Chondroitin Sulfate Protects the Liver in an Experimental Model of Extra-Hepatic Cholestasis Induced by Common Bile Duct Ligation
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Animals Subjected to BDL Model
2.2. Chondroitin Sulfate Improves Liver Fibrogenesis
2.3. Collagen Fibers Organization in the Hepatic Veins after Chondroitin Sulfate Treatment
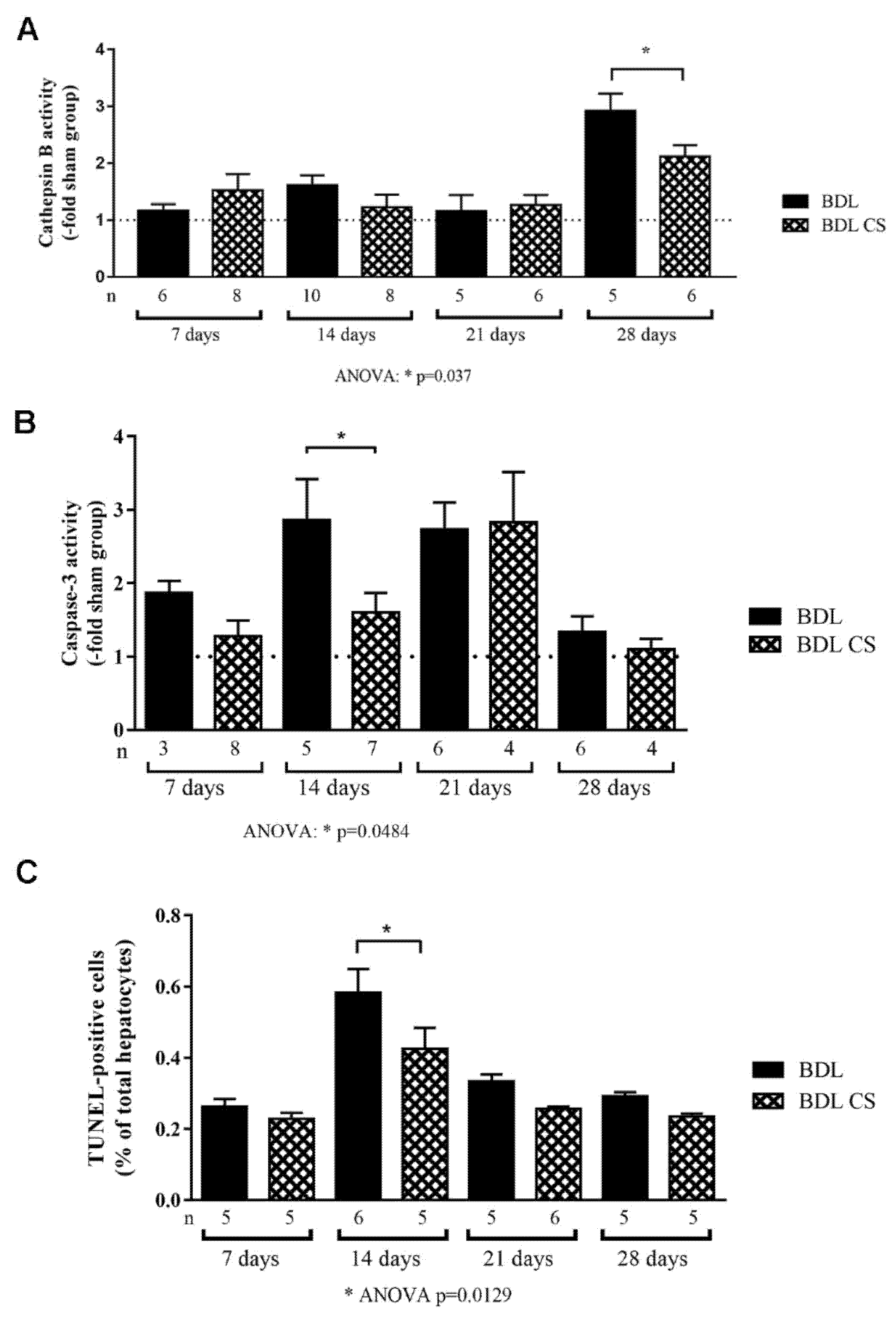
2.4. Chondroitin Sulfate Decreases Cell Death
2.5. Chondroitin Sulfate Promotes Hepatocyte Proliferation
3. Discussion
4. Materials and Methods
4.1. Animals
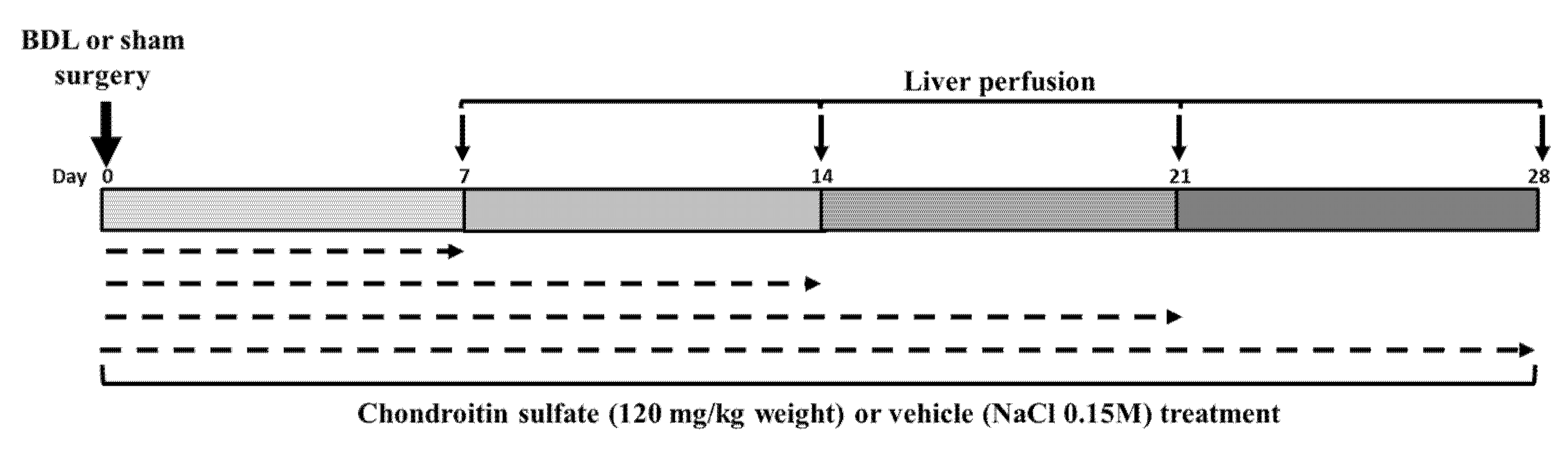
4.2. Common Bile Duct Ligation (BDL) and Treatment
4.3. Liver Perfusion
4.4. Histomorphometric Analysis of Liver Sections
4.5. TUNEL Assay
4.6. Anti-PCNA Immunohistochemistry
4.7. Cathepsin B and Caspase-3 Activity
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Iredale, J.P.; Pellicoro, A.; Fallowfield, J.A. Liver Fibrosis: Understanding the Dynamics of Bidirectional Wound Repair to Inform the Design of Markers and Therapies. Dig. Dis. 2017, 35, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Sepanlou, S.G.; Safiri, S.; Bisignano, C.; Ikuta, K.S.; Merat, S.; Saberifiroozi, M.; Poustchi, H.; Tsoi, D.; Colombara, D.V.; Abdoli, A.; et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef] [Green Version]
- Sodeman, T.; Bronk, S.F.; Roberts, P.J.; Miyoshi, H.; Gores, G.J. Bile salts mediate hepatocyte apoptosis by increasing cell surface trafficking of Fas. Am. J. Physiol. Liver Physiol. 2000, 278, G992–G999. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, H.; Gores, G.J., IV. Bile acids and death receptors. Am. J. Physiol. Liver Physiol. 2003, 284, G734–G738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.Z.; Lee, K.T.; Chern, C.L.; Cheng, J.T.; Stern, A.; Tsai, L.Y. Free Radical-Triggered Hepatic Injury of Experimental Obstructive Jaundice of Rats Involves Overproduction of Proinflammatory Cytokines and Enhanced Activation of Nuclear Factor κB. Ann. Clin. Lab. Sci. 2001, 31, 383–390. [Google Scholar] [PubMed]
- Gujral, J.S.; Farhood, A.; Bajt, M.L.; Jaeschke, H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology 2003, 38, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Arthur, M.J.P. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am. J. Physiol. Liver Physiol. 2000, 279, G245–G249. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of Liver Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 425–456. [Google Scholar] [CrossRef]
- Fausto, N.; Mead, J.E. Regulation of liver growth: Protooncogenes and transforming growth factors. Lab. Investig. 1989, 60, 4–13. [Google Scholar] [PubMed]
- Michalopoulos, G.K. Liver regeneration: Molecular mechanisms of growth control. FASEB J. 1990, 4, 176–187. [Google Scholar] [CrossRef]
- Greenbaum, L.E.; Wells, R.G. The role of stem cells in liver repair and fibrosis. Int. J. Biochem. Cell Biol. 2011, 43, 222–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuchweber, B.; Desmoulière, A.; Bochaton-Piallat, M.L.; Rubbia-Brandt, L.; Gabbiani, G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab. Investig. 1996, 74, 265–278. [Google Scholar]
- Desmoulière, A.; Darby, I.; Costa, A.M.; Raccurt, M.; Tuchweber, B.; Sommer, P.; Gabbiani, G. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab. Investig. 1997, 76, 765–778. [Google Scholar] [PubMed]
- Acharya, P.; Chouhan, K.; Weiskirchen, S.; Weiskirchen, R. Cellular Mechanisms of Liver Fibrosis. Front. Pharmacol. 2021, 12, 1072. [Google Scholar] [CrossRef]
- Silva, L.F. Isolation and Purification of Chondroitin Sulfate. Adv. Pharmacol. 2006, 53, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N. Anti-inflammatory activity of chondroitin sulphate: New functions from an old natural macromolecule. Inflammopharmacology 2011, 19, 299–306. [Google Scholar] [CrossRef]
- Guedes, P.L.R.; Castanon, M.C.M.N.; Nagaoka, M.R.; De Aguiar, J.A.K. Increase of glycosaminoglycans and metalloproteinases 2 and 9 in liver extracellular matrix on early stages of extrahepatic cholestasis. Arq. Gastroenterol. 2014, 51, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.J.; Lee, J.Y. The Effect of Chondroitin Sulfate against CCl4-Induced Hepatotoxicity. Biol. Pharm. Bull. 2003, 26, 622–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarcin, O.; Basaranoglu, M.; Tahan, V.; Tahan, G.; Sücüllü, I.; Yilmaz, N.; Sood, G.; Snyder, N.; Hilman, G.; Celikel, C.; et al. Time course of collagen peak in bile duct-ligated rats. BMC Gastroenterol. 2011, 11, 45. [Google Scholar] [CrossRef] [Green Version]
- Sharawy, M.H.; Abdel-Rahman, N.; Megahed, N.; El-Awady, M.S. Paclitaxel alleviates liver fibrosis induced by bile duct ligation in rats: Role of TGF-beta1, IL-10 and c-Myc. Life Sci. 2018, 211, 245–251. [Google Scholar] [CrossRef]
- Georgiev, P.; Jochum, W.; Heinrich, S.; Jang, J.H.; Nocito, A.; Dahm, F.; Clavien, P. Characterization of time-related changes after experimental bile duct ligation. BJS 2008, 95, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Abshagen, K.; König, M.; Hoppe, A.; Müller, I.; Ebert, M.; Weng, H.; Holzhütter, H.G.; Zanger, U.M.; Bode, J.; Vollmar, B.; et al. Pathobiochemical signatures of cholestatic liver disease in bile duct ligated mice. BMC Syst. Biol. 2015, 9, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Hatting, M.; Nevzorova, Y.A.; Peng, J.; Hu, W.; Boekschoten, M.V.; Roskams, T.; Muller, M.; Gassler, N.; Liedtke, C.; et al. Jnk1 in murine hepatic stellate cells is a crucial mediator of liver fibrogenesis. Gut 2014, 63, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.R.; Kouyoumdjian, M.; Borges, D.R. Hepatic clearance of tissue-type plasminogen activator and plasma kallikrein in experimental liver fibrosis. Liver Int. 2003, 23, 476–483. [Google Scholar] [CrossRef]
- Aycock, R.S.; Seyer, J.M. Collagens of Normal and Cirrhotic Human Liver. Connect. Tissue Res. 1989, 23, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.U.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J Histochem Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Ala-Kokko, L.; Pihlajaniemi, T.; Myers, J.C.; I Kivirikko, K.; Savolainen, E.R. Gene expression of type I, III and IV collagens in hepatic fibrosis induced by dimethylnitrosamine in the rat. Biochem. J. 1987, 244, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Shiba, M.; Shimizu, I.; Yasuda, M.; Ii, K.; Ito, S. Expression of type I and type III collagens during the course of dimethylnitrosamine-induced hepatic fibrosis in rats. Liver Int. 2008, 18, 196–204. [Google Scholar] [CrossRef]
- Königshofer, P.; Hofer, B.S.; Brusilovskaya, K.; Simbrunner, B.; Petrenko, O.; Wöran, K.; Herac, M.; Stift, J.; Lampichler, K.; Timelthaler, G.; et al. Distinct structural and dynamic components of portal hypertension in different animal models and human liver disease etiologies. Hepatology 2021. [Google Scholar] [CrossRef]
- Pinzani, M. Pathophysiology of Liver Fibrosis. Dig. Dis. 2015, 33, 492–497. [Google Scholar] [CrossRef]
- Jones, B.; Roberts, P.J.; Faubion, W.A.; Kominami, E.; Gores, G.J. Cystatin A expression reduces bile salt-induced apoptosis in a rat hepatoma cell line. Am. J. Physiol. Liver Physiol. 1998, 275, G723–G730. [Google Scholar] [CrossRef]
- Kahraman, A.; Barreyro, F.J.; Bronk, S.F.; Werneburg, N.W.; Mott, J.; Akazawa, Y.; Masuoka, H.C.; Howe, C.; Gores, G.J. TRAIL mediates liver injury by the innate immune system in the bile duct-ligated mouse. Hepatology 2008, 47, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Canbay, A.; Feldstein, A.; Baskin-Bey, E.; Bronk, S.F.; Gores, G.J. The Caspase Inhibitor IDN-6556 Attenuates Hepatic Injury and Fibrosis in the Bile Duct Ligated Mouse. J. Pharmacol. Exp. Ther. 2003, 308, 1191–1196. [Google Scholar] [CrossRef]
- Kahraman, A.; Mott, J.; Bronk, S.F.; Werneburg, N.W.; Barreyro, F.J.; Guicciardi, M.E.; Akazawa, Y.; Braley, K.; Craig, R.W.; Gores, G.J. Overexpression of Mcl-1 Attenuates Liver Injury and Fibrosis in the Bile Duct–Ligated Mouse. Am. J. Dig. Dis. 2009, 54, 1908–1917. [Google Scholar] [CrossRef]
- Greim, H.; Trülzsch, D.; Roboz, J.; Dressler, K.; Czygan, P.; Hutterer, F.; Schaffner, F.; Popper, H. Mechanism of cholestasis. 5. Bile acids in normal rat livers and in those after bile duct ligation. Gastroenterology 1972, 63, 837–845. [Google Scholar] [CrossRef]
- Chinnaiyan, A.M.; O’Rourke, K.; Yu, G.L.; Lyons, R.H.; Garg, M.; Duan, D.R.; Xing, L.; Gentz, R.; Ni, J.; Dixit, V.M. Signal Transduction by DR3, a Death Domain-Containing Receptor Related to TNFR-1 and CD95. Science 1996, 274, 990–992. [Google Scholar] [CrossRef]
- Graf, D.; Kurz, A.K.; Fischer, R.; Reinehr, R.; Häussinger, D. Taurolithocholic acid-3 sulfate induces CD95 trafficking and apoptosis in a c-Jun N-terminal kinase–dependent manner. Gastroenterology 2002, 122, 1411–1427. [Google Scholar] [CrossRef]
- Miyoshi, H.; Rust, C.; Roberts, P.J.; Burgart, L.J.; Gores, G.J. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology 1999, 117, 669–677. [Google Scholar] [CrossRef]
- Canbay, A.; Feldstein, A.E.; Higuchi, H.; Werneburg, N.; Grambihler, A.; Bronk, S.F.; Gores, G.J. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology 2003, 38, 1188–1198. [Google Scholar] [CrossRef]
- Beaussier, M.; Wendum, D.; Schiffer, E.; Dumont, S.; Rey, C.; Lienhart, A.; Housset, C. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab. Investig. 2007, 87, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Kossakowska, A.E.; Edwards, D.R.; Lee, S.S.; Urbanski, L.S.; Stabbler, A.L.; Zhang, C.-L.; Phillips, B.W.; Zhang, Y.; Urbanski, S.J. Altered Balance Between Matrix Metalloproteinases and Their Inhibitors in Experimental Biliary Fibrosis. Am. J. Pathol. 1998, 153, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Traina, P.; Sama, D.; Calatroni, A. Purified human plasma glycosaminoglycans reduced NF-κB activation, pro-inflammatory cytokine production and apoptosis in LPS-treated chondrocytes. Innate Immun. 2008, 14, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Cañas, N.; Gorina, R.; Planas, A.; Vergés, J.; Montell, E.; García, A.; López, M. Chondroitin sulfate inhibits lipopolysaccharide-induced inflammation in rat astrocytes by preventing nuclear factor kappa B activation. Neuroscience 2010, 167, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Vallières, M.; du Souich, P. Modulation of inflammation by chondroitin sulfate. Osteoarthr. Cartil. 2010, 18, S1–S6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero-Espinoza, L.; Huch, M. The balancing act of the liver: Tissue regeneration versus fibrosis. J. Clin. Investig. 2018, 128, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalopoulos, G.K. Liver regeneration. J. Cell. Physiol. 2007, 213, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Malato, Y.; Naqvi, S.; Schürmann, N.; Ng, R.; Wang, B.; Zape, J.; Kay, M.A.; Grimm, D.; Willenbring, H. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J. Clin. Investig. 2011, 121, 4850–4860. [Google Scholar] [CrossRef]
- Miyaoka, Y.; Ebato, K.; Kato, H.; Arakawa, S.; Shimizu, S.; Miyajima, A. Hypertrophy and Unconventional Cell Division of Hepatocytes Underlie Liver Regeneration. Curr. Biol. 2012, 22, 1166–1175. [Google Scholar] [CrossRef] [Green Version]
- Gilgenkrantz, H.; Collin de l’Hortet, A. Understanding Liver Regeneration: From Mechanisms to Regenerative Medicine. Am J Pathol. 2018, 188, 1316–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzalka, W.; Ziemienowicz, A. Proliferating cell nuclear antigen (PCNA): A key factor in DNA replication and cell cycle regulation. Ann. Bot. 2010, 107, 1127–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, M.; Maiorano, E.; Ladisa, R.; Pece, A.; Berloco, P.; Strazzabosco, M.; Caruso, M.L.; Valentini, A.M.; Ierardi, E.; Di Leo, A.; et al. Ursodeoxycholate further increases bile-duct cell proliferative response induced by partial bile-duct ligation in rats. Virchows. Archiv. 2004, 444, 554–560. [Google Scholar] [CrossRef]
- Glaser, S.; Lam, I.P.; Franchitto, A.; Gaudio, E.; Onori, P.; Chow, B.K.; Wise, C.; Kopriva, S.; Venter, J.; White, M.; et al. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology 2010, 52, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alatas, F.S.; Masumoto, K.; Matsuura, T.; Hayashida, M.; Saeki, I.; Kohashi, K.; Oda, Y.; Taguchi, T. Synchronized expressions of hepatic stellate cells and their transactivation and liver regeneration during liver injury in an animal model of cholestasis. J. Pediatr. Surg. 2011, 46, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Olteanu, D.; Nagy, A.; Dudea, M.; Filip, A.; Muresan, A.; Catoi, C.; A Mircea, P.; Clichici, S. Hepatic and systemic effects of rosuvastatin on an experimental model of bile duct ligation in rats. J. Physiol. Pharmacol. 2012, 63, 483–496. [Google Scholar]
- Edward, M.; Watson, H.H.; Williamson, F.B.; Long, W.F. Changes in glycosaminoglycans during regeneration of rat liver. Biochem. Soc. Trans. 1979, 7, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.P.; Sampaio, L.O.; Toledo, O.M.; Cássaro, C.M. Cell recognition and adhesiveness: A possible biological role for the sulfated mucopolysaccharides. Biochem. Biophys. Res. Commun. 1977, 75, 329–336. [Google Scholar] [CrossRef]
- Mizumoto, S.; Fongmoon, D.; Sugahara, K. Interaction of chondroitin sulfate and dermatan sulfate from various biological sources with heparin-binding growth factors and cytokines. Glycoconj. J. 2013, 30, 619–632. [Google Scholar] [CrossRef]
- Sakamoto, K.; Khai, N.C.; Wang, Y.; Irie, R.; Takamatsu, H.; Matsufuji, H.; Kosai, K.I. Heparin-binding epidermal growth factor-like growth factor and hepatocyte growth factor inhibit cholestatic liver injury in mice through different mechanisms. Int. J. Mol. Med. 2016, 38, 1673–1682. [Google Scholar] [CrossRef] [Green Version]
- Dao, D.T.; Anez-Bustillos, L.; Adam, R.M.; Puder, M.; Bielenberg, D.R. Heparin-Binding Epidermal Growth Factor–Like Growth Factor as a Critical Mediator of Tissue Repair and Regeneration. Am. J. Pathol. 2018, 188, 2446–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, P.; Henderson, N.C. Antifibrotics in chronic liver disease: Tractable targets and translational challenges. Lancet Gastroenterol. Hepatol. 2016, 1, 328–340. [Google Scholar] [CrossRef]
- Sodhi, H.; Panitch, A. Glycosaminoglycans in Tissue Engineering: A Review. Biomolecules 2020, 11, 29. [Google Scholar] [CrossRef]
- National Research Council (USA). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. In Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Le Sueur-Maluf, L.; Viana, M.B.; Nagaoka, M.R.; Amorim, A.L.B.; Cardoso, A.N.; Rodrigues, B.C.; Mendes, N.F.; Bittencourt, J.C.; Cespedes, I.C. Behavioral alterations and Fos protein immunoreactivity in brain regions of bile duct-ligated cirrhotic rats. Anais Acad. Brasil. Ciênc. 2015, 87, 331–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasso, G.R.D.S.; Florencio-Silva, R.; Da Fonseca, C.C.N.; Cezar, L.C.; Carbonel, A.A.F.; Gil, C.D.; Simões, M.D.J.; Girão, M.J.B.C. Effects of estrogen deficiency followed by streptozotocin-induced diabetes on periodontal tissues of female rats. J. Mol. Histol. 2020, 51, 353–365. [Google Scholar] [CrossRef]
- Koshimizu, J.Y.; Beltrame, F.L.; de Pizzol, J.P., Jr.; Cerri, P.S.; Caneguim, B.H.; Sasso-Cerri, E. NF-kB overexpression and decreased immunoexpression of AR in the muscular layer is related to structural damages and apoptosis in cimetidine-treated rat vas deferens. Reprod Biol Endocrinol. 2013, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Cotrin, S.S.; Puzer, L.; Judice, W.; Juliano, L.; Carmona, A.; Juliano, M.A. Positional-scanning combinatorial libraries of fluorescence resonance energy transfer peptides to define substrate specificity of carboxydipeptidases: Assays with human cathepsin B. Anal. Biochem. 2004, 335, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, H.; Bronk, S.F.; Taniai, M.; Canbay, A.; Gores, G.J. Cholestasis increases tumor necrosis factor-related apoptotis-inducing ligand (TRAIL)-R2/DR5 expression and sensitizes the liver to TRAIL-mediated cytotoxicity. J. Pharmacol. Exp. Ther. 2002, 303, 461–467. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, B.T.; Wu, Z.C.; Yu, W.T.; Lin, P.J.; Tsai, W.L.; Shiesh, S.C. Glycine ameliorates liver injury and vitamin D deficiency induced by bile duct ligation. Clin. Chim. Acta 2013, 420, 150–154. [Google Scholar] [CrossRef] [PubMed]



| Parameters | BDL | BDL-CS | ||||||
|---|---|---|---|---|---|---|---|---|
| 7d | 14d | 21d | 28d | 7d | 14d | 21d | 28d | |
| N | 6 | 11 | 5 | 7 | 9 | 9 | 6 | 6 |
| Age (week) | 6.93 ± 0.03 | 7.85 ± 0.24 a | 7.14 ± 0.29 | 7.28 ± 0.18 | 6.65 ± 0.02 | 6.68 ± 0.12 b | 7.14 ± 0.36 | 7.64 ± 0.21 a,c |
| Body weight(g) | 219 ± 6 | 264 ± 6 a | 292 ± 17 d | 282 ± 15 d | 193 ± 6 | 213 ± 10 b | 290 ± 6 b,e | 286 ± 3 b,e |
| Liver weight (g) | 15.8 ± 0.6 | 21.7 ± 0.8 d | 26.9 ± 1.8 e | 24.4 ± 1.8 e | 13.9 ± 0.5 | 16.6 ± 0.8 b | 26.0 ± 0.9 b,e | 27.9 ± 1.6 b,e |
| L/B weight ratio (%) | 7.2 ± 0.2 | 8.2 ± 0.2 | 9.2 ± 0.3 a | 8.7 ± 0.5 | 7.2 ± 0.1 | 7.9 ± 0.4 | 9.0 ± 0.2 a | 9.8 ± 0.6 c,e |
| ALT (U/L) | 111 ± 13 | 149 ± 24 | 134 ± 24 | 101 ± 23 | 78 ± 9 | 105 ± 15 | 113 ± 16 | 115 ± 18 |
| AST (U/L) | 493 ± 54 | 522 ± 77 | 478 ± 42 | 296 ± 62 | 343 ± 59 | 447 ± 42 | 334 ± 49 | 255 ± 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedes, P.L.R.; Carvalho, C.P.F.; Carbonel, A.A.F.; Simões, M.J.; Icimoto, M.Y.; Aguiar, J.A.K.; Kouyoumdjian, M.; Gazarini, M.L.; Nagaoka, M.R. Chondroitin Sulfate Protects the Liver in an Experimental Model of Extra-Hepatic Cholestasis Induced by Common Bile Duct Ligation. Molecules 2022, 27, 654. https://doi.org/10.3390/molecules27030654
Guedes PLR, Carvalho CPF, Carbonel AAF, Simões MJ, Icimoto MY, Aguiar JAK, Kouyoumdjian M, Gazarini ML, Nagaoka MR. Chondroitin Sulfate Protects the Liver in an Experimental Model of Extra-Hepatic Cholestasis Induced by Common Bile Duct Ligation. Molecules. 2022; 27(3):654. https://doi.org/10.3390/molecules27030654
Chicago/Turabian StyleGuedes, Pedro L. R., Carolina P. F. Carvalho, Adriana A. F. Carbonel, Manuel J. Simões, Marcelo Y. Icimoto, Jair A. K. Aguiar, Maria Kouyoumdjian, Marcos L. Gazarini, and Marcia R. Nagaoka. 2022. "Chondroitin Sulfate Protects the Liver in an Experimental Model of Extra-Hepatic Cholestasis Induced by Common Bile Duct Ligation" Molecules 27, no. 3: 654. https://doi.org/10.3390/molecules27030654
APA StyleGuedes, P. L. R., Carvalho, C. P. F., Carbonel, A. A. F., Simões, M. J., Icimoto, M. Y., Aguiar, J. A. K., Kouyoumdjian, M., Gazarini, M. L., & Nagaoka, M. R. (2022). Chondroitin Sulfate Protects the Liver in an Experimental Model of Extra-Hepatic Cholestasis Induced by Common Bile Duct Ligation. Molecules, 27(3), 654. https://doi.org/10.3390/molecules27030654







