Migration Testing of GPPS and HIPS Polymers: Swelling Effect Caused by Food Simulants Compared to Real Foods
Abstract
:1. Introduction
2. Results
2.1. Spiking Levels of Model Substances, Residual Content of Styrene Monomer and Styrene Oligomers
2.2. Migration, Weight Increase and Visual Changes of GPPS and HIPS with Food Simulants
2.2.1. Migration Kinetics and Weight Increase Using the Food Simulant Isooctane
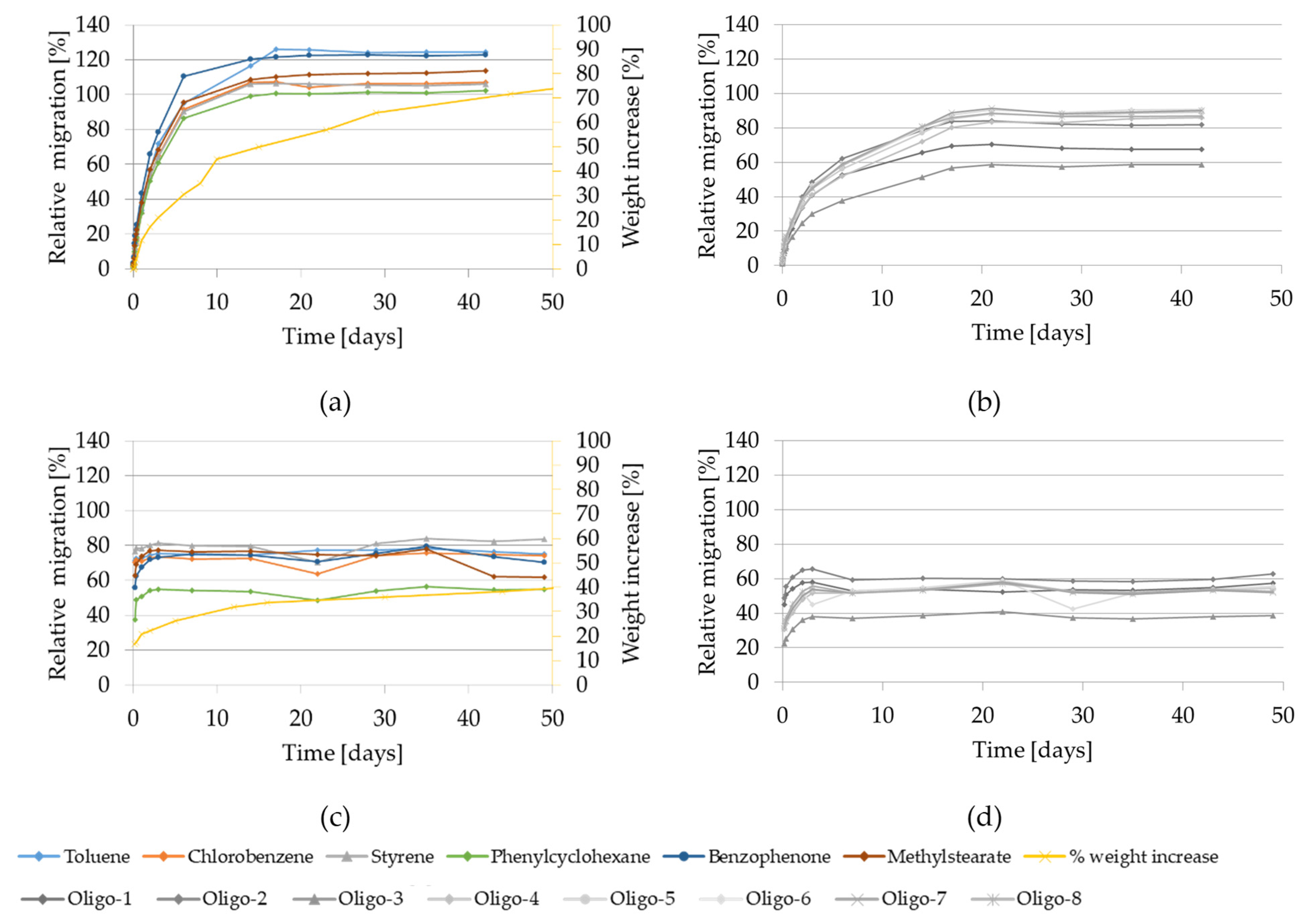
2.2.2. Migration Kinetics and Weight Increase Using the Food Simulant 95% Ethanol
2.2.3. Migration Kinetics and Weight Increase Using the Food Simulant 50% Ethanol
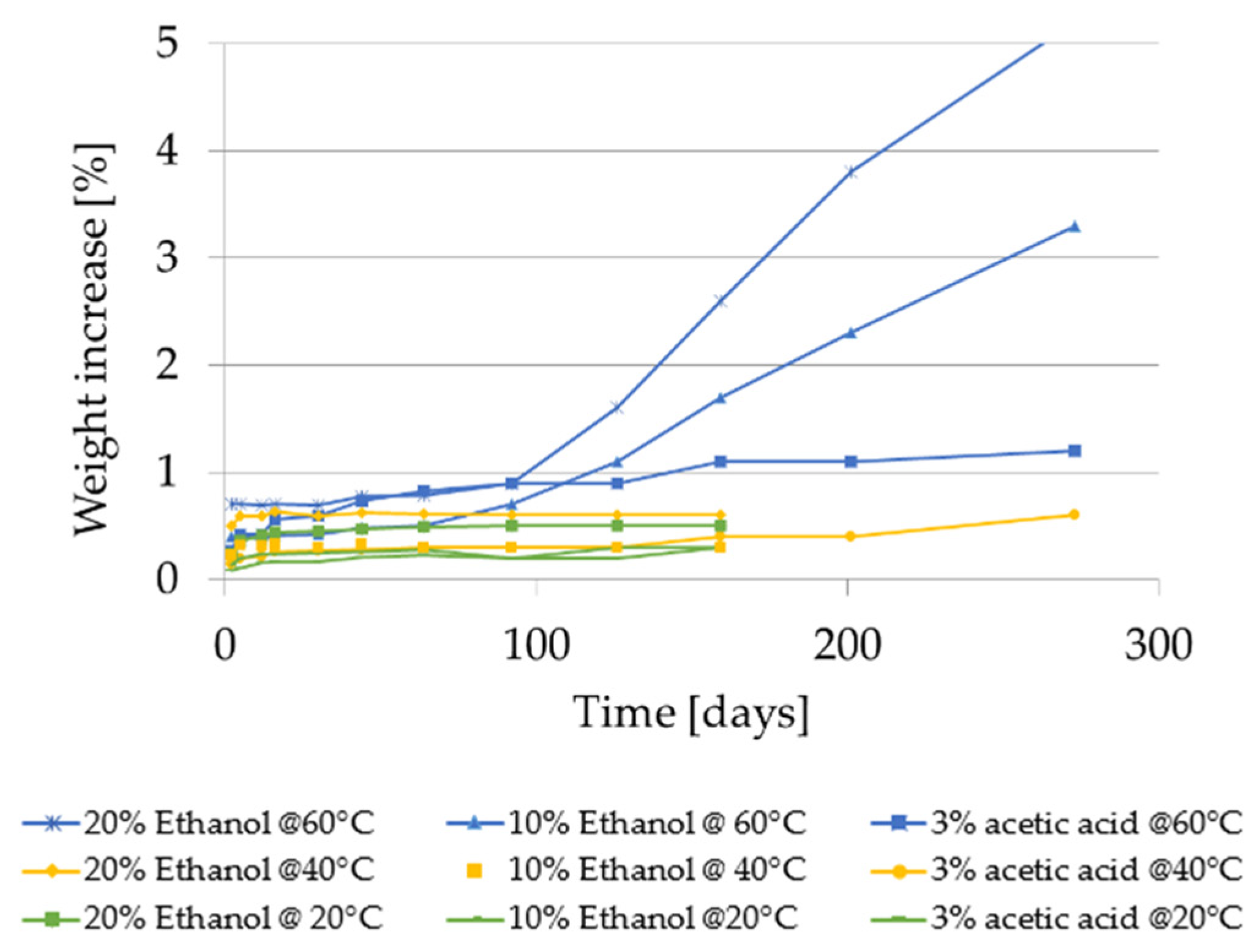
2.2.4. Weight Increase Using the Food Simulants 20% Ethanol, 10% Ethanol and 3% Acetic Acid
2.3. Migration and Weight Increase of GPPS and HIPS in Contact with Real Food
2.3.1. Migration and Weight Increase Using Milk, Cream and Olive Oil
2.3.2. Weight Increase of the Polymers after Contact with Several Foods under Real Application of Use
3. Discussion
4. Materials and Methods
4.1. Sample Materials
4.2. Determination of the Initial Concentration (Cp,0) of Spiked Substances, Styrene Monomer and Styrene Oligomers
4.3. Migration Kinetics in Food Simulants
4.4. Migration of Volatile Substances in Real Foods
4.5. Weight Increase with Food Simulants and Real Foods
4.6. Testing on Visual Changes of the Polymers after Contact with Food Simulants and Real Foods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Block, C.; Brands, B.; Gude, T. Packaging Materials 2. Polystyrene for Food Packaging Applications—Updated Version; International Life Sciences Institute: Brussels, Belgium, 2017; ISBN 9789078637448. [Google Scholar]
- Plastics Europe. Plastics—The Facts 2020. An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.plasticseurope.org/en/resources/publications/4312-plastics-facts-2020 (accessed on 15 October 2021).
- Baner, A.L.; Franz, R.; Piringer, O.G. Alternative fatty food simulants for polymer migration testing. Experimental confirmation. J. Polym. Eng. 1995, 15, 161–180. [Google Scholar] [CrossRef]
- Kirchkeszner, C.; Petrovics, N.; Tábi, T.; Magyar, N.; Kovács, J.; Szabó, B.S.; Nyiri, Z.; Eke, Z. Swelling as a promoter of migration of plastic additives in the interaction of fatty food simulants with polylactic acid- and polypropylene-based plastics. Food Control 2022, 132, 108354. [Google Scholar] [CrossRef]
- Stoffers, N.H.; Dekker, M.; Linssen, J.P.H.; Störmer, A.; Franz, R. Alternative fatty food simulants and diffusion kinetics of nylon 12 food packaging. Food Addit. Contam. 2003, 20, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.; Goodson, A.; O’Brien, A. Specific migration testing with alternative fatty food simulants. Food Addit. Contam. 1998, 15, 72–78. [Google Scholar] [CrossRef]
- Guazzotti, V.; Ebert, A.; Gruner, A.; Welle, F. Migration from acrylonitrile butadiene styrene (ABS) polymer: Swelling effect of food simulants compared to real foods. J. Consum. Prot. Food Saf. 2021, 16, 19–33. [Google Scholar] [CrossRef]
- Hoekstra, E.J. Technical Guidelines for Compliance Testing in the Framework of the Plastic FCM Regulation (EU) No 10/2011; Draft for Stakeholder Consultation; Version 24/08/2016; Joint Research Center: Luxembourg, 2016. [Google Scholar]
- Franz, R.; Welle, F. Migration measurement and modelling from poly(ethylene terephthalate) (PET) into soft drinks and fruit juices in comparison with food simulants. Food Addit. Contam. 2008, 25, 1033–1046. [Google Scholar] [CrossRef] [Green Version]
- Bradley, E.L.; Castle, L.; Day, J.S.; Ebner, I.; Ehlert, K.; Helling, R.; Koster, S.; Leak, J.; Pfaff, K. Comparison of the migration of melamine from melamineformaldehyde plastics (‘melaware’) into various food simulants and foods them-selves. Food Add. Contam. Part A 2010, 27, 1755–1764. [Google Scholar] [CrossRef]
- Driffield, M.; Garcia-Lopez, M.; Christy, J.; Lloyd, A.S.; Tarbin, J.A.; Hough, P.; Bradley, E.L.; Oldring, P.K.T. The determination of monomers and oligomers from polyester-based can coatings into foodstuffs over extended storage periods. Food Addit. Contam. Part A 2018, 35, 1200–1213. [Google Scholar] [CrossRef]
- Urbelis, J.H.; Cooper, J.R. Migration of food contact substances into dry foods: A review. Food Addit. Contam. Part A 2021, 38, 1044–1073. [Google Scholar] [CrossRef]
- IARC. Monograph Styrene, styrene-7,8-oxide, and quinoline. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC (International Agency for Research on Cancer): Lyon, France, 2019; Volume 121, pp. 83–341. [Google Scholar]
- EFSA. Scientific Opinion on the assessment of the impact of the IARC Monograph Vol. 121 on the safety of the substance styrene (FCM No 193) for its use in plastic food contact materials. Panel on Food Contact Materials, Enzymes and Processing Aids (CEP). EFSA J. 2020, 18, 6247. [Google Scholar]
- Withey, J.R.; Collins, P.G. Styrene monomer in foods: A limited Canadian survey. Bull. Environ. Contam. Toxicol. 1978, 19, 86–94. [Google Scholar] [CrossRef]
- Gilbert, J.; Startin, J.R. A survey of styrene monomer levels in foods and plastic packaging by coupled mass spectrometry automatic headspace gas chromatography. J. Sci. Food Agric. 1983, 34, 647–652. [Google Scholar] [CrossRef]
- MAFF. Survey of Styrene Levels in Food Contact Materials and in Foods; The Eleventh Report of the Steering Group on Food Surveillance; Food Surveillance Paper No. 1983-11; Ministry of Agriculture, Fisheries and Food: London, UK, 1983. [Google Scholar]
- Lickly, T.D.; Lehr, K.M.; Welsh, G.C. Migration of styrene from poly(styrene) foam food-contact articles. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1995, 33, 475–481. [Google Scholar] [CrossRef]
- Murphy, P.G.; MacDonald, D.A.; Lickly, T.D. Styrene migration from general- purpose and high-impact polystyrene into food-simulating solvents. Food. Chem. Toxicol. 1992, 30, 225–232. [Google Scholar] [CrossRef]
- Tawfik, M.S.; Huyghebaert, A. Polystyrene cups and containers: Styrene migration. Food Addit. Contam. 1998, 15, 592–599. [Google Scholar] [CrossRef]
- Khaksar, M.R.; Ghazi-Khansari, M. Determination of migration monomer styrene from GPPS (general purpose polystyrene) and HIPS (high impact polystyrene) cups to hot drinks. Toxicol. Mech. Methods 2009, 19, 257–261. [Google Scholar] [CrossRef]
- El-Ziney, M.G.; Tawfik, M.S. Migration levels of monostyrene from polystyrene containers to dairy products. MOJ Food Process. Technol. 2016, 3, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Naziruddin, M.A.; Sulaiman, R.; Abdul Halim Lim, S.; Jinap, S.; Nurulhuda, K.; Sanny, M. The effect of fat contents and conditions of contact in actual use on styrene monomer migrated from general-purpose polystyrene into selected fatty dishes and beverage. Food Packag. Shelf Life 2020, 23, 100461. [Google Scholar] [CrossRef]
- Genualdi, S.; Nyman, P.; Begley, T. Updated evaluation of the migration of styrene monomer and oligomers from polystyrene food contact materials to foods and food simulants. Food Addit. Contam. 2014, 4, 723–733. [Google Scholar] [CrossRef]
- O’Neill, E.T.; Tuohy, J.J.; Franz, R. Comparison of milk and ethanol/water mixtures with respect to monostyrene migration from a polystyrene packaging material. Int. Dairy J. 1994, 4, 271–283. [Google Scholar] [CrossRef]
- Till, D.E.; Ehntholt, D.J.; Reid, R.C.; Schwartz, P.S.; Sidman, K.R.; Schwope, A.D.; Whelan, R.H. Migration of styrene monomer from crystal polystyrene to foods and food simulating liquids. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 161–168. [Google Scholar] [CrossRef]
- Choi, J.O.; Jitsunari, F.; Asakawa, F.; Sun Lee, D. Migration of styrene monomer, dimers and trimers from polystyrene to food simulants. Food Addit. Contam. 2007, 22, 693–699. [Google Scholar] [CrossRef]
- Amirshaghaghi, Z.; Emam Djomeh, Z.; Oromiehie, A. Studies of Migration of Styrene Monomer from Polystyrene Packaging into the Food Simulant. Iran. J. Chem. Eng. 2011, 8, 43–49. [Google Scholar]
- Dawson, P.; Seydim, A.C.; Hirt, D. Styrene Monomer Migration from Expanded Polystyrene into Food Acids and Ethanol. J. Food Res. 2020, 9, 39–52. [Google Scholar] [CrossRef]
- Aja, A.; J’Bari, S.; Ononogbo, A.; Buonocore, F.; Bear, J.C.; Mayes, A.G.; Morgan, H. An Insight into the Growing Concerns of Styrene Monomer and Poly(Styrene) Fragment Migration into Food and Drink Simulants from Poly(Styrene) Packaging. Foods 2021, 10, 1136. [Google Scholar] [CrossRef]
- Welle, F. Diffusion Coefficients and Activation Energies of Diffusion of Organic Molecules in Polystyrene below and above Glass Transition Temperature. Polymers 2021, 13, 1317. [Google Scholar] [CrossRef]
- Kawamura, Y.; Nishi, K.; Sasaki, H.; Yamada, T. Determination Method of Styrene Dimers and Trimers in Instant Noodles Contained in Polystyrene Cups. J. Food Hyg. Soc. Jpn. 1998, 39, 310–314. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Hemm, I.; Eisenbrand, G. Estimation of human exposure to styrene and ethylbenzene. Toxicology 2000, 144, 39–50. [Google Scholar] [CrossRef]
- Nakada, Y.; Sakai, M.; Hinata, M.; Suzuki, T. Determination of the migration amount and total amount of styrene monomer, dimers and trimers in polystyrene food containers by GC/MS. Bunseki Kagaku 2000, 49, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Ewender, J.; Welle, F. Determination of the activation energies of diffusion of organic molecules in poly(ethylene terephthalate). J. Appl. Polym. Sci. 2012, 128, 3885–3892. [Google Scholar] [CrossRef]
- Welle, F. Activation energies of diffusion of organic migrants in cyclo olefin polymer. Int. J. Pharm. 2014, 473, 510–517. [Google Scholar] [CrossRef]
- Lajarrge, A.; Gontard, N.; Gaucel, S.; Peyron, S. Evaluation of the Food Contact Suitability of Aged Bio-Nanocomposite Materials Dedicated to Food Packaging Applications. Appl. Sci. 2020, 10, 877. [Google Scholar] [CrossRef] [Green Version]
- ILSI. Recycling of Plastics for Food Contact Use, Guidelines Prepared under the Responsibility of the International Life Sciences Institute (ILSI); ILSI European Packaging Task Force: Brussels, Belgium, 1998. [Google Scholar]
- Bayer, F.; Franz, R.; Welle, F. Guidance and Criteria for Safe Recycling of Post Consumer Polyethylene Terephthalate (PET) into New Food Packaging Applications; EU Report 21155; Publication office of the European Union: Luxembourg, 2004; ISBN 92-894-6776-2. [Google Scholar]
- FDA. Points to Consider for the Use of Recycled Plastics in Food Packaging: Chemistry Considerations; US Food and Drug Administration, Center for Food Safety and Applied Nutrition: Rockville, USA, 2021. [Google Scholar]
- Schwartz, P. Regulatory requirements for new packaging materials and processing technologies. Food Tech. 1985, 39, 61–63. [Google Scholar]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: New York, USA, 1975; ISBN 0-19-853411-6. [Google Scholar]
- Snyder, R.C.; Breder, C.V. New FDA migration cell used to study migration of styrene from polystyrene into various solvents. J. AOAC 1985, 68, 770–775. [Google Scholar] [CrossRef]
- Hopfenberg, H.B.; Frisch, H.L. Transport of organic micromolecules in amorphous polymers. J. Polym. Sci. Part B Polym. Lett. 1969, 7, 405–409. [Google Scholar] [CrossRef]
- Yoshida, R.; Watanabe, Y.; Sato, K.; Endo, F.; Yoshida, H. Migration behavior of acrylonitrile monomer from SAN food container. Annu. Rep. Tokyo Metrop. Res. Lab. Public Health 1983, 33, 238–241. [Google Scholar]
- Gehring, C.; Welle, F. Migration testing of polyethylene terephthalate: Comparison of regulated test conditions with migration into real food at the end of shelf life. Packag. Technol. Sci. 2018, 12, 755–790. [Google Scholar] [CrossRef]
- Linssen, J.P.H.; Reitsma, J.C.E. Comparison of migration of styrene monomer from high impact polystyrene in oil in water emulsions and fatty foods. J. Polym. Eng. 1995, 15, 133–138. [Google Scholar] [CrossRef]









| Average Concentration ± SD [mg/kg] | ||
|---|---|---|
| Substance | GPPS | HIPS |
| Artificially added “spiked” substances | ||
| Toluene | 417 ± 5 | 763 ± 11 |
| Chlorobenzene | 504 ± 5 | 821 ± 13 |
| Phenyl cyclohexane | 705 ± 8 | 1229 ± 33 |
| Benzophenone | 610 ± 5 | 1002 ± 27 |
| Methyl stearate | 718 ± 7 | 1179 ± 34 |
| Styrene | 352 ± 3 | 363 ± 6 |
| Styrene dimers (sum) 1: | 730 | 1743 |
| 1,3-Diphenylpropane: Oligo-1 | 180 ± 6 | 134 ± 4 |
| 2,4-Di-phenyl-1-butene: Oligo-2 | 550 ± 7 | 1609 ± 35 |
| Styrene trimers (sum) 1: | 5807 | 30290 |
| 2,4,6-Triphenyl-1-hexen: Oligo-3 | 1389 ± 23 | 4323 ± 152 |
| isomer of 1-Phenyl-4-(1-phenylethyl)-tetraline: Oligo-4 | 1092 ± 20 | 6740 ± 230 |
| isomer of 1-Phenyl-4-(1-phenylethyl)-tetraline: Oligo-5 | 2324 ± 44 | 13440 ± 455 |
| isomer of 1-Phenyl-4-(1-phenylethyl)-tetraline: Oligo-6 | 657 ± 14 | 3684 ± 146 |
| isomer of 1-Phenyl-4-(1-phenylethyl)-tetraline: Oligo-7 | 260 ± 5 | 1624 ± 60 |
| isomer of 1-Phenyl-4-(1-phenylethyl)-tetraline: Oligo-8 | 85 ± 2 | 479 ± 18 |
| Time/Temperature | Relative Migration [%] into Milk (3.5% Fat) | Migration of Styrene [µg/kg] | Weight Increase [%] | ||
|---|---|---|---|---|---|
| Toluene | Chlorobenzene | Styrene | |||
| 15 d/20 °C | 0.4 ± 0.2 | 1.3 ± 0.2 | 0.7 ± 0.1 | 32 ± 5 | <0.1 |
| 20 d/20 °C | 0.5 ± 0.3 | 1.3 ± 0.3 | 0.7 ± 0.3 | 32 ± 12 | <0.1 |
| 30 d/20 °C | 0.6 ± 0.1 | 1.4 ± 0.2 | 0.8 ± 0.1 | 37 ± 6 | <0.1 |
| 40 d/20 °C | 0.7 ± 0.2 | 1.6 ± 0.3 | 0.9 ± 0.2 | 40 ± 8 | <0.1 |
| 60 d/20 °C | 0.8 ± 0.2 | 1.6 ± 0.2 | 1.0 ± 0.2 | 43 ± 9 | <0.1 |
| 10 d/40 °C | 2.8 ± 0.4 | 3.2 ± 0.2 | 2.0 ± 0.1 | 92 ± 3 | 0.1 |
| Relative Migration [%] into Cream (30% Fat) | Migration of Styrene [µg/kg] | Weight Increase [%] 1 | |||
| Toluene | Chlorobenzene | Styrene | |||
| 15 d/20 °C | 4.0 ± 0.2 | 3.8 ± 0.2 | 2.8 ± 0.1 | 120 ± 4 | <0.1 |
| 20 d/20 °C | 4.2 ± 0.4 | 4.1 ± 0.4 | 2.9 ± 0.2 | 124 ± 9 | <0.1 |
| 30 d/20 °C | 5.2 ± 0.6 | 5.5 ± 0.4 | 3.7 ± 0.2 | 158 ± 8 | <0.1 |
| 40 d/20 °C | 5.5 ± 0.4 | 5.5 ± 0.3 | 3.5 ± 0.4 | 154 ± 19 | <0.1 |
| 60 d/20 °C | 6.3 ± 0.2 | 6.3 ± 0.3 | 4.1 ± 0.1 | 176 ± 3 | <0.1 |
| 10 d/40 °C | 6.7 ± 0.5 | 7.1 ± 0.6 | 5.1 ± 0.4 | 220 ± 19 | <0.1 |
| Relative Migration [%] into Olive Oil | Migration of Styrene [µg/kg] | Weight Increase [%] 1 | |||
| Toluene | Chlorobenzene | Styrene | |||
| 15 d/20 °C | 2.9 ± 0.2 | 2.4 ± 0.1 | 0.9 ± 0.3 | 55 ± 14 | 0.1 |
| 20 d/20 °C | 3.2 ± 0.1 | 3.1 ± 0.2 | 1.0 ± 0.1 | 55 ± 5 | 0.2 |
| 30 d/20 °C | 3.7 ± 0.2 | 3.6 ± 0.5 | 1.4 ± 0.3 | 63 ± 13 | 0.3 |
| 40 d/20 °C | 4.5 ± 0.3 | 4.3 ± 0.4 | 2.2 ± 0.2 | 97 ± 9 | 0.3 |
| 60 d/20 °C | 5.0 ± 0.2 | 5.0 ± 0.2 | 2.4 ± 03 | 109 ± 15 | 0.2 |
| 10 d/40 °C | 7.0 ± 0.8 | 7.1 ± 0.7 | 4.8 ± 0.5 | 211 ± 25 | 0.2 |
| Time/Temperature | Relative Migration [%] into Milk (3.5% Fat) | Migration of Styrene [µg/kg] | Weight Increase [%] | ||
|---|---|---|---|---|---|
| Toluene | Chlorobenzene | Styrene | |||
| 15 d/20 °C | 1.7 ± 0.3 | 2.8 ± 0.2 | 2.2 ± 0.2 | 91 ± 7 | 0.1 |
| 20 d/20 °C | 1.6 ± 0.1 | 2.5 ± 0.1 | 1.8 ± 0.1 | 79 ± 6 | 0.2 |
| 30 d/20 °C | 2.5 ± 0.5 | 3.7 ± 0.6 | 2.8 ± 0.5 | 118 ± 16 | 0.1 |
| 40 d/20 °C | 2.5 ± 0.4 | 3.7 ± 0.4 | 2.6 ± 0.3 | 111 ± 14 | 0.2 |
| 60 d/20 °C | 3.3 ± 0.1 | 4.8 ± 0.1 | 3.4 ± 0.1 | 145 ± 5 | 0.1 |
| 10 d/0 °C | 3.4 ± 0.2 | 4.6 ± 0.3 | 3.6 ± 0.3 | 152 ± 13 | 0.1 |
| Relative Migration [%] into Cream (30% Fat) | Migration of Styrene [µg/kg] | Weight Increase [%] | |||
| Toluene | Chlorobenzene | Styrene | |||
| 15 d/20 °C | 3.3 ± 0.2 | 3.6 ± 0.2 | 3.6 ± 0.4 | 149 ± 16 | 0.5 |
| 20 d/20 °C | 5.8 ± 1.1 | 5.9 ± 0.8 | 5.2 ± 0.7 | 221 ± 32 | 0.4 |
| 30 d/20 °C | 4.5 ± 0.3 | 4.8 ± 0.1 | 4.4 ± 0.3 | 185 ± 16 | 0.4 |
| 40 d/20 °C | 5.8 ± 0.8 | 6.5 ± 0.7 | 5.5 ± 0.5 | 233 ± 20 | 0.6 |
| 60 d/20 °C | 6.2 ± 0.4 | 7.2 ± 0.5 | 5.8 ± 0.4 | 241 ± 20 | 0.6 |
| 10 d/40 °C | 7.0 ± 0.6 | 8.0 ± 0.2 | 6.6 ± 0.3 | 278 ± 10 | 0.4 |
| Relative Migration [%] into Olive Oil | Migration of Styrene [µg/kg] | Weight Increase [%] | |||
| Toluene | Chlorobenzene | Styrene | |||
| 15 d/20 °C | 3.1 ± 0.2 | 3.3 ± 0.3 | 2.7 ± 0.4 | 115 ± 18 | 0.3 |
| 20 d/20 °C | 3.4 ± 0.3 | 3.7 ± 0.4 | 2.9 ± 0.3 | 122 ± 14 | 0.3 |
| 30 d/20 °C | 4.1 ± 0.3 | 4.3 ± 0.5 | 3.2 ± 0.2 | 137 ± 9 | 0.2 |
| 40 d/20 °C | 4.1 ± 0.1 | 4.4 ± 0.2 | 3.3 ± 0.2 | 139 ± 5 | 0.5 |
| 60 d/20 °C | 5.9 ± 1.1 | 6.5 ± 0.9 | 5.0 ± 1.0 | 213 ± 41 | 2.3 |
| 10 d/40 °C | 5.6 ± 0.4 | 6.9 ± 0.5 | 5.3 ± 0.6 | 222 ± 25 | 0.3 |
| Food/Storage Temperature | Weight Increase [%] | ||||||
|---|---|---|---|---|---|---|---|
| GPPS | HIPS | ||||||
| 10 Days | 20 Days | 30 Days | 50 Days | 10 Days | 20 Days | 30 Days | |
| Lard (100% fat)/5 °C | <0.1 | 0.1 | 0.1 | 0.2 | 0.5 | 0.5 | 0.7 |
| Butter/5 °C | <0.1 | 0.2 | 1.1 | 1.2 | 0.5 | 0.6 | 0.7 |
| Fish oil 1/20 °C | <0.1 | 0.2 | <0.1 | <0.1 | 0.5 | 0.5 | 0.9 |
| Miglyol® 812 2/20 °C | 0.1 | 0.4 4 | 0.4 4 | 0.4 4 | 3.6 | 6.1 | 8.0 |
| Clear orange juice/20 °C | 0.1 | 0.1 | <0.1 | <0.1 | 0.2 | 0.2 | 0.2 |
| Ground coffee beans/20 °C | <0.1 | <0.1 | <0.1 | <0.1 | 0.1 | 0.3 | 0.4 |
| Noodles 3/20 °C | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 0.1 | <0.1 |
| Oat flakes/20 °C | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Wheat loops (sugared)/20 °C | <0.1 | 0.1 | <0.1 | 0.1 | 0.1 | <0.1 | 0.1 |
| Water/20 °C | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Food/Temperature | Weight Increase [%] | |||||
|---|---|---|---|---|---|---|
| GPPS | HIPS | |||||
| 10 min | 20 min | 30 min | 10 min | 20 min | 30 min | |
| Boiling water (starting 100 °C, end 36 °C) | 0.1 | 0.1 | <0.1 | 0.1 | 0.2 | 0.3 |
| Hot water (starting 80 °C, end 36 °C) | <0.1 | <0.1 | <0.1 | 0.2 | 0.2 | 0.2 |
| Hot water (starting 60 °C, end 36 °C) | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Warm water (starting 40 °C, end 29 °C) | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Brewed coffee (starting 90 °C, end 28 °C) | 0.1 | 0.1 | 0.1 | 0.4 | 0.6 | 0.6 |
| Brewed coffee (100 °C constant) | 0.3 | 0.4 | 0.3 | 0.4 | 0.6 | 1.2 |
| Food (Simulant) Time-Temperature | GPPS | HIPS | ||||
|---|---|---|---|---|---|---|
| Styrene Migration a [µg/kg] | Weight Increase [%] | Visual Changes b | Styrene Migration a [µg/kg] | Weight Increase [%] | Visual Changes b | |
| Isooctane@10 d/20 °C c | 39 | <0.1 | no | 2665 | 3 | yes |
| Isooctane@10 d/40 °C c | 126 | 0.2 | no | 3492 | 12 | yes |
| Isooctane@10 d/60 °C c | 4248 | 45 | yes | 3425 | 30 | yes |
| Isooctane@1 d/20 °C d | 23 | <0.1 | no | 1788 | 1.6 | yes |
| Isooctane@1 d/40 °C d | 50 | 0.1 | no | 3156 | 5.6 | yes |
| Isooctane@1 d/60 °C d | 1423 | 12 | yes | 3366 | 21 | yes |
| 95% ethanol@10 d/20 °C c | <20 | 1.7 | no | 190 | 2.7 | no |
| 95% ethanol@10 d/40 °C c | 690 | 2.6 | no | 970 | 2.7 | no |
| 95% ethanol@10 d/60 °C c | 3329 | 4.1 | yes | 3617 | 3.2 | yes |
| 95% ethanol@1 d/40 °C d | 239 | 1.3 | no | 227 | 1.6 | no |
| 95% ethanol@1 d/60 °C d | 833 | 3 | yes | 980 | 3.2 | yes |
| 50% ethanol@10 d/20 °C c | <40 | 0.8 | no | 48 | 0.9 | no |
| 50% ethanol@10 d/40 °C c | <40 | 1.1 | no | 297 | 1.3 | no |
| 50% ethanol@10 d/60 °C c | 598 | 1.2 | no | 341 | 1.3 | yes |
| Milk@10 d/40 °C | 92 | 0.1 | no | 152 | 0.1 | no |
| Cream@10 d/40 °C | 220 e | <0.1 e | no | 278 | 0.4 | no |
| Olive oil@10 d/40 °C | 211 e | 0.2 e | yes | 222 | 0.3 | no |
| Substance | Limit of Detection in Food Simulants [µg/mL] and Real Foods [µg/g] | |||||
|---|---|---|---|---|---|---|
| 95% Ethanol | 50% Ethanol | Isooctane | Olive Oil | Cream | Milk | |
| Toluene | 0.58 | 1.95 | 1.29 | 0.06 | 0.02 | 0.01 |
| Chlorobenzene | 0.57 | 1.64 | 0.23 | 0.04 | 0.04 | 0.01 |
| Styrene | 0.22 | 1.43 | 4.40 | 0.03 | 0.06 | 0.01 |
| Phenyl cyclohexane | 0.60 | 0.74 | 2.14 | n.d. | n.d. | n.d. |
| Benzophenone | 0.20 | 0.18 | 1.96 | n.d. | n.d. | n.d. |
| Methyl stearate | 2.16 | 7.50 | 1.60 | n.d. | n.d. | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guazzotti, V.; Gruner, A.; Juric, M.; Hendrich, V.; Störmer, A.; Welle, F. Migration Testing of GPPS and HIPS Polymers: Swelling Effect Caused by Food Simulants Compared to Real Foods. Molecules 2022, 27, 823. https://doi.org/10.3390/molecules27030823
Guazzotti V, Gruner A, Juric M, Hendrich V, Störmer A, Welle F. Migration Testing of GPPS and HIPS Polymers: Swelling Effect Caused by Food Simulants Compared to Real Foods. Molecules. 2022; 27(3):823. https://doi.org/10.3390/molecules27030823
Chicago/Turabian StyleGuazzotti, Valeria, Anita Gruner, Mladen Juric, Veronika Hendrich, Angela Störmer, and Frank Welle. 2022. "Migration Testing of GPPS and HIPS Polymers: Swelling Effect Caused by Food Simulants Compared to Real Foods" Molecules 27, no. 3: 823. https://doi.org/10.3390/molecules27030823
APA StyleGuazzotti, V., Gruner, A., Juric, M., Hendrich, V., Störmer, A., & Welle, F. (2022). Migration Testing of GPPS and HIPS Polymers: Swelling Effect Caused by Food Simulants Compared to Real Foods. Molecules, 27(3), 823. https://doi.org/10.3390/molecules27030823







