Strengthening Anti-Glioblastoma Effect by Multi-Branched Dendrimers Design of a Scorpion Venom Tetrapeptide
Abstract
:1. Introduction
2. Results
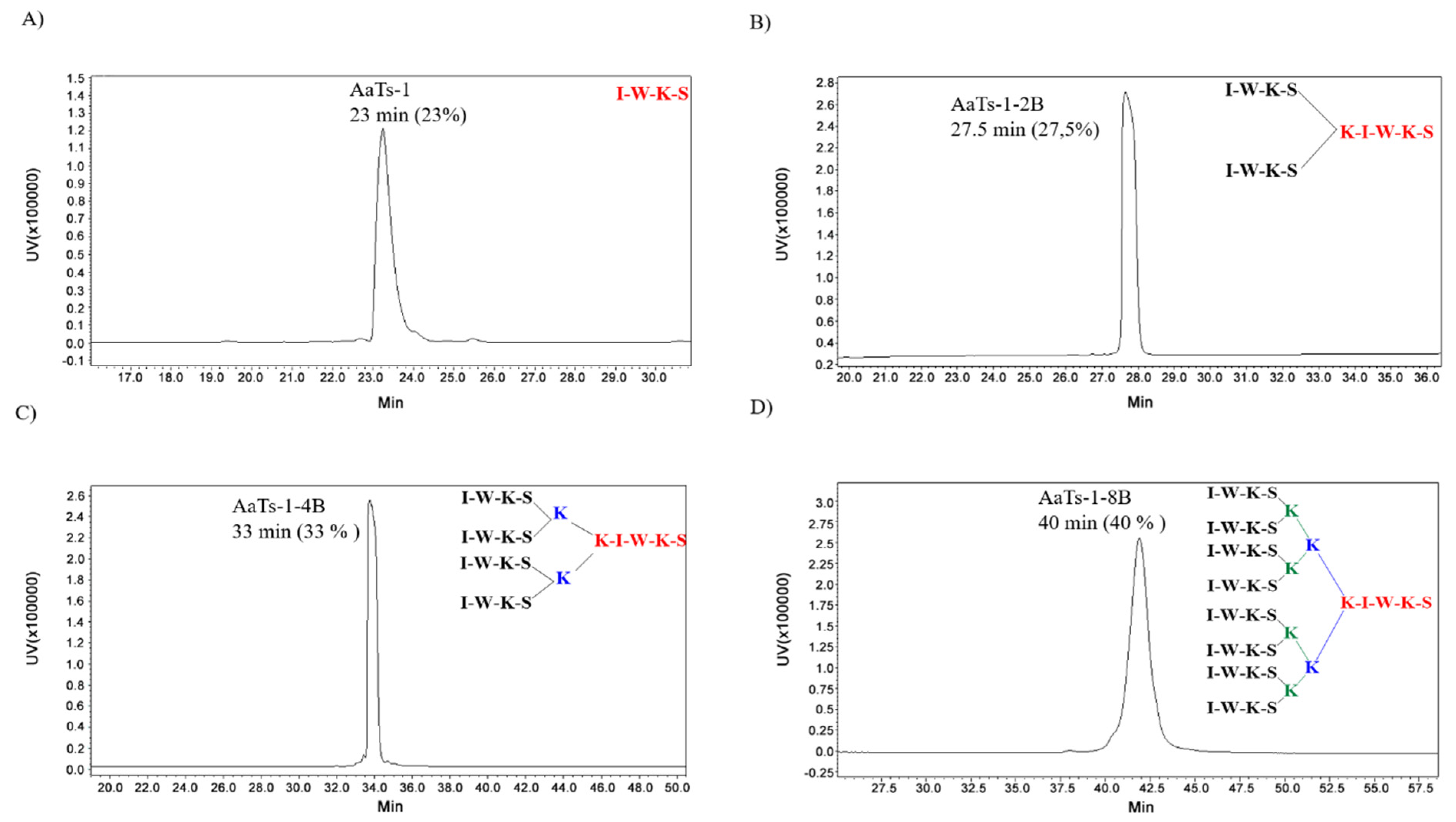
2.1. Synthesis of AaTs-1 and Dendritic Analogs
2.2. In Vivo Effect of AaTs-1 and Dendrimers
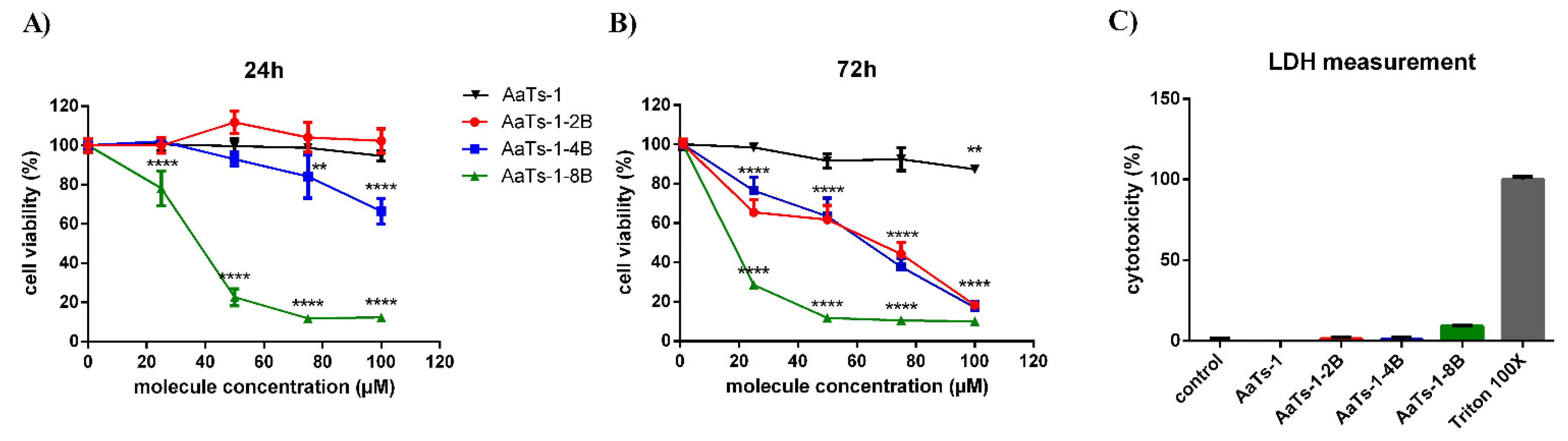
2.3. Effect of AaTs-1 and the Dendrimers on U87 Cells Viability
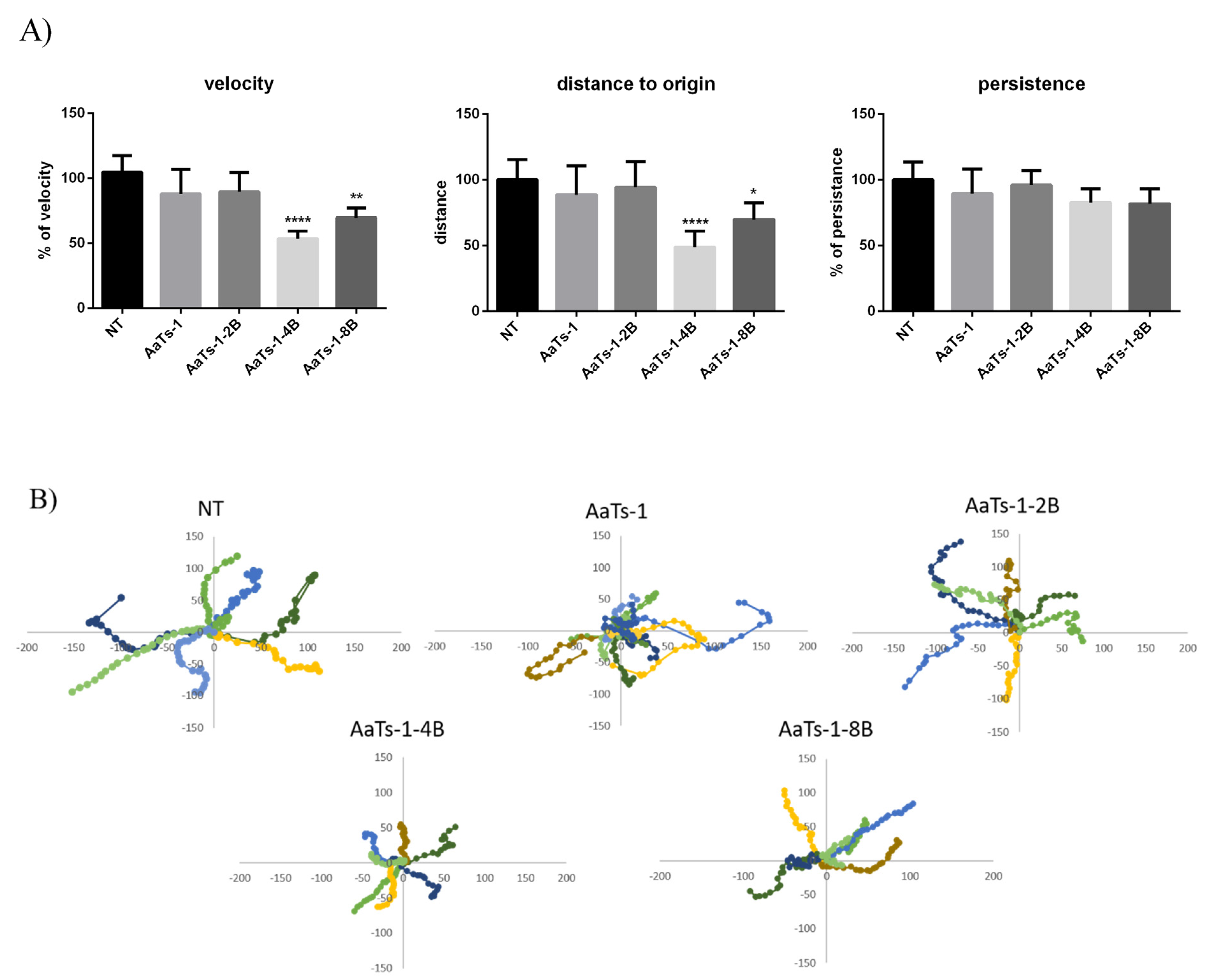
2.4. Videomicroscopy Migration Assay
2.5. Western Blot Analysis
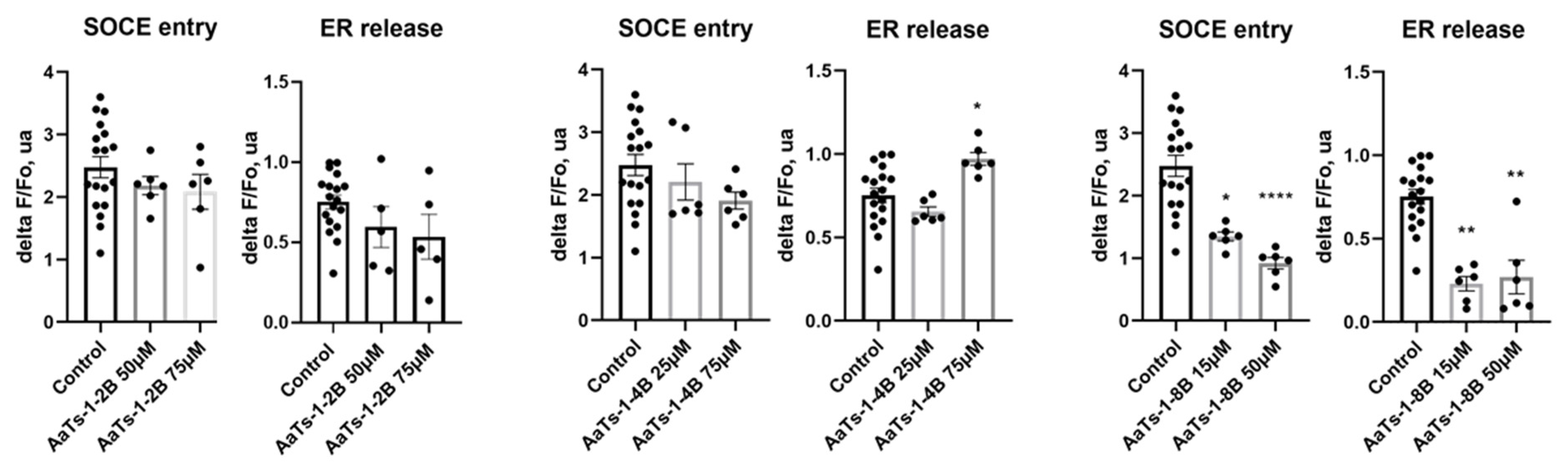
2.6. Effect of AaTs-1 and Dendrimers on Cytosolic Calcium Concentration
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Chemical Synthesis of AaTs-1 and MAPs
4.4. In Vivo Toxicity Assay
4.5. Cell Culture
4.6. Cell viability Assessment
4.7. Videomicroscopy Migration Assay
4.8. Cytosolic Ca2+ Measurements
4.9. Western Blot Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Alifieris, C.; Trafalis, D.T. Glioblastoma Multiforme: Pathogenesis and Treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Stoyanov, G.S.; Dzhenkov, D.; Ghenev, P.; Iliev, B.; Enchev, Y.; Tonchev, A.B. Cell Biology of Glioblastoma Multiforme: From Basic Science to Diagnosis and Treatment. Med. Oncol. Northwood Lond. Engl. 2018, 35, 27. [Google Scholar] [CrossRef]
- Velásquez, C.; Mansouri, S.; Mora, C.; Nassiri, F.; Suppiah, S.; Martino, J.; Zadeh, G.; Fernández-Luna, J.L. Molecular and Clinical Insights into the Invasive Capacity of Glioblastoma Cells. J. Oncol. 2019, 2019, 1740763. [Google Scholar] [CrossRef] [Green Version]
- Armento, A.; Ehlers, J.; Schötterl, S.; Naumann, U. Molecular Mechanisms of Glioma Cell Motility. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017; ISBN 978-0-9944381-2-6. [Google Scholar]
- Oberheim Bush, N.A.; Hervey-Jumper, S.L.; Berger, M.S. Management of Glioblastoma, Present and Future. World Neurosurg. 2019, 131, 328–338. [Google Scholar] [CrossRef]
- Batash, R.; Asna, N.; Schaffer, P.; Francis, N.; Schaffer, M. Glioblastoma Multiforme, Diagnosis and Treatment; Recent Literature Review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Franciolini, F. Role of KCa3.1 Channels in Modulating Ca2+ Oscillations during Glioblastoma Cell Migration and Invasion. Int. J. Mol. Sci. 2018, 19, 2970. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Bian, X.; Le, Y.; Gong, W.; Hu, J.; Zhang, X.; Wang, L.; Iribarren, P.; Salcedo, R.; Howard, O.M.Z.; et al. Formylpeptide Receptor FPR and the Rapid Growth of Malignant Human Gliomas. J. Natl. Cancer Inst. 2005, 97, 823–835. [Google Scholar] [CrossRef]
- Leclerc, C.; Haeich, J.; Aulestia, F.J.; Kilhoffer, M.-C.; Miller, A.L.; Néant, I.; Webb, S.E.; Schaeffer, E.; Junier, M.-P.; Chneiweiss, H.; et al. Calcium Signaling Orchestrates Glioblastoma Development: Facts and Conjunctures. Biochim. Biophys. Acta 2016, 1863, 1447–1459. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Dalavaikodihalli Nanjaiah, N.; Prasad, C.; Goswami, K. Transcriptional Modulation of Calcium-Permeable AMPA Receptor Subunits in Glioblastoma by MEK-ERK1/2 Inhibitors and Their Role in Invasion. Cell Biol. Int. 2020, 44, 830–837. [Google Scholar] [CrossRef]
- DeBin, J.A.; Maggio, J.E.; Strichartz, G.R. Purification and Characterization of Chlorotoxin, a Chloride Channel Ligand from the Venom of the Scorpion. Am. J. Physiol. 1993, 264, C361–C369. [Google Scholar] [CrossRef]
- McGonigle, S.; Majumder, U.; Kolber-Simonds, D.; Wu, J.; Hart, A.; Noland, T.; TenDyke, K.; Custar, D.; Li, D.; Du, H.; et al. Neuropilin-1 Drives Tumor-Specific Uptake of Chlorotoxin. Cell Commun. Signal. 2019, 17, 67. [Google Scholar] [CrossRef] [Green Version]
- Rjeibi, I.; Mabrouk, K.; Mosrati, H.; Berenguer, C.; Mejdoub, H.; Villard, C.; Laffitte, D.; Bertin, D.; Ouafik, L.; Luis, J.; et al. Purification, Synthesis and Characterization of AaCtx, the First Chlorotoxin-like Peptide from Androctonus Australis Scorpion Venom. Peptides 2011, 32, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui-Zid, D.; Saada, M.-C.; Moslah, W.; Potier-Cartereau, M.; Lemettre, A.; Othman, H.; Gaysinski, M.; Abdelkafi-Koubaa, Z.; Souid, S.; Marrakchi, N. AaTs-1: A Tetrapeptide from Androctonus australis Scorpion Venom, Inhibiting U87 Glioblastoma Cells Proliferation by p53 and FPRL-1 Up-Regulations. Molecules 2021, 26, 7610. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P. Synthetic Peptide Vaccine Design: Synthesis and Properties of a High-Density Multiple Antigenic Peptide System. Proc. Natl. Acad. Sci. USA 1988, 85, 5409–5413. [Google Scholar] [CrossRef] [Green Version]
- Mabrouk, K.; Van Rietschoten, J.; Rochat, H.; Loret, E.P. Correlation of Antiviral Activity with Beta-Turn Types for V3 Synthetic Multibranched Peptides from HIV-1 Gp120. Biochemistry 1995, 34, 8294–8298. [Google Scholar] [CrossRef]
- Yahi, N.; Fantini, J.; Baghdiguian, S.; Mabrouk, K.; Tamalet, C.; Rochat, H.; Van Rietschoten, J.; Sabatier, J.M. SPC3, a Synthetic Peptide Derived from the V3 Domain of Human Immunodeficiency Virus Type 1 (HIV-1) Gp120, Inhibits HIV-1 Entry into CD4+ and CD4- Cells by Two Distinct Mechanisms. Proc. Natl. Acad. Sci. USA 1995, 92, 4867–4871. [Google Scholar] [CrossRef] [Green Version]
- Michlewska, S.; Ionov, M.; Szwed, A.; Rogalska, A.; Sanz del Olmo, N.; Ortega, P.; Denel, M.; Jacenik, D.; Shcharbin, D.; de la Mata, F.J.; et al. Ruthenium Dendrimers against Human Lymphoblastic Leukemia 1301 Cells. Int. J. Mol. Sci. 2020, 21, 4119. [Google Scholar] [CrossRef]
- Marrakchi, N.; Mabrouk, K.; Regaya, I.; Sarray, S.; Fathallah, M.; Rochat, H.; El Ayeb, M. Lebetin Peptides: Potent Platelet Aggregation Inhibitors. Pathophysiol. Haemost. Thromb. 2001, 31, 207–210. [Google Scholar] [CrossRef]
- Clot-Faybesse, O.; Guieu, R.; Rochat, H.; Devaux, C. Toxicity during Early Development of the Mouse Nervous System of a Scorpion Neurotoxin Active on Sodium Channels. Life Sci. 1999, 66, 185–192. [Google Scholar] [CrossRef]
- Khurana, V.; P Patel, S.; Agrahari, V.; Pal, D.; K Mitra, A. Novel Pentablock Copolymer Based Nanoparticles Containing Pazopanib: A Potential Therapy for Ocular Neovascularization. Recent Pat. Nanomedicine 2014, 4, 57–68. [Google Scholar] [CrossRef]
- Krayem, N.; Abdelkefi-Koubaa, Z.; Gargouri, Y.; Luis, J. Integrin-Mediated Human Glioblastoma Cells Adhesion, Migration and Invasion by Native and Recombinant Phospholipases of Scorpio Maurus Venom Glands. Arch. Biochem. Biophys. 2018, 645, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Morjen, M.; Kallech-ziri, O.; Bazaa, A.; Othman, H.; Mabrouk, K.; Zouari-kessentini, R.; Sanz, L.; Calvete, J.J.; Srairi-Abid, N.; El Ayeb, M.; et al. PIVL, a New Serine Protease Inhibitor from Macrovipera Lebetina Transmediterranea Venom, Impairs Motility of Human Glioblastoma Cells. Matrix Biol. 2013, 32, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Spetzler, J.C. Multiple Antigen Peptide System. Methods Enzymol. 1997, 289, 612–637. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapra, R.; Verma, R.P.; Maurya, G.P.; Dhawan, S.; Babu, J.; Haridas, V. Designer Peptide and Protein Dendrimers: A Cross-Sectional Analysis. Chem. Rev. 2019, 119, 11391–11441. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Martinho, N.; Florindo, H.; Silva, L.; Brocchini, S.; Zloh, M.; Barata, T. Molecular Modeling to Study Dendrimers for Biomedical Applications. Review. Molecules 2014, 19, 20424–20467. [Google Scholar] [CrossRef] [Green Version]
- Evangelista-Lara, A.; Guadarrama, P. Theoretical evaluation of the nanocarrier properties of two families of functionalized dendrimers. Int. J. Quantum Chem. 2005, 103, 460–470. [Google Scholar] [CrossRef]
- Lacroix, M.; Riscal, R.; Arena, G.; Linares, L.K.; Le Cam, L. Metabolic Functions of the Tumor Suppressor P53: Implications in normal physiology, metabolic disorders, and cancer. Mol. Metab. 2020, 33, 2–22. [Google Scholar] [CrossRef]
- Dutra-Oliveira, A.; Monteiro, R.Q.; Mariano-Oliveira, A. Protease-Activated Receptor-2 (PAR2) Mediates VEGF Production through the ERK1/2 Pathway in Human Glioblastoma Cell Lines. Biochem. Biophys. Res. Commun. 2012, 421, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Liao, Q.; Su, M.; Huang, K.; Jin, J.; Cao, D. AKT and ERK Dual Inhibitors: The Way Forward? Cancer Lett. 2019, 459, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. PI3K/Akt/MTOR and Ras/Raf/MEK/ERK Signaling Pathways Inhibitors as Anticancer Agents: Structural and Pharmacological Perspectives. Eur. J. Med. Chem. 2016, 109, 314–341. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, V.; Park, Y.; Chen, C.-C.; Xu, P.-Z.; Chen, M.-L.; Tonic, I.; Unterman, T.; Hay, N. Akt Determines Replicative Senescence and Oxidative or Oncogenic Premature Senescence and Sensitizes Cells to Oxidative Apoptosis. Cancer Cell 2008, 14, 458–470. [Google Scholar] [CrossRef] [Green Version]
- Krishna-Subramanian, S.; Hanski, M.L.; Loddenkemper, C.; Choudhary, B.; Pagès, G.; Zeitz, M.; Hanski, C. UDCA Slows down Intestinal Cell Proliferation by Inducing High and Sustained ERK Phosphorylation. Int. J. Cancer 2012, 130, 2771–2782. [Google Scholar] [CrossRef]
- Cagnol, S.; Chambard, J.-C. ERK and Cell Death: Mechanisms of ERK-Induced Cell Death—Apoptosis, Autophagy and Senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef]
- Zhang, D.; Li, C.; Zhang, L.; Li, B.; Wang, Y.; Wang, R.; Chen, Z.; Xu, L.; Liu, T. Cannabisin D from Sinomenium Acutum Inhibits Proliferation and Migration of Glioblastoma Cells through MAPKs Signaling. Nutr. Cancer 2021, 73, 2491–2501. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Sforna, L.; Esposito, V.; Limatola, C.; Franciolini, F. Ion Channels in Glioma Malignancy. Rev. Physiol. Biochem. Pharmacol. 2021, 181, 223–267. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Jiang, D.; Zhang, D.; Qian, Z.; Liu, C.; Tao, J. Inhibition of T-Type Ca2+ Channels by Endostatin Attenuates Human Glioblastoma Cell Proliferation and Migration. Br. J. Pharmacol. 2012, 166, 1247–1260. [Google Scholar] [CrossRef] [Green Version]
- Kwan, D.H.T.; Kam, A.Y.F.; Wong, Y.H. Activation of the Human FPRL-1 Receptor Promotes Ca2+ Mobilization in U87 Astrocytoma Cells. Neurochem. Res. 2008, 33, 125–133. [Google Scholar] [CrossRef]
- Morrone, F.B.; Gehring, M.P.; Nicoletti, N.F. Calcium Channels and Associated Receptors in Malignant Brain Tumor Therapy. Mol. Pharmacol. 2016, 90, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fantini, J.; Yahi, N.; Mabrouk, K.; Van Rietschoten, J.; Rochat, H.; Sabatier, J.M. Multi-Branched Peptides Based on the HIV-1 V3 Loop Consensus Motif Inhibit HIV-1 and HIV-2 Infection in CD4+ and CD4− Cells. C. R. Acad. Sci. III 1993, 316, 1381–1387. [Google Scholar] [PubMed]
- Galeotti, N.; Bartolini, A.; Ghelardini, C. The Phospholipase C-IP3 Pathway Is Involved in Muscarinic Antinociception. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2003, 28, 888–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadok, A.; Bourgarel-Rey, V.; Gattacceca, F.; Penel, C.; Lehmann, M.; Kovacic, H. Nox1-Dependent Superoxide Production Controls Colon Adenocarcinoma Cell Migration. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2008, 1783, 23–33. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moslah, W.; Aissaoui-Zid, D.; Aboudou, S.; Abdelkafi-Koubaa, Z.; Potier-Cartereau, M.; Lemettre, A.; ELBini-Dhouib, I.; Marrakchi, N.; Gigmes, D.; Vandier, C.; et al. Strengthening Anti-Glioblastoma Effect by Multi-Branched Dendrimers Design of a Scorpion Venom Tetrapeptide. Molecules 2022, 27, 806. https://doi.org/10.3390/molecules27030806
Moslah W, Aissaoui-Zid D, Aboudou S, Abdelkafi-Koubaa Z, Potier-Cartereau M, Lemettre A, ELBini-Dhouib I, Marrakchi N, Gigmes D, Vandier C, et al. Strengthening Anti-Glioblastoma Effect by Multi-Branched Dendrimers Design of a Scorpion Venom Tetrapeptide. Molecules. 2022; 27(3):806. https://doi.org/10.3390/molecules27030806
Chicago/Turabian StyleMoslah, Wassim, Dorra Aissaoui-Zid, Soioulata Aboudou, Zaineb Abdelkafi-Koubaa, Marie Potier-Cartereau, Aude Lemettre, Ines ELBini-Dhouib, Naziha Marrakchi, Didier Gigmes, Christophe Vandier, and et al. 2022. "Strengthening Anti-Glioblastoma Effect by Multi-Branched Dendrimers Design of a Scorpion Venom Tetrapeptide" Molecules 27, no. 3: 806. https://doi.org/10.3390/molecules27030806





