The Study of Chemical Profile and Antioxidant Properties of Poplar-Type Polish Propolis Considering Local Flora Diversity in Relation to Antibacterial and Anticancer Activities in Human Breast Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mineral Composition
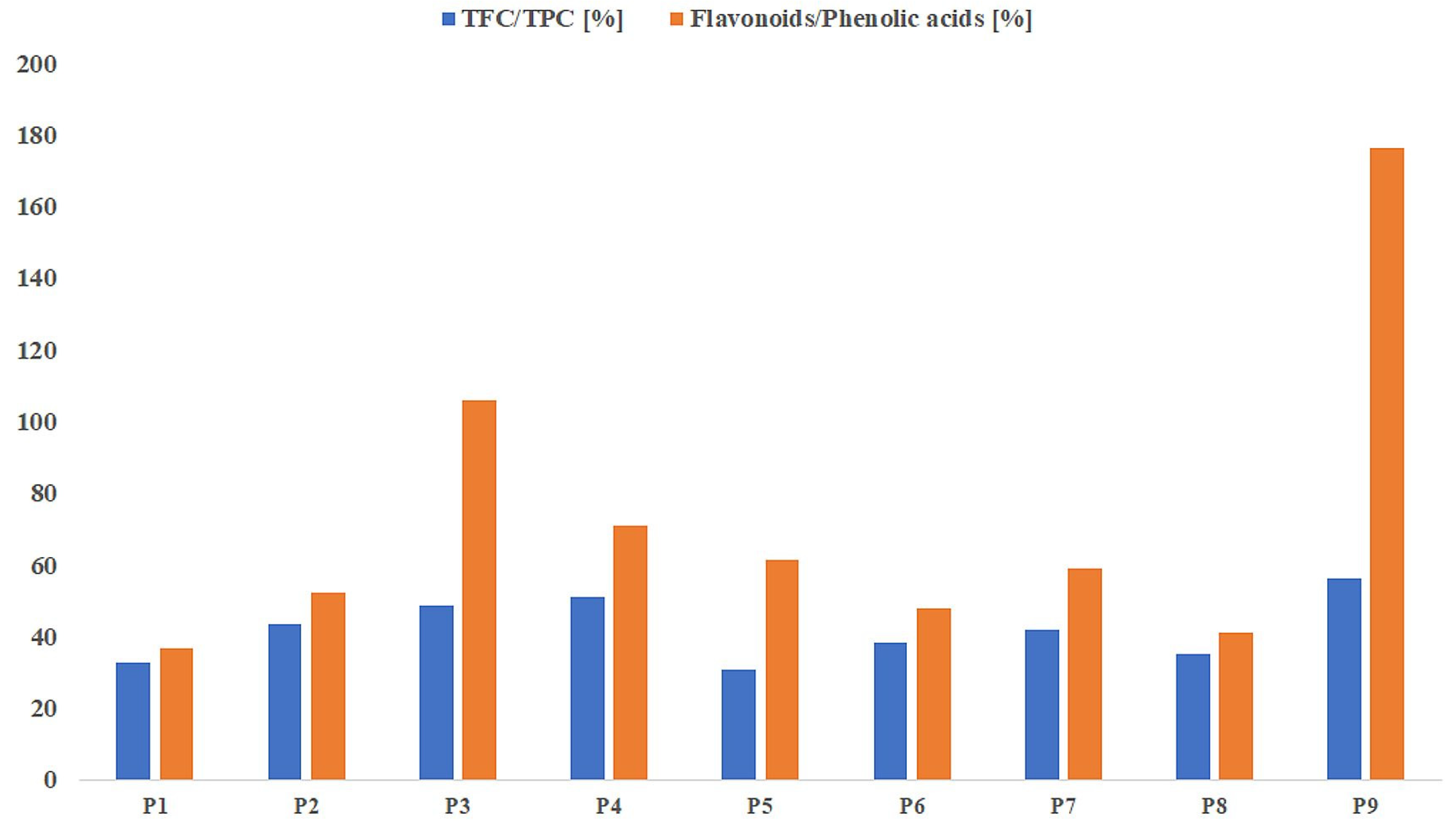
2.2. Antioxidant Capacity
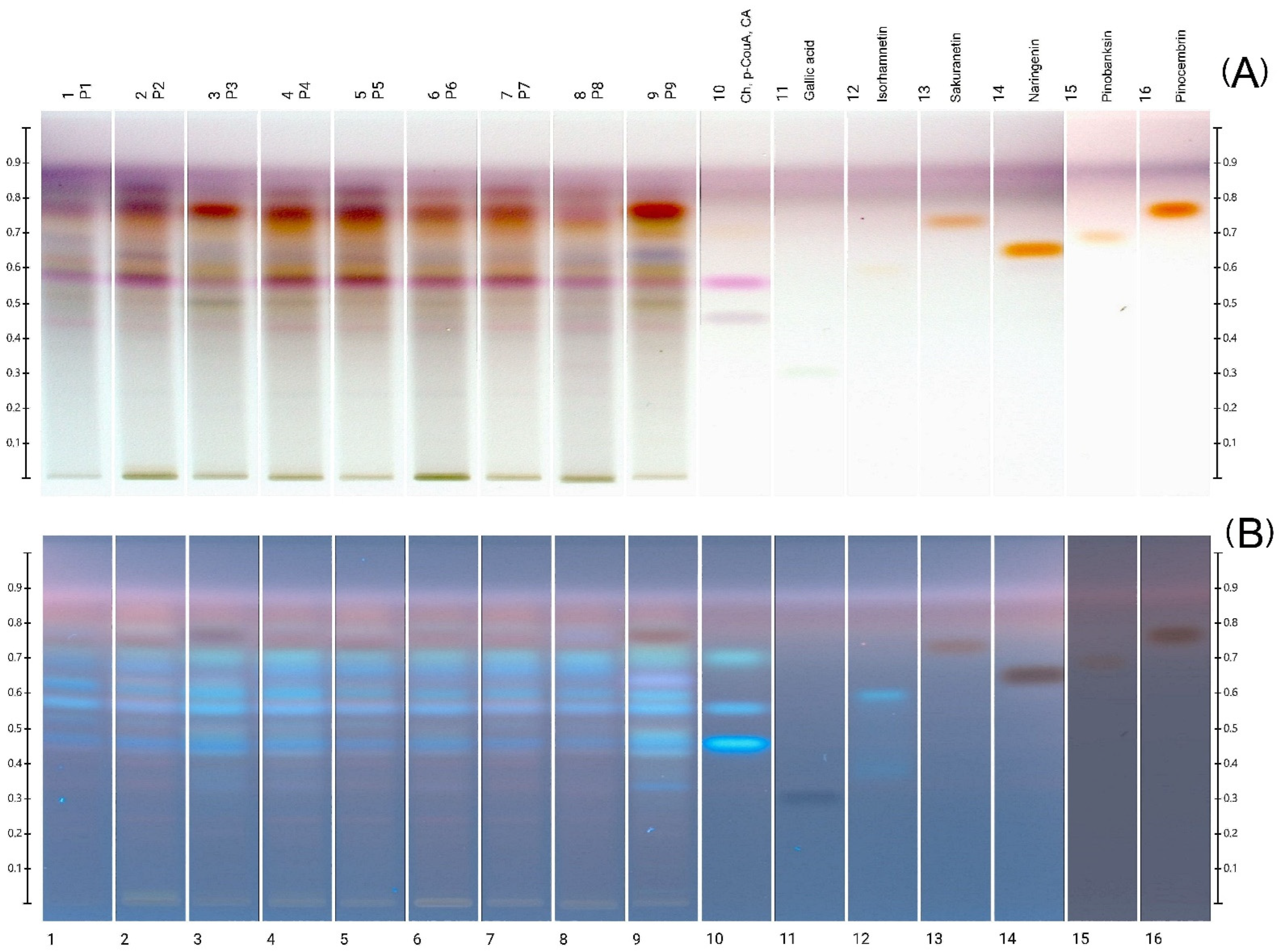
2.3. HPTLC and HPLC-DAD Polyphenolic Profiles
2.4. Antibacterial Activity Assays
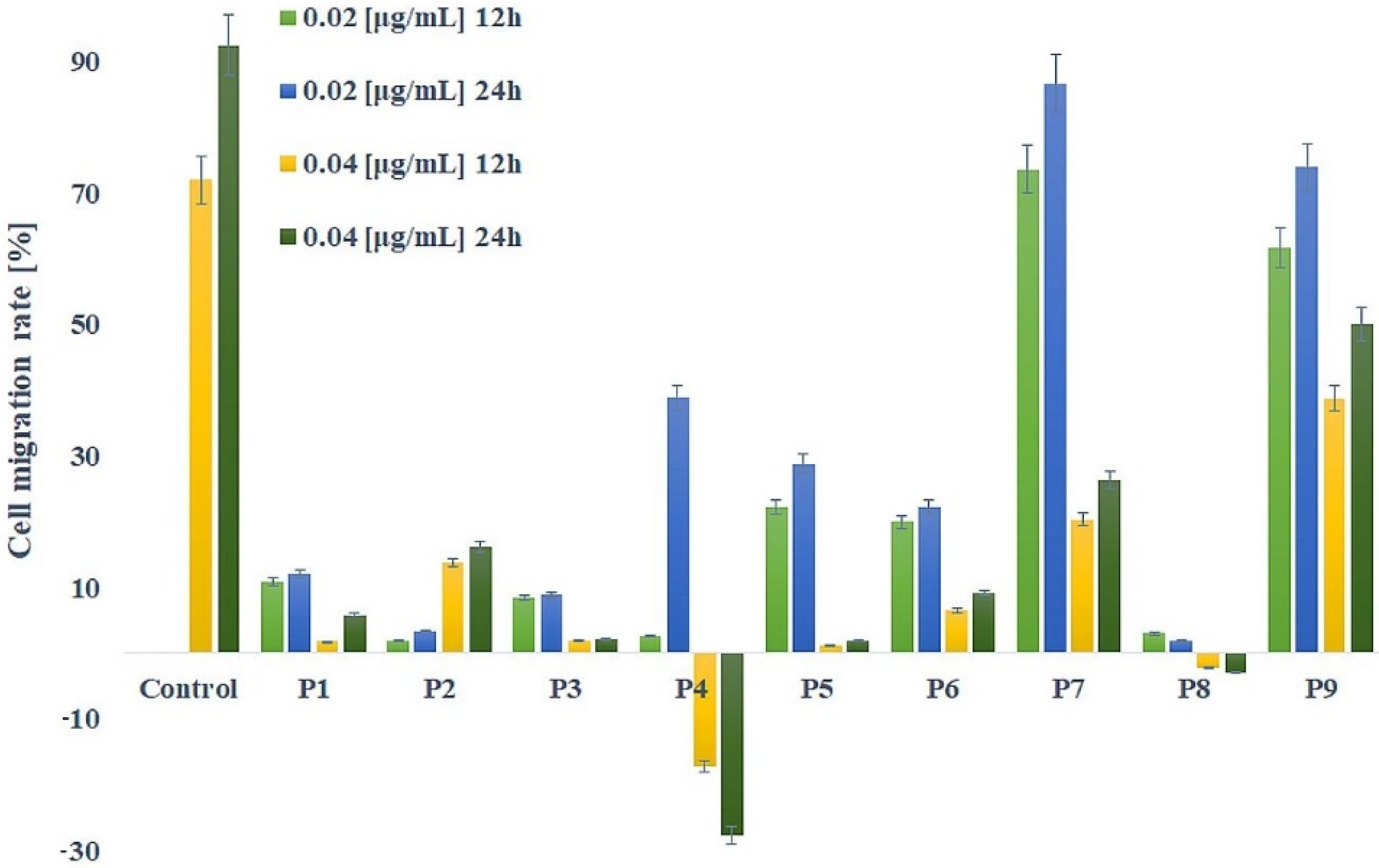
2.5. Cancer Cells Migration Inhibition Assay
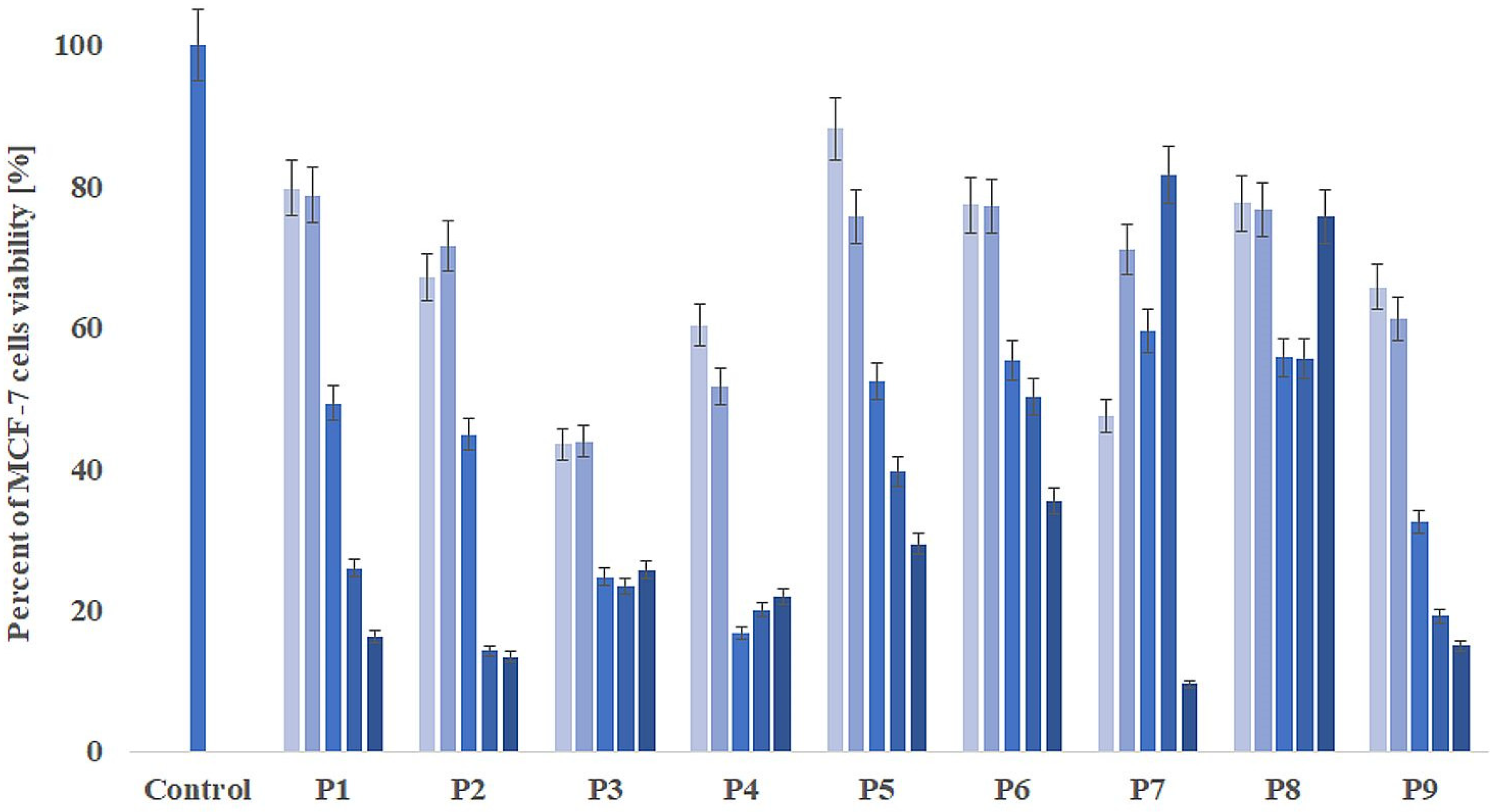
2.6. Cell Proliferation Assay
3. Materials and Methods
3.1. Chemicals
3.2. Media and Bacterial Strains
3.3. Material Collection
3.4. Preparation of Propolis Dry Extract
3.5. Mineral Composition of Bioelements Using the ICP-OES Method
3.6. Antioxidants Assay
3.7. Total Phenolic (TPC) and Flavonoid (TFC) Content
3.8. Polyphenolic Profile by the HPTLC Method
3.9. Identification of Polyphenols by HPLC-DAD Method
3.10. Antibacterial Activity Assays
3.11. Cancer Cell Culture and Propolis Treatment
3.12. Cancer Cells Migration Inhibition Assay
3.13. Cell Proliferation Assay
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ristivojević, P.; Trifković, J.; Andrić, F.; Milojković-Opsenica, D. Poplar-type propolis: Chemical composition, botanical origin and biological activity. Nat. Prod. Commun. 2015, 10, 1869–1876. [Google Scholar] [CrossRef] [Green Version]
- El-Guendouz, S.; Lyoussi, B.; Miguel, M.G. Insight on Propolis from Mediterranean Countries: Chemical Composition, Biological Activities and Application Fields. Chem. Biodivers. 2019, 16, e1900094. [Google Scholar] [CrossRef]
- Vera, N.; Solorzano, E.; Ordoñez, R.; Maldonado, L.; Bedascarrasbure, E.; Isla, M.I. Chemical composition of Argentinean propolis collected in extreme regions and its relation with antimicrobial and antioxidant activities. Nat. Prod. Commun. 2011, 6, 823–827. [Google Scholar] [CrossRef] [Green Version]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, Z.; Naseri, M.; Vatandoost, F. Propolis: Chemical composition and its applications in endodontics. Iran. Endod. J. 2018, 13, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- De Groot, A.C. Propolis: A review of properties, applications, chemical composition, contact allergy, and other adverse effects. Dermatitis 2013, 24, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Cantarelli, M.Á.; Camiña, J.M.; Pettenati, E.M.; Marchevsky, E.J.; Pellerano, R.G. Trace mineral content of Argentinean raw propolis by neutron activation analysis (NAA): Assessment of geographical provenance by chemometrics. LWT Food Sci. Technol. 2011, 44, 256–260. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [Green Version]
- Pellati, F.; Orlandini, G.; Pinetti, D.; Benvenuti, S. HPLC-DAD and HPLC-ESI-MS/MS methods for metabolite profiling of propolis extracts. J. Pharm. Biomed. Anal. 2011, 55, 934–948. [Google Scholar] [CrossRef]
- Bertrams, J.; Müller, M.B.; Kunz, N.; Kammerer, D.R.; Stintzing, F.C. Phenolic compounds as marker compounds for botanical origin determination of German propolis samples based on TLC and TLC-MS. J. Appl. Bot. Food Qual. 2013, 86, 143–153. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical composition and antioxidant activity of propolis prepared in different forms and in different solvents useful for finished products. Foods 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avula, B.; Sagi, S.; Masoodi, M.H.; Bae, J.Y.; Wali, A.F.; Khan, I.A. Quantification and characterization of phenolic compounds from Northern Indian propolis extracts and dietary supplements. J. AOAC Int. 2021, 103, 1378–1393. [Google Scholar] [CrossRef] [PubMed]
- Irigoiti, Y.; Navarro, A.; Yamul, D.; Libonatti, C.; Tabera, A.; Basualdo, M. The use of propolis as a functional food ingredient: A review. Trends Food Sci. Technol. 2021, 115, 297–306. [Google Scholar] [CrossRef]
- Grecka, K.; Kuś, P.M.; Okińczyc, P.; Worobo, R.W.; Walkusz, J.; Szweda, P. The anti-staphylococcal potential of ethanolic Polish propolis extracts. Molecules 2019, 24, 1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pobiega, K.; Gniewosz, M.; Kraśniewska, K. Antimicrobial and antiviral properties of different types of propolis. Zesz. Probl. Postępów Nauk Rol. 2018, 69–79. [Google Scholar] [CrossRef]
- Zulhendri, F.; Ravalia, M.; Kripal, K.; Chandrasekaran, K.; Fearnley, J.; Perera, C.O. Propolis in metabolic syndrome and its associated chronic diseases: A narrative review. Antioxidants 2021, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Zampini, I.C.; Salas, A.L.; Maldonado, L.M.; Simirgiotis, M.J.; Inés Isla, M. Propolis from the Monte region in Argentina: A potential phytotherapic and food functional ingredient. Metabolites 2021, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Ratajczak, I.; Kędzia, B.; Rogoziński, T. The content of some elements in propolis and its ethanolic extract. Postępy Fitoterapii 2016, 1, 3–7. (In Polish) [Google Scholar]
- Dogan, M.; Silici, S.; Saraymen, R.; Ilhan, I.O. Element content of propolis from different regions of Turkey. Acta Aliment. 2006, 35, 127–130. [Google Scholar] [CrossRef]
- Tosic, S.; Stojanovic, G.; Mitic, S.; Pavlovic, A.; Alagic, S. Mineral composition of selected serbian propolis samples. J. Apic. Sci. 2017, 61, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, N.A.; Ja’afar, F.; Yasin, H.M.; Taha, H.; Petalcorin, M.I.R.; Mamit, M.H.; Kusrini, E.; Usman, A. Physicochemical analyses, antioxidant, antibacterial, and toxicity of propolis particles produced by stingless bee Heterotrigona itama found in Brunei Darussalam. Heliyon 2019, 5, e02476. [Google Scholar] [CrossRef] [Green Version]
- Formicki, G.; Greń, A.; Stawarz, R.; Zyśk, B.; Gał, A. Metal content in honey, propolis, wax, and bee pollen and implications for metal pollution monitoring. Polish J. Environ. Stud. 2013, 22, 99–106. [Google Scholar]
- Gong, S.; Luo, L.; Gong, W.; Gao, Y.; Xie, M. Multivariate analyses of element concentrations revealed the groupings of propolis from different regions in China. Food Chem. 2012, 134, 583–588. [Google Scholar] [CrossRef]
- Walker, P.; Crane, E. Constituents of propolis. Apidologie 1987, 18, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Touzani, S.; Imtara, H.; Katekhaye, S.; Mechchate, H.; Ouassou, H.; Alqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Fearnley, H.; Fearnley, J.; et al. Determination of phenolic compounds in various propolis samples collected from an african and an asian region and their impact on antioxidant and antibacterial activities. Molecules 2021, 26, 4589. [Google Scholar] [CrossRef]
- Papotti, G.; Bertelli, D.; Bortolotti, L.; Plessi, M. Chemical and functional characterization of Italian propolis obtained by different harvesting methods. J. Agric. Food Chem. 2012, 60, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Pobiega, K.; Kraśniewska, K.; Derewiaka, D.; Gniewosz, M. Comparison of the antimicrobial activity of propolis extracts obtained by means of various extraction methods. J. Food Sci. Technol. 2019, 56, 5386–5395. [Google Scholar] [CrossRef] [Green Version]
- Moskwa, J.; Naliwajko, S.K.; Markiewicz-Żukowska, R.; Gromkowska-Kępka, K.J.; Nowakowski, P.; Strawa, J.W.; Borawska, M.H.; Tomczyk, M.; Socha, K. Chemical composition of Polish propolis and its antiproliferative effect in combination with Bacopa monnieri on glioblastoma cell lines. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Wezgowiec, J.; Wieczynska, A.; Wieckiewicz, W.; Kulbacka, J.; Saczko, J.; Pachura, N.; Wieckiewicz, M.; Gancarz, R.; Wilk, K.A. Polish propolis-Chemical composition and biological effects in tongue cancer cells and macrophages. Molecules 2020, 25, 2426. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Waśkiewicz, A.; Rogoziński, T.; Ratajczak, I. The role of seasonality on the chemical composition, antioxidant activity and cytotoxicity of Polish propolis in human erythrocytes. Rev. Bras. Farmacogn. 2019, 29, 301–308. [Google Scholar] [CrossRef]
- Hernández Zarate, M.S.; Abraham del Juárez, M.R.; Cerón García, A.; Ozuna López, C.; Gutiérrez Chávez, A.J.; Segoviano de Garfias, J.J.N.; Avila Ramos, F. Flavonoids, phenolic content, and antioxidant activity of propolis from various areas of Guanajuato, Mexico. Food Sci. Technol. 2018, 38, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Kuś, P.M.; Jerković, I. Mediterranean propolis from the adriatic sea islands as a source of natural antioxidants: Comprehensive chemical biodiversity determined by GC-MS, ftiratr, UHPLC-DAD-QQTOF-MS, DPPH and FRAP assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef] [Green Version]
- Martinello, M.; Mutinelli, F. Antioxidant activity in bee products: A review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Mura, F.; Valenzuela, G.; Figueroa, C.; Salinas, R.; Zuñiga, M.C.; Torres, J.L.; Fuguet, E.; Delporte, C. Identification of phenolic compounds by HPLC-ESI-MS/MS and antioxidant activity from Chilean propolis. Food Res. Int. 2014, 64, 873–879. [Google Scholar] [CrossRef]
- Escriche, I.; Juan-Borrás, M. Standardizing the analysis of phenolic profile in propolis. Food Res. Int. 2018, 106, 834–841. [Google Scholar] [CrossRef]
- Calegari, M.A.; Ayres, B.B.; dos Santos Tonial, L.M.; de Alencar, S.M.; Oldoni, T.L.C. Fourier transform near infrared spectroscopy as a tool for predicting antioxidant activity of propolis. J. King Saud. Univ. Sci. 2020, 32, 784–790. [Google Scholar] [CrossRef]
- Cavalaro, R.I.; da Cruz, R.G.; Dupont, S.; de Moura Bell, J.M.L.N.; de Vieira, T.M.F. In vitro and in vivo antioxidant properties of bioactive compounds from green propolis obtained by ultrasound-assisted extraction. Food Chem. X 2019, 4, 100054. [Google Scholar] [CrossRef]
- Milojković Opsenica, D.; Ristivojević, P.; Trifković, J.; Vovk, I.; Lušić, D.; Tešić, Ž. TLC Fingerprinting and Pattern Recognition Methods in the Assessment of Authenticity of Poplar-Type Propolis. J. Chromatogr. Sci. 2016, 54, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- Isidorov, V.A.; Szczepaniak, L.; Bakier, S. Rapid gc/ms determination of botanical precursors of eurasian propolis. Food Chem. 2014, 142, 101–106. [Google Scholar] [CrossRef]
- Okinczyc, P.; Szumny, A.; Szperlik, J.; Kulma, A.; Franiczek, R.; Zbikowska, B.; Krzyzanowska, B.; Sroka, Z. Profile of polyphenolic and essential oil composition of polish propolis, black poplar and aspens buds. Molecules 2018, 23, 1262. [Google Scholar] [CrossRef] [Green Version]
- Turrini, F.; Donno, D.; Beccaro, G.L.; Pittaluga, A.; Grilli, M.; Zunin, P.; Boggia, R. Bud-derivatives, a novel source of polyphenols and how different extraction processes affect their composition. Foods 2020, 9, 1343. [Google Scholar] [CrossRef] [PubMed]
- Dimkić, I.; Ristivojević, P.; Janakiev, T.; Berić, T.; Trifković, J.; Milojković-Opsenica, D.; Stanković, S. Phenolic profiles and antimicrobial activity of various plant resins as potential botanical sources of Serbian propolis. Ind. Crops Prod. 2016, 94, 856–871. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Ligaj, M.; Stuper-Szablewska, K.; Szymanowska, D.; Tichoniuk, M.; Szulc, P. Polyphenol content and antioxidant activities of Prunus padus L. And Prunus serotina L. Leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties. Open Chem. 2020, 18, 1125–1135. [Google Scholar] [CrossRef]
- Szwajkowska-Michałek, L.; Przybylska-Balcerek, A.; Rogoziński, T.; Stuper-Szablewska, K. Phenolic compounds in trees and shrubs of central Europe. Appl. Sci. 2020, 10, 6907. [Google Scholar] [CrossRef]
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664. [Google Scholar] [CrossRef] [Green Version]
- Charpentier, T.; Boisard, S.; Le Ray, A.; Breard, D.; Chabreier, A.; Esselin, H.; Guilet, D.; Ripoll, C.; Richomme, P. A Descriptive Chemical Composition of Concentrated Bud Macerates through an Optimized SPE-HPLC-UV-MS 2 Method—Application to Alnus glutinosa, Ribes nigrum, Rosa canina, Rosmarinus officinalis and Tilia tomentosa. Plants 2022, 11, 144. [Google Scholar] [CrossRef]
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; da Silva Cunha, I.B.; Danert, C.; Eberlin, M.N.; Falcão, S.I.; Isla, M.I.; Moreno, M.I.N.; et al. Standard methods for Apis mellifera propolis research. J. Apic. Res. 2019, 58, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Tegos, G.; Stermitz, F.R.; Lomovskaya, O.; Lewis, K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3133–3141. [Google Scholar] [CrossRef] [Green Version]
- Fernandes-Silva, C.C.; Salatino, A.; Salatino, M.L.F.; Breyer, E.D.H.; Negri, G. Chemical profiling of six samples of brazilian propolis. Quim. Nova 2013, 36, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Al-Waili, N.; Al-Ghamdi, A.; Ansari, M.J.; Al-Attal, Y.; Salom, K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus Aureus, Escherichia coli and Candida Albicans isolates in single and polymicrobial cultures. Int. J. Med. Sci. 2012, 9, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Adomavičiute, E.; Stanys, S.; Žilius, M.; Juškaite, V.; Pavilonis, A.; Briedis, V. Formation and Biopharmaceutical Characterization of Electrospun PVP Mats with Propolis and Silver Nanoparticles for Fast Releasing Wound Dressing. Biomed. Res. Int. 2016, 2016, 4648287. [Google Scholar] [CrossRef]
- Benhanifia, M.; Shimomura, K.; Tsuchiya, I.; Inui, S.; Kumazawa, S.; Mohamed, W.; Boukraa, L.; Sakharkar, M.; Benbarek, H. Chemical composition and antimicrobial activity of propolis collected from some localities of Western Algeria. Acta Aliment. 2014, 43, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Kubiliene, L.; Laugaliene, V.; Pavilonis, A.; Maruska, A.; Majiene, D.; Barcauskaite, K.; Kubilius, R.; Kasparaviciene, G.; Savickas, A. Alternative preparation of propolis extracts: Comparison of their composition and biological activities. BMC Complement. Altern. Med. 2015, 15, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almuhayawi, M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef]
- Berretta, A.A.; Silveira, M.A.D.; Cóndor Capcha, J.M.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharmacother. 2020, 131. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Propolis, bee honey, and their components protect against coronavirus disease 2019 (Covid-19): A review of in silico, in vitro, and clinical studies. Molecules 2021, 26, 1232. [Google Scholar] [CrossRef]
- Wang, X.; Decker, C.C.; Zechner, L.; Krstin, S.; Wink, M. In vitro wound healing of tumor cells: Inhibition of cell migration by selected cytotoxic alkaloids. BMC Pharmacol. Toxicol. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In vitro cell migration, invasion, and adhesion assays: From cell imaging to data analysis. Front. Cell Dev. Biol. 2019, 7, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, B.; Kim, M.; Kim, H.A.; Kim, D.; Yang, J.; Her, S.; Song, Y.S. Caffeic Acid Phenethyl Ester, a Component of Beehive Propolis, is a Novel Selective Estrogen Receptor Modulators. Phytoter. Res. 2010, 24, 295–300. [Google Scholar] [CrossRef]
- Begnini, K.R.; Moura De Leon, P.M.; Thurow, H.; Schultze, E.; Campos, V.F.; Martins Rodrigues, F.; Borsuk, S.; Dellagostin, O.A.; Savegnago, L.; Roesch-Ely, M.; et al. Brazilian red propolis induces apoptosis-like cell death and decreases migration potential in bladder cancer cells. Evidence-based Complement. Altern. Med. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Borawska, M.H.; Naliwajko, S.K.; Moskwa, J.; Markiewicz-Zukowska, R.; Puscion-Jakubik, A.; Soroczynska, J. Anti-proliferative and anti-migration effects of Polish propolis combined with Hypericum perforatum L. on glioblastoma multiforme cell line U87MG. BMC Complement. Altern. Med. 2016, 16, 367. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Wang, Y.; Yin, X.; Liu, X.; Xuan, H. Ethanol extract of propolis and its constituent caffeic acid phenethyl ester inhibit breast cancer cells proliferation in inflammatory microenvironment by inhibiting TLR4 signal pathway and inducing apoptosis and autophagy. BMC Complement. Altern. Med. 2017, 17, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabala-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Caffeic Acid Versus Caffeic Acid Phenethyl Ester in the Treatment of Breast Cancer MCF-7 Cells: Migration Rate Inhibition. Integr. Cancer Ther. 2018, 17, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Zingue, S.; Maxeiner, S.; Rutz, J.; Ndinteh, D.T.; Chun, F.K.H.; Fohouo, F.N.T.; Njamen, D.; Blaheta, R.A. Ethanol-extracted Cameroonian propolis: Antiproliferative effects and potential mechanism of action in prostate cancer. Andrologia 2020, 52, 1–11. [Google Scholar] [CrossRef]
- Frión-Herrera, Y.; Díaz-García, A.; Ruiz-Fuentes, J.; Rodríguez-Sánchez, H.; Sforcin, J.M. The cytotoxic effects of propolis on breast cancer cells involve PI3K/Akt and ERK1/2 pathways, mitochondrial membrane potential, and reactive oxygen species generation. Inflammopharmacology 2019, 27, 1081–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darbre, P.D.; Bakir, A.; Iskakova, E. Effect of aluminium on migratory and invasive properties of MCF-7 human breast cancer cells in culture. J. Inorg. Biochem. 2013, 128, 245–249. [Google Scholar] [CrossRef]
- Roszak, J.; Smok-Pieniążek, A.; Spryszyńska, S.; Kowalczyk, K.; Domeradzka-Gajda, K.; Świercz, R.; Grobelny, J.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Celichowski, G.; et al. Cytotoxic effects in transformed and non-transformed human breast cell lines after exposure to silver nanoparticles in combination with selected aluminium compounds, parabens or phthalates. J. Hazard. Mater. 2020, 392, 122442. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A.; Majeed Khan, M.A.; Ahamed, M. Aluminum doping tunes band gap energy level as well as oxidative stress-mediated cytotoxicity of ZnO nanoparticles in MCF-7 cells. Sci. Rep. 2015, 5, 122442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adham, A.N.; Abdelfatah, S.; Naqishbandi, A.M.; Mahmoud, N.; Efferth, T. Cytotoxicity of apigenin toward multiple myeloma cell lines and suppression of iNOS and COX-2 expression in STAT1-transfected HEK293 cells. Phytomedicine 2021, 80, 153371. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Simal-Gandara, J.; Kopustinskiene, D.M.; Bernatoniene, J.; Samarghandian, S. Emerging cellular and molecular mechanisms underlying anticancer indications of chrysin. Cancer Cell Int. 2021, 21, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.A.E.; Amarante, M.K.; Conti, B.J.; Sforcin, J.M. Cytotoxic constituents of propolis inducing anticancer effects: A review. J. Pharm. Pharmacol. 2011, 63, 1378–1386. [Google Scholar] [CrossRef]
- Turan, I.; Demir, S.; Misir, S.; Kilinc, K.; Mentese, A.; Aliyazicioglu, Y.; Deger, O. Cytotoxic effect of Turkish propolis on liver, colon, breast, cervix and prostate cancer cell lines. Trop. J. Pharm. Res. 2015, 14, 777–782. [Google Scholar] [CrossRef] [Green Version]
- Aru, B.; Güzelmeric, E.; Akgül, A.; Demirel, G.Y.; Kırmızıbekmez, H. Antiproliferative Activity of Chemically Characterized Propolis from Turkey and Its Mechanisms of Action. Chem. Biodivers. 2019, 16, e1900189. [Google Scholar] [CrossRef]
- Cetin, E.O.; Salmanoglu, D.S.; Ozden, I.; Ors-Kumoglu, G.; Akar, S.; Demirozer, M.; Karabey, F.; Kilic, K.D.; Kirilmaz, L.; Uyanikgil, Y.; et al. Preparation of Ethanol Extract of Propolis Loaded Niosome Formulation and Evaluation of Effects on Different Cancer Cell Lines. Nutr. Cancer 2021, 1–13. [Google Scholar] [CrossRef]
- Campoccia, D.; Ravaioli, S.; Santi, S.; Mariani, V.; Santarcangelo, C.; De Filippis, A.; Montanaro, L.; Arciola, C.R.; Daglia, M. Exploring the anticancer effects of standardized extracts of poplar-type propolis: In vitro cytotoxicity toward cancer and normal cell lines. Biomed. Pharmacother. 2021, 141, 111895. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Biju, J.; Reddy, V.; Sulaiman, C.T. Total Phenolics and Flavonoids in Selected Justicia Species. J. Pharmacogn. Phytochem. 2013, 2, 51–52. [Google Scholar]
- Karcz, D.; Starzak, K.; Ciszkowicz, E.; Lecka-Szlachta, K.; Kamiński, D.; Creaven, B.; Jenkins, H.; Radomski, P.; Miłoś, A.; Ślusarczyk, L.; et al. Novel coumarin-thiadiazole hybrids and their Cu(II) and Zn(II) complexes as potential antimicrobial agents and acetylcholinesterase inhibitors. Int. J. Mol. Sci. 2021, 22, 9709. [Google Scholar] [CrossRef]
- Bocian, A.; Ciszkowicz, E.; Hus, K.K.; Buczkowicz, J.; Lecka-Szlachta, K.; Pietrowska, M.; Petrilla, V.; Petrillova, M.; Legáth, Ľ.; Legáth, J. Antimicrobial activity of protein fraction from Naja ashei venom against Staphylococcus epidermidis. Molecules 2020, 25, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Governa, P.; Carullo, G.; Biagi, M.; Rago, V.; Aiello, F. Evaluation of the in vitro wound-healing activity of calabrian honeys. Antioxidants 2019, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]

| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | Min | Max | Mean | SD | Variability% | F-Value | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| microelements [mg/100 g] | ||||||||||||||||
| Mo | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.03 * | 0.01 | 0.01 | 0.02 | 0.01 | 0.03 | 0.02 | 0.01 | 42.54 | 14.4 | 0.000 |
| Sr | 0.04 * | 0.07 * | 0.09 * | 0.10 | 0.07 * | 0.11 | 0.16 * | 0.18 * | 0.18 * | 0.04 | 0.18 | 0.11 | 0.05 | 45.45 | 2315.6 | 0.000 |
| Cu | 0.13 * | 0.13 * | 0.18 | 0.20 | 0.17 | 0.25 | 0.21 | 0.20 | 0.45 * | 0.13 | 0.45 | 0.21 | 0.10 | 44.69 | 117.4 | 0.000 |
| Cr | 0.78 | 0.81 | 0.68 | 0.64 | 0.75 | 0.69 | 0.73 | 0.74 | 0.79 | 0.64 | 0.81 | 0.73 | 0.06 | 7.53 | 8.3 | 0.000 |
| Mn | 0.37 * | 1.05 | 0.71 * | 0.96 | 0.87 | 0.65 * | 1.12 | 1.82 * | 1.20 * | 0.37 | 1.82 | 0.97 | 0.41 | 41.96 | 269.0 | 0.000 |
| Zn | 0.09 * | 1.04 * | 0.80 * | 0.99 * | 0.38 * | 0.73 * | 0.76 * | 5.42 * | 2.14 * | 0.09 | 5.42 | 1.37 | 1.62 | 118.15 | 148,834.5 | 0.000 |
| macroelements [mg/100 g] | ||||||||||||||||
| Na | 0.34 * | 1.19 * | 0.62 * | 0.97 * | 5.62 * | 0.77 * | 1.49 * | 5.06 * | 3.53 * | 0.34 | 5.62 | 2.18 | 2.02 | 92.88 | 1122.5 | 0.000 |
| Mg | 4.29 * | 9.65 * | 12.82 | 12.09 * | 12.95 | 12.98 | 13.23 | 19.08 * | 19.65 * | 4.29 | 19.65 | 12.97 | 4.60 | 35.46 | 3896.2 | 0.000 |
| Fe | 12.05 * | 11.55 * | 10.75 * | 11.32 * | 13.97 | 16.84 | 13.62 | 17.20 | 30.03 * | 10.75 | 30.03 | 15.26 | 6.01 | 39.36 | 392.5 | 0.000 |
| S | 4.85 * | 10.65 * | 13.92 * | 13.83 * | 15.45 * | 15.23 * | 22.73 * | 22.86 * | 28.27 * | 4.85 | 28.27 | 16.42 | 7.11 | 43.29 | 4402.8 | 0.000 |
| Ca | 7.24 * | 17.31 * | 28.53 * | 37.01 | 33.57 | 46.27 * | 40.51 | 56.17 * | 59.22 * | 7.24 | 59.22 | 36.20 | 16.99 | 46.94 | 1248.9 | 0.000 |
| P | 8.89 * | 27.06 * | 27.86 * | 24.24 * | 48.26 | 13.30 * | 45.00 | 61.87 * | 71.19 * | 8.89 | 71.19 | 36.41 | 21.43 | 58.85 | 281.8 | 0.000 |
| K | 15.99 * | 54.21 * | 67.89 * | 60.19 | 61.79 | 89.13 * | 62.86 | 68.66 * | 82.64 * | 15.99 | 89.13 | 62.60 | 20.68 | 33.03 | 2764.9 | 0.000 |
| toxic elements [mg/100 g] | ||||||||||||||||
| Cd | n.d. | 0.01 | 0.01 | 0.01 | 0.01 | n.d. | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 36.77 | 15.5 | 0.000 |
| Pb | 0.03 * | 0.04 * | 0.03 * | 0.03 * | 0.03 * | 0.05 * | 0.03 * | 0.52 * | 0.17 * | 0.03 | 0.52 | 0.10 | 0.16 | 157.58 | 1482.7 | 0.000 |
| Ni | 0.54 | 0.51 | 0.53 | 0.51 | 0.58 | 0.51 | 0.52 | 0.50 | 0.70 * | 0.50 | 0.70 | 0.54 | 0.06 | 11.47 | 503.3 | 0.000 |
| Al | 4.54 * | 4.95 * | 5.82 * | 5.30 * | 6.95 | 7.85 | 7.51 | 8.22 | 17.07 * | 4.54 | 17.07 | 7.58 | 3.80 | 50.12 | 1847.5 | 0.000 |
| Total | 37.23 | 104.08 | 130.66 | 127.29 | 151.19 | 157.12 | 156.45 | 195.95 | 231.00 | |||||||
| Sample Number | TPC [mg GAE/g d.e.] | TFC [mg QE/g d.e.] | FRAP [μmol TE/g d.e.] | DPPH [μmol TE/g d.e.] | ABTS [mmol TE/g d.e.] |
|---|---|---|---|---|---|
| P1 | 289.29 * | 94.42 * | 681.25 * | 351.40 * | 7.77 * |
| P2 | 263.84 * | 114.05 * | 485.53 * | 219.48 * | 5.72 * |
| P3 | 319.20 * | 155.11 * | 699.34 * | 357.63 * | 6.31 * |
| P4 | 285.12 * | 144.96 * | 581.25 * | 252.56 * | 6.35 * |
| P5 | 278.72 * | 85.04 * | 566.12 * | 231.55 * | 6.00 |
| P6 | 252.38 * | 96.87 * | 489.14 * | 212.09 * | 4.75 * |
| P7 | 267.86 * | 112.14 * | 493.09 * | 230.38 * | 6.02 |
| P8 | 268.90 * | 93.82 * | 533.22 * | 224.15 * | 5.25 * |
| P9 | 326.34 * | 183.62 * | 725.99 * | 342.84 * | 6.02 |
| Min | 252.38 | 85.04 | 485.53 | 212.09 | 4.75 |
| Max | 326.34 | 183.82 | 725.99 | 357.63 | 7.77 |
| Mean | 283.52 | 120.03 | 583.88 | 269.12 | 6.02 |
| SD | 24.95 | 33.77 | 95.38 | 62.22 | 0.83 |
| Variability [%] | 8.80 | 28.13 | 16.34 | 23.12 | 13.83 |
| F-value | 6.52 | 73.36 | 14.61 | 28.13 | 11.77 |
| p-value | 0.005 | 0.000 | 0.000 | 0.000 | 0.001 |
| Sample Number | Caffeic Acid [µg/g of Extract] | p-Coumaric Acid [µg/g of Extract] | Ferulic Acid [µg/g of Extract] | Benzoic Acid [µg/g of Extract] | Sum of Phenolic Acids [µg/g of Extract] | Chrysin [µg/g of Extract] | Naringenin [µg/g of Extract] | Sakuranetin [µg/g of Extract] | Pinobanksin [µg/g of Extract] | Pinocembrin [µg/g of Extract] | Sum of Flavonoids [µg/g of Extract] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 0.44 * | 38.36 * | 15.95 * | 22.65 * | 77.4 | nd * | 0.84 * | 20.46 * | 2.13 * | 5.04 * | 28.47 |
| P2 | 1.83 * | 46.75 * | 10.73 * | 12.10 * | 71.41 | nd * | 1.10 * | 21.97 * | 7.03 * | 7.12 * | 37.22 |
| P3 | 2.16 * | 22.78 * | 13.08 * | 19.33 * | 57.35 | 39.61 * | 0.15 * | nd * | 7.29 * | 13.57 * | 60.62 |
| P4 | 2.88 * | 42.54 * | 12.90 * | 16.42 * | 74.74 | nd * | 2.04 * | 31.02 * | 9.79 * | 10.17 * | 53.02 |
| P5 | 1.43 * | 37.88 * | 10.62 * | 18.65 * | 68.58 | nd * | 1.54 * | 28.17 * | 5.00 * | 7.92 * | 42.06 |
| P6 | 1.57 | 39.88 * | 10.81 * | 15.37 * | 67.63 | nd * | 0.63 * | 17.63 * | 6.57 * | 7.45 * | 32.28 |
| P7 | 1.28 * | 38.31 * | 9.83 * | 9.36 * | 58.78 | nd * | 0.75 * | 18.98 * | 7.84 * | 7.17 * | 34.74 |
| P8 | 0.07 * | 30.27 * | 8.31 * | 17.29 * | 55.94 | nd * | 0.16 * | 16.04 * | 1.11 * | 5.58 * | 22.89 |
| P9 | 2.52 * | 13.88 * | 12.26 * | 17.36 * | 46.02 | 61.49 * | nd | nd * | 8.54 * | 11.14 * | 81.17 |
| Min | 0.07 | 13.88 | 8.31 | 9.36 | 0.00 | 0.00 | 0.00 | 1.11 | 5.04 | ||
| Max | 2.88 | 46.75 | 15.95 | 22.65 | 61.49 | 2.04 | 31.02 | 9.79 | 13.57 | ||
| Mean | 1.58 | 34.52 | 11.61 | 16.50 | 11.23 | 0.80 | 17.14 | 6.14 | 8.35 | ||
| SD | 0.91 | 10.40 | 2.22 | 3.93 | 22.95 | 0.68 | 10.85 | 2.89 | 2.76 | ||
| Variability% | 57.93 | 30.12 | 19.15 | 23.80 | 204.32 | 84.66 | 63.27 | 47.08 | 33.03 | ||
| F-value | 33,323.11 | 4,324,609.00 | 197,680.00 | 617,202.00 | 21,071,411.00 | 18,401.44 | 4,705,307.44 | 334,762.11 | 304,370.44 | ||
| p-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| EEP Sample | E. coli | S. aureus | S. epidermidis | |||
|---|---|---|---|---|---|---|
| 6538 | MRSA 4236 a | 12228 | 35984 | MRCNS 2452 a | ||
| MIC (μg/mL) | ||||||
| P1 | 390.6 | 195.3/390.6 b | 781 | 195.3 | 195.3/781 b | 48.8 |
| P2 | 195.3 | 24.4/390.6 b | 781 | 24.4 | 48.8/48.8 b | 24.4 |
| P3 | 195.3 | 24.4/390.6 b | 781 | 48.8 | 48.8/48.8 b | 390.6 |
| P4 | 390.6 | 24.4/390.6 b | 390.6 | 48.8 | 48.8/24.4 b | 24.4 |
| P5 | 781 | 390.6/- | 390.6 | 48.8 | 390.6/- | 390.6 |
| P6 | 390.6 | 390.6/- | 390.6 | 48.8 | 195/- | 48.8 |
| P7 | 781 | 390.6/- | 390.6 | 48.8 | 390.6/- | 6.1 |
| P8 | 390.6 | 390.6/- | 390.6 | 195 | 390.6/- | 24.4 |
| P9 | 390.6 | 195.3/- | 390.6 | 48.8 | 195.3/- | 24.4 |
| Tetracycline (positive control) | 0.5 | 0.12/0.12 | 7.8 | 62.5 | 0.12/0.24 | 62.5 |
| Sample Number | Localization of Apiary | Most Abundant Plants near the Apiary |
|---|---|---|
| P1 | rural area (49°50′ N, 21°69′ E) | Malus domestica, Pyrus communis, Ribes nigrum, Aronia melanocarpa, Alnus sp., Betula sp., Salix sp., Padus avium, Robinia pseudoacacia, Tilia sp., Brassica napus, Solidago sp., Impatiens sp. |
| P2 | rural area (49°79′ N, 21°94′ E) | Abies sp., Picea sp., Quercus sp., Salix sp., Frangula sp., Tilia sp., Populus tremula, Betula sp., fruit trees, Taraxacum officinale, Mentha sp., Centaurea sp., Rubus sp., Eupatorium cannabinum, Solidago sp., Thymus pulegoides |
| P3 | rural area (49°54′ N, 21°97′ E) | Abies sp., Pinus sp., Larix sp., Acer sp., Tilia sp., Betula sp., Fagus sp., Salix sp., Rubus sp., meadow flowers, forest shrubs |
| P4 | urban area (49°68′ N, 21°77′ E) | Picea pungens, Thuja sp., Chamaecyparis sp., Tilia sp., Acer sp., Taraxacum officinale, Solidago sp. |
| P5 | rural area (49°67′ N, 21°80′ E) | Pinus sp., Tilia sp., Carpinus sp., Salix sp., Robinia pseudoacacia, Quercus sp., Abies sp., Picea sp., Prunus avium, Thuja sp., Trifolium repens, Solidago sp., meadow flowers, garden plants |
| P6 | rural area (49°70′ N, 21°77′ E) | not specified |
| P7 | rural area (49°78′ N, 22°54′ E) | not specified |
| P8 | rural area (51°19′ N, 22°61′ E) | not specified |
| P9 | rural area (49°95′ N, 20°81′ E) | not specified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miłek, M.; Ciszkowicz, E.; Tomczyk, M.; Sidor, E.; Zaguła, G.; Lecka-Szlachta, K.; Pasternakiewicz, A.; Dżugan, M. The Study of Chemical Profile and Antioxidant Properties of Poplar-Type Polish Propolis Considering Local Flora Diversity in Relation to Antibacterial and Anticancer Activities in Human Breast Cancer Cells. Molecules 2022, 27, 725. https://doi.org/10.3390/molecules27030725
Miłek M, Ciszkowicz E, Tomczyk M, Sidor E, Zaguła G, Lecka-Szlachta K, Pasternakiewicz A, Dżugan M. The Study of Chemical Profile and Antioxidant Properties of Poplar-Type Polish Propolis Considering Local Flora Diversity in Relation to Antibacterial and Anticancer Activities in Human Breast Cancer Cells. Molecules. 2022; 27(3):725. https://doi.org/10.3390/molecules27030725
Chicago/Turabian StyleMiłek, Michał, Ewa Ciszkowicz, Monika Tomczyk, Ewelina Sidor, Grzegorz Zaguła, Katarzyna Lecka-Szlachta, Anna Pasternakiewicz, and Małgorzata Dżugan. 2022. "The Study of Chemical Profile and Antioxidant Properties of Poplar-Type Polish Propolis Considering Local Flora Diversity in Relation to Antibacterial and Anticancer Activities in Human Breast Cancer Cells" Molecules 27, no. 3: 725. https://doi.org/10.3390/molecules27030725






