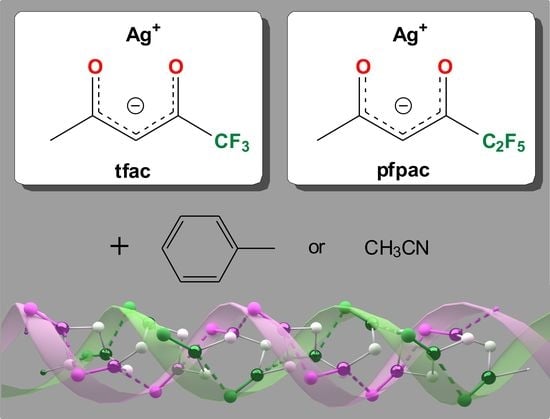
Structural Diversity of Silver Fluorinated β-Diketonates: Effect of the Terminal Substituent and Solvent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sourses and Analitical Methods
2.2. Synthesis of [Ag(tfac)]∞ 1
2.3. Synthesis of [Ag(pfpac)]∞ 2
2.4. Synthesis of Ag(I) β-Diketonate Adducts
2.5. Single-Crystal XRD Study
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Structural Features of Ag(I) Fluorinated β-Diketonates and Their Adducts
- [Ag2 (hfac)2 (C7H8)] 3t [20] (hfac = 1,1,1,5,5,5-hexafluoro-3,5-pentanedionate, R1 = R2 = CF3);
- [Ag2 (btfac)2 (CH3CN)] 4a [21] (btfac = 4,4,4-trifluoro-1-phenyl-1,3-butanedionate, R1 = CF3, R2 = C6H5);
- [Ag2 (nphtfac)2 (CH3CN)] 5a [21] (nphtfac = 4,4,4-trifluoro-1-(2-naphthyl)-1,3-butanedione, R1 = CF3, R2 = C10H7).
3.3. Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Condorelli, G.G.; Malandrino, G.; Fragalà, I.L. Engineering of molecular architectures of β-diketonate precursors toward new advanced materials. Coord. Chem. Rev. 2007, 251, 1931–1950. [Google Scholar] [CrossRef]
- Vigato, P.A.; Peruzzo, V.; Tamburini, S. The evolution of β-diketone or β-diketophenol ligands and related complexes. Coord. Chem. Rev. 2009, 253, 1099–1201. [Google Scholar] [CrossRef]
- Mishra, S.; Daniele, S. Metal–organic derivatives with fluorinated ligands as precursors for inorganic nanomaterials. Chem. Rev. 2015, 115, 8379–8448. [Google Scholar] [CrossRef]
- Kremer, M.; Englert, U.N. Donor substituted acetylacetones—Versatile ditopic ligands. Z. Kristallogr. Cryst. Mater. 2018, 233, 437–452. [Google Scholar] [CrossRef]
- Blake, A.J.; Champness, N.R.; Howdle, S.M.; Webb, P.B. Silver (I) coordination polymers using thioether macrocycle building blocks. Inorg. Chem. 2000, 39, 1035–1038. [Google Scholar] [CrossRef]
- Blake, A.J.; Champness, N.R.; Howdle, S.M.; Morley, K.S.; Webb, P.B.; Wilson, C. Silver (I)–thioether coordination polymers constructed using asymmetric diketonate anions. CrystEngComm 2002, 4, 88–92. [Google Scholar] [CrossRef]
- Pointillart, F.; Herson, P.; Boubekeur, K.; Train, C. Square-planar and trigonal prismatic silver (I) in bipyrimidine and oxalate bridged tetranuclear complexes and one-dimensional compounds: Synthesis and crystal structures. Inorg. Chim. Acta 2008, 361, 373–379. [Google Scholar] [CrossRef]
- Marandi, F.; Ghadermazi, M.; Marandi, A.; Pantenburg, I.; Meyer, G. Two new silver (I) coordination polymers with 4,4′-bipyridine and two perfluoro-β-diketonates. J. Mol. Struct. 2011, 1006, 136–141. [Google Scholar] [CrossRef]
- Jin, J.L.; Xie, Y.P.; Cui, H.; Duan, G.X.; Lu, X.; Mak, T.C. Structure-directing role of phosphonate in the synthesis of high-nuclearity silver (I) sulfide-ethynide-thiolate clusters. Inorg. Chem. 2017, 56, 10412–10417. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.L.; Shen, Y.L.; Xie, Y.P.; Lu, X. Silver ethynide clusters constructed with fluorinated β-diketonate ligands. CrystEngComm 2018, 20, 2036–2042. [Google Scholar] [CrossRef]
- Shen, Y.L.; Jin, J.L.; Duan, G.X.; Yu, P.Y.; Xie, Y.P.; Lu, X. Nestlike Silver (I) Thiolate Clusters with Tunable Emission Color Templated by Heteroanions. Chem. A Eur. J. 2021, 27, 1122–1126. [Google Scholar] [CrossRef]
- Zanotto, L.; Benetollo, F.; Natali, M.; Rossetto, G.; Zanella, P.; Kaciulis, S.; Mezzi, A. Facile Synthesis and Characterization of New β-Diketonate Silver Complexes. Single-Crystal Structures of (1,1,1,5,5,5-Hexafluoro-2,4-pentadionato)(2,2′-bipyridine) silver (I) and (1,1,1,5,5,5-Hexafluoro-2,4-pentadionato)(N,N,N′,N′-tetramethylethylenediamine) silver (I) and Their Use as MOCVD Precursors for Silver Films. Chem. Vapor Depos. 2004, 10, 207–213. [Google Scholar]
- Liu, H.; Battiato, S.; Pellegrino, A.L.; Paoli, P.; Rossi, P.; Jiménez, C.; Malandrino, G.; Muñoz-Rojas, D. Deposition of metallic silver coatings by Aerosol Assisted MOCVD using two new silver β-diketonate adduct metalorganic precursors. Dalton Trans. 2017, 46, 10986–10995. [Google Scholar] [CrossRef]
- Piszczek, P.; Radtke, A. Silver nanoparticles fabricated using chemical vapor deposition and atomic layer deposition techniques: Properties, applications and perspectives: Review. In Noble and Precious Metals—Properties, Nanoscale Effects and Applications; Seehra, M.S., Bristow, A.D., Eds.; IntechOpen: London, UK, 2018; pp. 187–213. [Google Scholar]
- Ruan, H.X. 1,1,1-(trifluoroacetylacetonato)silver(I) Used for Photochemical and Thermal Deposition of Silver and Silver Oxide Film and Its Kinetics. Master’s Thesis, Simon Fraser University, Bumaby, BC, Canada, 2007. [Google Scholar]
- Fedoseev, I.S.; Vikulova, E.S.; Il’in, I.Y.; Smolentsev, A.I.; Gallyamov, M.R.; Morozova, N.B. Crystal structure of (1,1,1-trifluoro-5,5-dimethylhexan-2,4-dionato) silver (I). J. Struct. Chem. 2016, 57, 1667–1670. [Google Scholar] [CrossRef]
- Gulyaev, S.A.; Vikulova, E.S.; Sukhikh, T.S.; Ilyin, I.Y.; Morozova, N.B. Structures and thermal properties of silver (I) β-diketonates with bulky terminal substituents. J. Struct. Chem. 2021, 62, 1836–1845. [Google Scholar] [CrossRef]
- Patterson, S.; Henderson, D.; Tasker, P. CCDC 1410235: Experimental Crystal Structure Determination. 2015. Available online: https://research.utwente.nl/en/publications/ccdc-1483947-experimental-crystal-structure-determination-2 (accessed on 29 December 2021).
- Xu, C.; Corbitt, T.S.; Hampden-Smith, M.J.; Kodas, T.T.; Duesler, E.N. Synthesis and characterization of Lewis-base adducts of 1,1,1,5,5,5-hexafluoroacetylacetonatosilver (I). J. Chem. Soc. Dalton Trans. 1994, 19, 2841–2849. [Google Scholar] [CrossRef]
- Evans, W.J.; Giarikos, D.G.; Josell, D.; Ziller, J.W. Synthesis and structure of polymeric networks of silver hexafluoroacetylacetonate complexes of THF, toluene, and vinyltrimethylsilane. Inorg. Chem. 2003, 42, 8255–8261. [Google Scholar] [CrossRef] [PubMed]
- Akhbari, K.; Morsali, A. One-dimensional Corrugated Tape AgI coordination polymers constructed of Ag−C bonds. Cryst. Growth Des. 2007, 7, 2024–2030. [Google Scholar] [CrossRef]
- Rybaltovskii, A.O.; Arakcheev, V.G.; Bekin, A.N.; Danilyuk, A.F.; Gerasimova, V.I.; Minaev, N.V.; Golubeva, E.N.; Parenago, O.O.; Bagratashvili, V.N. Photo–induced processes in Ag and Eu β-diketonates incorporated into aerogel matrix of silicon dioxide by supercritical fluid impregnation. Russ. J. Phys. Chem. B 2015, 9, 1137–1142. [Google Scholar] [CrossRef]
- Wisser, F.M.; Schumm, B.; Mondin, G.; Grothe, J.; Kaskel, S. Precursor strategies for metallic nano-and micropatterns using soft lithography. J. Mater. Chem. C 2015, 3, 2717–2731. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, G.; Petralia, S.; Franco, D.; Nocito, G.; Fabbi, C.; Forte, L.; Guglielmino, S.; Squarzoni, S.; Train, F.; Conoci, S. A new Ag-nanostructured hydroxyapatite porous scaffold: Antibacterial effect and cytotoxicity study. Mater. Sci. Eng. C 2021, 118, 111394. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, C.; Pettinari, R.; Di Nicola, C.; Tombesi, A.; Scuri, S.; Marchetti, F. Antimicrobial MOFs. Coord. Chem. Rev. 2021, 446, 214121. [Google Scholar] [CrossRef]
- Jaros, S.W.; Krogul-Sobczak, A.; Bażanów, B.; Florek, M.; Poradowski, D.; Nesterov, D.S.; Śliwińska-Hill, U.; Kirillov, A.M.; Smoleński, P. Self-assembly and multifaceted bioactivity of a silver (I) quinolinate coordination polymer. Inorg. Chem. 2021, 60, 15435–15444. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Costa, I.F.; Jorge, P.; Sousa, A.C.; André, V.; Cerca, N.; Kirillov, A.M. Silver (I) coordination polymers immobilized into biopolymer films for antimicrobial applications. ACS Appl. Mater. Interfaces 2021, 13, 12836–12844. [Google Scholar] [CrossRef] [PubMed]
- Mikhailovskaya, T.F.; Makarov, A.G.; Selikhova, N.Y.; Makarov, A.Y.; Pritchina, E.A.; Bagryanskaya, I.Y.; Vorontsova, E.V.; Ivanov, I.G.; Tikhova, V.D.; Gritsan, N.P.; et al. Carbocyclic functionalization of quinoxalines, their chalcogen congeners 2,1,3-benzothia/selenadiazoles, and related 1,2-diaminobenzenes based on nucleophilic substitution of fluorine. J. Fluor. Chem. 2016, 183, 44–58. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Organics and Impurities: Common Laboratory Solvents and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Gamer, M.T.; Scheer, M.; Konchenko, S.N.; Roesky, P.W. Intramolecular Phosphorus–Phosphorus Bond Formation within a Co2P4 Core. Chem. Comm. 2013, 49, 2183–2185. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Powder Diffraction File, Release 2010, International Centre for Diffraction Data, Pennsylvania, USA. Available online: http://www.icdd.com/products/pdf2.htm (accessed on 20 December 2021).










| Coordination Polymer | [Ag (tfac)]∞ | [Ag (pfpac)]∞ |
|---|---|---|
| Identification code | 1 | 2 |
| Distance, Å | ||
| Ag–Oβ-dik (chelate, non-bridging) | 2.328 (6), 2.374 (6) | 2.37 (1)–2.46 (2) |
| Ag–Oβ-dik (chelate, bridging) | 2.374 (6) | 2.37 (1)–2.43 (1) |
| Ag–Oβ-dik (non-chelate, bridging) | 2.466 (6) | 2.26 (1)–2.37 (1) |
| Ag–Cα | 2.336 (8) | 2.25 (2)–2.32 (2) |
| Ag···Ag (min., in the chain) | 3.666 (2) | 3.43 (1)–4.25 (1) |
| Ag···Ag (min., between the chains) | 2.861 (2) | 7.80(1) |
| Angle, ° | ||
| O–Ag–Oβ-dik (chelate) | 77.0 (3) | 71.9 (5)–76.2 (5) |
| Deformation of chelate metallocycle AgO2C3: angle between the planes, ° | ||
| (Ag, O, Oβ) & (O, C, Cβ, Oβ) | 162.2 | 156.4–159.8 |
| (C, Cα, Cβ) & (O, C, Cβ, Oβ) | 170.1 | 168.5–172.2 |
| General Formula | [Ag2 (L)2 (Q)]∞ | |||
|---|---|---|---|---|
| L | tfac | pfpac | ||
| Q | CH3CN, X = N | C7H8, X = C | CH3CN, X = N | C7H8, X = C |
| Identification Code | 1a | 1t | 2a | 2t |
| Distance, Å | ||||
| Ag (S)–X | – | 2.650 (2), 2.548 (2) | 2.180 (3) | 2.481 (5) |
| Ag (NS)–Oβ-dik (chelate, non-bridging) | – | 2.344 (2) | 2.370 (2) | 2.346 (3) |
| Ag (S)–Oβ-dik (chelate, non-bridging) | 2.395 (2) | – | – | – |
| Ag (NS)–Oβ-dik (chelate, bridging) | – | 2.412 (2)–2.455 (2) | 2.358 (2)–2.472 (2) | 2.365 (3)–2.515 (4) |
| Ag (S)–Oβ-dik (chelate, bridging) | 2.439 (2) | 2.388 (2), 2.714 (2) | 2.474 (2), 2.596 (2) | 2.484 (4), 2.732 (3) |
| Ag (S)–Oβ-dik (non-chelate, bridging) | 2.459 (2) | 2.397 (2), 2.404 (2) | 2.326 (2), 2.488 (2) | 2.292 (3), 2.432 (4) |
| Ag (NS)–Cα | – | 2.369 (2) | 2.390 (3) | 2.487 (5) |
| Ag (S)–Cα | 2.468 (2) | – | – | – |
| Ag···Ag (min., in the chain) | 3.820 (2) | 3.1235 (4) | 3.2379 (3) | 3.1087 (6) |
| Angle, ° | ||||
| O–Ag–Oβ-dik (chelate) | 75.40 (6) | 67.53 (5)–76.67 (6) | 66.79 (7)–75.82 (8) | 69.7 (1)–77.1 (1) |
| Ag···Ag···Ag (in the chain) | 71.9, 108.1 | 69.8, 110.2 | 64.0, 116.0 | 72.4, 107.6 |
| Deformation of chelate metallocycle AgO2C3: angle between the planes, ° | ||||
| (Ag, O, Oβ) & (O, C, Cβ, Oβ) | 160.8 | 130.3–147.3 | 132.0, 148.4 | 123.4–153.4 |
| (C, Cα, Cβ) & (O, C, Cβ, Oβ) | 173.2 | 169.4, 178.3 | 172.6, 179.0 | 175.4, 176.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikulova, E.S.; Sukhikh, T.S.; Gulyaev, S.A.; Ilyin, I.Y.; Morozova, N.B. Structural Diversity of Silver Fluorinated β-Diketonates: Effect of the Terminal Substituent and Solvent. Molecules 2022, 27, 677. https://doi.org/10.3390/molecules27030677
Vikulova ES, Sukhikh TS, Gulyaev SA, Ilyin IY, Morozova NB. Structural Diversity of Silver Fluorinated β-Diketonates: Effect of the Terminal Substituent and Solvent. Molecules. 2022; 27(3):677. https://doi.org/10.3390/molecules27030677
Chicago/Turabian StyleVikulova, Evgeniia S., Taisiya S. Sukhikh, Sergey A. Gulyaev, Igor Yu. Ilyin, and Natalia B. Morozova. 2022. "Structural Diversity of Silver Fluorinated β-Diketonates: Effect of the Terminal Substituent and Solvent" Molecules 27, no. 3: 677. https://doi.org/10.3390/molecules27030677
APA StyleVikulova, E. S., Sukhikh, T. S., Gulyaev, S. A., Ilyin, I. Y., & Morozova, N. B. (2022). Structural Diversity of Silver Fluorinated β-Diketonates: Effect of the Terminal Substituent and Solvent. Molecules, 27(3), 677. https://doi.org/10.3390/molecules27030677