskal, K.; Carmouche, R.; Newman, S.; Salbaum, J.M.; Raskin, I. A dietary isothiocyanate-enriched moringa (Moringa oleifera) seed extract improves glucose tolerance in a high-fat-diet mouse model and modulates the gut microbiome. J. Funct. Foods 2018, 47, 376–385. [Google Scholar] [CrossRef]
- Hannan, M.A.; Kang, J.-Y.; Mohibbullah, M.; Hong, Y.-K.; Lee, H.; Choi, J.-S.; Choi, I.S.; Moon, I.S. Moringa oleifera with promising neuronal survival and neurite outgrowth promoting potentials. J. Ethnopharmacol. 2014, 152, 142–150. [Google Scholar] [CrossRef]
- Kirisattayakul, W.; Wattanathorn, J.; Tong-Un, T.; Muchimapura, S.; Wannanon, P.; Jittiwat, J. Cerebroprotective Effect of Moringa oleifera against Focal Ischemic Stroke Induced by Middle Cerebral Artery Occlusion. Oxid. Med. Cell. Longev. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Guha, D. Alteration of brain monoamines & EEG wave pattern in rat model of Alzheimer’s disease & protection by Moringa oleifera. Indian J. Med. Res. 2008, 128, 744–751. [Google Scholar]
- Silvestro, S.; Chiricosta, L.; Gugliandolo, A.; Iori, R.; Rollin, P.; Perenzoni, D.; Mattivi, F.; Bramanti, P.; Mazzon, E. The Moringin/α-CD Pretreatment Induces Neuroprotection in an In Vitro Model of Alzheimer’s Disease: A Transcriptomic Study. Curr. Issues Mol. Biol. 2021, 43, 17. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Rajan, T.S.; De Nicola, G.R.; Iori, R.; Bramanti, P.; Mazzon, E. Moringin activates Wnt canonical pathway by inhibiting GSK3 β in a mouse model of experimental autoimmune encephalomyelitis. Drug Des. Devel. Ther. 2016, 10, 3291–3304. [Google Scholar] [CrossRef]
- Giacoppo, S.; Rajan, T.S.; De Nicola, G.R.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The isothiocyanate isolated from Moringa oleifera shows potent anti-inflammatory activity in the treatment of murine sub-acute Parkinson’s disease. Rejuvenation Res. 2017, 20, 50–63. [Google Scholar] [CrossRef]
- Giacoppo, S.; Galuppo, M.; De Nicola, G.R.; Iori, R.; Bramanti, P.; Mazzon, E. 4(α-L-Rhamnosyloxy)-benzyl isothiocyanate, a bioactive phytochemical that attenuates secondary damage in an experimental model of spinal cord injury. Bioorg. Med. Chem. 2015, 23, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Mammana, S.; Gugliandolo, A.; Cavalli, E.; Diomede, F.; Iori, R.; Zappacosta, R.; Bramanti, P.; Conti, P.; Fontana, A.; Pizzicannella, J.; et al. Human gingival mesenchymal stem cells pretreated with vesicular moringin nanostructures as a new therapeutic approach in a mouse model of spinal cord injury. J. Tissue Eng. Regen. Med. 2019, 13, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Galuppo, M.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Bramanti, P.; Mazzon, E. Administration of 4-(α-L-Rhamnosyloxy)-benzyl Isothiocyanate Delays Disease Phenotype in SOD1 G93A Rats: A Transgenic Model of Amyotrophic Lateral Sclerosis. Biomed. Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Rajan, T.S.; De Nicola, G.R.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Anticancer activity of glucomoringin isothiocyanate in human malignant astrocytoma cells. Fitoterapia 2016, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Azarenko, O.; Jordan, M.A.; Wilson, L. Erucin, the Major Isothiocyanate in Arugula (Eruca sativa), Inhibits Proliferation of MCF7 Tumor Cells by Suppressing Microtubule Dynamics. PLoS ONE 2014, 9, 1–12. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Giacoppo, S.; Ficicchia, M.; Aliquò, A.; Bramanti, P.; Mazzon, E. Eruca sativa seed extract: A novel natural product able to counteract neuroinflammation. Mol. Med. Rep. 2018, 17, 6235–6244. [Google Scholar] [CrossRef]
- Piragine, E.; Flori, L.; Mannelli, L.D.C.; Ghelardini, C.; Pagnotta, E.; Matteo, R.; Lazzeri, L.; Martelli, A.; Miragliotta, V.; Pirone, A.; et al. Eruca sativa Mill. seed extract promotes anti-obesity and hypoglycemic effects in mice fed with a high-fat diet. Phyther. Res. 2020, 35, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Piragine, E.; Citi, V.; Testai, L.; Pagnotta, E.; Manzo, O.L.; Ugolini, L.; Lazzeri, L.; Mannelli, L.D.C.; Bucci, M.; et al. Erucin exhibits vasorelaxing effects and antihypertensive activity by H2S-releasing properties. Br. J. Pharmacol. 2020, 177, 824–835. [Google Scholar] [CrossRef]
- Martelli, A.; Piragine, E.; Gorica, E.; Citi, V.; Testai, L.; Pagnotta, E.; Lazzeri, L.; Pecchioni, N.; Ciccone, V.; Montanaro, R.; et al. The H2S-Donor Erucin Exhibits Protective Effects against Vascular Inflammation in Human Endothelial and Smooth Muscle Cells. Antioxidants 2021, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, B.; El-masry, T.A.; Tousson, E.; Alarfaj, S.J.; Saleh, A. Therapeutic effect of rocket seeds (Eruca sativa L.) against hydroxyapatite nanoparticles injection induced cardiac toxicity in rats. Pak. J. Pharm. Sci. 2020, 33, 1839–1845. [Google Scholar]
- Morroni, F.; Sita, G.; Djemil, A.; Amico, M.D.; Pruccoli, L.; Cantelli-forti, G.; Hrelia, P.; Tarozzi, A. Comparison of Adaptive Neuroprotective Mechanisms of Sulforaphane and its Interconversion Product Erucin in in Vitro and in Vivo Models of Parkinson’s Disease. J. Agric. Food Chem. 2018, 66, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Oowatari, Y.; Ogawa, T.; Katsube, T.; Iinuma, K.; Yoshitomi, H.; Gao, M. Wasabi leaf extracts attenuate adipocyte hypertrophy through PPARγ and AMPK. Biosci. Biotechnol. Biochem. 2016, 80, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Im, S.W.; Jung, C.H.; Ha, T.Y. Allyl isothiocyanate ameliorates insulin resistance through the regulation of mitochondrial function. J. Nutr. Biochem. 2014, 25, 1026–1034. [Google Scholar] [CrossRef]
- Hansted, A.K.; Bhatt, D.K.; Olesen, J.; Jensen, L.J.; Jansen-Olesen, I. Effect of TRPA1 activator allyl isothiocyanate (AITC) on rat dural and pial arteries. Pharmacol. Rep. 2019, 71, 565–572. [Google Scholar] [CrossRef]
- Caglayan, B.; Kilic, E.; Dalay, A.; Altunay, S.; Tuzcu, M.; Erten, F.; Orhan, C.; Gunal, M.Y.; Yulug, B.; Juturu, V.; et al. Allyl isothiocyanate attenuates oxidative stress and inflammation by modulating Nrf2/HO-1 and NF-κB pathways in traumatic brain injury in mice. Mol. Biol. Rep. 2019, 46, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Wei, L.; Zong, J.; Bian, Z.; Zhou, H.; Zhang, R.; Tang, Q. Attenuation of cardiac remodeling by indole-3-carbinol in mice is associated with improved energy metabolism. Int. J. Cardiol. 2014, 172, e531–e533. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, S.; Kanala, S.R.; Khatri, S.; Kumar, V.; Mallela, V.; Peraman, R. Cardioprotective potential of indol-3-carbinol against high salt induced myocardial stress and hypertrophy in Sprague Dawley rats besides molecular docking on muscarinic receptor-2. Nat. Prod. Res. 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, P.; Pugalendi, K.V.; Sankaran, M. Attenuation of hyperglycemia-mediated oxidative stress by indole-3-carbinol and its metabolite 3, 3 ′ - diindolylmethane in C57BL/6J mice. J. Physiol. Biochem. 2014, 70, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, G.; Liao, Q.; Zou, Y.; Du, Y.; Huang, J. Indole-3-carbinol inhibits lipid deposition and promotes autophagy in hyperlipidemia zebra fish larvae. Environ. Toxicol. Pharmacol. 2019, 70, 103205. [Google Scholar] [CrossRef]
- Lee, B.D.; Yoo, J.-M.; Baek, S.Y.; Li, F.Y.; Sok, D.-E.; Kim, M.R. 3,3’-Diindolylmethane Promotes BDNF and Antioxidant Enzyme Formation via TrkB/Akt Pathway Activation for Neuroprotection against Oxidative Stress-Induced Apoptosis in Hippocampal Neuronal Cells. Antioxidants 2019, 9, 3. [Google Scholar] [CrossRef]
- Saini, N.; Akhtar, A.; Chauhan, M.; Dhingra, N.; Sah, S.P. Protective effect of Indole-3-carbinol, an NF-κB inhibitor in experimental paradigm of Parkinson’s disease: In silico and in vivo studies. Brain Behav. Immun. 2020, 90, 108–137. [Google Scholar] [CrossRef]
- Cohen, T.; Frydman-Marom, A.; Rechter, M.; Gazit, E. Inhibition of Amyloid Fibril Formation and Cytotoxicity by Hydroxyindole Derivatives. Biochemistry 2006, 45, 4727–4735. [Google Scholar] [CrossRef]
- Qian, C.; Yang, C.; Lu, M.; Bao, J.; Shen, H.; Deng, B.; Li, S.; Li, W.; Zhang, M.; Cao, C. Activating AhR alleviates cognitive deficits of Alzheimer’s disease model mice by upregulating endogenous A β catabolic enzyme Neprilysin. Theranostics 2021, 11, 8797–8812. [Google Scholar] [CrossRef] [PubMed]
- Rouse, M.; Singh, N.P.; Nagarkatti, P.S.; Nagarkatti, M. Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and Th17 cells. Br. J. Pharmacol. 2013, 169, 1305–1321. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, P.; Chauhan, G.; Gautam, D.; Dash, D.; Patne, S.C.U.; Krishnamurthy, S. Indole-3-carbinol improves neurobehavioral symptoms in a cerebral ischemic stroke model. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 613–625. [Google Scholar] [CrossRef]
- Chuang, H.-Y.; Hsu, L.-Y.; Pan, C.-M.; Pikatan, N.W.; Yadav, V.K.; Fong, I.-H.; Chen, C.-H.; Yeh, C.-T.; Chiu, S.-C. The E3 Ubiquitin Ligase NEDD4-1 Mediates Temozolomide-Resistant Glioblastoma through PTEN Attenuation and Redox Imbalance in Nrf2 – HO-1 Axis. Int. J. Mol. Sci. 2021, 22, 247. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic Inflammation and Oxidative Stress as a Major Cause of Age- Related Diseases and Cancer. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxid. Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stresses and their classifications. Ukr. Biochem. J. 2015, 87, 11–18. [Google Scholar] [CrossRef]
- Katsiari, C.G.; Bogdanos, D.P.; Sakkas, L.I. Inflammation and cardiovascular disease. World J Transl Med 2019, 8, 1–8. [Google Scholar] [CrossRef]
- Sorriento, D.; Iaccarino, G. Inflammation and Cardiovascular Diseases: The Most Recent Findings. Int. J. Mol. Sci. 2019, 20, 3879. [Google Scholar] [CrossRef] [PubMed]
- Sethwala, A.M.; Goh, I.; Amerena, J.V. Combatting Inflammation in Cardiovascular Disease. Hear. Lung Circ. 2020, 1–10. [Google Scholar] [CrossRef]
- Citi, V.; Corvino, A.; Fiorino, F.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Brogi, S.; Flori, L.; Gorica, E.; et al. Structure-activity relationships study of isothiocyanates for H2S-releasing properties: 3-Pyridyl-isothiocyanate as a new promising cardioprotective agent. J. Adv. Res. 2021, 27, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Citi, V.; Testai, L.; Brogi, S.; Calderone, V. Organic Isothiocyanates as H2S-donors. Antioxid. Redox Signal. 2019, 1, 1–99. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, X.; Lu, Y.; Liang, D.; Huang, D. Isothiocyanates as H2S Donors Triggered by Cysteine: Reaction Mechanism and Structure and Activity Relationship. Org. Lett. 2019, 21, 5977–5980. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, X.; Pu, H.; Lin, Y.; Li, D.; Liu, S.-Q.; Huang, D. Moringin and its structural analogues as slow H2S donors, their mechanisms and bioactivity. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Liu, X.; Lin, Y.; Zheng, X.; Lu, Y. Hydrogen Sulfide (H2S) Releasing Capacity of Isothiocyanates from Moringa oleifera Lam. Molecules 2018, 23, 2809. [Google Scholar] [CrossRef]
- Testai, L.; Marino, A.; Piano, I.; Brancaleone, V.; Tomita, K.; Mannelli, L.D.C.; Martelli, A.; Citi, V.; Breschi, M.C.; Levi, R.; et al. The novel H2S-donor 4-carboxyphenyl isothiocyanate promotes cardioprotective effects against ischemia / reperfusion injury through activation of mitoK ATP channels and reduction of oxidative stress. Pharmacol. Res. 2016, 113, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Lu, X.; Song, J.; Li, H.; Wang, S. Molecular mechanisms of hydrogen sulfide against uremic accelerated atherosclerosis through cPKCβII/Akt signal pathway. BMC Nephrol. 2019, 20, 1–7. [Google Scholar] [CrossRef]
- Arauna, D.; Furrianca, M.; Espinosa-Parrilla, Y.; Fuentes, E.; Alarcón, M.; Palomo, I. Natural Bioactive Compounds As Protectors Of Mitochondrial Dysfunction In Cardiovascular Diseases And Aging. Molecules 2019, 24, 4259. [Google Scholar] [CrossRef] [PubMed]
- Corssac, G.B.; Campos-Carraro, C.; Hickmann, A.; da Rosa Araujo, A.S.; Fernandes, R.O.; Belló-Klein, A. Sulforaphane effects on oxidative stress parameters in culture of adult cardiomyocytes. Biomed. Pharmacother. 2018, 104, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mangla, B.; Javed, S.; Hadi, M.; Pankaj, S.; Kohli, K.; Najmi, A.; Alhazmi, H.A.; Al Bratty, M.; Ahsan, W. Sulforaphane: A review of its therapeutic potentials, advances in its nanodelivery, recent patents, and clinical trials. Phyther. Res. 2021, 35, 5440–5458. [Google Scholar] [CrossRef]
- Fernandes, R.O.; De Castro, A.L.; Bonetto, J.H.P.; Ortiz, V.D.; Müller, D.D.; Campos-Carraro, C.; Barbosa, S.; Neves, L.T.; Xavier, L.L.; Schenkel, P.C.; et al. Sulforaphane effects on postinfarction cardiac remodeling in rats: Modulation of redox-sensitive prosurvival and proapoptotic proteins. J. Nutr. Biochem. 2016, 34, 106–117. [Google Scholar] [CrossRef]
- Angeloni, C.; Hrelia, S.; Malaguti, M. Neuroprotective Effects of Glucosinolates. In Glucosinolates; Mérillon, J., Ramawat, K., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 275–299. [Google Scholar]
- Babu, P.V.A.; Petersen, C.; Jia, Z. Sulforaphane and Atherosclerosis. In Glucosinolates; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 319–337. ISBN 9783319254616. [Google Scholar]
- Chen, X.-L.; Dodd, G.; Kunsch, C. Sulforaphane inhibits TNF-α-induced activation of p38 MAP kinase and VCAM-1 and MCP-1 expression in endothelial cells. Inflamm. Res. 2009, 58, 513–521. [Google Scholar] [CrossRef]
- Huang, C.-S.; Lin, A.-H.; Liu, C.-T.; Tsai, C.-W.; Chang, I.-S.; Chen, H.-W.; Lii, C.-K. Isothiocyanates protect against oxidized LDL-induced endothelial dysfunction by upregulating Nrf2-dependent antioxidation and suppressing NF-kB activation. Mol. Nutr. Food Res. 2013, 57, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, H.; Hee, S.; Sohn, E.; Kim, B.; Moon, E.; Rhee, D.; Pyo, S. Sulforaphane suppresses vascular adhesion molecule-1 expression in TNF-α-stimulated mouse vascular smooth muscle cells: Involvement of the MAPK, NF-κB and AP-1 signaling pathways. Vascul. Pharmacol. 2012, 56, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Lee, Y.; Kim, W.; Jung, S.; Shin, K.; Yu, J.; Kyeong, M.; Lee, Y.; Tae, J.; Yun, Y.; et al. Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. J. Nutr. Biochem. 2014, 25, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Lee, Y.; Sin, D.; Lee, S.; Lee, M.K.; Lee, Y.; Hong, J.; Yun, Y.; Yoo, H. Sulforaphane Inhibits Mitotic Clonal Expansion During Adipogenesis Through Cell Cycle Arrest. Obesity 2012, 20, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Shawky, N.M.; Pichavaram, P.; Shehatou, G.S.G.; Suddek, G.M.; Gameil, N.M.; Jun, J.Y.; Segar, L. Sulforaphane improves dysregulated metabolic profile and inhibits leptin-induced VSMC proliferation: Implications toward suppression of neointima formation after arterial injury in western diet-fed obese mice. J. Nutr. Biochem. 2017, 32, 73–84. [Google Scholar] [CrossRef]
- Liu, Y.; Hsieh, C.; Weng, Y.; Chuang, S.; Hsieh, C.-Y.; Wung, B.-S. Sulforaphane inhibition of monocyte adhesion via the suppression of ICAM-1 and NF-κB is dependent upon glutathione depletion in endothelial cells. Vascul. Pharmacol. 2008, 48, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, P.; Si, H.; Babu, P.V.A.; Pan, D.; Fu, Y.; Brooke, E.A.S.; Shah, H.; Zhen, W.; Zhu, H.; Liu, D.; et al. Sulforaphane reduces vascular inflammation in mice and prevents TNF-α-induced monocyte adhesion to primary endothelial cells through interfering with the NF-κB pathway. J. Nutr. Biochem. 2014, 25, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.; Huang, H.; Wang, C.; Liu, K.; Lii, C. Sulforaphane Inhibits TNF-α-Induced Adhesion Molecule Expression Through the Rho A/ROCK/NF-κB Signaling Pathway. J. Med. Food 2014, 17, 1095–1102. [Google Scholar] [CrossRef]
- Kwon, J.-S.; Joung, H.; Kim, Y.S.; Shim, Y.-S.; Ahn, Y.; Jeong, M.H.; Kee, H.J. Sulforaphane inhibits restenosis by suppressing inflammation and the proliferation of vascular smooth muscle cells. Atherosclerosis 2012, 225, 41–49. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Qiu, Z.; Jiang, L. Sulforaphane Attenuates Angiotensin II-Induced Vascular Smooth Muscle Cell Migration via Suppression of NOX4/ROS/Nrf2 Signaling. Int. J. Biol. Sci. 2019, 15, 148–157. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Lim, Y.; Kim, S.-J.; Yoo, K.-D.; Yoo, H.-S.; Hong, J.-T.; Lee, M.-Y.; Yun, Y.-P. Sulforaphane inhibits PDGF-induced proliferation of rat aortic vascular smooth muscle cell by up-regulation of p53 leading to G1/S cell cycle arrest. Vascul. Pharmacol. 2013, 59, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Shehatou, G.S.G.; Suddek, G.M. Sulforaphane attenuates the development of atherosclerosis and improves endothelial dysfunction in hypercholesterolemic rabbits. Exp. Biol. Med. 2016, 241, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, A.M.; Mäkinen, P.I.; Jyrkkänen, H.; Mella-aho, E.; Xia, Y.; Kansanen, E.; Leinonen, H.; Verma, I.M.; Ylä-herttuala, S.; Levonen, A. Sulforaphane inhibits endothelial lipase expression through NF-κB in endothelial cells. Atherosclerosis 2010, 213, 122–128. [Google Scholar] [CrossRef]
- Peng, N.; Jin, L.; He, A.; Deng, C.; Wang, X. Effect of sulphoraphane on newborn mouse cardiomyocytes undergoing ischaemia/reperfusion injury. Pharm. Biol. 2019, 57, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Kee, H.J.; Kim, G.R.; Kim, I.K.; Jeong, M.H. Sulforaphane suppresses cardiac hypertrophy by inhibiting GATA4/GATA6 expression and MAPK signaling pathways. Mol. Nutr. Food Res. 2015, 59, 221–230. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, Q.; Sun, Y.-P.; Wang, X.; Lv, L.; Zhang, L.-P.; Liu, J.-S.; Zhao, S.; Wang, X.-L. Sulforaphane protection against the development of induced chronic heart failure is associated with Nrf2 Upregulation. Cardiovasc. Ther. 2017, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, F.; Rezaee, R.; Karimi, G. Natural compounds against doxorubicin-induced cardiotoxicity: A review on the involvement of Nrf2/ARE signaling pathway. Phyther. Res. 2020, 35, 1163–1175. [Google Scholar] [CrossRef]
- Evans, L.W.; Ferguson, B.S. Food Bioactive HDAC Inhibitors in the Epigenetic Regulation of Heart Failure. Nutrients 2018, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, S.; Zhang, H.; Yang, G.; Bai, Y.; Liu, P.; Meng, L.; Jiang, X.; Xin, Y. Sulforaphane prevents angiotensin II- induced cardiomyopathy by activation of Nrf2 through epigenetic modification. J. Cell Mol. Med. 2021, 25, 4408–4419. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Wang, W.; Chen, J.; Zhang, Z.; Zheng, Q.; Liu, Q.; Cai, L. Protection against diabetic cardiomyopathy is achieved using a combination of sulforaphane and zinc in type 1 diabetic OVE26 mice. J. Cell Mol. Med. 2019, 223, 6319–6330. [Google Scholar] [CrossRef]
- Yang, D.; Han, B.; Baiyun, R.; Lv, Z.; Wang, X.; Li, S.; Lv, Y.; Xue, J.; Liu, Y.; Zhang, Z. Sulforaphane Attenuates Hexavalent Chromium-Induced Cardiotoxicity via Activation of the Sesn2/AMPK/Nrf2 Signaling Pathway. Metallomics 2020, 12, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Bertl, E.; Bartsch, H.; Gerhauser, C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol. Cancer Ther. 2006, 5, 575–586. [Google Scholar] [CrossRef]
- Rhoden, A.; Friedrich, F.W.; Brandt, T.; Raabe, J.; Schweizer, M.; Meisterknecht, J.; Wittig, I.; Ulmer, M.; Klampe, B.; Uebeler, J.; et al. Sulforaphane exposure impairs contractility and mitochondrial function in three-dimensional engineered heart tissue. Redox Biol. 2021, 41, 101951. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Singh, S.V. Phenethyl Isothiocyanate Inhibits Angiogenesis In vitro and Ex vivo. Cancer Res. 2007, 67, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.F.; Li, J.; Qi, D.; Li, Y.; Li, C. Phenethyl Isothiocyanate Decrease Neovascularization and Alleviates Inflammatory Damage Throught Rescuing the Autophagy Impairment Mediated by Nrf2/Keap1. Atherosclerosis 2019, 287, 123–288. [Google Scholar] [CrossRef]
- Dey, M.; Ribnicky, D.; Kurmukov, A.G.; Raskin, I. In vitro and in vivo anti-inflammatory activity of a seed preparation containing phenethylisothiocyanate. Am. Soc. Pharmacol. Exp. Ther. 2005, 317, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Liu, H.; Chen, Y. Suppression of Inflammatory Mediators by Cruciferous Vegetable-Derived Indole-3-Carbinol and Phenylethyl Isothiocyanate in Lipopolysaccharide-Activated Macrophages. Mediat. Inflamm. 2010, 2010, 293642. [Google Scholar] [CrossRef]
- Keum, Y.-S.; Owuor, E.D.; Kim, B.-R.; Hu, R.; Tony Kong, A.N. Involvement of Nrf2 and JNK1 in the Activation of Antioxidant Responsive Element (ARE) by Chemopreventive Agent Phenethyl Isothiocyanate (PEITC). Pharm. Res. 2003, 20, 1351–1356. [Google Scholar] [CrossRef]
- Ernst, I.M.A.; Wagner, A.E.; Schuemann, C.; Storm, N.; Höppner, W.; Döring, F.; Stocker, A.; Rimbach, G. Allyl-, butyl- and phenylethyl-isothiocyanate activate Nrf2 in cultured fibroblasts. Pharmacol. Res. 2011, 63, 233–240. [Google Scholar] [CrossRef]
- Chuang, W.; Liu, Y.; Huang, C.; Lo, C.; Yao, H.; Chen, H.; Lii, C. Benzyl Isothiocyanate and Phenethyl Isothiocyanate Inhibit Adipogenesis and Hepatosteatosis in Mice with Obesity Induced by a High-Fat Diet. J. Agric. Food Chem. 2019, 67, 7136–7146. [Google Scholar] [CrossRef] [PubMed]
- Im, Y.-S.; Gwon, M.-H.; Yun, J.-M. Protective effects of phenethyl isothiocyanate on foam cell formation by combined treatment of oxidized low-density lipoprotein and lipopolysaccharide in THP-1 macrophage. Food Sci Nutr. 2021, 9, 3269–3279. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Lopez-rodriguez, N.A.; Gaytán-martínez, M.; Reyes-vega, M.D.L.; Loarca-piña, G. Glucosinolates and Isothiocyanates from Moringa oleifera: Chemical and Biological Approaches. Plant Foods Hum. Nutr. 2020, 75, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Auta, R.; Waziri, P.; Mohammed, J.S.; Abubakar, R.I.; Wayah, S.; Alhassan, Y. Characterization of GMG-ITC Isolated From Aerial Parts of Moringa Oleifera Tree. Sci. World J. 2020, 15, 162–165. [Google Scholar]
- Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Shi, Y.; Liu, H.; Panjwani, A.A.; Warrick, C.R.; Olson, M.E. A Strategy to Deliver Precise Oral Doses of the Glucosinolates or Isothiocyanates from Moringa oleifera Leaves for Use in Clinical Studies. Nutrients 2019, 11, 1547. [Google Scholar] [CrossRef]
- Waterman, C.; Cheng, D.M.; Rojas-Silva, P.; Poulev, A.; Dreifus, J.; Lila, M.A.; Raskin, I. Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry. 2014, 103, 114–122. [Google Scholar] [CrossRef]
- Waterman, C.; Rojas-silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W.; et al. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance and hepatic gluconeogenesis in mice. Mol. Nutr. Food Res. 2016, 59, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Michl, C.; Vivarelli, F.; Weigl, J.; De Nicola, G.R.; Canistro, D.; Paolini, M.; Iori, R.; Rascle, A. The Chemopreventive Phytochemical Moringin Isolated from Moringa oleifera Seeds Inhibits JAK/STAT Signaling. PLoS ONE 2016, 11, 1–20. [Google Scholar] [CrossRef]
- Giacoppo, S.; Rajan, T.S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The α-cyclodextrin complex of the Moringa isothiocyanate suppresses lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells through Akt and p38 inhibition. Inflamm. Res. 2017, 66, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Gao, L.; Su, S.; Sargsyan, D.; Wu, R.; Raskin, I. Moringa isothiocyanate activates Nrf2: Potential role in diabetic nephropathy. AAPS J. 2020, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tumer, T.B.; Rojas-silva, P.; Poulev, A.; Raskin, I.; Waterman, C. Direct and Indirect Antioxidant Activity of Polyphenol- and Isothiocyanate-Enriched Fractions from Moringa oleifera. J. Agric. Food Chem. 2016, 63, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, B.S.; Aita, R.; Maledatu, S.; Ribnicky, D.; Verzi, M.P.; Raskin, I. Moringa isothiocyanate-1 regulates Nrf2 and NF-κB pathway in response to LPS-driven sepsis and inflammation. PLoS ONE 2021, 16, 1–18. [Google Scholar] [CrossRef]
- Galuppo, M.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Milardi, D.; Bramanti, P.; Mazzon, E. 4(α-L-rhamnosyloxy)-benzyl isothiocyanate, a bioactive phytochemical that defends cerebral tissue and prevents severe damage induced by focal ischemia/reperfusion. J. Biol. Regul. Homeost. Agents 2015, 29, 343–356. [Google Scholar]
- Giacoppo, S.; Iori, R.; Bramanti, P.; Mazzon, E. Topical moringin cream relieves neuropathic pain by suppression of inflammatory pathway and voltage-gated ion channels in murine model of multiple sclerosis. Mol. Pain 2017, 13, 1–17. [Google Scholar] [CrossRef]
- Giacoppo, S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Moringa isothiocyanate complexed with α-cyclodextrin: A new perspective in neuroblastoma treatment. BMC Complement. Altern. Med. 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, R.; Arora, R.; Arora, S.; Singh, B.; Sharma, U. Quantitative and Qualitative Analysis of Eruca sativa and Brassica juncea Seeds by UPLC-DAD and UPLC-ESI-QTOF. Nat. Prod. Commun. 2017, 12, 1485–1489. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, Y.; Zou, L.; Sun, J.; Song, X.; Mao, J.; Wu, Y. Simultaneous Hydrolysis and Extraction Increased Erucin Yield from Broccoli Seeds. ACS Omega 2021, 6, 6385–6392. [Google Scholar] [CrossRef] [PubMed]
- Jakubıkova, J.; Sedlak, J.; Mithen, R.; Bao, Y. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2M arrest and cell death in Caco-2 cells. Biochem. Pharmacol. 2005, 69, 1543–1552. [Google Scholar] [CrossRef]
- Hichri, A.O.; Mosbah, H.; Majouli, K.; Hlila, M.B.; Ben Jannet, H.; Flamini, G.; Aouni, M.; Selmi, B. Chemical composition and biological activities of Eruca vesicaria subsp. longirostris essential oils. Pharm. Biol. 2016, 54, 2236–2243. [Google Scholar] [CrossRef]
- Cedrowski, J.; Dabrowa, K.; Krogul-Sobczak, A.; Litwinienko, G. A Lesson Learnt from Food Chemistry— Elevated Temperature Triggers the Antioxidant Action of Two Edible Isothiocyanates: Erucin and Sulforaphane. Antioxidants 2020, 9, 1090. [Google Scholar] [CrossRef] [PubMed]
- Cedrowski, J.; Dąbrowa, K.; Przybylski, P.; Krogul-Sobczak, A.; Litwinienko, G. Antioxidant activity of two edible isothiocyanates: Sulforaphane and erucin is due to their thermal decomposition to sulfenic acids and methylsulfinyl radicals. Food Chem. 2021, 353, 129213. [Google Scholar] [CrossRef]
- Wagner, A.E.; Sturm, C.; Piegholdt, S.; Wolf, I.M.A.; Esatbeyoglu, T.; De Nicola, G.R.; Iori, R.; Rimbach, G. Myrosinase-treated glucoerucin is a potent inducer of the Nrf2 target gene heme oxygenase 1 — studies in cultured HT-29 cells and mice. J. Nutr. Biochem. 2015, 26, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, N.; Okpara, A.; Coldham, N.; Sauer, M.J.; Ioannides, C. Modulation of Rat Hepatic and Pulmonary Cytochromes P450 and Phase II Enzyme Systems by Erucin, an Isothiocyanate Structurally Related to Sulforaphane. J. Agric. Food Chem. 2008, 56, 7866–7871. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Lee, K.W.; Park, J.H.Y. Erucin Exerts Anti-Inflammatory Properties in Murine Macrophages and Mouse Skin: Possible Mediation through the Inhibition of NFκB Signaling. Int. J. Mol. Sci. 2013, 14, 20564–20577. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.Y.; Seo, S.G.; Yang, H.; Yu, J.G.; Suk, S.J.; Jung, E.S.; Ji, H.; Kwon, J.Y.; Lee, H.J.; Lee, K.W. Anti-adipogenic effect of erucin in early stage of adipogenesis by regulating Ras activity in 3T3-L1 preadipocytes. J. Funct. Foods 2015, 19, 700–709. [Google Scholar] [CrossRef]
- Salma, U.; Khan, T.; Shah, A.J. Antihypertensive effect of the methanolic extract from Eruca sativa Mill., (Brassicaceae) in rats: Muscarinic receptor-linked vasorelaxant and cardiotonic effects. J. Ethnopharmacol. 2018, 224, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Alarcón, M.; Fuentes, M.; Carrasco, G.; Palomo, I. A Novel Role of Eruca sativa Mill. (Rocket) Extract: Antiplatelet (NF-κB Inhibition) and Antithrombotic Activities. Nutrients 2014, 6, 5839–5852. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-X.; Gao, J.-G.; Wan, X.-Y.; Chen, Y.; Xu, C.-F.; Feng, Z.-M.; Zeng, H.; Lin, Y.-M.; Ma, H.; Xu, P.; et al. Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways. World J. Gastroenterol. 2019, 25, 5120–5133. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Inoue, J.; Shimizu, M.; Sato, R. Allyl isothiocyanate suppresses the proteolytic activation of sterol regulatory element-binding proteins and de novo fatty acid and cholesterol synthesis. Biosci. Biotechnol. Biochem. 2016, 80, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-R.; Kim, H.-J.; Lee, Y.-J.; Ku, S.-K. Anti-Diabetic Obesity Effects of Wasabia Japonica Matsum Leaf Extract on 45% Kcal High-Fat Diet-Fed Mice. Nutrients 2020, 12, 2837. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Kato, T.; Momoi, S.; OKunishi, I.; Minami, M.; Oishi, Y.; Osawa, T.; Naito, M. Anti-obesity Effects of Wasabi Leaf Extract on Rats Fed a High-fat Diet are Related to Upregulation of mRNA Expression of β3-adrenergic Receptors in Interscapular Brown Adipose Tissue. Food Sci. Technol. Res. 2016, 22, 665–671. [Google Scholar] [CrossRef]
- Yoshida, S.; Hosoya, T.; Inui, S.; Masuda, H.; Kumazawa, S. Component Analysis of Wasabi Leaves and an Evaluation of their Anti-inflammatory Activity. Food Sci. Technol. Res. 2015, 21, 247–253. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Ahn, J.; Chung, W.; Jang, Y.J.; Seong, K.-S.; Moon, J.-H.; Ha, T.Y.; Jung, C.H. Pharmacokinetics, Tissue Distribution, and Anti-Lipogenic/Adipogenic Effects of Allyl Isothiocyanate Metabolites. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Chen, C.; Chen, Y.; Hsu, Y.; Huang, C.; Chang, C.; Lin, C.; Lin, C.; Lin, H.; Liu, F.; et al. Allyl Isothiocyanate Ameliorates Obesity by Inhibiting. Mol. Nutr. Food Res. 2018, 62, 1–11. [Google Scholar] [CrossRef]
- Mori, N.; Kurata, M.; Yamazaki, H.; Hosokawa, H.; Nadamoto, T.; Inoue, K.; Fushiki, T. Intragastric Administration of Allyl Isothiocyanate Reduces Hyperglycemia in Intraperitoneal Glucose Tolerance Test (IPGTT) by Enhancing Blood Glucose Consumption in Mice. J. Nutr. Sci. Vitaminol. 2013, 59, 56–63. [Google Scholar] [CrossRef][Green Version]
- Oguri, G.; Nakajima, T.; Kikuchi, H.; Obi, S.; Nakamura, F.; Komuro, I. Allyl isothiocyanate (AITC) activates nonselective cation currents in human cardiac fibroblasts: Possible involvement of TRPA1. Heliyon 2021, 7, e05816. [Google Scholar] [CrossRef] [PubMed]
- Joseph, V.; Yang, X.; Gao, S.S.; Elstrott, J.; Robby, M.; Theess, W.; Thrasher, C.; Singh, N.; Lin, J.; Rebecca, N. Development of AITC-induced dermal blood flow as a translational in vivo biomarker of TRPA1 activity in human and rodent skin. Br. J. Clin. Pharmacology. 2021, 87, 129–139. [Google Scholar] [CrossRef]
- Thejass, P.; Kuttan, G. Inhibition of Endothelial Cell Differentiation and Proinflammatory Cytokine Production During Angiogenesis by Allyl Isothiocyanate and Phenyl Isothiocyanate. Integr. Cancer Ther. 2007, 6, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, T.; Pugalendhi, P.; Jayaganesh, R.; Ananthakrishnan, D.; Gunasekaran, K. Effect of allyl isothiocyanate on NF-κB signaling in 7,12-dimethylbenz(a)anthracene and-methyl-N-nitrosourea-induced mammary carcinogenesis. Breast Cancer 2018, 25, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Chen, B.; Inbaraj, B.S.; Chien, J. Preparation of allyl isothiocyanate nanoparticles, their anti-inflammatory activity towards RAW 264. 7 macrophage cells and anti-proliferative effect on HT1376 bladder cancer cells. J. Sci. Food Agric. 2019, 99, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Boesch-Saadatmandi, C.; Dose, J.; Schultheiss, G.; Rimbach, G. Anti-inflammatory potential of allyl isothiocyanate – role of Nrf2, NF-κB and microRNA-155. J. Cell. Mol. Med. 2012, 16, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Lu, C.; Tang, Y.; Chiang, J.; Kuo, D.; Chen, F.; Chen, I.; Yang, J. Allyl isothiocyanate inhibits cell metastasis through suppression of the MAPK pathways in epidermal growth factor - stimulated HT29 human colorectal adenocarcinoma cells. Oncol. Rep. 2014, 31, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, T.; Pugalendhi, P.; Thilagavathi, S.; Ananthakrishnan, D.; Gunasekaran, K. Allyl isothiocyanate, a potent chemopreventive agent targets AhR/Nrf2 signaling pathway in chemically induced mammary carcinogenesis. Mol. Cell Biochem. 2017, 437, 1–12. [Google Scholar] [CrossRef]
- Hasegawa, K.; Miwa, S.; Tsutsumiuchi, K.; Miwa, J. Allyl isothiocyanate that induces GST and UGT expression confers oxidative stress resistance on C. elegans, as demonstrated by nematode biosensor. PLoS ONE 2010, 5, e9267. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.S.; Hadley, S.H.; Morris, K.F.; Breslin, J.W.; Dean, J.B.; Taylor-Clark, T.E. Characterization of cardiovascular reflexes evoked by airway stimulation with allyl isothiocyanate, capsaicin, and ATP in Sprague-Dawley rats. J. Appl. Physiol. 2016, 120, 580–591. [Google Scholar] [CrossRef]
- Hooper, J.S.; Taylor-clark, T.E. Morphological changes in spontaneously hypertensive rats via reflex modulation of the autonomic nervous system. Front. Physiol. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Licznerska, B.; Baer-dubowska, W. Indole-3-Carbinol and Its Role in Chronic Diseases. In Anti-inflammatory Nutraceuticals and Chronic Diseases; Gupta, S.C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 131–154. ISBN 9783319413341. [Google Scholar]
- López-Vázquez, A.; Garcia-Bañuelos, J.J.; González-Garibay, A.S.; Del Toro-Arreola, S.; Bueno-Topete, M.R.; Sánchez-Enríquez, S.; Muñoz-Valle, J.F.; Jave-Suárez, L.F.; Armendáriz-Borunda, J.; Bastidas-Ramírez, B.E. IRS-1 pY612 and Akt-1/PKB pT308 Phosphorylation and Anti- inflammatory Effect of Diindolylmethane in Adipocytes Cocultured with Macrophages. Med. Chem. 2017, 13, 727–733. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zong, J.; Wu, Q.Q.; Zhou, H.; Zhang, J.-Y.; Yuan, Y.; Bian, Z.-Y.; Deng, W.; Dai, J.; Li, F.-F.; Xu, M.; et al. 3,3′-Diindolylmethane attenuates cardiac H9c2 cell hypertrophy through 5′-adenosine monophosphate-activated protein kinase-α. Mol. Med. Rep. 2015, 12, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Adwas, A.A.; Elkhoely, A.A.; Kabel, A.M.; Abdel-Rahman, M.N.; Eissa, A.A. Anti-cancer and cardioprotective effects of indol-3-carbinol in doxorubicin-treated mice. J. Infect. Chemother. 2016, 22, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zong, J.; Bian, Z.; Zhou, H.; Yuan, Y.; Zhang, R.; Guo, H.; Zhang, Y.; Shen, D.; Li, H.; et al. Indole-3-carbinol protects against pressure overload induced cardiac remodeling via activating AMPK-α. Mol. Nutr. Food Res. 2013, 57, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Hajra, S.; Patra, A.R.; Basu, A.; Bhattacharya, S. Prevention of doxorubicin (DOX)-induced genotoxicity and cardiotoxicity: Effect of plant derived small molecule indole-3-carbinol (I3C) on oxidative stress and inflammation. Biomed. Pharmacother. 2018, 101, 228–243. [Google Scholar] [CrossRef]
- Maiyoh, G.K.; Kuh, J.E.; Casaschi, A.; Theriault, A.G. Cruciferous Indole-3-Carbinol Inhibits Apolipoprotein B Secretion in HepG2 Cells 1 – 3. J. Nutr. 2007, 137, 2185–2189. [Google Scholar] [CrossRef][Green Version]
- Chang, H.-P.; Wang, M.-L.; Chan, M.-H.; Chiu, Y.-S.; Chen, Y.-H. Antiobesity activities of indole-3-carbinol in high-fat-diet – induced obese mice. Nutrients 2011, 27, 463–470. [Google Scholar] [CrossRef]
- Chang, H.-P.; Wang, M.-L.; Hsu, C.-Y.; Liu, M.-E.; Chan, M.-H.; Chen, Y.-H. Suppression of inflammation-associated factors by indole-3-carbinol in mice fed high-fat diets and in isolated, co-cultured macrophages and adipocytes. Int. J. Obes. 2011, 35, 1530–1538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, Y.; Kim, Y.; Park, S.; Won, K.; Park, T. Indole-3-carbinol prevents diet-induced obesity through modulation of multiple genes related to adipogenesis, thermogenesis or inflammation in the visceral adipose tissue of mice. J. Nutr. Biochem. 2012, 23, 1732–1739. [Google Scholar] [CrossRef]
- Choi, Y.; Um, S.-J.; Park, T. Indole-3-carbinol directly targets SIRT1 to inhibit adipocyte differentiation. Int. J. Obes. 2013, 37, 881–884. [Google Scholar] [CrossRef]
- Choi, H.; Jeon, H.; Lee, O.; Lee, B. Indole-3-carbinol, a vegetable phytochemical, inhibits adipogenesis by regulating cell cycle and AMPK-α signaling. Biochimie 2014, 104, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-L.; Lin, S.-H.; Hou, Y.-Y.; Chen, Y.-H. Suppression of Lipid Accumulation by Indole-3-Carbinol Is Associated with Increased Expression of the Aryl Hydrocarbon Receptor and CYP1B1 Proteins in Adipocytes and with Decreased Adipocyte-Stimulated Endothelial Tube Formation. Int. J. Mol. Sci. 2016, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’ s Disease, Parkinson’ s Disease, and Huntington’ s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a Potential Protective Phytochemical against Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F. Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci. 2000, 23, 298–304. [Google Scholar] [CrossRef]
- Ahmad, S.; Hafeez, A.; Sharma, S.K.; Ahmad, M.; Kumar, A. Natural product for the prevention and treatment of Alzheimer’s disease. Glocal J. Sci. Technol. 2021, 8, 71–85. [Google Scholar]
- Ghimire, S.; Subedi, L.; Acharya, N.; Gaire, B.P. Moringa oleifera: A Tree of Life as a Promising Medicinal Plant for Neurodegenerative Diseases. J. Agric. Food Chem. 2021, 69, 14358–14371. [Google Scholar] [CrossRef]
- Mizuno, K.; Kume, T.; Muto, C.; Takada-Takatori, Y.; Izumi, Y.; Sugimoto, H.; Akaike, A. Glutathione Biosynthesis via Activation of the Nuclear Factor E2 – Related Factor 2 (Nrf2) – Antioxidant-Response Element (ARE) Pathway Is Essential for Neuroprotective Effects of Sulforaphane and 6-(Methylsulfinyl)Hexyl Isothiocyanate. J. Pharmacol. Sci. 2011, 115, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Trio, P.Z.; Fujisaki, S.; Tanigawa, S.; Hisanaga, A.; Sakao, K.; Hou, D.-X. DNA Microarray Highlights Nrf2-Mediated Neuron Protection Targeted by Wasabi-Derived Isothiocyanates in IMR-32 Cells. Gene Regul. Syst. Biol. 2016, 10, 73–83. [Google Scholar] [CrossRef]
- Eren, E.; Tufekci, K.U.; Isci, K.B.; Tastan, B.; Genc, K.; Genc, S. Sulforaphane Inhibits Inflammation, Cytotoxicity, Oxidative Stress, and miR-155 Expression and Switches to Mox Phenotype through Activating Extracellular Signal-Regulated Kinase 1/2 – Nuclear Factor Erythroid 2-Related Factor 2/Antioxidant Response Elemen. Front. Immunol. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Qin, S.; Yang, C.; Huang, W.; Du, S.; Mai, H.; Xiao, J.; Lü, T. Sulforaphane attenuates microglia-mediated neuronal necroptosis through down-regulation of MAPK / NF-κB signaling pathways in LPS-activated BV-2 microglia. Pharmacol. Res. 2018, 133, 218–235. [Google Scholar] [CrossRef]
- Subedi, L.; Cho, K.; Park, Y.U.; Choi, H.J.; Kim, S.Y. Sulforaphane-Enriched Broccoli Sprouts Pretreated by Pulsed Electric Fields Reduces Neuroinflammation and Ameliorates Scopolamine-Induced Amnesia in Mouse Brain through Its Antioxidant Ability via Nrf2-HO-1 Activation. Oxid. Med. Cell. Longev. 2019, 2019, 3549274. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.V.; Kim, H.Y.; Ehrlich, H.Y.; Choi, S.Y.; Kim, D.J.; Kim, Y. Amelioration of Alzheimer’s disease by neuroprotective effect of sulforaphane in animal model. Amyloid 2013, 20, 7–12. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, J.; Fang, L.; Li, X.; Zhao, Y.; Shi, W.; An, L. Neuroprotective Effects of Sulforaphane on Cholinergic Neurons in Mice with Alzheimer’s Disease-Like Lesions. Int. J. Mol. Sci. 2014, 15, 14396–14410. [Google Scholar] [CrossRef]
- Kim, J. Pre-Clinical Neuroprotective Evidences and Plausible Mechanisms of Sulforaphane in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2929. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Gwon, S.; Choi, B.; Han, J.; Won, K.; Kim, J. Sulforaphane alleviates scopolamine-induced memory impairment in mice. Pharmacol. Res. 2014, 85, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Park, G.H.; Lee, S.-R.; Jang, J.-H. Attenuation of β-Amyloid-Induced Oxidative Cell Death by Sulforaphane via Activation of NF-E2-Related Factor 2. Oxid. Med. Cell. Longev. 2013, 2013, 313510. [Google Scholar] [CrossRef]
- Lee, S.; Choi, B.-R.; Kim, J.; LaFerla, F.M.; Park, J.H.Y.; Han, J.; Lee, K.W.; Kim, J. Sulforaphane upregulates the heat shock protein co-chaperone CHIP and clears amyloid-β and tau in a mouse model of Alzheimer’s disease. Mol. Nutr. Food Res. 2018, 62, 1–33. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, J.; Chang, N. Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer’s disease. Eur. J. Pharmacol. 2018, 824, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jhang, K.A.; Park, J.; Kim, H.; Chong, Y.H. Sulforaphane rescues amyloid- β peptide- mediated decrease in MerTK expression through its anti-inflammatory effect in human THP-1 macrophages. J. Neuroinflammation 2018, 15, 1–12. [Google Scholar] [CrossRef]
- An, Y.W.; Jhang, K.A.; Woo, S.-Y.; Kang, J.L.; Chong, Y.H. Sulforaphane exerts its anti-inflammatory effect against amyloid-β peptide via STAT-1 dephosphorylation and activation of Nrf2 / HO-1 cascade in human THP-1 macrophages. Neurobiol. Aging 2016, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chilakala, R.R.; Manchikalapudi, A.L.; Kumar, A.; Sunkaria, A. Sulforaphane Attenuates Aβ Oligomers Mediated Decrease in Phagocytic Activity of Microglial Cells. Neuroscience 2020, 429, 225–234. [Google Scholar] [CrossRef]
- Park, H.-M.; Kim, J.-A.; Kwak, M.-K. Protection against amyloid beta cytotoxicity by sulforaphane: Role of the proteasome. Arch. Pharm. Res. 2009, 32, 109–115. [Google Scholar] [CrossRef]
- Sunkaria, A.; Bhardwaj, S.; Yadav, A.; Halder, A.; Sandhir, R. Sulforaphane attenuates postnatal proteasome inhibition and improves spatial learning in adult mice. J. Nutr. Biochem. 2018, 51, 69–79. [Google Scholar] [CrossRef]
- Tarozzi, A.; Morroni, F.; Merlicco, A.; Hrelia, S.; Angeloni, C.; Cantelli-Forti, G.; Hrelia, P. Sulforaphane as an inducer of glutathione prevents oxidative stress-induced cell death in a dopaminergic-like neuroblastoma cell line. J. Neurochem. 2009, 111, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Lee, Y.J.; Lee, S.Y.; Kim, E.M.; Moon, Y.; Kim, H.W.; Hwang, O. Protective Effect of Sulforaphane against Dopaminergic Cell Death. J. Pharmacol. Exp. Ther. 2007, 321, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Tao, R.; Yu, S.-Z.; Jin, H. Sulforaphane protects against 6-hydroxydopamine-induced cytotoxicity by increasing expression of heme oxygenase-1 in a PI3K/Akt-dependent manner. Mol. Med. Rep. 2012, 5, 847–851. [Google Scholar] [CrossRef][Green Version]
- Deng, C.; Tao, R.; Yu, S.-Z.; Jin, H. Inhibition of 6-hydroxydopamine-induced endoplasmic reticulum stress by sulforaphane through the activation of Nrf2 nuclear translocation. Mol. Med. Rep. 2012, 6, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Jazwa, A.; Rojo, A.I.; Innamorato, N.G.; Hesse, M.; Fernandez-Ruiz, J.; Cuadrado, A. Pharmacological Targeting of the Transcription Factor Nrf2 at the Basal Ganglia Provides Disease Modifying Therapy for Experimental Parkinsonism. Antioxid. Redox Signal. 2011, 14, 2347–2360. [Google Scholar] [CrossRef]
- Galuppo, M.; Iori, R.; De Nicola, G.R.; Bramanti, P.; Mazzon, E. Anti-inflammatory and anti-apoptotic effects of (Rs)-glucoraphanin bioactivated with myrosinase in murine sub-acute and acute MPTP-induced Parkinson’s disease. Bioorg. Med. Chem. 2013, 21, 5532–5547. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Galuppo, M.; Iori, R.; De Nicola, G.R.; Bramanti, P.; Mazzon, E. (Rs)-glucoraphanin purified from Tuscan black kale and bioactivated with myrosinase enzyme protects against cerebral ischemia/reperfusion injury in rats. Fitoterapia 2014, 99, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; He, Q.; Zheng, J.; Li, L.Y.; Hou, Y.H.; Song, F.Z. Sulforaphane improves outcomes and slows cerebral ischemic/reperfusion injury via inhibition of NLRP3 inflammasome activation in rats. Int. Immunopharmacol. 2017, 45, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Galuppo, M.; De Nicola, G.R.; Iori, R.; Bramanti, P.; Mazzon, E. Tuscan black kale sprout extract bioactivated with myrosinase: A novel natural product for neuroprotection by inflammatory and oxidative response during cerebral ischemia/reperfusion injury in rat. BMC Complement. Altern. Med. 2015, 15, 1–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Danilov, C.A.; Chandrasekaran, K.; Racz, J.; Soane, L.; Zielke, C.; Fiskum, G. Sulforaphane Protects Astrocytes Against Oxidative Stress and Delayed Death Caused by Oxygen and Glucose Deprivation. Glia 2009, 57, 645–656. [Google Scholar] [CrossRef]
- Thwaini, M.H.; Abu Ragif, A.R.; Hadi, N.R. Effects of Sulforaphane in brain ischemic reperfusion injury in rats. Int. J. Pharm. Res. 2020, 12, 3687–3694. [Google Scholar]
- Chang, G.; Guo, Y.; Jia, Y.; Duan, W.; Bin, L.; Jixu, Y.; Li, C. Protective Effect of Combination of Sulforaphane and Riluzole on Glutamate-Mediated Excitotoxicity. Biol. Pharm. Bull. 2010, 33, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Nobre, M.S.C.; Albino, S.L.; Lócio, L.L.; Nascimento, A.P.S.; Scotti, L.; Scotti, M.T.; Oshiro-junior, J.A.; Lima, M.C.A.; Mendonça-junior, F.J.B.; et al. Secondary Metabolites with Antioxidant Activities for the Putative Treatment of Amyotrophic Lateral Sclerosis (ALS): “Experimental Evidences". Oxid. Med. Cell. Longev. 2020, 2020, 5642029. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cui, W.; Liu, J.; Li, R.; Liu, Q.; Xie, X.-H.; Ge, X.-L.; Zhang, J.; Song, X.-J.; Wang, Y.; et al. Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp. Neurol. 2013, 250, 239–249. [Google Scholar] [CrossRef]
- Giacoppo, S.; Galuppo, M.; Iori, R.; De Nicola, G.R.; Bramanti, P.; Mazzon, E. The protective effects of bioactive (Rs)-glucoraphanin on the permeability of the mice blood-brain barrier following experimental autoimmune encephalomyelitis. Eur. Rev. Med. Pharmacol. Sci. 2013, 18, 194–204. [Google Scholar]
- Giacoppo, S.; Galuppo, M.; Iori, R.; De Nicola, G.R.; Cassata, G.; Bramanti, P.; Mazzon, E. Protective Role of (Rs)-glucoraphanin Bioactivated with Myrosinase in an Experimental Model of Multiple Sclerosis. CNS Neurosci. Ther. 2013, 19, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, J.; Park, S. Sulforaphane-induced autophagy flux prevents prion protein-mediated neurotoxicity through AMPK pathway. Neuroscience 2014, 278, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, W.; Li, B.; Chen, X.; Zhang, Y.; Chen, C.; Yu, M.; Xiu, Y.; Li, W.; Cao, J.; et al. PEITC Promotes Neurite Growth in Primary Sensory Neurons via the miR-17-5p/STAT3/GAP-43 Axis. J. Drug Target. 2019, 27, 82–93. [Google Scholar] [CrossRef]
- Thejass, P.; Kuttan, G. Allyl isothiocyanate (AITC) and phenyl isothiocyanate (PITC) inhibit tumour-specific angiogenesis by downregulating nitric oxide (NO) and tumour necrosis factor-a (TNF - a) production. Nitric Oxide 2007, 16, 247–257. [Google Scholar] [CrossRef]
- Okubo, T.; Washida, K.; Murakami, A. Phenethyl isothiocyanate suppresses nitric oxide production via inhibition of phosphoinositide 3-kinase / Akt-induced IFN- c secretion in LPS-activated peritoneal macrophages. Mol. Nutr. Food Res. 2010, 54, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-S.; Hsiao, Y.-T.; Lin, J.-J.; Liao, C.-L.; Lin, C.-C.; Chung, J.-G. Phenethyl Isothiocyanate (PEITC) and Benzyl Isothiocyanate (BITC) Inhibit Human Melanoma A375.S2 Cell Migration and Invasion by Affecting MAPK Signaling Pathway In Vitro. Anticancer Res. 2017, 37, 6223–6234. [Google Scholar] [CrossRef]
- Qin, C.; Zhang, X.; Wu, L.; Wen, C.; Hu, L.; Lv, Q.; Shen, D.; Zhou, H. Advances in Molecular Signaling Mechanisms of β-Phenethyl Isothiocyanate Antitumor Effects. J. Agric. Food Chem. 2015, 63, 3311–3322. [Google Scholar] [CrossRef]
- Xu, K.; Thornalley, P.J. Signal transduction activated by the cancer chemopreventive isothiocyanates: Cleavage of BID protein, tyrosine phosphorylation and activation of JNK. Br. J. Cancer 2001, 84, 670–673. [Google Scholar] [CrossRef]
- Brown, K.K.; Blaikie, F.H.; Smith, R.A.J.; Tyndall, J.D.A.; Lue, H.; Winterbourn, C.C.; Hampton, M.B. Direct Modification of the Proinflammatory Cytokine Macrophage Migration Inhibitory Factor by Dietary Isothiocyanates. J. Biol. Chem. 2009, 284, 32425–32433. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Kim, S.-J.; Park, S.-J.; Eom, S.-H.; Gu, G.-J.; Hwan, S.; Youn, H.-S. Phenethyl isothiocyanate regulates inflammation through suppression of the TRIF-dependent signaling pathway of Toll-like receptors. Life Sci. 2013, 92, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O. How Functional Is Moringa oleifera? A Review of Its Nutritive, Medicinal, and Socioeconomic Potential. Food Nutr. Bull. 2018, 39, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Ekong, M.B.; Ekpo, M.M.; Akpanyung, E.O.; Nwaokonko, D.U. Neuroprotective effect of Moringa oleifera leaf extract on aluminium-induced temporal cortical degeneration. Metab. Brain Dis. 2017, 32, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.G.; Wopara, I.; Aduema, W.; Ebo, O.T.; Umoren, E.B. Long-term consumption of Moringa oleifera-supplemented diet enhanced neurocognition, suppressed oxidative stress, acetylcholinesterase activity and neuronal degeneration in rat’s hippocampus. Drug Metabol. Pers. Ther. 2021, 36, 223–231. [Google Scholar] [CrossRef]
- Onasanwo, S.A.; Adamaigbo, V.O.; Adebayo, O.G.; Eleazer, S.E. Moringa oleifera-supplemented diet protect against cortico-hippocampal neuronal degeneration in scopolamine-induced spatial memory deficit in mice: Role of oxido-inflammatory and cholinergic neurotransmission pathway. Metab. Brain Dis. 2021, 36, 2445–2460. [Google Scholar] [CrossRef]
- Rajan, T.S.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia 2016, 112, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Jaja-Chimedza, A.; Graf, B.L.; Simmler, C.; Kim, Y.; Kuhn, P.; Pauli, G.F.; Raskin, I. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS ONE 2017, 12, 1–21. [Google Scholar] [CrossRef]
- Jaafaru, M.S.; Nordin, N.; Rosli, R.; Shaari, K.; Bako, H.Y.; Noor, N.M.; Abdull Razis, A.F. Prospective role of mitochondrial apoptotic pathway in mediating GMG-ITC to reduce cytotoxicity in H2O2 -induced oxidative stress in differentiated SH-SY5Y cells. Biomed. Pharmacother. 2019, 119, 109445. [Google Scholar] [CrossRef] [PubMed]
- Galuppo, M.; Giacoppo, S.; De Nicola, G.R.; Iori, R.; Navarra, M.; Lombardo, G.E.; Bramanti, P.; Mazzon, E. Antiinflammatory activity of glucomoringin isothiocyanate in a mouse model of experimental autoimmune encephalomyelitis. Fitoterapia 2014, 95, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Tiloke, C.; Anand, K.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera and their phytonanoparticles: Potential antiproliferative agents against cancer. Biomed. Pharmacother. 2018, 108, 457–466. [Google Scholar] [CrossRef]
- Chiricosta, L.; Silvestro, S.; Pizzicannella, J.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Transcriptomic Analysis of Stem Cells Treated with Moringin or Cannabidiol: Analogies and Differences in Inflammation Pathways. Int. J. Mol. Sci. 2019, 20, 6039. [Google Scholar] [CrossRef]
- Mathiron, D.; Iori, R.; Pilard, S.; Rajan, S.T.; Landy, D.; Mazzon, E.; Rollin, P.; Djedaïni-Pilard, F. A Combined Approach of NMR and Mass Spectrometry Techniques Applied to the α-Cyclodextrin/Moringin Complex for a Novel Bioactive Formulation. Molecules 2018, 23, 1714. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Suzuki, R.; Murata, I.; Nomura, H.; Isshiki, Y.; Kanamoto, I. Evaluation of Antibacterial Activity Expression of the Hinokitiol/Cyclodextrin Complex Against Bacteria. ACS Omega 2020, 5, 27180–27187. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Ferlazzo, N.; Gugliandolo, A.; Musumeci, L.; Mazzon, E.; Bramanti, A.; Navarra, M. Moringin from Moringa oleifera Seeds Inhibits Growth, Arrests Cell-Cycle, and Induces Apoptosis of SH-SY5Y Human Neuroblastoma Cells through the Modulation of NF-κB and Apoptotic Related Factors. Int. J. Mol. Sci. 2019, 20, 1930. [Google Scholar] [CrossRef]
- Lu, Y.; Dorothea, R.; Vos, M.; Zhang, Y.; Zhang, M.; Liu, Y.; Fu, C.; Quan, S.; Huang, D. The degradation kinetics and mechanism of moringin in aqueous solution and the cytotoxicity of degraded products. Food Chem. 2021, 364, 130424. [Google Scholar] [CrossRef]
- Tarozzi, A.; Morroni, F.; Bolondi, C.; Sita, G.; Hrelia, P.; Djemil, A.; Cantelli-forti, G. Neuroprotective Effects of Erucin against 6-Hydroxydopamine-Induced Oxidative Damage in a Dopaminergic-like Neuroblastoma Cell Line. Int. J. Mol. Sci. 2012, 13, 10899–10910. [Google Scholar] [CrossRef]
- Subedi, L.; Venkatesan, R.; Kim, S.Y. Neuroprotective and Anti-Inflammatory Activities of Allyl Isothiocyanate through Attenuation of JNK/NF-κB/TNF-α Signaling. Int. J. Mol. Sci 2017, 18, 1423. [Google Scholar] [CrossRef] [PubMed]
- Latronico, T.; Larocca, M.; Milella, S.; Fasano, A.; Rossano, R.; Liuzzi, G.M. Neuroprotective potential of isothiocyanates in an in vitro model of neuroinflammation. Inflammopharmacology 2021, 29, 561–571. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Stetter, R.; Herz, C.; Spöttel, J.; Krell, M.; Hanschen, F.S.; Schreiner, M.; Rohn, S.; Behrens, M.; Lamy, E. Allyl isothiocyanate: A TAS2R38 receptor-dependent immune modulator at the interface between personalized medicine and nutrition. Front. Immunol. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Marques, C.S.; Dias, M.V.; Soares, N.d.F.F.; Borges, S.V.; de Oliveira, I.R.N.; Pires, A.C.d.S.; Medeiros, E.A.A.; Alves, E. Ultrastructural and antimicrobial impacts of allyl isothiocyanate incorporated in cellulose, β-cyclodextrin, and carbon nanotubes nanocomposites. J. Vinyl Addit. Technol. 2021, 27, 1–11. [Google Scholar] [CrossRef]
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.N.V.R. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release. 2006, 113, 189–207. [Google Scholar] [CrossRef]
- Kim, J.K.; Shin, E.; Kim, C.R.; Park, G.G.; Choi, S.J.; Park, C.-S.; Shin, D.-H. Effects of Brussels Sprouts and Their Phytochemical Components on Oxidative Stress–Induced Neuronal Damages in PC12 Cells and ICR Mice. J. Med. Food 2013, 16, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, J.; Kim, J.; Lee, S.; Choi, B.-R.; Han, J.-S.; Lee, K.W.; Lee, H.J. 3,3′-Diindolylmethane Inhibits Lipopolysaccharide-Induced Microglial Hyperactivation and Attenuates Brain Inflammation. Toxicol. Sci. 2014, 137, 158–167. [Google Scholar] [CrossRef]
- Rostoka, E.; Isajevs, S.; Baumane, L.; Line, A.; Silina, K.; Dzintare, M.; Sharipova, J.; Svirina, D.; Kalvinsh, I.; Sjakste, N. Effects of lycopene, indole-3-carbinol, and luteolin on nitric oxide production and iNOS expression are organ-specific in rats. Arh. Hig. Rada Toksikol. 2012, 61, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Lin, T.-Y.; Yang, H.C.; Hung, C.F.; Weng, J.R.; Chang, D.C.; Wang, S.J. [1-(4-chloro-3-nitrobenzenesulfonyl)-1H-indol-3-yl]-methanol, an indole-3-carbinol derivative, inhibits glutamate release in rat cerebrocortical nerve terminals by suppressing the P/Q-type Ca2+ channels and Ca2+/calmodulin/protein kinase A pathway. Neurochem. Int. 2020, 140, 104845. [Google Scholar] [CrossRef]
- Ghobeh, M.; Ahmadian, S.; Meratan, A.A.; Ebrahim-Habibi, A.; Ghasemi, A.; Shafizadeh, M.; Nemat-Gorgani, M. Interaction of Aβ(25–35) Fibrillation Products with Mitochondria: Effect of Small-Molecule Natural Products. Biopolym. Pept. Sci. 2014, 102, 473–486. [Google Scholar] [CrossRef]
- Sherer, C.; Tolaymat, I.; Rowther, F.; Warr, T.; Snape, T.J. Preliminary SAR on indole-3-carbinol and related fragments reveals a novel anticancer lead compound against resistant glioblastoma cells. Bioorg. Med. Chem. Lett. 2017, 27, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, J.; Bak, D.H.; Yu, K.S.; Lee, J.H.; Lee, N.S.; Jeong, Y.G.; Kim, D.K.; Kim, D.-K.; Han, S.-Y. Protective Effects of Indole-3-Carbinol-Loaded Poly(lactic-co-glycolic acid) Nanoparticles Against Glutamate-Induced Neurotoxicity. J. Nanosci. Nanotechnol. 2015, 15, 7922–7928. [Google Scholar] [CrossRef] [PubMed]
- Donkin, S.G.; Eiteman, M.A.; Williams, P.L. Toxicity of Glucosinolates and Their Enzymatic Decomposition Products to Caenorhabditis elegans. J. Nematol. 1995, 27, 258–262. [Google Scholar] [PubMed]
- Canistro, D.; Barillari, J.; Melega, S.; Sapone, A.; Iori, R.; Speroni, E. Black cabbage seed extract affects rat Cyp-mediated biotransformation: Organ and sex related differences. Food Chem. Toxicol. 2012, 50, 2612–2621. [Google Scholar] [CrossRef]
- Paolini, M.; Perocco, P.; Canistro, D.; Valgimigli, L.; Pedulli, G.F.; Iori, R.; Della Croce, C.; Cantelli-Forti, G.; Legator, M.S.; Abdel-Rahman, S.Z. Induction of cytochrome P450, generation of oxidative stress, in vitro cell-transforming and DNA-damaging activities by glucoraphanin, the bioprecursor of the chemopreventive agent sulforaphane found in broccoli. Carcinogenesis 2004, 25, 61–67. [Google Scholar] [CrossRef]
- Nugon-Baudon, L.; Rabot, S.; Szylit, O.; Raibaud, P. Glucosinolates toxicity in growing rats: Interactions with the hepatic detoxification system. Xenobiotica 1990, 20, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Mondal, C.; Chandra, A.K. Goitrogenic/antithyroidal potential of moringa leaves (Moringa oleifera) and spinach (Spinacia oleracea) of Indian origin on thyroid status in male albino rats. Braz. J. Pharm. Sci. 2004, 55, 1–11. [Google Scholar] [CrossRef]
- Prieto, M.A.; López, C.J.; Simal-gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [PubMed]
- Stohs, S.J.; Hartman, M.J. Review of the Safety and Efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pehrsson, P.R. Current Knowledge and Challenges on the Development of a Dietary Glucosinolate Database in the United States. Curr. Dev. Nutr. 2021, 5, 1–21. [Google Scholar] [CrossRef] [PubMed]
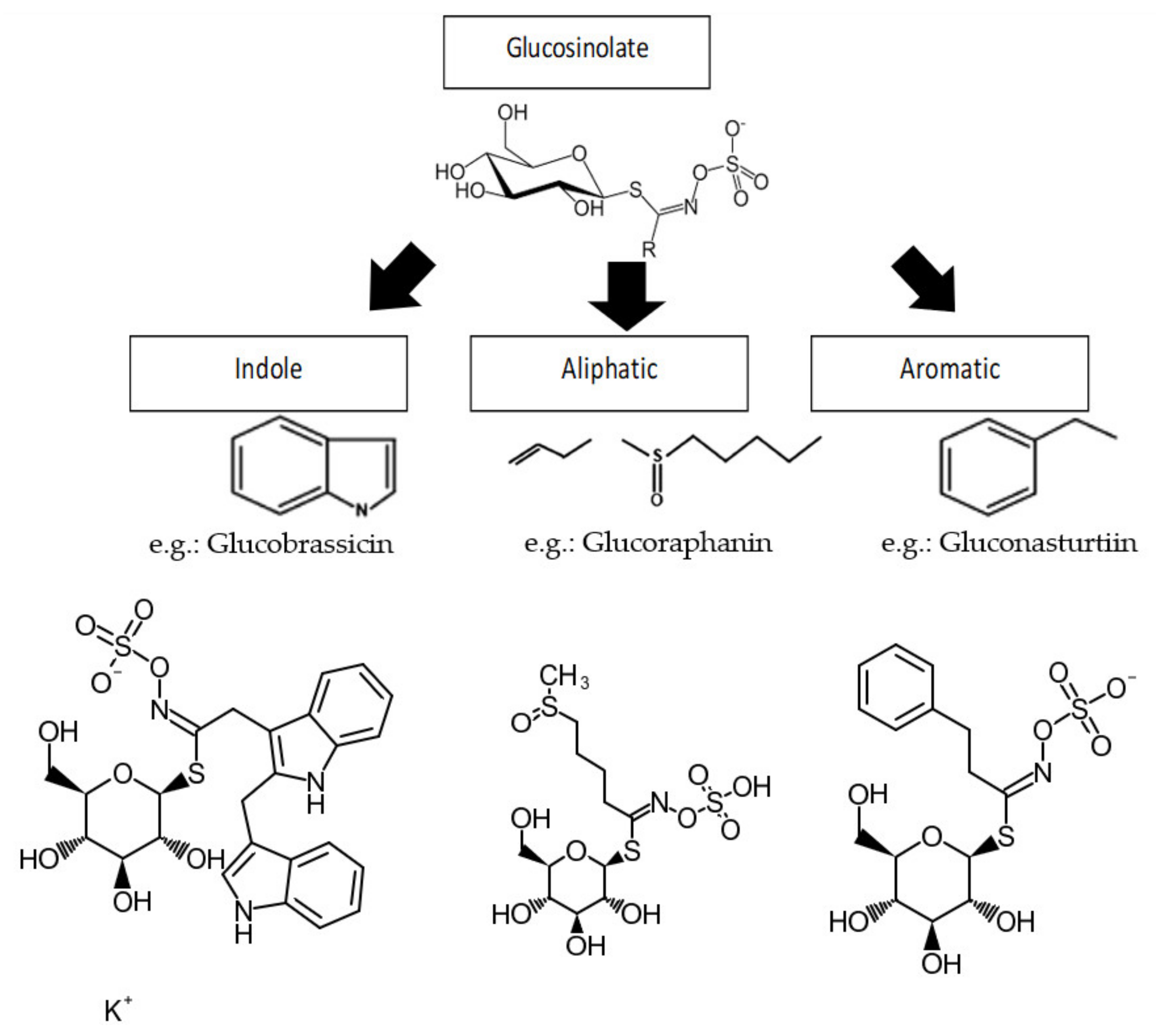
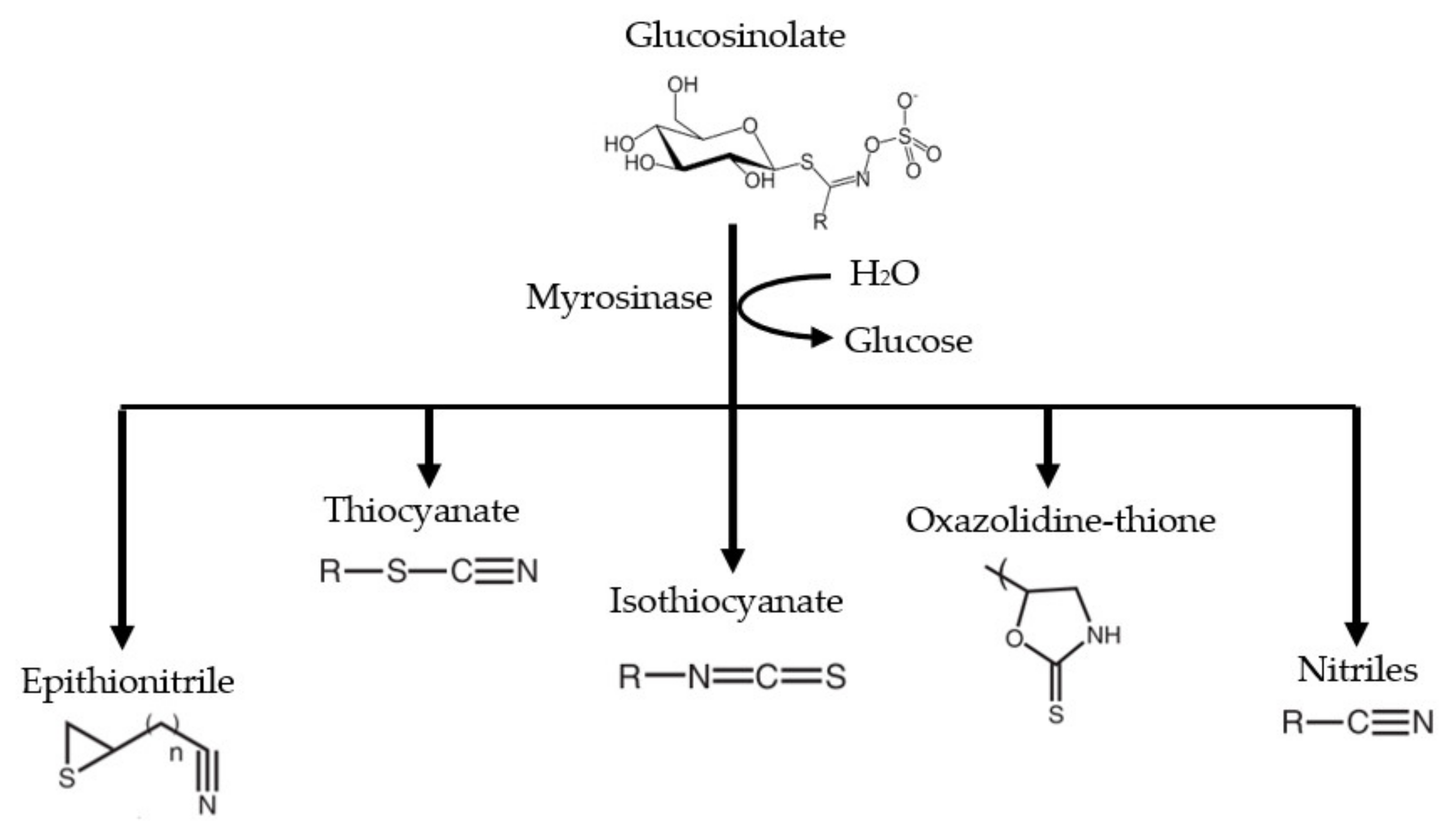
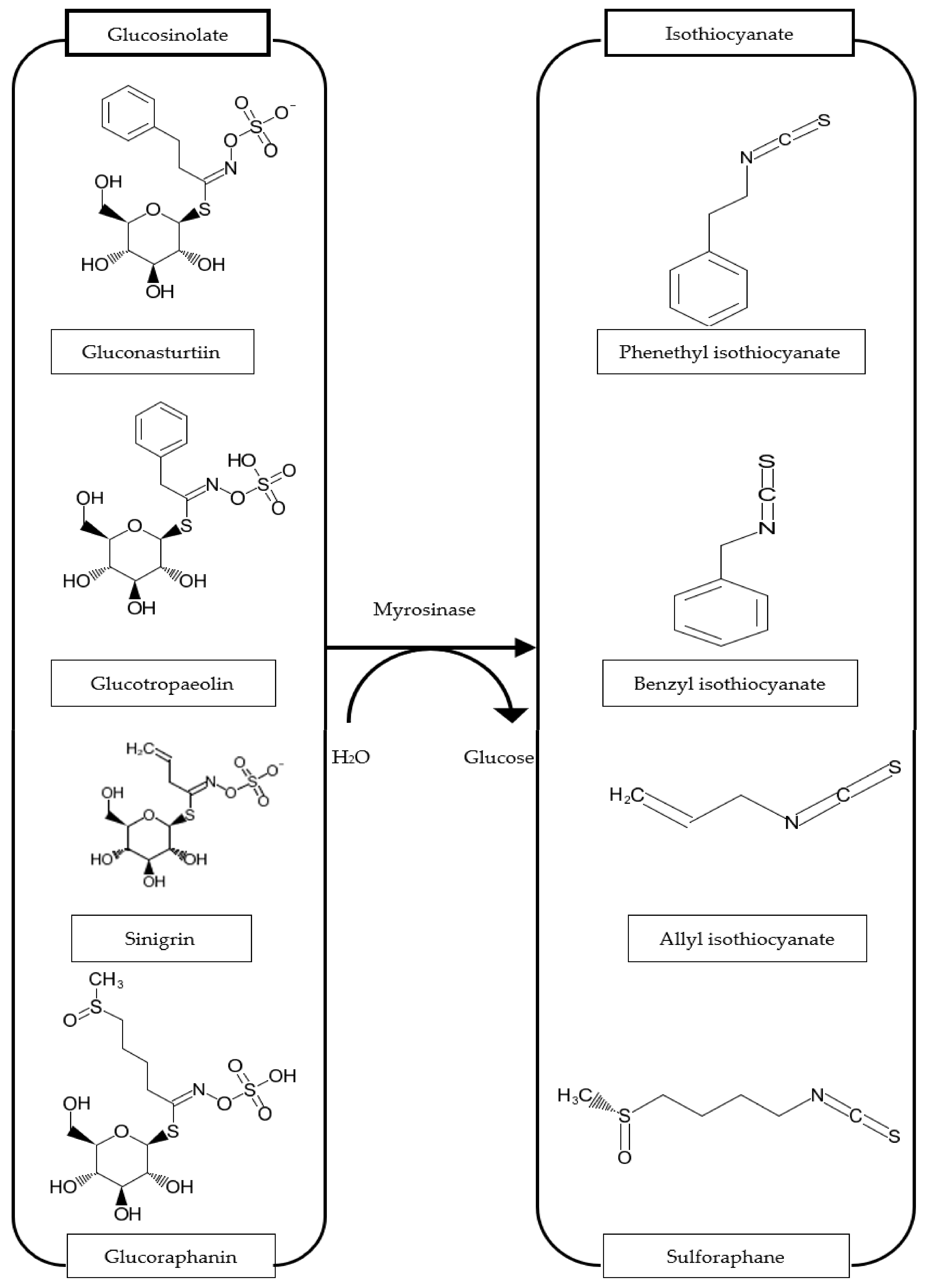
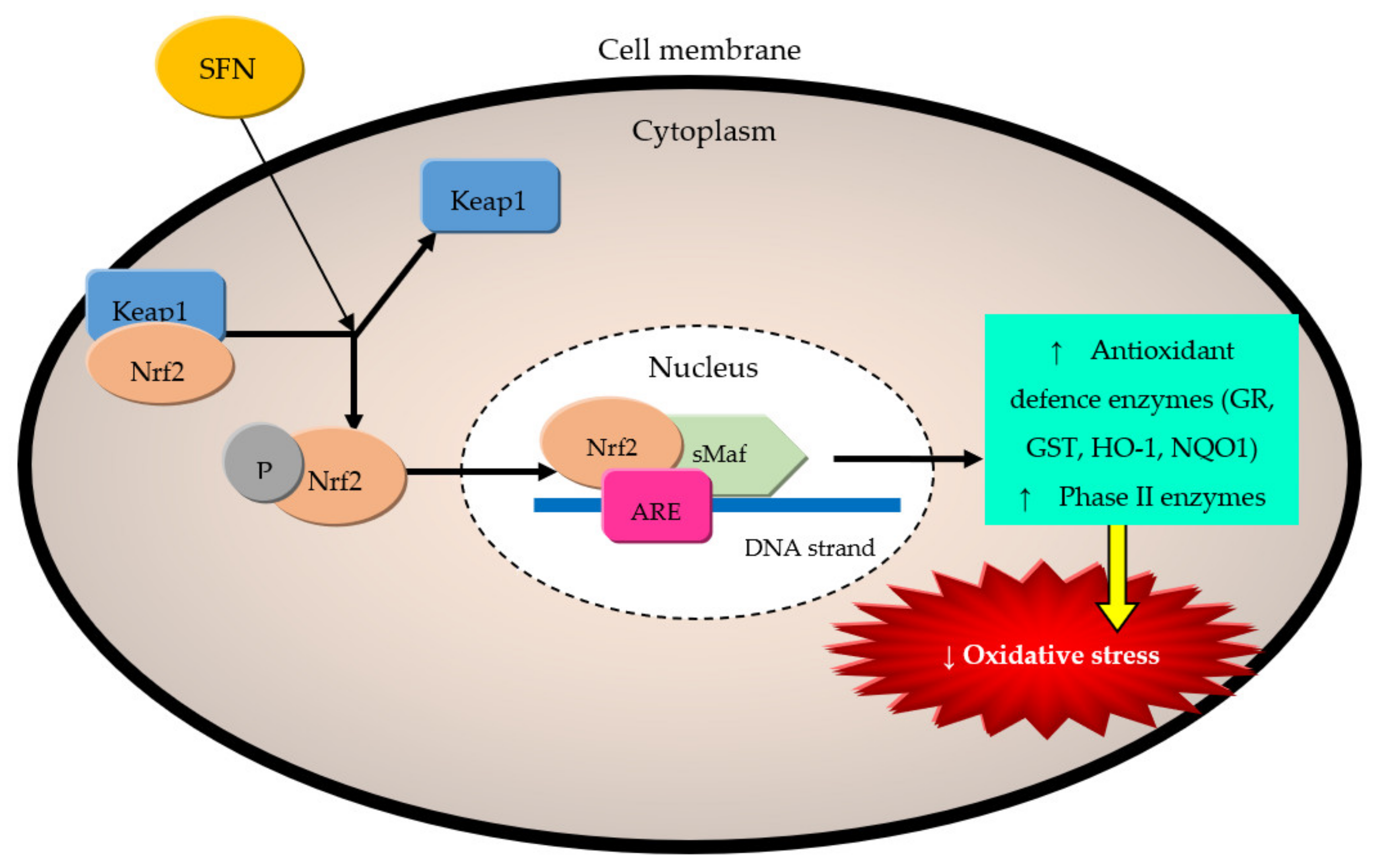
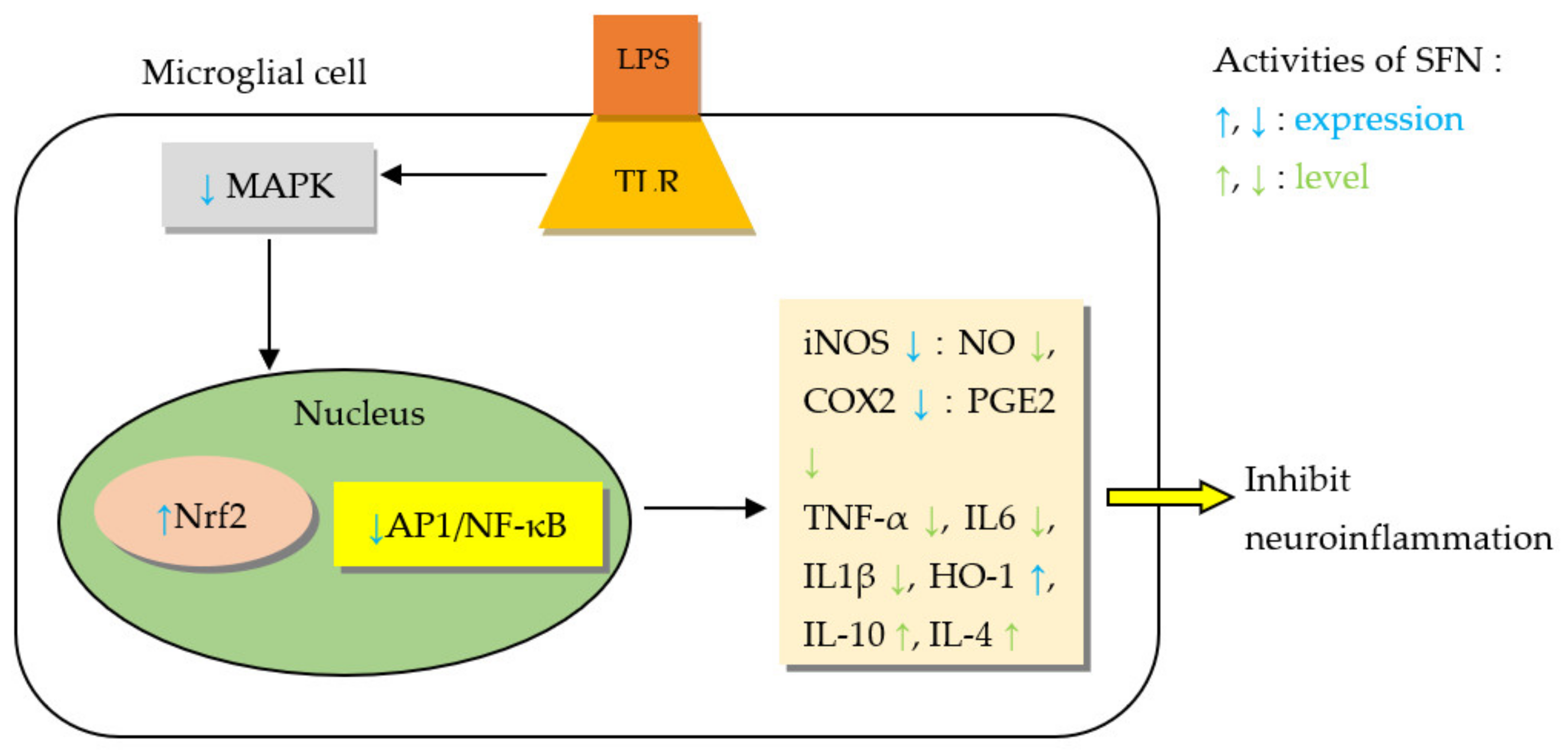
| Phytochemical | Major Food Sources | Main Effects on CVDs/Risk Factors | Main Effects on NDDs |
|---|---|---|---|
| SFN | Broccoli, cauliflower, kale, brussels sprouts, cabbage [15,16] | Reduced obesity, normalized serum lipids, increased plasma insulin, decreased blood pressure, slowed progression of atherosclerosis, and prevented vascular complications in diabetes mellitus (DM) [61] Suppressed myocardial damage, decreased infarct area in myocardial infarction (MI)[62] Improved cardiac function in arrhythmia, MI, and heart failure [62,63,64] Regularized heart rate in arrhythmia [64] Reduced severity of right heart failure/pulmonary hypertension [65] Decreased heart muscle dysfunction in the elderly [66] | Reduced cholinergic neurons’ apoptosis, improved cholinergic neurotransmission, and neurobehavioral responses [67,68] Downregulated amyloidogenesis [68,69] Inhibited dopaminergic neuron death, increased tyrosine hydroxylase (TH) formation in Parkinson’s disease (PD) [70,71] Decreased brain infarct in ischemic stroke and neonatal hypoxia-ischemia injury [72,73] Prevented motor neuron death, decreased severity and incidence of multiple sclerosis (MS), epilepsy and, depression [74,75,76] Reduced side effects of schizophrenia medications [77] |
| PEITC | Turnips, radish, watercress, broccoli [78] | Reduced food intake, body weight, fat deposition, and atherosclerosis [79,80] Normalized plasma insulin and blood glucose [81] Suppressed left ventricular dysfunction in HIV/AIDS [82] | Inhibited acetylcholinesterase activity [39] |
| MG | Moringa seeds and leaves [22] | Minimized MI size, decreased creatine kinase MB (CK-MB), improved cardiac function, and reduced mortality after MI [83,84] Reduced disease severity in heart failure [85] Decreased fat accumulation, increased lean body mass, improved blood glucose, and gut microbiome in DM [86] | Promoted neurogenesis and viability [87] Improved neurological score and decreased infarct size in stroke [88] Improved neurocognition, normalized catecholamines and electroencephalogram (EEG) wave pattern, suppressed disease progression, and enhanced neuronal repair in AD [89,90] Decreased demyelination and improved remyelination in MS [91] Increased TH production, decreased dopaminergic neuron apoptosis, improved behavior and motor symptoms in PD [92] Suppressed progression of spinal cord injury and hastened motor function recovery [93,94] Delayed development of motor deficits in amyotrophic lateral sclerosis (ALS) [95] Induced apoptosis in astrocytoma and myeloma [22,96] |
| ER | Arugula, kohlrabi, Chinese cabbage kohlrabi, broccoli seeds [97,98] | Reduced body mass index, lipid accumulation, serum triglycerides, fasting blood glucose, hemoglobin A1C [99] Decreased blood pressure [100,101] Suppressed platelet aggregation and thrombosis, improved coronary blood flow [100] Decreased CK-MB and lactate dehydrogenase (LDH) [102] | Increased resistance of dopaminergic neurons to apoptosis and increased TH secretion in PD [103] |
| AITC | Wasabi, mustard, horse radish [104] | Suppressed insulin resistance, decreased blood glucose, reduced obesity, decreased cholesterol synthesis, reduced MABP [105,106] | Decreased infarct volume from traumatic brain injury [107] |
| I3C | Broccoli, brussels sprouts, cabbage [15,16] | Anti-platelet, anti-thrombotic activity [108] Prevented myocardial hypertrophy, stimulated parasympathetic effect [109] Normalized cardiac nitric oxide, decreased CK-MB [109] Increased insulin, decreased blood glucose, HbA1C, and cholesterol [110] Suppressed lipid deposition in blood vessels [111] | Improved cholinergic neurotransmission and memory [112] Decreased dopaminergic neuron loss [113] Ameliorated cognitive dysfunction, inhibited formation and aggregation, and also promoted degradation of amyloid beta plaques [114,115] Decreased severity of MS [116] Increased neurological score and cerebral blood flow, and decreased infarct volume [117] Increased sensitivity of temozolomide-resistant glioblastoma cells [118] |
| ITC or EAxtract | CVD and/or Model | Effect on CVDs | Mechanism of Action | References |
|---|---|---|---|---|
| SFN | Myocardial infarction (MI)/surgical left coronary artery occlusion in rats | Decreased heart congestion and remodeling | Upregulated MAPK/Akt/ERK pathway and downregulated p38 and Bax/Bcl-2-caspase-3 pathways | [135] |
| Preserved cardiac function and reduced infarct size more than postC | Balanced Nrf2/AhR activation | [62] | ||
| MI/Hypoxia/reoxygenation (H/R) myocardial cells model | Restored cardiac anti-oxidant status, reduced apoptosis | Activated Nrf2/HO-1 pathway | [152] | |
| CVD/Mutated GATA cardiomyocytes (in vitro)/isoproterenol-induced cardiac hypertrophy in mice (in vivo) | Suppressed cardiac hypertrophy | Inhibited GATA4/GATA6 expression and MAPK signaling pathway | [153] | |
| Chronic heart failure (CHF)/Doxorubicin (DOX)-induced CHF | Retarded disease progression and improved heart function | Stimulate Nrf2 transcription, inhibited PAI-1 and CTGF expression | [73] | |
| Arrhythmia/Isoproterenol-induced cardiac stress in rat | Normalized heart rate and improved left ventricular function | Normalized cardiac autonomic drive | [64] | |
| Pulmonary arterial hypertension (PAH)/VEGFR inhibitor (SU5416)-induced PAH in mice | Prevented right ventricular and pulmonary vascular dysfunction and remodeling | Reduced NLRP3 expression and upregulated Nrf2/NQO-1 pathway | [65] | |
| Cardiomyopathy (CM)/Angiotensin II induced cardiomyopathy in mice | Suppressed cardiac oxidative stress, inflammation, remodeling, and dysfunction | Epigenetic modification of Nrf2 activation with HDAC and DNMT inhibition | [157] | |
| Diabetic CM/Type I DM OVE 26 (OVE) mice | Improved cardiac function and ameliorated fibrosis | Increased Nrf2 activity and metallothionein expression | [158] | |
| CM/Aged-mice cardiac muscle dysfunction | Improved cardiac and mitochondrial function | Upregulated Nrf2 signaling | [66] | |
| Chromium heart toxicity/Chromium (CrVI)-induced cardiotoxicity | Ameliorated cardiac physiological and morphological alterations | Activated Sesn2/AMPK/Nrf2 signaling pathway | [159] | |
| Diabetic vascular injury/AGEs-exposed HUVECs and AGEs-injected rat aorta | Antioxidative, anti-inflammatory | Inhibited AGE/RAGE pathway | [61] | |
| CVD/Rat aortic smooth muscle cells (RASMCs)- in vitro; rat carotid artery balloon injury model – in vivo | Inhibited neointima formation | Inhibited PDGF-BB-stimulated proliferation of RASMCs, by causing cell cycle arrest through downregulating the p53 signaling pathway | [149] | |
| CVD/H2O2-exposed adult cardiomyocytes | Antioxidative: reduced ROS and raised SOD | Induced Nrf2 and PGC-1α protein expression | [133] | |
| CHF/aortic constriction in rabbits | Improved heart function and remodeling | Inhibited oxidative stress and inflammation (↓TNF-α, ↓IL-6) and decreased BNP and ANP | [63] | |
| M. oleifera extract | MI/left coronary artery ligation in mice | Minimized infarct sizes, alleviated contractile dysfunction, prevented ventricular failure, and reduced mortality | Repressed oxidative/nitrosative stress, apoptosis, and fibrosis | [83] |
| CHF/DOX-induced CHF | Reduced serum LDH, CK-MB, normalized ECG parameters, and reduced mortality | Increased cardiotonicity | [85] | |
| MI/isoproterenol-induced myocardial damage in rats | Improves cardiac performance, antioxidative, antiperoxidative, and myocardial preservative effects | Restores hemodynamic parameters, prevents leakage of LDH and CK-MB from the myocardium, SOD, CAT, and GSHPx | [84] | |
| ER | Hypertension/HASMCs, noradrenaline-induced vasoconstriction endothelium-intact or -denuded rat aortic rings, coronary arteries of Langendorff-perfused rat hearts and normotensive and SHRs | Vasorelaxant, antihypertensive effect | H2S-releasing | [100] |
| E. sativa | Hydroxyapatite cardiac toxicity/Hydroxyapatite-induced cardiac damage | Lowered CK-MB, LDH, and myoglobin | - | [102] |
| I3C | CVD/DOX-induced cardiotoxicity | Raised cardiac antioxidant status Reduced oxidative stress, inflammation, and apoptosis | - | [218] |
| Upregulated Nrf2/ARE pathway, downregulated NF-kB pathway, modified apoptotic genes’ expression | [220] | |||
| Heart failure/Aortic banding in mice | Prevented pressure overload-induced cardiac remodeling | Activated AMPK-α signaling and improved energy metabolism | [108,219] | |
| Hypertension/High salt-induced myocardial stress and hypertrophy | Anti-hypertensive, anti-hypertrophic, and anti-apoptotic effects | Stimulation of muscarinic receptor-2 | [109] |
| Isothiocyanate (ITC) or Extract | Neurodegenerative Diseases (NDD) and/or Model | Effect on NDDs | Mechanism of Action | References |
|---|---|---|---|---|
| SFN | Alzheimer’s disease (AD)-like mouse model | Abolished apoptosis of cholinergic neurons, reduced cognitive impairment | Probably neurogenesis and aluminum load reduction | [239] |
| Amyloid bete (Aβ)-induced AD acute mouse model | Improved cognitive function | - | [238] | |
| Transgenic AD mouse model | Ameliorated neurobehavioral deficits and reduced Aβ burden | Increased expression of p75NTR | [67] | |
| D-galactose and aluminum-induced AD-lesion mouse model | Improved cognitive and locomotor function | Suppressed Aβ deposition | [68] | |
| Scopolamine-induced memory impairment in C57BL/6 mice (in vivo), scopolamine-treated primary cortical neurons (in vitro) | Improved cholinergic neurotransmission, memory, and cognition. | Inhibited acetylcholinesterse (AChE) activity | [241] | |
| AD/ Aβ-induced-SH-SY5Y cells | Antiapoptotic | Stimulated Nrf2 pathway | [242] | |
| AD transgenic mouse (PS1V97L) | Improved spatial learning and memory | Inhibited Aβ oligomer formation | [69] | |
| Triple transgenic AD mouse model (3×Tg-AD) | - | Enhanced Aβ and tau degradation via increased CHIP and HSP70 expression | [243] | |
| AD/Mouse neuroblastoma N2a cells expressing human Swedish mutant amyloid precursor protein (N2a/APPswe cells) | Inhibited oxidative and inflammatory effects of Aβ | Epigenetic modification of Nrf2 | [244] | |
| Aβ1–42 induced-human THP-1 macrophages (in vitro AD model) | Suppressed neuroinflammation | Preserved MerTK expression via NF-κB pathway downregulation | [245] | |
| AD/ Aβ1–42 monomers induced human THP-1 microglia-like cells | Anti-inflammatory effect (decreased IL-1β) | Inhibited activation of STAT-1 and NLRP3 inflammasome, decreased microRNA-146a and upregulated Nrf2 pathway | [246] | |
| AD/Aβ oligomer-induced microglial cells | Anti-inflammatory effect | Improved microglial phagocytic activity | [247] | |
| AD/Aβ1–42-induced cytotoxicity in Neuro2A and N1E115 cells | Anti-oxidant effect | Increased proteasome (PSMB5) Aβ degradation | [248] | |
| NDD/MG132-induced proteasome inhibition in Balb/c mice | Improved spatial learning | Induced catalytic activity of proteasome | [249] | |
| Parkinson’s disease (PD)/MPTP-induced sub-acute model | Prevented dopaminergic neuron loss, micro- and astrogliosis | Upregulated Nrf2 mediated phase II enzymes expression | [254] | |
| PD/6-OHDA- induced PC12 cells | Anti-apoptotic | Enhanced PI3K/Akt-dependent HO-1 expression | [213] | |
| PD/6-OHDA- induced ER stress in PC12 cells | Anti-oxidative | Improved Nrf2 inhibition of endoplasmic reticulum (ER) stress | [253] | |
| PD/ 6-OHDA- and BH4- induced SK-N- BE(2)C, CATH.a and mesencephalic neurons | Prevented dopaminergic cell death | Removal of dopamine quinone from neuronal cells | [251] | |
| ALS/Threo-hydroxyaspartate (THA)-induced glutamate excitotoxicity on spinal cord explant model | Decreased motor neuron death | Induction of phase II enzymes via Nrf2/ARE signaling | [261] | |
| PD/CysDA-induced primary cortical neurons injury | Abolished oxidative stress and apoptosis | Upregulated ERK/Keap1/Nrf2 pathway | [70] | |
| PD/6-OHDA-induced mouse model | Improved behavior and motor coordination | Downregulated phosphorylation of ERK1/2, increased GSH and GR, and blocked expression of caspase-3 | [71] | |
| PD/H2O2 or 6-OHDA-induced cytotoxicity in SH-SY5Y cells | Anti-apoptotic | Induced GSH-mediated antioxidative response | [250] | |
| PD/Acute and sub-acute MPTP models in C57BL/6 mice | Improved behavior, coordination, and motor function | Reduced dopamine transporter degradation, increased tyrosine hydroxylase (TH) expression. Normalized expression of neurotrophic factors; GAP-43, NGF, and BDNF | [255] | |
| * Stroke/rat common carotid/middle cerebral artery (CCA/MCA) occlusion model | Reduced infarct volume | Increased HO-1 expression | [72] | |
| * Stroke/carotid artery occlusion CIR injury in rats | Reduced infarct volume, restored BBB integrity | Decreased ERK1/2, NF-kB, and casp3 expression, increased Nrf2 activity | [256,258] | |
| * Stroke/rat MCAO model | Improved neurological scores and minimized infarct volume | Inhibited NLRP3 inflammasome and caspase-1 activation | [257] | |
| Perinatal hypoxia-ischemia/Neonatal HI rat model (left common carotid artery ligation and hypoxia) | Reduced infarct ratio | Induction of phase II enzymes through Nrf2 signaling | [73] | |
| * Stroke/bilateral common carotid artery occlusion (BCCAO) injury in rat | Lowered extent of acute cerebral injury | - | [260] | |
| NDD/oxygen and glucose deprivation OGD in rat cortical astrocytes | Suppressed astrocyte death | Stimulated Nrf2 pathway | [259] | |
| Multiple sclerosis (MS)/MOG35-55-induced EAE in C57BL/6 mice | Inhibited disease development and severity, suppressed spinal cord demyelination | Inhibited Th17 autoimmune response, upregulated Nrf2 pathway | [263] | |
| MS/MOG35-55-induced EAE mouse model | Improved BBB integrity | Increased expression of TJ-proteins, decreased Foxp3, ERK1/2 and caspase 3 expression | [264] | |
| Suppressed symptoms | Modulated inflammatory pathways, reduced apoptosis | [265] | ||
| Prion diseases/PrP exposed-SH-SY5Y cells | Antiapoptotic | Induced autophagy by stimulating AMPK pathway | [266] | |
| Schizophrenia/anti-psychotics-induced SK-N-SH cells | Suppressed dopaminergic neuron toxicity | Decreased protein-bound quinones | [77] | |
| Epilepsy/Amygdala chronic kindling model | Suppressed amygdala kindling and cognitive impairment | Activation of Nrf2-ARE signal pathway | [75] | |
| Depression/Acute and chronic stress mouse model | Antidepressant- and anxiolytic-like activities | Inhibited HPA axis activity | [76] | |
| PEITC | Spinal cord injury (SCI)/Dorsal column/Sciatic nerve injury in rats | Promoted neurite outgrowth | Modulated miR-17-5p/STAT3/GAP-43 | [267] |
| M. oleifera extract | NDD/Primary culture of hippocampal neurons | Promoted neuronal survival and neurite outgrowth | - | [87] |
| NDD/Al-induced temporo-cortical degeneration in mice | Reduced degenerative features | Increased NSE, decreased GFAP | [276] | |
| * Stroke/right MCAO model in rat | Improved clinical score, reduced infarct volume | Decreased MDA levels, increased SOD and GSHPx activity | [88] | |
| NDD/Hippocampal neurodegeneration rat model | Enhanced memory and cognition | Inhibited AChE activity | [277] | |
| AD/Colchicine-induced AD model | Improvement of RAM task and EEG wave pattern, normalization of serotonin, norepinephrine, and dopamine | - | [89] | |
| AD/Scopolamine-induced spatial memory deficit in mice | Improved spatial memory function | Maintained cholinergic transmission and neuron integrity | [278] | |
| MG | MS/MOG35-55-induced EAE in C57BL/6 mice | Stopped TNF-α inflammation | Inhibited phospho-ERK p42/44 signaling pathway | [282] |
| Decreased clinical disease score and inflammatory markers | Modulated Wnt–β-catenin signaling | [91] | ||
| AD/Aβ-induced-SH-SY5Y cells | Slowed disease progression, promoted neuronal repair | Downregulated pathways involved in senescence, autophagy, and mitophagy | [90] | |
| PD/MPTP-induced sub-acute PD mouse model | Reduced bradykinesia | Suppressed inflammatory response, increased TH expression | [92] | |
| * Stroke/left carotid artery occlusion model in rat | Reduced infarct size, improved neurologic symptoms | Downregulated NF-κB pathway | [181] | |
| SCI/extradural spinal cord compression in ICR (CD-1) mice | Restored motor function, spinal cord morphology, and promoted regenerative effects | Increased expression of TGF-β and IL-10 | [94] | |
| Reduced disease severity, prevented secondary spinal cord damage after injury | Downregulated NF-κB pathway | [93] | ||
| Amyotrophic lateral sclerosis (ALS)/ALS transgenic model (SOD1G93A rat) | Delayed appearance of motor dysfunction | Downregulated TLR4 and CD8α mediated inflammation, oxidative stress, and apoptosis | [95] | |
| Neuroblastoma (NBL)/ SH-SY5Y human NBL cell line | Stimulates cancer cell apoptosis | Inhibited PI3K/Akt/mTOR pathway | [183] | |
| ER | PD/6-OHDA induced SH-SY5Y cells’ model | Antioxidative, antiapoptotic effects | Increased GSH level, prevented loss of mitochondrial membrane potential | [103,289]. |
| PD/6-OHDA induced mouse model | Counteracted asymmetric motor behavior | Increased TH expression | [103] | |
| AITC | NDD/LPS-induced neuroinflammation model (BV2 murine microglia, C6 glioma, and N2a mouse neuroblastoma cells) | Antiapoptotic, improved neurite outgrowth | Suppressed JNK/NF-κB/TNF-α signaling | [290] |
| Traumatic brain injury (TBI)/Cryogenic TBI model in mice | Improved infarct volume and BBB permeability | Modulated Nrf2/HO-1 and NF-κB pathways | [107] | |
| AITC, PEITC and SFN | NDD/LPS-activated primary cultures of rat astrocytes | Anti-inflammatory | Modulated MMP transcription via downregulating MAPK/ERK signaling | [291] |
| I3C/DIM | NDD/LPS-induced microglial hyperactivation in BV-2 Microglia (in vitro)/mice (in vivo) | Suppressed neuroinflammation and apoptosis Reduced activated microglial cells in the hippocampus | Inhibited NF-κB | [296] |
| NDD/glutamate-treated HT-22 Cells/Scopolamine-induced memory impairment in mice | Anti-apoptotic Improved cognition; reduced AChE activity and enhanced choline acetyltransferase (ChAT) activity | Activated TrkB/Akt pathway (increased BDNF and antioxidants) | [112] | |
| NDD/ 4-aminopyridine-treated synaptosomes | Inhibited glutamate release from nerve terminals | Downregulated Ca2+/calmodulin/protein kinase A pathway and P/Q-type Ca2+ channels | [298] | |
| NDD/glutamate excitotoxicity (GE) in PC12 neuronal cells | Anti-apoptotic | Scavenged ROS, inhibit caspase-3 and -8 | [301] | |
| PD/intranigral LPS-induced neuroinflammation in rats | Improved motor functions, coordination, learning, and memory | NF-κB pathway inhibition | [113] | |
| AD/Aβ-induced PC12 cells | Inhibition of amyloid fibril formation, aggregation, and cytotoxicity | - | [114] | |
| AD/Aβ(25–35)-induced rat brain mitochondria | Inhibited amyloid fibrils formation, destroys amyloid aggregates | Inhibited mitochondrial membranes damage | [299] | |
| AD/Small interfering RNA knockdown and plasmid transfection model (in vitro)/ Adeno-associated virus-mediated RNAi in mice (in vivo) | Improved cognition and Aβ catabolism | Stimulated AhR-induced Neprilysin expression | [115] | |
| MS/MOG35-55-induced EAE in C57BL/6 mice | Reduced disease severity and T-cell infiltration in the CNS | Increased Treg cell/FoxP3 formation and decreased Th17 by activating AhR | [116] | |
| * Stroke/MCAO in rat | Improved neurological score and mean cerebral blood flow. Reduced platelet aggregation and infarct volume | - | [117] | |
| Glioblastoma/Temozolomide-resistant U87MG and U251 cells | Improved sensitivity of resistant cells to temozolomide | Inhibited upregulation of NEDD4-1- (induces PTEN, suppresses Akt/Nrf2/HO-1) | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, R.M.; Abdull Razis, A.F.; Mohd Sukri, N.S.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases. Molecules 2022, 27, 624. https://doi.org/10.3390/molecules27030624
Kamal RM, Abdull Razis AF, Mohd Sukri NS, Perimal EK, Ahmad H, Patrick R, Djedaini-Pilard F, Mazzon E, Rigaud S. Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases. Molecules. 2022; 27(3):624. https://doi.org/10.3390/molecules27030624
Chicago/Turabian StyleKamal, Ramla Muhammad, Ahmad Faizal Abdull Razis, Nurul Syafuhah Mohd Sukri, Enoch Kumar Perimal, Hafandi Ahmad, Rollin Patrick, Florence Djedaini-Pilard, Emanuela Mazzon, and Sébastien Rigaud. 2022. "Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases" Molecules 27, no. 3: 624. https://doi.org/10.3390/molecules27030624
APA StyleKamal, R. M., Abdull Razis, A. F., Mohd Sukri, N. S., Perimal, E. K., Ahmad, H., Patrick, R., Djedaini-Pilard, F., Mazzon, E., & Rigaud, S. (2022). Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases. Molecules, 27(3), 624. https://doi.org/10.3390/molecules27030624









