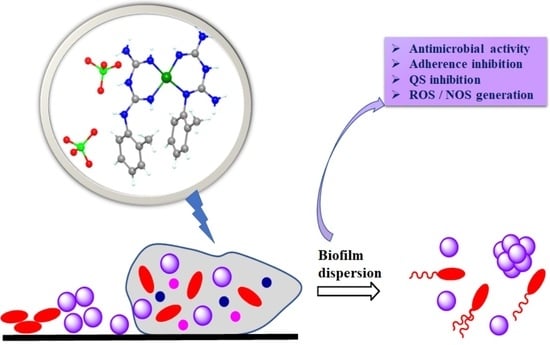
Metal Complexes—A Promising Approach to Target Biofilm Associated Infections
Abstract
:1. Introduction
2. Aspects Concerning Biofilm Formation and Disruption
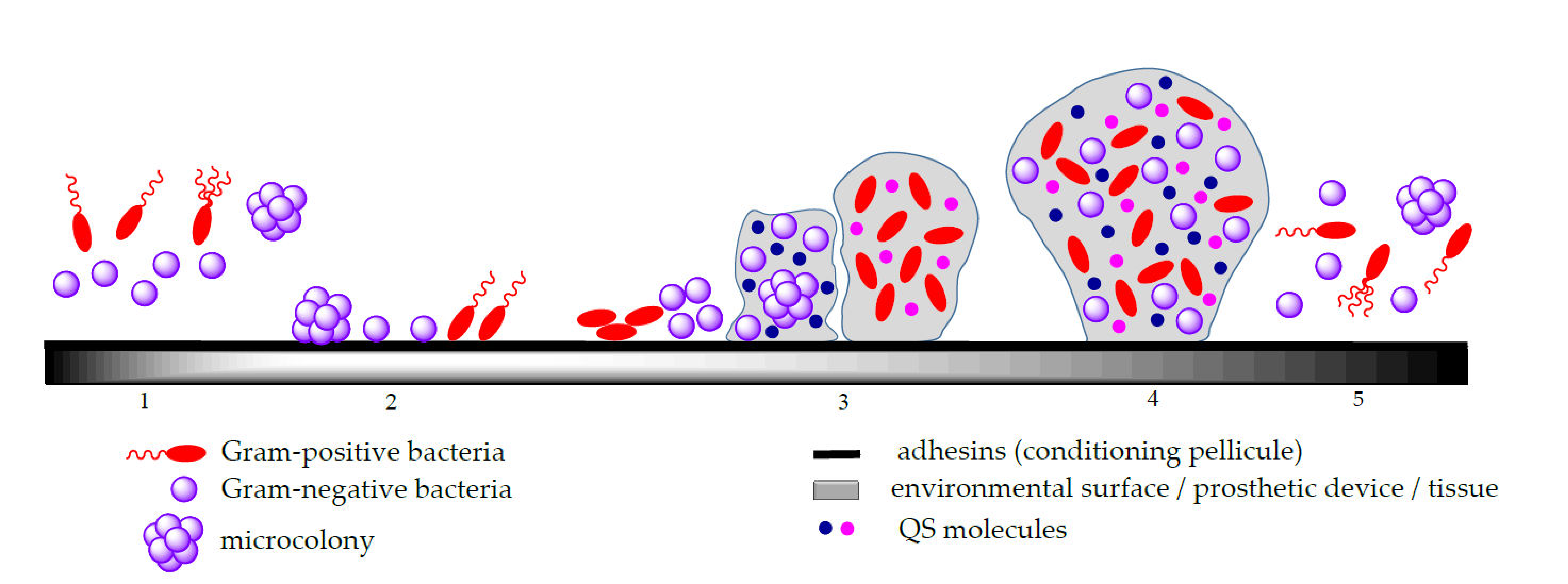
2.1. Biofilm Formation
- (i)
- Adhesion in which the planktonic cells are reversibly attached to the solid biotic/abiotic surfaces; the bacterial attachment on the biotic/abiotic surfaces involves both physical and chemical interactions such as Brownian movements, van der Waals and electrostatic attraction that contribute to the initial phase of microbial adhesion, as well as interactions between these surfaces and the bacterial adhesins, represented by polymeric species exposed on the surface of cells that enable the formation of a “key-lock” bond between the cell and the surface and result in a stronger interfacial adhesion [27,34,35,36].
- (ii)
- Microcolony formation following initial microorganism adhesion and proliferation, with the generation of a multi-layered biofilm embedded in self-produced EPS, a complex and viscous matrix composed mainly from polysaccharides, proteins and lipids; this matrix represents the greatest barrier to diffusion for both antimicrobials and their delivery systems. In soil microbial communities, the EPS production contributes significantly to the improvement of soil quality due to its electrostatic charge that is attracting and aggregates the soil particles exerting a positive effect on the soils’ mechanical conductivity [37]. Moreover, biofilm embedded microorganisms can also produce small, diffusible signalling molecules involved in the density-dependent intercellular communication mechanism called QS; this system allows microorganisms to detect a critical density and assures a coordinated behaviour within the biofilm, such as iron chelation and antibiotic defensive activities [6].
- (iii)
- Biofilm maturation consisting of the development of a three-dimensional structure (3D) with a thickness typically less than 100 mm and a network that assures the efficient transportation of both nutrients and signaling particles in the biofilm;
- (iv)
- Detachment or dispersion corresponding to microcolonies or single cell separation that colonize other surfaces; after maturation, the migration of cells to the environment or dispersion is a result of a too dense layer formation [34].
2.2. Role of the Extracellular Polymeric Substances in Protecting Biofilm Cells
2.3. Quorum Sensing Role in Biofilm Development
2.4. Anti-Biofilm Strategies
- Inhibition of the initial microbial adhesion to the substrate, by designing materials or coatings exhibiting electrochemical repulsion or by eluting substrata loaded with antimicrobial substances [54];
- Inhibition of adhesion by blocking the expression of adhesins or their recognition sites;
- Inhibition of adhesion by blocking flagellar motility and impairing bacteria to reach the adhesion sites;
- Inhibition of biofilm formation by interfering with the QS mechanisms; the quorum quenching (QQ) by using QS inhibitors can attenuate the production of bacterial toxin or biofilm formation and provide a novel therapeutic approach to control bacterial infections. The QQ can be achieved either by blocking the QS molecules biosynthesis, by destruction of QS molecules, or by inhibition of the binding of AIs to QS receptors [55]. QSIs often exhibit a synergic anti-biofilm activity with antibiotics [56].
- Inhibition of biofilm maturation by blocking the production of extracellular polymeric substances (EPS);
- Inhibition of maturation by inhibiting the growth and multiplication of cells in biofilm by using different approaches such as application of infrared and light pulsing, direct-current electrical stimulation, ultrasound and alternating electric fields [57]; use of drug delivery systems [58]; local delivery of catheter locks, intratracheal locks etc. [59];
- Targeting non-growing dormant and persister biofilm cells (by metabolic interference or lytic substances) or interfering with the formation of persister cells (by inhibiting the bacterial regulatory tetra and penta-guanosine phosphate nucleotides, which activate the inhibitors of cell growth) [60].
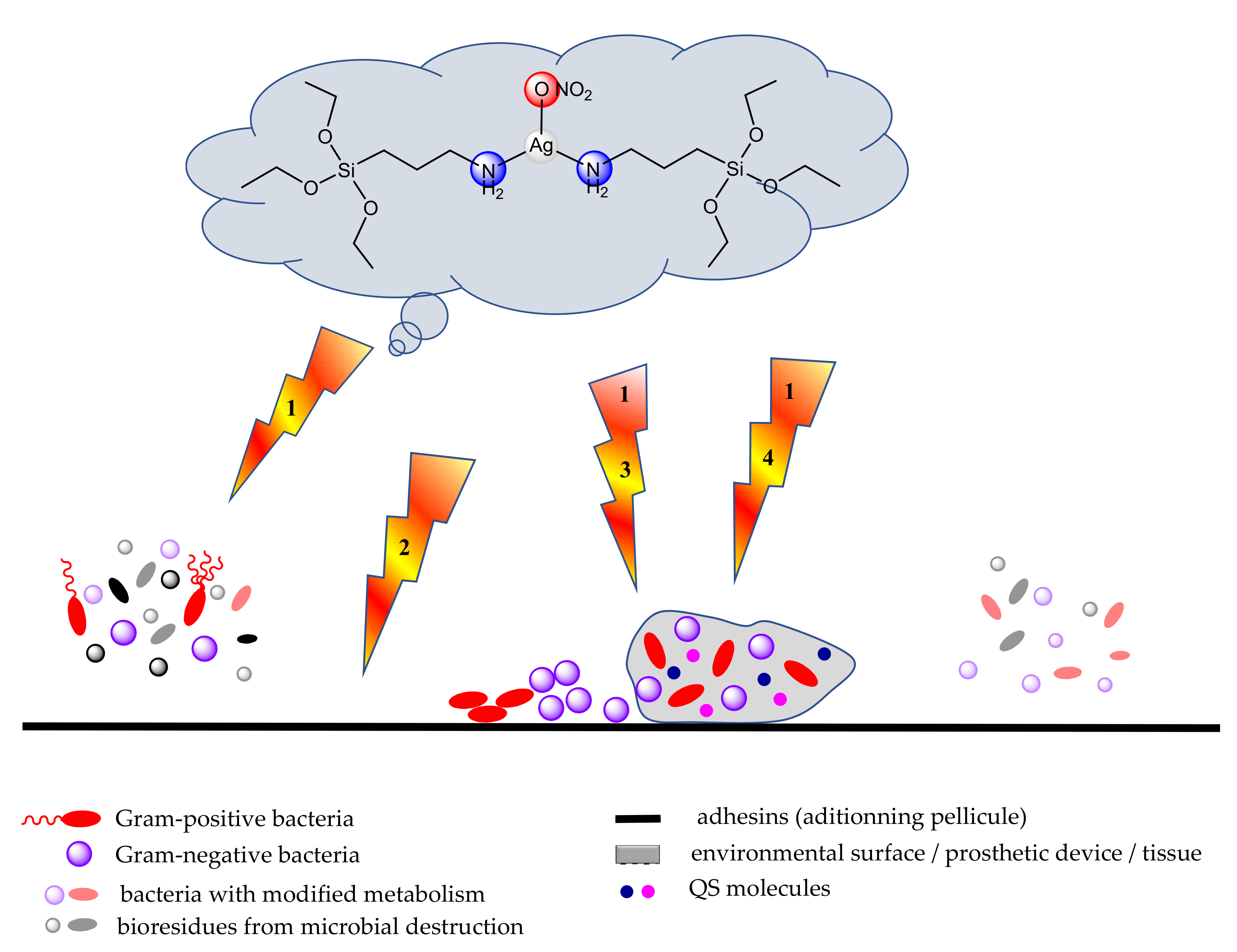
- Elimination of the biofilm by disorganization of the protective extracellular matrix based on EPS-degrading enzymes, anti-EPS antibodies, and nucleic acid binding proteins, matrix destabilizing agents such as ethylenediaminetetraacetic acid (H4EDTA), which are targeting the biofilm extracellular polymeric substance, leading to biofilm dispersion or by the mechanical debridement of biofilms by using ultrasound and surgical procedures [61,62,63].
3. Complexes with Antibiofilm Activity
3.1. Complexes with Antibiotics
3.2. Complexes with Heterocyclic Derivatives
3.3. Complexes with Schiff Bases
3.4. Complexes with Biguanide Derivatives
3.5. Complexes with Macrocyclic Ligands
3.6. Complexes with Miscellaneous Ligands
3.7. Materials as Carriers for Metal Ions or Complexes with Anti-Biofilm Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| amx | amoxicillin |
| aptes | 3-aminopropyltriethoxysilane |
| bedtcm | N-(benzyl)-(ethyl)-dithio carbamate |
| bigR | biguanide derivatives |
| bmc | bismacrocycle |
| bpa | 1,2-bis(4-pyridyl)ethane |
| bpy | 2,2′-bipyridine |
| btmpp | 2,6-bis(3,4,5-trimethylpyrazolyl)pyridine) |
| cbl | β-carboline |
| c-di-GMP | cyclic-di-guanosine monophosphate |
| CFU | colony forming units |
| cpR | substituted cyclopentadienyl |
| cur | curcumin |
| cv | crystal violet |
| dach | 1S,2S- or 1R,2R-diaminocyclohexane |
| dfx | deferasirox |
| DMF | N,N-dimethylformamide |
| dmtp | 5,7-dimethyl-1,2,4-triazolo[1,5-a]pyrimidine |
| dttct | dibenzo[e,k]-2,3,8,9-tetraphenyl-1,4,7,10-tetraaza-cyclododeca-1,3,7,9-tetraene |
| edtaod | EDTA-based phenylene macrocycle |
| edtp | N,N,N′,N′-tetrakis(2-hydroxypropyl)ethylenediamine |
| EPS | extracellular polymeric substances |
| fcz | fluconazole |
| fphen | functionalized 1,10-phenanthrolines |
| H2bmic | 1-benzimidazole-5-carboxylic acid |
| H2dmbgMc | ligand resulted from Hdmbg condensation with ammonia/hydrazine and formaldehyde |
| H2kg | α-ketoglutaric acid |
| H2muco | trans, trans-muconic acid |
| H3BTTri | 1,3,5-tris(1H-1,2,3-triazol-5-yl)benzene |
| H4bptc | 3,3,5,5-biphenyltetracarboxylate |
| H4EDTA | ethylenediaminetetraacetic acid |
| Hacr | acrylic acid |
| Hapa | 2-aminopyridine-4-carboxylic acid |
| Hbza | benzoic acid |
| Hbzim | benzimidazole |
| Hcip | ciprofloxacin |
| Hdmbg | N,N′-dimethylbiguanide |
| Him | imidazole |
| Hphtt | (E)-2-(2-(pyridin-2-ylmethylene)hydrazinyl)-4-(p-tolyl)thiazole |
| Htbg | 1-(o-tolyl)biguanide |
| Htsa | thiosalicylic acid; |
| imdtcm | N-(4-isopropyl-benzyl)-(4-methoxy-benzyl)-dithiocarbamate |
| mcim | 4-methyl-5-carboxyaldehyde-imidazole |
| Me2bzim | 5,6-dimethylbenzimidazole |
| MIC | minimum inhibitory concentration |
| mmphi | (E)-7-(4-methoxybenzylidene)-3-(4-methoxyphenyl)-2-pyridyl-3,3a,4,5,6,7-hexahydro-2H-indazole |
| MOF | metal-organic framework |
| MRSA | methicillin resistant S. aureus |
| MSN | mesoporous silica nanoparticles |
| nhc | N-heterocyclic carbene |
| PAMAM | polyamidoamine |
| PEU | poly(ether urethane) |
| Ph | phenyl |
| phen | 1,10-phenantroline |
| phendione | 1,10-phenanthroline-5,6-dione |
| pmtp | 5-phenyl-7-methyl-1,2,4-triazolo[1,5-a]pyrimidine |
| PVC | poly(vinyl chloride) |
| py | pyridine |
| pym | pyrimidine |
| QS | quorum sensing |
| quz | quinazoline |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| S-bu-thiosal | S-butyl derivative of thiosalicylic acid |
| S-et-thiosal | S-ethyl derivative of thiosalicylic acid; |
| smx | sulfamethoxazole |
| snh | substituted nitrogen heterocycle |
| tbpp | 1-thiocarbamoyl-5-(4-bromophenyl)-3-phenyl-4,5-dihydro-1H-pyrazole |
| tcpp | 1-thiocarbamoyl-5-(4-chlorophenyl/)-3-phenyl-4,5-dihydro-1H-pyrazole |
| tea | triethanolamine |
| tet | tetrazole |
| tmeda | N,N,N′,N′-tetramethylethylenediamine |
| XTT | 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide |
References
- Kamaruzzaman, N.F.; Tan, L.P.; Yazid, K.A.M.; Saeed, S.I.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Chivu, A.; Gibson, A.J. Targeting the Bacterial Protective Armour; Challenges and Novel Strategies in the Treatment of Microbial Biofilm. Materials 2018, 11, 1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pircalabioru, G.G.; Chifiriuc, M.C. Nanoparticulate drug-delivery systems for fighting microbial biofilms–from bench to bedside. Future Microbiol. 2020, 15, 679–698. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Andes, D.R.; Krysan, D.J. Candida–streptococcal interactions in biofilm associated oral diseases. PLoS Pathog. 2018, 14, e1007342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costerton, J.W.; Montanaro, L.; Arciola, C.R. Biofilm in Implant Infections: Its Production and Regulation. Int. J. Artif. Organ. 2005, 28, 1062–1068. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 248, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; Low, W.; Gupta, A.; Amin, M.; Radecka, I.; Britland, S.; Raj, P.; Kenward, K. Strategies for Antimicrobial Drug Delivery to Biofilm. Curr. Pharm. Des. 2014, 21, 43–66. [Google Scholar] [CrossRef]
- Mihai, M.M.; Preda, M.; Lungu, I.; Cartelle Gestal, M.; Popa, M.I.; Holban, A.M. Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation. Int. J. Mol. Sci. 2018, 19, 1179. [Google Scholar] [CrossRef] [Green Version]
- Pallavicini, P.; Dacarro, G.; Diaz-Fernandez, Y.A.; Taglietti, A. Coordination chemistry of surface-grafted ligands for antibacterial materials. Coord. Chem. Rev. 2014, 275, 37–53. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Dąbrowski, J.M.; Mazuryk, O.; Śpiewak, K.; Kyzioł, A. Bioinorganic antimicrobial strategies in the resistance era. Coord. Chem. Rev. 2017, 351, 76–117. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef]
- Sadekuzzaman, M.; Yang, S.; Mizan, M.F.R.; Ha, S.D. Current and Recent Advanced Strategies for Combating Biofilms. Compr. Rev. Food Sci. Food Saf. 2015, 14, 491–509. [Google Scholar] [CrossRef]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010, 65, 1955–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd El-Hamid, M.I.; El-Naenaeey, E.Y.; Kandeel, T.M.; Hegazy, W.A.H.; Mosbah, R.A.; Nassar, M.S.; Bakhrebah, M.A.; Abdulaal, W.H.; Alhakamy, N.A.; Bendary, M.M. Promising Antibiofilm Agents: Recent Breakthrough against Biofilm Producing Methicillin-Resistant Staphylococcus aureus. Antibiotics 2020, 9, 667. [Google Scholar] [CrossRef]
- Mosallam, F.M.; Helmy, E.A.; Bendary, M.M.; El-Batal, I.A. Potency of a novel synthesized Ag-eugenol nanoemulsion for treating some bacterial and fungal pathogens. J. Mater. Res. 2021, 36, 1524–1537. [Google Scholar] [CrossRef]
- Bi, Y.; Xia, G.; Shi, C.; Wan, J.; Liu, L.; Chen, Y.; Wu, Y.; Zhang, W.; Zhou, M.; He, H.; et al. Therapeutic strategies against bacterial biofilms. Fundam. Res. 2021, 1, 193–212. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Anti-biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Sajid, M.; Khan, M.S.A.; Cameotra, S.S.; Ahmad, I. Drug Delivery Systems That Eradicate and/or Prevent Biofilm Formation. In Antibiofilm Agents: From Diagnosis to Treatment and Prevention; Rumbaugh, K.P., Ahmad, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 407–424. [Google Scholar]
- Dharmaprakash, A.; Thandavarayan, R.; Joseph, I.; Thomas, S. Development of broad-spectrum antibiofilm drugs: Strategies and challenges. Future Microbiol. 2015, 10, 1035–1048. [Google Scholar] [CrossRef]
- Blackman, L.D.; Qu, Y.; Cass, P.; Locock, K.E.S. Approaches for the inhibition and elimination of microbial biofilms using macromolecular agents. Chem. Soc. Rev. 2021, 50, 1587–1616. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, I.B.; Simões, M.; Simões, L.C. Copper Surfaces in Biofilm Control. Nanomaterials 2020, 10, 2491. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Libardo, M.D.J.; Bahar, A.A.; Ma, B.; Fu, R.; McCormick, L.E.; Zhao, J.; McCallum, S.A.; Nussinov, R.; Ren, D.; Angeles-Boza, A.M.; et al. Nuclease activity gives an edge to host-defense peptide piscidin 3 over piscidin 1, rendering it more effective against persisters and biofilms. FEBS J. 2017, 284, 3662–3683. [Google Scholar] [CrossRef] [Green Version]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Fan, X.; Yahia, L.; Sacher, E. Antimicrobial Properties of the Ag, Cu Nanoparticle System. Biology 2021, 10, 137. [Google Scholar] [CrossRef]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle-Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Hemdan, B.A.; El-Taweel, G.E.; Goswami, P.; Pant, D.; Sevda, S. The role of bioflm in the development and dissemination of ubiquitous pathogens in drinking water distribution systems: An overview of surveillance, outbreaks, and prevention. World J. Microbiol. Biotechnol. 2021, 37, 1–16. [Google Scholar] [CrossRef]
- Sharahi, J.Y.; Azimi, T.; Shariati, A.; Safari, H.; Tehrani, M.K.; Hashemi, A. Advanced strategies for combating bacterial bioflms. J. Cell. Physiol. 2019, 234, 14689–14708. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 2015, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Kocot, A.M.; Olszewska, M.A. Bioflm formation and microscopic analysis of biofilms formed by Listeria monocytogenes in a food processing context. LWT Food Sci. Technol. 2017, 84, 47–57. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cell Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Int. Sci. 2020, 283, 102228. [Google Scholar] [CrossRef]
- Thakkar, R.B.; Shah, K.; Mehta, A.; Shah, M.V.; Singh, S.; Upadhyay, T.K.; Upadhye, V.J.; Panchal, R.R. Investigations on Presence of Biopolymer Producing Microorganism in Marine Soils & Enhanced Potential of Marine Soil by Using Isolated Microorganism. Biointerface Res. Appl. Chem. 2022, 12, 3135–3149. [Google Scholar] [CrossRef]
- Kragh, K.N.; Hutchison, J.B.; Melaugh, G.; Rodesney, C.; Roberts, A.E.L.; Irie, Y.; Jensen, P.Ø.; Diggle, S.P.; Allen, R.J.; Gordon, V.; et al. Role of Multicellular Aggregates in Biofilm Formation. mBio 2016, 7, e00237-16. [Google Scholar] [CrossRef] [Green Version]
- Kreve, S.; Reis, A.C.D. Bacterial adhesion to biomaterials: What regulates this attachment? A review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef]
- Fang, L.; Yang, S.; Huang, Q.; Xue, A.; Cai, P. Biosorption mechanisms of Cu(II) by extracellular polymeric substances from Bacillus subtilis. Chem. Geol. 2014, 386, 143–151. [Google Scholar] [CrossRef]
- Jefferson, K.K. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef] [PubMed]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wuertz, S. Bacteria and archaea on earth and their abundance in bioflms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Mann, E.E.; Wozniak, D.J. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol. Rev. 2012, 36, 893–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svenningsen, N.B.; Martínez-García, E.; Nicolaisen, M.H.; de Lorenzo, V.; Nybroe, O. The biofilm matrix polysaccharides cellulose and alginate both protect Pseudomonas putida mt-2 against reactive oxygen species generated under matric stress and copper exposure. Microbiology 2018, 164, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Nkoh, J.N.; Yana, J.; Honga, Z.-N.; Xua, R.-K.; Kamrana, M.A.; Juna, J.; Li, J.-Y. An electrokinetic perspective into the mechanism of divalent and trivalent cation sorption by extracellular polymeric substances of Pseudomonas fluorescen. Colloids Surf. Biointerfaces 2019, 183, 110450. [Google Scholar] [CrossRef]
- Sheng, G.P.; Xu, J.; Luo, H.W.; Li, W.W.; Li, W.H.; Yu, H.Q.; Xie, Z.; Wei, S.Q.; Hu, F.C. Thermodynamic analysis on the binding of heavy metals onto extracellular polymeric substances (EPS) of activated sludge. Water Res. 2013, 47, 607–614. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Xu, R.-K.; Yan, J.; Jiang, J.; Li, J.-Y.; Kamran, M.A. Mechanism of Cu(II) and Cd(II) immobilization by extracellular polymeric substances (Escherichia coli) on variable charge soils. Environ. Pollut. 2019, 247, 136–145. [Google Scholar] [CrossRef]
- Kim, M.K.; Zhao, A.; Wang, A.; Brown, Z.Z.; Muir, T.W.; Stone, H.A.; Bassler, B.L. Surface-attached molecules control Staphylococcus aureus quorum sensing and biofilm development. Nat. Microbiol. 2017, 22, 17080. [Google Scholar] [CrossRef] [Green Version]
- Haque, S.; Yadav, D.K.; Bisht, S.C.; Yadav, N.; Singh, V.; Dubey, K.K.; Jawed, A.; Wahid, M.; Dar, S.A. Quorum sensing pathways in Gram-positive and -negative bacteria: Potential of their interruption in abating drug resistance. J. Chemother. 2019, 31, 161–187. [Google Scholar] [CrossRef]
- Lal, A. Quorum sensing: How bacteria talk to each other? Resonance 2009, 14, 866–871. [Google Scholar] [CrossRef]
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-Associated Infection: Locating the Finish Line in the Race for the Surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef] [Green Version]
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front. Microbiol. 2014, 5, 405. [Google Scholar] [CrossRef] [Green Version]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum Sensing Inhibitors Increase the Susceptibility of Bacterial Biofilms to Antibiotics In Vitro and In Vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-H. Nanoparticle-based Therapies for Wound Biofilm Infection: Opportunities and Challenges. IEEE Trans. Nanobiosci. 2016, 15, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Kasimanickam, R.; Ranjan, A.; Asokan, G.V.; Kasimanickam, R.V.; Kastelic, J.P. Prevention and treatment of biofilms by hybrid- and nanotechnologies. Int. J. Nanomed. 2013, 8, 2809–2819. [Google Scholar] [CrossRef]
- Chauhan, A.; Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Full and broad-spectrum in vivo eradication of catheter-associated biofilms using gentamicin-EDTA antibiotic lock therapy. Antimicrob. Agents Chemother. 2012, 56, 6310–6318. [Google Scholar] [CrossRef] [Green Version]
- Caly, D.L.; Bellini, D.; Walsh, M.A.; Dow, J.M.; Ryan, R.P. Targeting cyclic di-GMP signalling: A strategy to control biofilm formation? Curr. Pharm. Des. 2015, 21, 12–24. [Google Scholar] [CrossRef]
- Kolderman, E.; Bettampadi, D.; Samarian, D.; Dowd, S.E.; Foxman, B.; Jakubovics, N.S.; Rickard, A.H. L-Arginine Destabilizes Oral Multi-Species Biofilm Communities Developed in Human Saliva. PLoS ONE 2015, 10, e0121835. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Moreau-Marquis, S.; O’Toole, G.A.; Stanton, B.A. Tobramycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells. Am. J. Respir. Cell. Mol. Biol. 2009, 41, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisch, T. Resistance in antimicrobial photodynamic inactivation of bacteria. Photochem. Photobiol. Sci. 2015, 14, 1518–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tewes, F.; Bahamondez-Canas, T.F.; Smyth, H.D.C. Efficacy of Ciprofloxacin and Its Copper Complex against Pseudomonas aeruginosa Biofilms. AAPS PharmSciTech 2019, 20, 205. [Google Scholar] [CrossRef]
- Rafiee, F.; Haghi, F.; Bikas, R.; Heidari, A.; Gholami, M.; Kozakiewicz, A.; Zeighami, H. Synthesis, characterization and assessment of anti-quorum sensing activity of copper(II)-ciprofloxacin complex against Pseudomonas aeruginosa PAO1. AMB Expr. 2020, 10, 82. [Google Scholar] [CrossRef] [Green Version]
- Stevanović, N.L.J.; Aleksic, I.; Kljun, J.; Skaro Bogojevic, S.; Veselinovic, A.; Nikodinovic-Runic, J.; Turel, I.; Djuran, M.I.; Glišić, B.Ð. Copper(II) and Zinc(II) Complexes with the Clinically Used Fluconazole: Comparison of Antifungal Activity and Therapeutic Potential. Pharmaceuticals 2021, 14, 24. [Google Scholar] [CrossRef]
- Di Santo, A.; Gil, D.M.; Pomiro, F.; Piro, O.E.; Echeverría, G.A.; Arena, M.; Luciardi, C.; Carbonio, R.E.; Ben Altabef, A. Biofilm inhibition by a new Mn(II) complex with sulfamethoxazole: Synthesis, spectroscopic characterization and crystal structure. Inorg. Chim. Acta 2015, 436, 16–22. [Google Scholar] [CrossRef]
- Mizdal, C.; Stefanello, S.; Bonez, P.; Agertt, V.; Flores, V.; Rossi, G.; Siqueira, F.; Marques, L.; Campos, M. Anti-biofilm and Antibacterial Effects of Novel Metal-coordinated Sulfamethoxazole Against Escherichia coli. Lett. Drug Des. Discov. 2017, 14, 339–344. [Google Scholar] [CrossRef]
- Cordenonsi Bonez, P.; Agertt, V.A.; Guidolin Rossi, G.; dos Santos Siqueira, F.; Siqueira, J.D.; Marques, L.L.; Manzoni de Oliveira, G.N.; Vianna Santos, R.C.; Anraku de Campos, M.M. Sulfonamides complexed with metals as mycobacterial biofilms inhibitors. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 23, 100217. [Google Scholar] [CrossRef]
- Poole, K. At the nexus of antibiotics and metals: The impact of Cu and Zn on antibiotic activity and resistance. Trends Microbiol. 2017, 25, 820–832. [Google Scholar] [CrossRef]
- Božić, B.; Korać, J.; Stanković, D.M.; Stanić, M.; Romanović, M.; Bogdanović Pristov, J.; Spasić, S.; Popović-Bijelić, A.; Spasojević, I.; Bajčetić, M. Coordination and redox interactions of b-lactam antibiotics with Cu2+ in physiological settings and the impact on antibacterial activity. J. Free Radic. Biol. Med. 2018, 129, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Hrioua, A.; Loudiki, A.; Farahi, A.; Laghrib, F.; Bakasse, M.; Lahrich, S.; Saqrane, S.; El Mhammedi, M.A. Complexation of amoxicillin by transition metals: Physico-chemical and antibacterial activity evaluation. Bioelectrochemistry 2021, 142, 107936. [Google Scholar] [CrossRef]
- Calu, L.; Badea, M.; Cerc Korošec, R.; Bukovec, P.; Daniliuc, C.C.; Chifiriuc, M.C.; Măruţescu, L.; Ciulică, C.; Şerban, G.; Olar, R. Thermal behaviour of some novel biologically active complexes with a triazolopyrimidine pharmacophore. J. Therm. Anal. Calorim. 2017, 127, 697–708. [Google Scholar] [CrossRef]
- Olar, R.; Calu, L.; Badea, M.; Chifiriuc, M.C.; Bleotu, C.; Velescu, B.; Stoica, O.; Ioniţă, G.; Stanică, N.; Silvestro, L.; et al. Thermal behaviour of some biologically active species based on complexes with a triazolopyrimidine pharmacophore. J. Therm. Anal. Calorim. 2017, 127, 685–696. [Google Scholar] [CrossRef]
- Badea, M.; Calu, L.; Celan Korosin, N.; David, I.G.; Chifiriuc, M.C.; Bleotu, C.; Ioniţă, G.; Silvestro, L.; Maurer, M.; Olar, R. Thermal behaviour of some biological active perchlorate complexes with a triazolopyrimidine derivative. J. Therm. Anal. Calorim. 2018, 134, 665–677. [Google Scholar] [CrossRef]
- Maruțescu, L.; Calu, L.; Chifiriuc, M.C.; Bleotu, C.; Daniliuc, C.-G.; Falcescu, D.; Kamerzan, C.M.; Badea, M.; Olar, R. Synthesis, Physico-chemical Characterization, Crystal Structure and Influence on Microbial and Tumor Cells of Some Co(II) Complexes with 5,7-Dimethyl-1,2,4-triazolo[1,5-a]pyrimidine. Molecules 2017, 22, 1233. [Google Scholar] [CrossRef] [Green Version]
- Ruta, L.L.; Farcasanu, I.C.; Bacalum, M.; Raileanu, M.; Rostas, A.M.; Daniliuc, C.G.; Chifiriuc, M.C.; Maru-tescu, L.; Popa, M.; Badea, M.; et al. Biological activity of N-N-helating heterocycle copper(II) complexes modulated by an auxiliary triazolopyrimidine ligand. Molecules 2021, 26, 6772. [Google Scholar] [CrossRef]
- Khan, M.S.; Hayat, M.U.; Khanam, M.; Saeed, H.; Owais, M.; Khalid, M.; Shahid, M.; Ahmad, M. Role of biologically important imidazole moiety on the antimicrobial and anticancer activity of Fe(III) and Mn(II) complexes. J. Biomol. Struct. Dyn. 2021, 39, 4037–4050. [Google Scholar] [CrossRef]
- Badea, M.; Vlaicu, I.D.; Olar, R.; Constand, M.; Bleotu, C.; Chifiriuc, M.C.; Marutescu, L.; Lazar, V.; Grecu, M.N.; Marinescu, D. Thermal behaviour and characterisation of new biologically active Cu(II) complexes with benzimidazole as main ligand. J. Therm. Anal. Calorim. 2014, 118, 1119–1133. [Google Scholar] [CrossRef]
- Vlaicu, I.D.; Constand, M.; Olar, R.; Marinescu, D.; Grecu, M.N.; Lazar, V.; Chifiriuc, M.C.; Badea, M. Thermal stability of new biologic active copper(II) complexes with 5,6-dimethylbenzimidazole. J. Therm. Anal. Calorim. 2013, 113, 1369–1377. [Google Scholar] [CrossRef]
- Boni Dias, B.; Gomes da Silva Dantas, F.; Galvão, F.; Cupozak-Pinheiro, W.J.; Wender, H.; Pizzuti, L.; Rosa, P.P.; Tenório, K.V.; Gatto, C.C.; Negri, M.; et al. Synthesis, structural characterization, and prospects for new cobalt (II) complexes with thiocarbamoyl-pyrazoline ligands as promising antifungal agents. J. Inorg. Biochem. 2020, 213, 111277. [Google Scholar] [CrossRef]
- Glišić, B.Đ.; Aleksic, I.; Comba, P.; Wadepohl, H.; Ilic-Tomic, T.; Nikodinovic-Runic, J.; Djuran, M.I. Copper(II) complexes with aromatic nitrogen-containing heterocycles as effective inhibitors of quorum sensing activity in Pseudomonas aeruginosa. RSC Advances 2013, 6, 86695–86709. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Khan, R.A.; Khan, M.S.; Alam, M.Z.; Ansari, F.A.; Laeeq, S.; Zubair, M.; Shahzad, S.A.; Khan, J.M.; et al. Interference of phosphane copper (I) complexes of b-carboline with quorum sensing regulated virulence functions and biofilm in foodborne pathogenic bacteria: A first report. Saudi J. Biol. Sci. 2019, 26, 308–316. [Google Scholar] [CrossRef]
- Bernardi, T.; Badel, S.; Mayer, P.; Groelly, J.; de Frémont, P.; Jacques, B.; Braunstein, P.; Teyssot, M.-L.; Gaulier, C.; Cisnetti, F.; et al. High-Throughput Screening of Metal-N-Heterocyclic Carbene Complexes against Biofilm Formation by Pathogenic Bacteria. Chem. Med. Chem. 2014, 9, 1140–1144. [Google Scholar] [CrossRef]
- Pan, T.; Wang, Y.; Liu, F.-S.; Lin, H.; Zhou, Y. Copper(I)–NHCs complexes: Synthesis, characterization and their inhibition against the biofilm formation of Streptococcus mutans. Polyhedron 2021, 197, 115033. [Google Scholar] [CrossRef]
- Soto-Aguilera, N.; Üstün, E.; Tutar, U.; Çelik, C.; Gürbüz, N.; Özdemir, İ. Antimicrobial activity, inhibition of biofilm formation, and molecular docking study of novel Ag-NHC complexes. J. Organomet. Chem. 2021, 954–955, 122082. [Google Scholar] [CrossRef]
- Fu, D.; Yang, S.; Lu, J.; Lian, H.; Qin, K. Two Cu(II) Coordination Polymers: Treatment Activity on Spine Surgery Incision Infection by Inhibiting the Staphylococcus aureus Biofilm Formation. J. Clust. Sci. 2021, 1–8. [Google Scholar] [CrossRef]
- Olar, R.; Badea, M.; Bacalum, M.; Raileanu, M.; Ruta, L.L.; Farcasanu, I.C.; Rostas, A.M.; Vlaicu, I.D.; Popa, M.; Chifiriuc, M.C. Antiproliferative and antibacterial properties of biocompatible copper(II) complexes bearing chelating N,N-heterocycle ligands and potential mechanisms of action. Biometals 2021, 34, 1155–1172. [Google Scholar] [CrossRef] [PubMed]
- Rostas, A.M.; Badea, M.; Ruţă, L.L.; Farcaşanu, I.C.; Maxim, C.; Chifiriuc, M.C.; Popa, M.; Luca, M.; Čelan Korošin, N.; Cerc Korošec, R.; et al. Copper(II) complexes with mixed heterocycle ligands as promising antibacterial and antitumor species. Molecules 2020, 25, 3777. [Google Scholar] [CrossRef] [PubMed]
- Beeton, M.L.; Aldrich-Wright, J.R.; Bolhuis, A. The antimicrobial and antibiofilm activities of copper(II) complexes. J. Inorg. Biochem. 2014, 140, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Matiadis, D.; Karagiaouri, M.; Mavroidi, B.; Nowak, K.E.; Katsipis, G.; Pelecanou, M.; Pantazaki, A.; Sagnou, M. Synthesis and antimicrobial evaluation of a pyrazolinepyridine silver(I) complex: DNA-interaction and antibiofilm activity. Biometals 2021, 34, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Wawrzycka, A.; Rogala, P.; Czerwonka, G.; Michałkiewicz, S.; Hodorowicz, M.; Gałczyńska, K.; Cieślak, B.; Kowalczyk, P. Tuning Anti-Biofilm Activity of Manganese(II) Complexes: Linking Biological Effectiveness of Heteroaromatic Complexes of Alcohol, Aldehyde, Ketone, and Carboxylic Acid with Structural Effects and Redox Activity. Int. J. Mol. Sci. 2021, 22, 4847. [Google Scholar] [CrossRef]
- Ventura, R.F.; Galdino, A.C.M.; Viganor, L.; Schuenck, R.P.; Devereux, M.; McCann, M.; Santos, A.L.S.; Nunes, A.P.F. Antimicrobial action of 1,10-phenanthroline-based compounds on carbapenemase-producing Acinetobacter baumannii clinical strains: Efficacy against planktonic- and biofilm-growing cells. Braz. J. Microbiol. 2020, 51, 1703–1710. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Ji, P. A Microporous Zn(II)–MOF for Solvent-Free Cyanosilylation and Treatment Effect Against Bacterial Infection on Burn Patients Via Inhibiting the Staphylococcus aureus Biofilm Formation. J. Inorg. Organ. Polym. Mater. 2021, 31, 492–499. [Google Scholar] [CrossRef]
- Borges, A.; Simões, M.; Todorović, T.R.; Filipović, N.R.; García-Sosa, A.T. Cobalt Complex with Thiazole-Based Ligand as New Pseudomonas aeruginosa Quorum Quencher, Biofilm Inhibitor and Virulence Attenuator. Molecules 2018, 23, 1385. [Google Scholar] [CrossRef] [Green Version]
- Hopa, C.; Kara, H.; Aybey, A. Synthesis, structural characterization and biological evaluation of novel mixed-ligand Co(II) complexes as quorum sensing inhibitory agent. J. Mol. Struct. 2020, 1202, 127322. [Google Scholar] [CrossRef]
- Zandvakili, T.; Fatemi, S.J.; Ebrahimipour, S.Y.; Ebrahimnejad, H.; Castro, J.; Dusek, M.; Eigner, V. Deferasirox pyridine solvate and its Cu(II) complex: Synthesis, crystal structure, Hirshfeld surface analysis, antimicrobial assays and antioxidant activity. J. Mol. Struct. 2022, 1249, 131525. [Google Scholar] [CrossRef]
- Calu, L.; Badea, M.; Chifiriuc, M.C.; Bleotu, C.; David, G.-I.; Ioniţă, G.; Măruțescu, L.; Lazăr, V.; Stănică, N.; Soponaru, I.; et al. Synthesis, spectral, thermal, magnetic and biological characterization of Co(II), Ni(II), Cu(II) and Zn(II) complexes with a Schiff base bearing a 1,2,4-triazole pharmacophore. J. Therm. Anal. Calorim. 2014, 120, 375–386. [Google Scholar] [CrossRef]
- Badea, M.; Calu, L.; Chifiriuc, M.C.; Bleotu, C.; Marin, A.; Ion, S.; Ioniță, G.; Stănică, N.; Măruțescu, L.; Lazăr, V.; et al. Thermal behaviour of some novel antimicrobials based on complexes with a Schiff base bearing 1,2,4-triazole pharmacophore. J. Therm. Anal. Calorim. 2014, 118, 1145–1157. [Google Scholar] [CrossRef]
- Calu, L.; Badea, M.; Čelan Korošin, N.; Chifiriuc, M.C.; Bleotu, C.; Stanică, N.; Silvestro, L.; Maurer, M.; Olar, R. Spectral, thermal and biological characterization of complexes with a Schiff base bearing triazole moiety as potential antimicrobial species. J. Therm. Anal. Calorim. 2018, 134, 1839–1850. [Google Scholar] [CrossRef]
- Reiss, A.; Chifiriuc, M.C.; Amzoiu, E.; Spînu, C.I. Transition metal(II) complexes with cefotaxime-derived Schiff base: Synthesis, characterization and antimicrobial studies. Bioinorg. Chem. Appl. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Reiss, A.; Chifiriuc, M.C.; Amzoiu, E.; Cioateră, N.; Dăbuleanu, I.; Rotaru, P. New metal(II) complexes with ceftazidime Schiff base. J. Therm. Anal. Calorim. 2017, 131, 2073–2085. [Google Scholar] [CrossRef]
- Zarafu, I.; Badea, M.; Ioniţă, G.; Ioniţă, P.; Păun, A.; Bucur, M.; Chifiriuc, M.C.; Bleotu, C.; Olar, R. Spectral, magnetic, thermal and biological studies on Ca(II) and Cu(II) complexes with a novel crowned Schiff base. J. Therm. Anal. Calorim. 2016, 127, 1511–1521. [Google Scholar] [CrossRef]
- Zarafu, I.; Olar, R.; Chifiriuc, M.C.; Bleotu, C.; Ioniţă, P.; Mulţescu, M.; Ioniță, G.; Grădișteanu, G.; Tatibouët, A.; Badea, M. Synthesis, thermal, spectral, antimicrobial and cytotoxicity profile of the Schiff bases bearing pyrazolone moiety and their Cu(II) complexes. J. Therm. Anal. Calorim. 2018, 134, 1851–1861. [Google Scholar] [CrossRef]
- Zarafu, I.; Badea, M.; Ioniţă, G.; Chifiriuc, M.C.; Bleotu, C.; Popa, M.; Ioniţă, P.; Tatibouët, A.; Olar, R. Thermal, spectral and biological characterisation of copper(II) complexes with isoniazid-based hydrazones. J. Therm. Anal. Calorim. 2019, 136, 1977–1987. [Google Scholar] [CrossRef]
- Usman, M.; Arjmand, F.; Ahmad, M.; Khan, M.S.; Ahmad, I.; Tabassum, S. A comparative analyses of bioactive Cu(II) complexes using Hirshfeld surface and density functional theory (DFT) methods: DNA binding studies, cleavage and antibiofilm activities. Inorg. Chim. Acta 2016, 453, 193–201. [Google Scholar] [CrossRef]
- Ebrahimipour, S.Y.; Sheikhshoaie, I.; Mohamadi, M.; Suarez, S.; Baggio, R.; Khaleghi, M.; Torkzadeh-Mahani, M.; Mostafavi, A. Synthesis, characterization, X-ray crystal structure, DFT calculation, DNA binding, and antimicrobial assays of two new mixed-ligand copper(II) complexes. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 142, 410–422. [Google Scholar] [CrossRef]
- Hubberstey, P.; Suksangpanya, U. Hydrogen-Bonded Supramolecular Chain and Sheet Formation by Coordinated Guanidine Derivatives. Struct. Bond. 2004, 111, 33–83. [Google Scholar] [CrossRef]
- Gabel, S.A.; Duff, M.R.; Pedersen, L.C.; DeRose, E.F.; Krahn, J.M.; Howell, E.E.; London, R.E. A Structural Basis for Biguanide Activity. Biochemistry 2017, 56, 4786–4798. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Liu, L.; Yang, H.; Zhou, R.; Che, C.; Li, X.; Li, C.; Luan, S.; Yin, J.; Shi, H. Water-Insoluble Polymeric Guanidine Derivative and Application in the Preparation of Antibacterial Coating of Catheter. ACS Appl. Mater. Interfaces 2018, 10, 39257–39267. [Google Scholar] [CrossRef]
- Li, J.; Zhong, W.; Zhang, K.; Wang, D.; Hu, J.; Chan-Park, M.B. Biguanide-Derived Polymeric Nanoparticles Kill MRSA Biofilm and Suppress Infection In Vivo. ACS Appl. Mater. Interfaces 2020, 12, 21231. [Google Scholar] [CrossRef]
- Olar, R.; Badea, M.; Marinescu, D.; Chifiriuc, C.-M.; Bleotu, C.; Grecu, M.N.; Iorgulescu, E.E.; Bucur, M.; Lazar, V.; Finaru, A. Prospects for new antimicrobials based on N,N-dimethylbiguanide complexes as effective agents on both planktonic and adhered microbial strains. Eur. J. Med. Chem. 2010, 45, 2868–2875. [Google Scholar] [CrossRef] [PubMed]
- Olar, R.; Badea, M.; Marinescu, D.; Chifiriuc, M.; Bleotu, C.; Grecu, M.N.; Iorgulescu, E.; Lazar, V. N,N-dimethylbiguanide complexes displaying low cytotoxicity as potential large spectrum antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Badea, M.; Crasanda, A.-M.; Chifiriuc, M.C.; Marutescu, L.; Lazar, V.; Marinescu, D.; Olar, R. Synthesis, spectral and thermal study on new Fe(III) complexes with N,N-dimethylbiguanide as antibacterial agents. J. Therm. Anal. Calorim. 2012, 111, 1743–1751. [Google Scholar] [CrossRef]
- Badea, M.; Iosub, E.; Chifiriuc, C.M.; Marutescu, L.; Iorgulescu, E.E.; Lazar, V.; Marinescu, D.; Bleotu, C.; Olar, R. Thermal, spectral, electrochemical and biologic characterization of new Pd(II) complexes with ligands bearing biguanide moieties. J. Therm. Anal. Calorim. 2012, 111, 1753–1761. [Google Scholar] [CrossRef]
- Olar, R.; Badea, M.; Marinescu, D.; Iorgulescu, E.E.; Frunza, E.; Lazar, V.; Chifiriuc, C. Thermal, spectral and antimicrobial study on some Cu(II) complexes with ligands bearing biguanide moieties. J. Therm. Anal. Calorim. 2009, 99, 815–821. [Google Scholar] [CrossRef]
- Maxim, C.; Badea, M.; Rostas, A.M.; Chifiriuc, M.C.; Gradisteanu Pircalabioru, G.; Avram, S.; Olar, R. Copper(II) species with 1-(o-tolyl)biguanide: Structural characterization, ROS scavenging, antibacterial activity, biocompatibility and in silico studies. Appl. Organomet. Chem. 2021, e6471. [Google Scholar] [CrossRef]
- Nuţă, I.; Badea, M.; Chifiriuc, M.C.; Bleotu, C.; Popa, M.; Daniliuc, C.-G.; Olar, R. Synthesis, physico-chemical characterization and bioevaluation of Ni(II), Pd(II), and Pt(II) complexes with 1-(o-tolyl)biguanide: Antimicrobial and antitumor studies. Appl. Organ. Chem. 2020, 34, e5807. [Google Scholar] [CrossRef]
- Mihalache, M.; Oprea, O.; Guran, C.; Holban, A.M. Synthesis, characterization, and biological activity of some complex combinations of nickel with α-ketoglutaric acid and 1-(o-tolyl)biguanide. Comptes Rendus Chim. 2018, 21, 32–40. [Google Scholar] [CrossRef]
- Mihalache, M.; Guran, C.; Meghea, A.; Bercu, V.; Motelica, L.; Holban, A.M. Complexes of Cu (II) with α-Ketoglutaric Acid and 1-(o-tolyl)Biguanide. Synthesis, Characterization and Biological Activity. Rev. Chim. 2019, 70, 3603–3610. [Google Scholar] [CrossRef]
- Chen, F.; Moat, J.; McFeely, D.; Clarkson, G.; Hands-Portman, I.J.; Furner-Pardoe, J.P.; Harrison, F.; Dowson, C.G.; Sadler, P.J. Biguanide Iridium(III) Complexes with Potent Antimicrobial Activity. J. Med. Chem. 2018, 61, 7330–7344. [Google Scholar] [CrossRef]
- Badea, M.; Grecu, M.N.; Chifiriuc, M.C.; Bleotu, C.; Popa, M.; Iorgulescu, E.E.; Avram, S.; Uivarosi, V.; Munteanu, A.-C.; Ghica, D.; et al. Insight on Ni(II) and Cu(II) complexes of biguanide derivatives developed as effective antimicrobial and antitumor agents. Appl. Organomet. Chem. 2021, 35, e6155. [Google Scholar] [CrossRef]
- Olar, R.; Pătraşcu, F.; Chifiriuc, M.C.; Iorgulescu, E.E.; Bleotu, C.; Măruţescu, L.; Lazăr, V.; Marinescu, D.; Stănică, N.; Badea, M. Insight on thermal, spectral, magnetic and biological behaviour of new Ni(II), Cu(II) and Zn(II) complexes with a pentaazamacrocyclic ligand derived from nicotinamide. J. Therm. Anal. Calorim. 2014, 118, 1159–1168. [Google Scholar] [CrossRef]
- Badea, M.; Pătraşcu, F.; Cerc Korošec, R.; Bukovec, P.; Raita, M.; Chifiriuc, M.C.; Măruțescu, L.; Bleotu, C.; Velescu, B.; Marinescu, D.; et al. Thermal, spectral, magnetic and biologic characterization of new Ni(II), Cu(II) and Zn(II) complexes with a hexaazamacrocyclic ligand bearing ketopyridine moieties. J. Therm. Anal. Calorim. 2014, 118, 1183–1193. [Google Scholar] [CrossRef]
- Brahma, U.; Kothari, R.; Sharma, P.; Bhandari, V. Antimicrobial and anti-biofilm activity of hexadentated macrocyclic complex of copper (II) derived from thiosemicarbazide against Staphylococcus aureus. Sci. Rep. 2018, 8, 8050. [Google Scholar] [CrossRef]
- Vázquez-Armenta, F.J.; Beltrán-Torres, M.; Ayala-Zavala, J.F.; Velázquez-Contreras, E.F.; Rocha-Alonzo, F.; González-Aguilar, G.A.; Sugich-Miranda, R. Antibiofilm properties of copper (II) and iron (III) complexes with an EDTA-based phenylene macrocycle and its acyclic analogue against food and clinical related pathogens. Polyhedron 2021, 198, 115076. [Google Scholar] [CrossRef]
- Bucur, C.; Badea, M.; Larisa, C.; Marinescu, D.; Grecu, M.N.; Stanica, N.; Chifiriuc, M.C.; Olar, R. Thermal behaviour of some new complexes with decaaza bismacrocyclic ligand as potential antimicrobial species. J. Therm. Anal. Calorim. 2012, 110, 235–241. [Google Scholar] [CrossRef]
- Bucur, C.; Korošec, R.C.; Badea, M.; Calu, L.; Chifiriuc, M.C.; Grecu, N.; Stănică, N.; Marinescu, D.; Olar, R. Investigation of thermal stability, spectral, magnetic, and antimicrobial behavior for new complexes of Ni(II), Cu(II), and Zn(II) with a bismacrocyclic ligand. J. Therm. Anal. Calorim. 2013, 113, 1287–1295. [Google Scholar] [CrossRef]
- Badea, M.; Bucur, C.; Chifiriuc, M.C.; Bleotu, C.; Grecu, M.-N.; Lazar, V.; Marinescu, D.; Olar, R. Insight on thermal behaviour of new complexes of Ni(II), Cu(II) and Zn(II) with a bismacrocyclic ligand developed as biologically active species. J. Therm. Anal. Calorim. 2016, 127, 487–497. [Google Scholar] [CrossRef]
- Bucur, C.; Badea, M.; Chifiriuc, M.C.; Bleotu, C.; Iorgulescu, E.E.; Badea, I.A.; Grecu, M.N.; Lazăr, V.; Patriciu, O.-I.; Marinescu, D.; et al. Studies on thermal, spectral, magnetic and biological properties of new Ni(II), Cu(II) and Zn(II) complexes with a bismacrocyclic ligand bearing an aromatic linker. J. Therm. Anal. Calorim. 2013, 115, 2179–2189. [Google Scholar] [CrossRef]
- Bano, N.; Rauf, M.A.; Owais, M.; Shakir, M. Pharmacologically bio-relevant N-functionalized homo-binuclear macrocyclic complexes: Synthesis, spectral studies, biological screening, HSA binding, and molecular docking. Inorg. Nano-Met. Chem. 2019, 49, 413–430. [Google Scholar] [CrossRef]
- Arouri, A.; Essghaier, B.; Dridi, R.; Zid, M.F. Crystal structure, spectral investigation, thermal properties and evaluation of the antimicrobial behavior of a new 2D polymeric Mn(II): (C6H9N2)2[Mn(C2O4)2]·H2O. J. Mol. Struct. 2021, 1244, 131251. [Google Scholar] [CrossRef]
- Essghaier, B.; Dridi, R.; Arouri, A.; Zid, M.F. Synthesis, structural characterization and prospects for a new tris (5-methylbenzimidazole) tris (oxalato) ferrate(III) trihydrate complex as a promising antibacterial and antifungal agent. Polyhedron 2021, 208, 115420. [Google Scholar] [CrossRef]
- Li, J.-Y.; Li, B.; Liu, X.-M. A New Co(II)-Based Metal–Organic Framework: Photocatalytic Dye Degradation and Treatment Activity Against Renal Failure Patients Combined with Staphylococcus aureus Biofilm Formation. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1836–1845. [Google Scholar] [CrossRef]
- Gholami, M.; Zeighami, H.; Bikas, R.; Heidari, A.; Rafiee, F.; Haghi, F. Inhibitory activity of metal-curcumin complexes on quorum sensing related virulence factors of Pseudomonas aeruginosa PAO1. AMB Express 2020, 10, 111. [Google Scholar] [CrossRef]
- Rubab, M.; Akhtar, M.N.; Zierkiewicz, W.; Michalczyk, M.; Nadeem, R.; Shahid, M.; Tahir, M.N.; Akram, M.; Hanif, M.A.; AlDamen, M.A. The role of hydrogen bonding in π···π stacking interactions in Ni(II) complex derived from triethanolamine: Synthesis, crystal structure, antimicrobial, and DFT studies. Res. Chem. Intermed. 2019, 45, 5649–5664. [Google Scholar] [CrossRef]
- Drzewiecka-Antonik, A.; Rejmak, P.; Klepka, M.T.; Wolska, A.; Pietrzyk, P.; Stępień, K.; Sanna, G.; Struga, M. Synthesis, structural studies and biological activity of novel Cu(II) complexes with thiourea derivatives of 4-azatricyclo[5.2.1.0 2,6 ]dec-8-ene-3,5-dione. J. Inorg. Biochem. 2017, 176, 8–16. [Google Scholar] [CrossRef]
- Bukonjić, A.M.; Tomović, D.L.; Nikolić, M.V.; Mijajlović, M.Ž.; Jevtić, V.V.; Ratković, Z.R.; Novaković, S.B.; Bogdanović, G.A.; Radojević, I.D.; Maksimović, J.Z.; et al. Antibacterial, antibiofilm and antioxidant screening of copper(II)-complexes with some S-alkyl derivatives of thiosalicylic acid. Crystal structure of the binuclear copper(II)-complex with S-propyl derivative of thiosalicylic acid. J. Mol. Struct. 2017, 1128, 330–337. [Google Scholar] [CrossRef]
- Bielenica, A.; Drzewiecka-Antonik, A.; Rejmak, P.; Stefańska, J.; Koliński, M.; Kmiecik, S.; Lesyngd, B.; Włodarczyk, M.; Pietrzyk, P.; Struga, M. Synthesis, structural and antimicrobial studies of type II topoisomerase-targeted copper(II) complexes of 1,3-disubstituted thiourea ligands. J. Inorg. Biochem. 2018, 182, 61–70. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Harrison, W.T.A.; Shahid, M.; Khan, I.-U.; Ejaz, I.-U.K.; Iqbal, J. Synthesis, crystal structure and biological activity of a cobalt(II) complex of N,N,N′,N′-tetrakis(2-hydroxypropyl) ethylenediamine. Trans. Met. Chem. 2016, 41, 325–330. [Google Scholar] [CrossRef]
- Nayak, M.; Kumar Singh, A.; Prakash, P.; Kant, R.; Bhattacharya, S. Strucutral studies on thiosalicylate complexes of Zn(II) & Hg(II). First insight into Zn(II)-thiosalicylate complex as potential antibacterial, antibiofilm and anti-tumour agent. Inorg. Chim. Acta 2020, 501, 119263. [Google Scholar] [CrossRef]
- Maurya, V.K.; Singh, A.K.; Singh, R.P.; Yadav, S.; Kumar, K.; Prakash, P.; Prasad, L.B. Synthesis and evaluation of Zn(II) dithiocarbamate complexes as potential antibacterial, antibiofilm, and antitumor agents. J. Coord. Chem. 2019, 72, 3338–3358. [Google Scholar] [CrossRef]
- Mihsen, H.H.; Shareef, N.K.; Alwazni, W.S. Synthesis, Characterization and Antibacterial Studies of Silver Complex of 3-Aminopropyltriethoxysilane. Asian J. Chem. 2018, 30, 1465–1468. [Google Scholar] [CrossRef]
- Klrissa, S.; Katouli, M. Pseudomonas aeruginosa: A review of their pathogenesis and prevalence in clinical settings and the environment. Infect. Epidemiol. Med. 2016, 2, 25–32. [Google Scholar] [CrossRef]
- Bahamondez-Canas, T.F.; Zhang, H.; Tewes, F.; Leal, J.; Smyth, H.D. PEGylation of tobramycin improves mucus penetration and antimicrobial activity against Pseudomonas aeruginosa biofilms in vitro. Mol. Pharm. 2018, 15, 1643–1652. [Google Scholar] [CrossRef]
- Badea, M.; Uivarosi, V.; Olar, R. Improvement in the Pharmacological Profile of Copper Biological Active Complexes by Their Incorporation into Organic or Inorganic Matrix. Molecules 2020, 25, 5830. [Google Scholar] [CrossRef]
- Bosch, P.; Staneva, D.; Vasileva-Tonkova, E.; Grozdanov, P.; Nikolova, I.; Kukeva, R.; Stoyanova, R.; Grabchev, I. New Poly(Propylene Imine) Dendrimer Modified with Acridine and Its Cu(II) Complex: Synthesis, Characterization and Antimicrobial Activity. Materials 2019, 12, 3020. [Google Scholar] [CrossRef] [Green Version]
- Grabchev, I.; Vasileva-Tonkova, E.; Staneva, D.; Bosch, P.; Kukeva, R.; Stoyanova, R. Synthesis, spectral characterization and in vitro antimicrobial activity in liquid medium and applied on cotton fabric of a new PAMAM metallodendrimer. Int. J. Polym. Anal. Char. 2018, 23, 45–57. [Google Scholar] [CrossRef]
- Grabcheva, I.; Vasileva-Tonkovab, E.; Stanevac, D.; Boschd, P.; Kukevae, R.; Stoyanova, R. Impact of the Cu(II) and Zn(II) ions on the functional properties of new PAMAM metallodendrimers. New J. Chem. 2018, 42, 7853–7862. [Google Scholar] [CrossRef]
- Llamazares, C.; Sanz del Olmo, N.; Ortega, P.; Gómez, R.; Soliveri, J.; Javier de la Mata, F.; García-Gallego, S.; Copa-Patiño, J.L. Antibacterial Effect of Carbosilane Metallodendrimers in Planktonic Cells of Gram-Positive and Gram-Negative Bacteria and Staphylococcus aureus Biofilm. Biomolecules 2019, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Wonoputri, V.; Gunawan, C.; Liu, S.; Barraud, N.; Yee, L.H.; Lim, M.; Amal, R. Copper Complex in Poly(vinyl chloride) as a Nitric Oxide-Generating Catalyst for the Control of Nitrifying Bacterial Biofilms. ACS Appl. Mater. Interfaces 2015, 7, 22148–22156. [Google Scholar] [CrossRef]
- Wonoputri, V.; Gunawan, C.; Liu, S.; Barraud, N.; Yee, L.H.; Lim, M.; Amal, R. Iron Complex Facilitated Copper Redox Cycling for Nitric Oxide Generation as Nontoxic Nitrifying Biofilm Inhibitor. ACS Appl. Mater. Interfaces 2016, 8, 30502–30510. [Google Scholar] [CrossRef] [Green Version]
- Saleh, S.; Sweileh, B.; Taha, S.O.; Mahmoud, R.; Taha, M.O. Preparation of Polyester-Based Metal-Cross Linked Polymeric Composites as Novel Materials Resistant to Bacterial Adhesion and Biofilm Formation. Molecules 2011, 16, 933–950. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Huang, Y.; Duan, Y.; Liu, Q.; Xu, Q.; Jia, J.; Wang, J.; Tong, Q.; Luo, P.; Wen, Y.; et al. Schiff-base silver nanocomplexes formation on natural biopolymer coated mesoporous silica contributed to the improved curative effect on infectious microbes. Nano Res. 2021, 14, 2735–2748. [Google Scholar] [CrossRef]
- Ma, H.; Darmawan, E.T.; Zhang, M.; Zhang, L.; Bryers, J.D. Development of a poly(ether urethane) system for the controlled release of two novel anti-biofilm agents based on gallium or zinc and its efficacy to prevent bacterial biofilm formation. J. Control. Release 2013, 172, 1035–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiros, J.; Boltes, K.; Aguado, S.; de Villoria, R.G.; Vilatela, J.J.; Rosal, R. Antimicrobial metal-organic frameworks incorporated into electrospun fibers. Chem. Eng. J. 2015, 262, 189–197. [Google Scholar] [CrossRef]
- Neufeld, B.H.; Neufeld, M.J.; Lutzke, A.; Schweickart, S.M.; Reynolds, M.M. Metal-organic framework material inhibits biofilm formation of Pseudomonas aeruginosa. Adv. Funct. Mater. 2017, 27, 1702255. [Google Scholar] [CrossRef]
- Duan, F.; Feng, X.; Jin, Y.; Liu, D.; Yang, X.; Zhou, G.; Liu, D.; Li, Z.; Liang, X.-J.; Zhang, J. Metal-carbenicillin framework-based nanoantibiotics with enhanced penetration and highly efficient inhibition of MRSA. Biomaterials 2017, 144, 155–165. [Google Scholar] [CrossRef] [PubMed]
| Complex | Metallic Ion | Microorganism/ Biofilm Assay Method | Mechanism | Ref. |
|---|---|---|---|---|
| Antibiotic Ligand | ||||
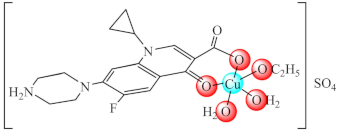 (1·EtOH) | Cu(II) | P. aeruginosa/crystal violet (CV) | QS inhibition (lasI and lasR genes modified expression) | [65,66] |
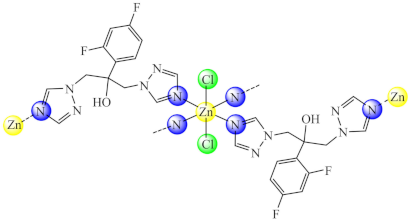 (2) | Cu(II), Zn(II) | C. albicans, P. aeruginosa/CV | ND | [67] |
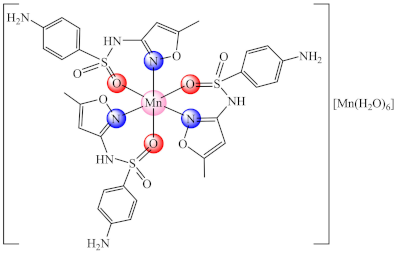 (3) | Mn(II) | S. aureus/CV | ND | [68] |
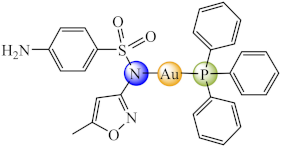 (4) | Au(I), Cu(II), Ag(I), Hg(II), Cd(II) | M. abscessus, M. fortuitum, M. massiliense/CV | ND | [70] |
| N-based Heterocycle Ligand | ||||
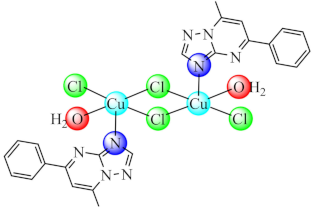 (5) | Co(II), Ni(II), Cu(II), Zn(II) | E. coli, K. pneumoniae, P. aeruginosa, S. aureus, B. subtilis/CV | ND | [74] |
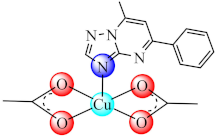 (6) | Co(II), Ni(II), Cu(II), Zn(II) | E. coli, K. pneumoniae, P. aeruginosa, S. aureus, B. subtilis, C. albicans/CV | ND | [75] |
 (7) | Co(II), Ni(II), Cu(II), Zn(II) | E. coli, K. pneumoniae, P. aeruginosa, S. aureus, B. subtilis, C. albicans/CV | ND | [76] |
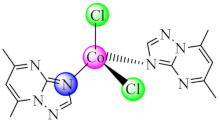 (8) | Co(II) | E. coli, K. pneumoniae, P. aeruginosa, S. aureus, B. subtilis, C. albicans/CV | ND | [77] |
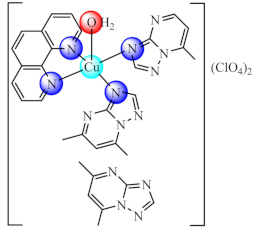 (10) | Cu(II) | E. coli, P. aeruginosa, S. aureus, MRSA/CV | ROS generation | [78] |
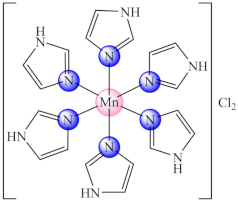 (11) | Mn(II) | E. coli/Fluorescence microscopy and XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide) | ND | [79] |
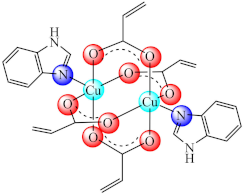 (12) | Cu(II) | E. coli, S. aureus, B. subtilis, E. faecium, C. albicans/CV | ND | [80] |
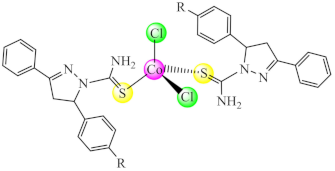 (14) R: Cl, Br | Co(II) | C. glabrata/XTT | ND | [82] |
 (15) | Cu(II) | P. aeruginosa/CV | QS inhibition | [83] |
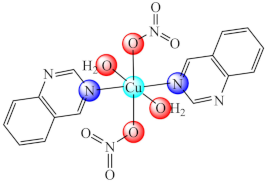 (16) | Cu(II) | P. aeruginosa/CV | QS inhibition | [83] |
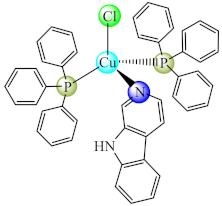 (17) | Cu(I) | P. aeruginosa, E. coli, C. violaceum, S. marcescens, K. pneumoniae, L. monocytogenes/CV | QS inhibition (violacein, elastase, pyocyanin, alginate, prodigiosin production inhibition) | [84] |
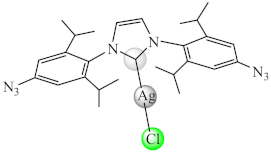 (18) | Cu(I), Ag(I) | L. monocytogenes, S. aureus, S. epidermidis, P. aeruginosa, E. coli/CV | ND | [85] |
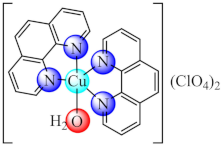 (20) | Cu(II) | S. aureus, E. coli, P. aeruginosa/CV | ND | [89] |
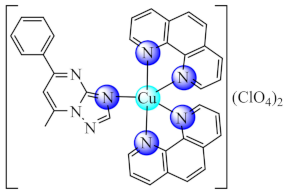 (22) | Cu(II) | S. aureus, E. coli, P. aeruginosa/CV | ROS generation | [91] |
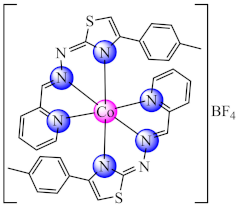 (29) | Co(III) | P. aeruginosa/CV alamar blue | QS inhibition (lasI and lasR inhibition) reduced level of pyocyanin and pyoverdine virulence factors | [96] |
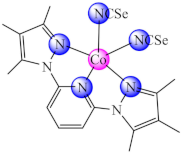 (30) | Co(II) | B. substilis, P. aeruginosa, S. typhimurium, S. sonnei, Y. enterocolitica/CV | swarming bacterial motility inhibition | [97] |
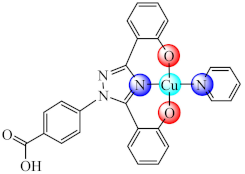 (31) | Cu(II) | S. aureus, P. aeruginosa/CV | ND | [98] |
| Schiff Base Ligands | ||||
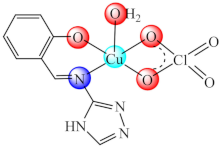 (32) | Co(II), Ni(II), Cu(II), Zn(II) | E. coli, K. pneumoniae, S. aureus, B. subtilis, C. albicans/CV | ND | [101] |
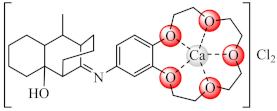 (34) | Ca(II), Cu(II) | S. aureus, B. subtilis, P. aeruginosa, E. coli, C. albicans/CV | ND | [104] |
 R: C2H5, n-C3H7, i-C3H7, C6H5 (36) | Cu(II) | S. aureus, B. subtilis, P. aeruginosa, E. coli, C. albicans/CV | ND | [105] |
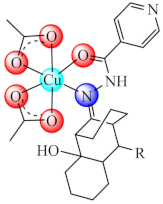 R: CH3, C2H5, n-C3H7, i-C3H7, (37) | Cu(II) | S. aureus, P. aeruginosa, E. coli, C. albicans/CV | ND | [106] |
 (40) | Cu(II) | P. aeruginosa, S. aureus/CV | ND | [108] |
| Biguanide Ligands | ||||
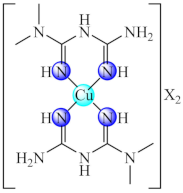 (41) X: CH3COO, ClO4 | Mn(II), Ni(II), Cu(II), Zn(II) | S. aureus, P. aeruginosa/CV | ND | [113,114] |
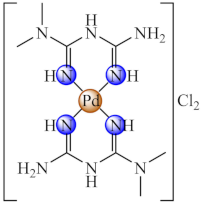 (43) | Pd(II) | S. aureus, B. subtilis, E. coli, C. albicans/CV | ND | [116] |
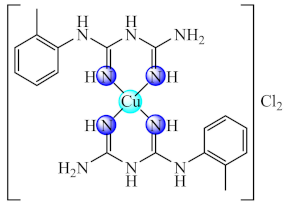 (44) | Cu(II) | S. aureus, P. aeruginosa/CV | ND | [117] |
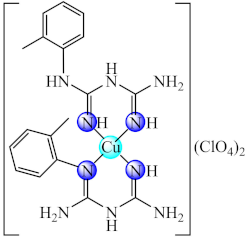 (45) | Cu(II) | K. pneumoniae, E. cloacae, B. subtilis, L. monocytogenes/CV | ROS generation | [118] |
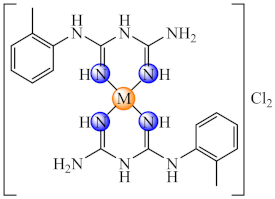 (46) | Ni(II), Pd(II), Pt(II) | S. aureus, B. subtilis, E. coli/CV | ND | [119] |
 (48) | Ir(III) | S. aureus/CV | ND | [122] |
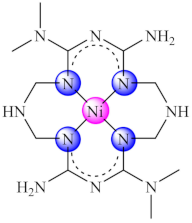 (49) | Ni(II), Cu(II) | P. aeruginosa, E. faecium, E. faecalis, C. albicans, C. parapsilosis/CV | ND | [123] |
| Macrocyclic Ligands | ||||
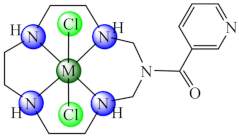 (50) | Ni(II), Cu(II), Zn(II) | P. aeruginosa, E. coli, K. pneumoniae, S. aureus, B. subtilis, C. albicans/CV | ND | [124] |
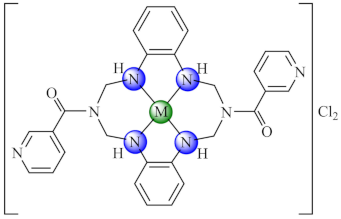 (51) | Ni(II), Cu(II), Zn(II) | P. aeruginosa, E. coli, K. pneumoniae, S. aureus, B. subtilis, C. albicans/CV | ND | [125] |
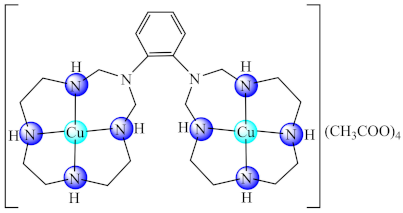 (53) | Ni(II), Cu(II), Zn(II) | S. aureus, B. subtilis, E. faecalis, K. pneumoniae, E. coli, E. cloacae, P. aeruginosa, C. krusei/CV | ND | [128] |
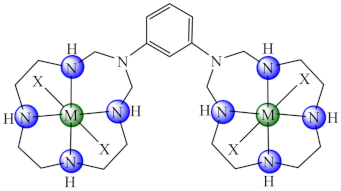 (54) X: Cl, (55) X: CH3COO | Ni(II), Cu(II), Zn(II) | B. subtilis, E. faecalis, S. aureus, S. epidermidis, E. cloacae, E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa, C. albicans, C. krusei/CV | ND | [129,130] |
 (56) | Ni(II), Cu(II), Zn(II) | B. subtilis, E. faecalis, S. aureus, S. epidermis, E. cloacae, E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa, C. albicans, C. krusei/CV | ND | [131] |
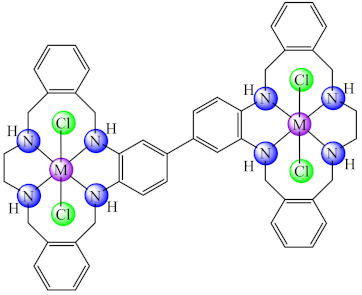 (57) | Co(II), Ni(II), Cu(II), Zn(II) | S. aureus/XTT | ND | [132] |
| Miscellaneous Ligands | ||||
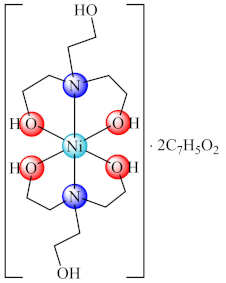 (61) | Ni(II) | E. coli, P. multocida, S. aureus/CV | ND | [137] |
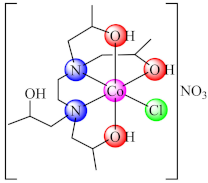 (64) | Co(II) | B. subtilis, E. coli/CV | ND | [141] |
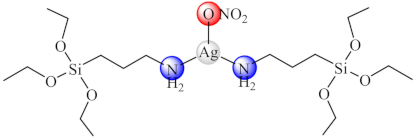 (68) | Ag(I) | P. mirablis/CV | ND | [144] |
| Carriers for Metal Ions or Complexes | ||||
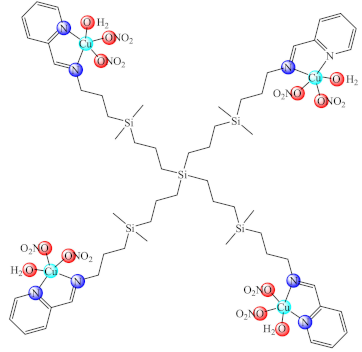 (69) | S. aureus, E. coli/CV | ND | [151] | |
 (70) | Cu(II) | Bacillus sp. | [152] | |
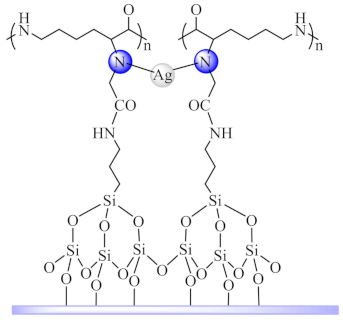 (72) | Ag(I) | E. coli, S. aureus, M. tuberculosis, C. albicans/XTT | NO generation | [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olar, R.; Badea, M.; Chifiriuc, M.C. Metal Complexes—A Promising Approach to Target Biofilm Associated Infections. Molecules 2022, 27, 758. https://doi.org/10.3390/molecules27030758
Olar R, Badea M, Chifiriuc MC. Metal Complexes—A Promising Approach to Target Biofilm Associated Infections. Molecules. 2022; 27(3):758. https://doi.org/10.3390/molecules27030758
Chicago/Turabian StyleOlar, Rodica, Mihaela Badea, and Mariana Carmen Chifiriuc. 2022. "Metal Complexes—A Promising Approach to Target Biofilm Associated Infections" Molecules 27, no. 3: 758. https://doi.org/10.3390/molecules27030758
APA StyleOlar, R., Badea, M., & Chifiriuc, M. C. (2022). Metal Complexes—A Promising Approach to Target Biofilm Associated Infections. Molecules, 27(3), 758. https://doi.org/10.3390/molecules27030758