Design, Synthesis of New 1,2,4-Triazole/1,3,4-Thiadiazole with Spiroindoline, Imidazo[4,5-b]quinoxaline and Thieno[2,3-d]pyrimidine from Isatin Derivatives as Anticancer Agents
Abstract
1. Introduction
2. Results and Discussion
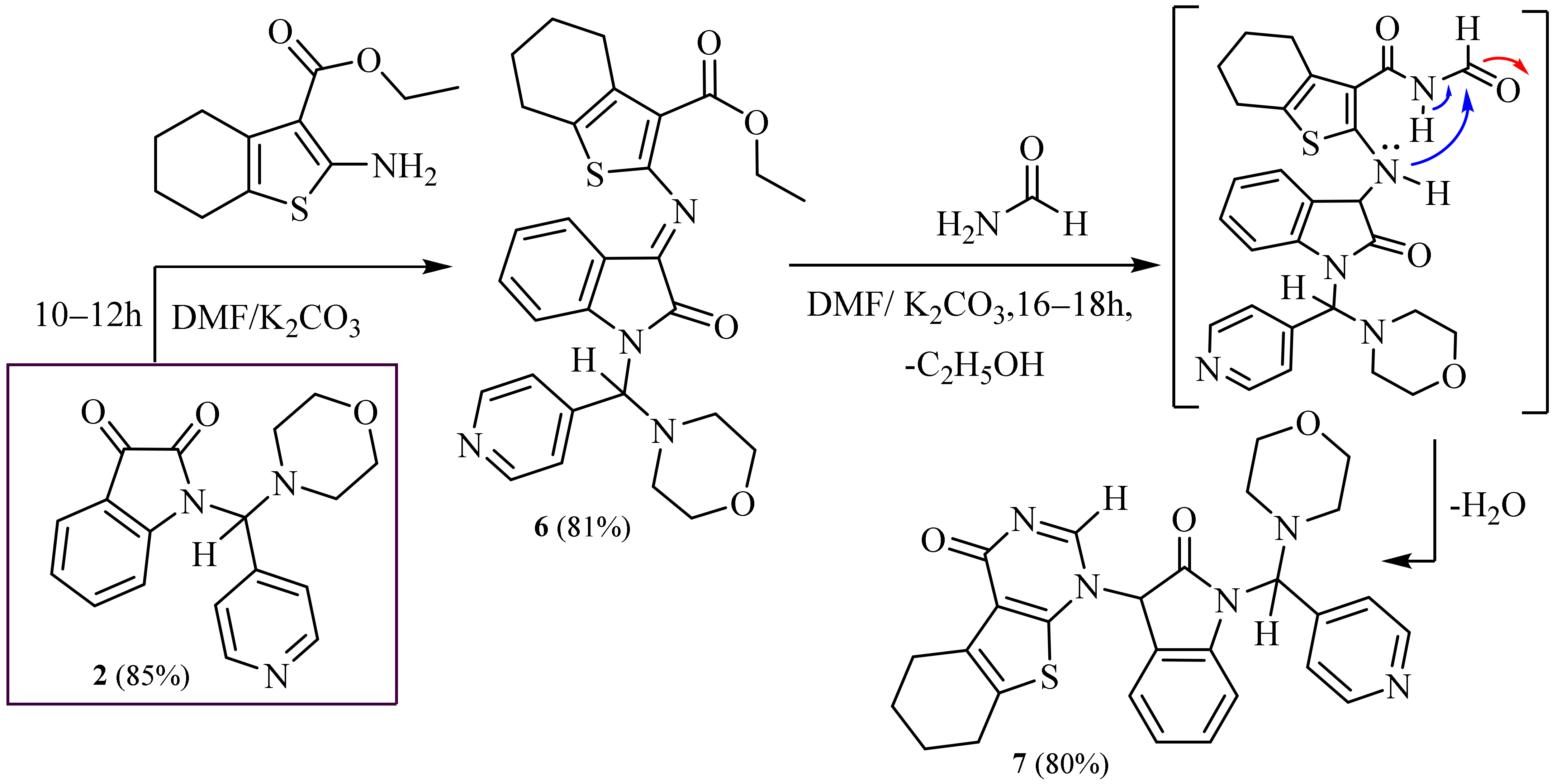
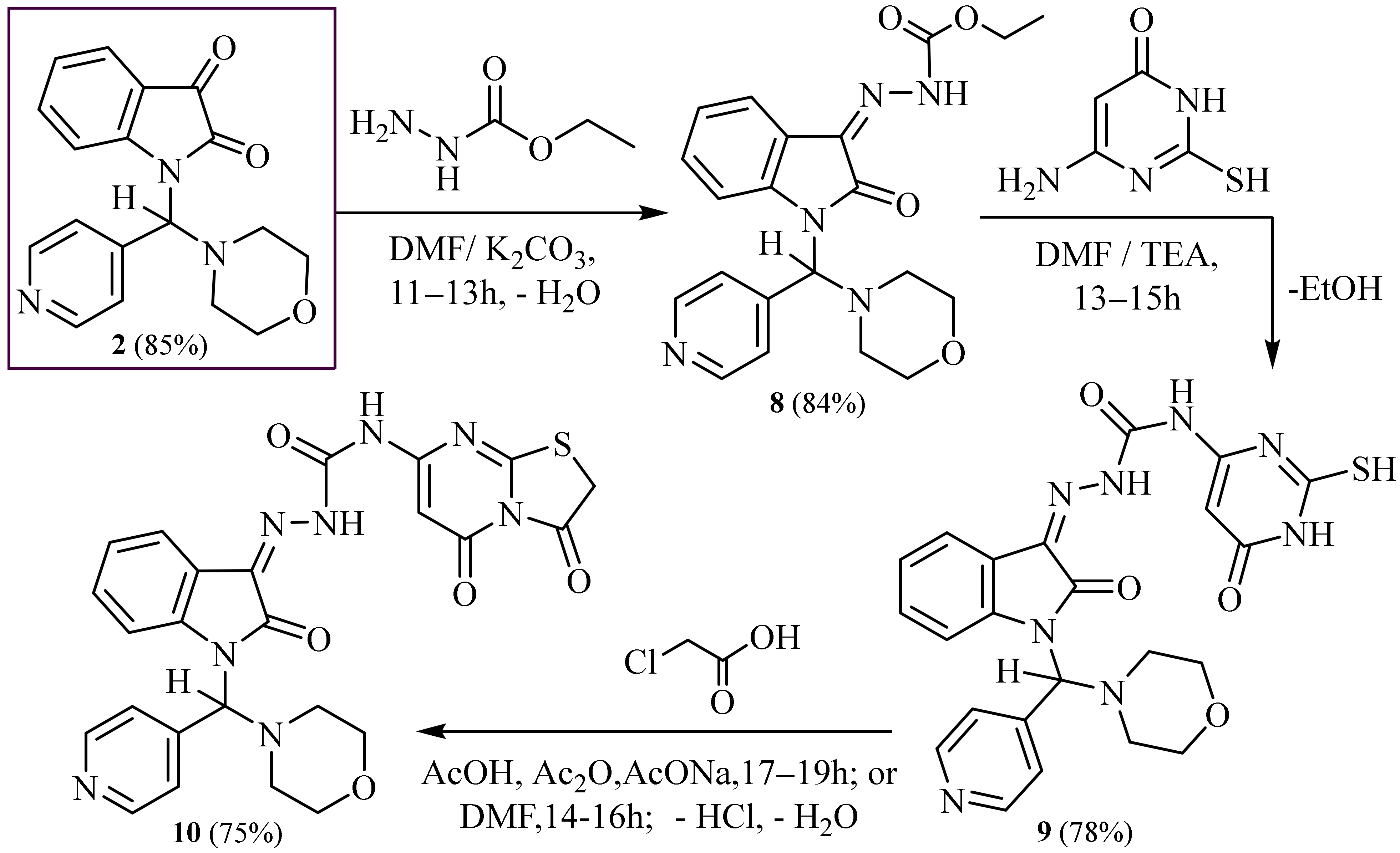
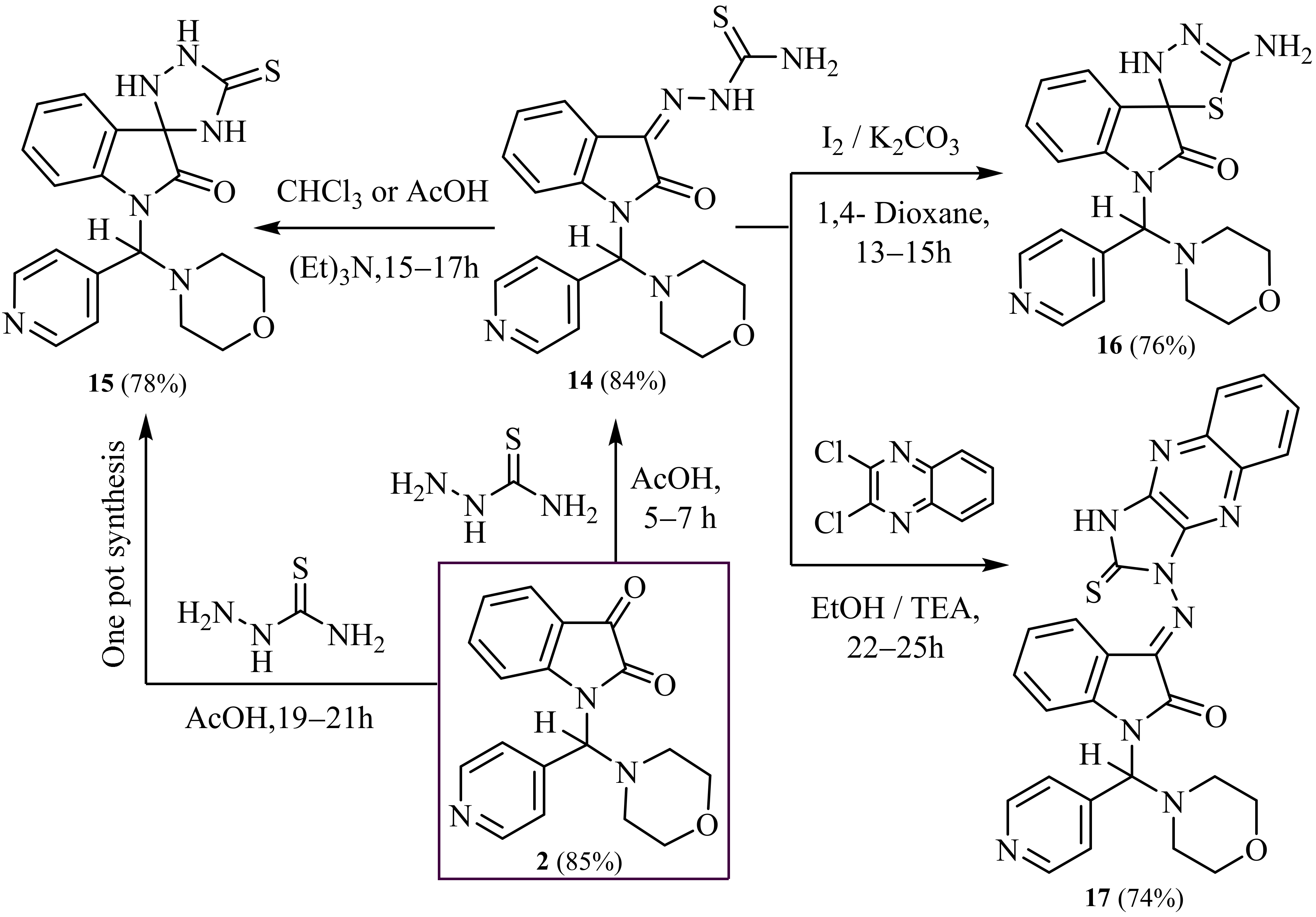
2.1. Synthesis
2.2. Pharmacological Activities
2.2.1. Anticancer Screening
In Vitro Cytotoxic Activity
2.2.2. Structural Activity Relationship (SAR)
- Isatin derivatives are used as free ligands or coordinated bonds with many metal and organic ions. Hence, they provide promising anti-proliferative properties against different cancer cells.
- Isatin-containing ligands have been fused with drug delivery systems for anti-tu- mor application.
- Isatin and its derivatives have the ability to bind to nucleic acid (DNA) and generate different types of ions that act as antioxidants and also inhibit selected proteins.
- In addition, isatin has been detected in the human body as a metabolite of tryptophan or epinephrine, and it is widely distributed in the central nervous system (CNS) [55].
3. Experimental Section
3.1. General Information
3.2. Synthesis of 1-(Morpholino (Pyridin-4-yl) Methyl) Indoline-2,3-Dione (2)
3.3. Synthesis of 3-((4-Bromophenyl) Imino)-1-(Morpholino (Pyridin-4-yl) Methyl) Indolin-2-One (3)
3.4. Synthesis of 2-((1-(Morpholino (Pyridin-4-yl) Methyl)-2-Oxoindolin-3-Ylidene) Amino) Benzoic Acid (4)
3.5. Synthesis of 1-(Morpholino (Pyridin-4-yl) Methyl) -3-(Thiazol-2-Ylimino) Indolin-2-One (5)
3.6. Synthesis of Ethyl -2-((1-(Morpholino (Pyridin-4-yl) Methyl)-2-Oxoindolin-3-Ylidene) amino)-4,5,6,7-Tetrahydrobenzo[b]thiophene-3-Carboxylate (6)
3.7. Synthesis of 1-(1-(Morpholino(Pyridin-4-yl)Methyl)-2-Oxoindolin-3-yl)-5,6,7,8-Tetrahydro benzo[4,5]thieno[2,3-d]pyrimidin-4(1H)-One (7)
3.8. Synthesis of Ethyl -2-(1-(Morpholino (Pyridin-4-yl) Methyl)-2-Oxoindolin-3-Ylidene) Hydrazine-1-Carboxylate (8)
3.9. Synthesis of N-(2-Mercapto-6-oxo-1,6-Dihydropyrimidin-4-yl)-2-(1-(Morpholino (Pyridin-4- yl) methyl)-2-Oxoindolin-3-Ylidene) Hydrazine-1-Carboxamide (9)
3.10. Synthesis of N-(3,5-Dioxo-2,3-Dihydro-5H-Thiazolo [3,2-a] Pyrimidin-7-yl)-2-(1- (Morphol- ino (Pyridin-4-yl) Methyl)-2-Oxoindolin-3-Ylidene) Hydrazine-1-Carboxamide (10)
3.11. Synthesis of 3-((2-Aminophenyl) Amino)-3-Hydroxy-1-(Morpholino (Pyridin-4-yl) Methyl) Indolin-2-One (11)
3.12. Synthesis of 3-((2-Aminophenyl) Imino)-1-(Morpholino (Pyridin-4-yl) Methyl) Indolin-2-one (12)
3.13. Synthesis of 2-(2-((1-(Morpholino (Pyridin-4-yl) Methyl)-2-Oxoindolin-3-Ylidene) Amino) phenyl) Isoindoline-1,3-Dione (13)
3.14. Synthesis of 2-(1-(Morpholino (Pyridin-4-yl) Methyl)-2-Oxoindolin-3-Ylidene) Hydrazine-1-Carbothioamide (14)
3.15. Synthesis of 1- (Morpholino (Pyridin-4-yl) Methyl)-5′-Thioxospiro [Indoline-3,3′-[1,2,4] Triazolidin]-2-One (15)
3.16. Synthesis of 5′-Amino-1-(Morpholino (Pyridin-4-yl) Methyl) -3′H-Spiro [Indoline-3,2′-[1,3,4] Thiadiazol]-2-One (16)
3.17. Synthesis of 1-(Morpholino (Pyridin-4-yl) Methyl)-3-((2-Thioxo-2,3-Dihydro-1H- Imidazo [4,5-b] Quinoxalin-1-yl) Imino) Indolin-2-One (17)
3.18. Pharmacological Screening
3.18.1. Ethics Approval and Consent to Participate
3.18.2. Human and Animal Rights
3.18.3. Chemicals and Drugs
3.18.4. Materials and Methods (In Vitro Cytotoxicity)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sagar, S.; Esau, L.; Moosa, B.; Khashab, N.M.; Bajic, V.B.; Kaur, M. Cytotoxicity and apoptosis induced by a plumbagin derivative in estrogen positive MCF-7 breast cancer cells. Anti-Cancer Agents Med. Chem. 2014, 14, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Plackal, B.; George, A.; Abrahamse, H. A review on novel breast cancer therapies: Photodynamic therapy and plant derived agent induced cell death mechanisms. Anti-Cancer Agents Med. Chem. 2016, 16, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.; Buneeva, O.; Gnedenko, O.; Ershov, P.; Ivanov, A. Isatin, an endogenous nonpeptide biofactor: A review of its molecular targets, mechanismsof actions, and their biomedical implications. BioFactors 2018, 44, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Blowers, E.C.; Tebbe, C.; Contreras, J.I.; Radhakrishnan, P.; Kizhake, S.; Zhou, T.; Rajule, R.N.; Arnst, J.; Munkarah, A.R.; et al. Isatin derived spirocyclic analogs with α-methylene-γ-butyrolactone as anticancer agents: A structure activity relationship study. J. Med. Chem. 2016, 59, 5121–5127. [Google Scholar] [CrossRef]
- Abo-Salem, H.M.; Nassrallah, A.; Soliman, A.A.F.; Ebied, M.S.; Elawady, M.E.; Abdelhamid, S.A.; El-Sawy, E.R.; Al-Sheikh, Y.A.; Aboul-Soud, M.A.M. Synthesis and bioactivity assessment of novel spiro pyrazole-oxindole congeners exhibiting potent and selective in vitro anticancer effects. Molecules 2020, 25, 1124. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Shia, K.S.; Song, J.S.; Wu, M.H.; Li, W.T. Synthesis of (E)-oxindolylidene acetate using tandem palladium-catalyzed Heck and alkoxycarbonylation reactions. Org. Biomol. Chem. 2016, 14, 220–228. [Google Scholar] [CrossRef]
- Singh, A.; Raghuwanshi, K.; Patel, V.K.; Jain, D.K.; Veerasamy, R.; Dixit, A.; Rajak, H. Assessment of 5-substituted isatin as surface recognition group: Design, synthesis, and anti-proliferative evaluation of hydroxamates as novel histone deacetylase inhibitors. Pharm. Chem. J. 2017, 51, 366–374. [Google Scholar] [CrossRef]
- Varun, C.; Sonam, N.; Kakkar, R. Isatin and its derivatives: A survey of recent syntheses, reactions, and applications. Med. Chem. Commun. 2019, 10, 351–368. [Google Scholar] [CrossRef]
- Webber, S.E.; Tikhe, J.; Worland, S.T.; Fuhrman, S.A.; Hendrickson, T.F.; Matthews, D.A.; Love, R.A.; Patick, A.K.; Meador, J.W.; Ferre, R.A.; et al. Design, synthesis, and evaluation of nonpeptidic inhibitors of human rhinovirus 3C protease. J. Med. Chem. 1996, 39, 5072–5082. [Google Scholar] [CrossRef]
- Quenelle, D.C.; Keith, K.A.; Kern, E.R. In vitro and in vivo evaluation of isatin-beta-thiosemicarbazone and marboran against vaccinia and cowpox virus infections. Antivir. Res. 2006, 71, 24–30. [Google Scholar] [CrossRef]
- De Clercq, E. Historical perspectives in the development of antiviral agents against poxviruses. Viruses 2010, 2, 1322–1339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Y.; Zhang, W.; Wei, P.; Huang, C.; Pei, J.; Yuan, Y.; Lai, L. Isatin compounds as noncovalent SARS coronavirus 3Clike protease inhibitors. J. Med. Chem. 2006, 49, 3440–3443. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhu, H.M.; Niu, G.J.; Shi, E.Z.; Chen, J.; Sun, B.; Chen, W.Q.; Zhou, H.G.; Yang, C. Synthesis, modification and docking studies of 5-sulfonyl isatin derivatives as SARS-CoV 3C-like protease inhibitors. Bioorg. Med. Chem. 2014, 22, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Ronen, D.; Sherman, L.; Bar-Nun, S.; Teitz, Y. N-methylisatin-beta-4′, 4′-diethylthiosemi-carbazone, an inhibitor of moloney leukemia virus protein production: Characterization and in vitro translation of viral mRNA. Antimicrob. Agents Chemother. 1987, 31, 1798–1802. [Google Scholar] [CrossRef][Green Version]
- Meleddu, R.; Distinto, S.; Corona, A.; Tramontano, E.; Bianco, G.; Melis, C.; Cottiglia, F.; Maccioni, E. Isatin thiazoline hybrids as dual inhibitors of HIV-1 reverse transcriptase. J. Enzyme Inhib. Med. Chem. 2017, 32, 130–136. [Google Scholar] [CrossRef]
- Zhang, H.M.; Dai, H.; Hanson, P.J.; Li, H.; Guo, H.; Ye, X.; Hemida, M.G.; Wang, L.; Tong, Y.; Qiu, Y.; et al. Antiviral activity of an isatin derivative via induction of PERK-Nrf2-mediated suppression of cap-independent translation. ACS Chem. Biol. 2014, 9, 1015–1024. [Google Scholar] [CrossRef]
- Mishra, P.; Kumar, A.; Mamidi, P.; Kumar, S.; Basantray, I.; Saswat, T.; Das, I.; Nayak, T.K.; Chattopadhyay, S.; Subudhi, B.B.; et al. Inhibition of chikungunya virus replication by 1-[(2-methylbenzimidazol-1-yl) methyl]-2-oxo-indolin-3-ylidene] amino] thiourea (MBZM-N-IBT). Sci. Rep. 2016, 6, 20122. [Google Scholar] [CrossRef]
- Aboul-Fadl, T.; Abdel-Hamid, M.K.; Youssef, A.F. Schiff bases of indoline-2, 3-dione (isatin) derivatives as efficient agents against resistant strains of mycobacterium tuberculosis. Der Pharma Chem. 2015, 7, 217–225. [Google Scholar]
- Hu, Y.Q.; Meng, L.D.; Qiang, M.; Song, X.F. Design, Synthesis, and in vitro anti-mycobacterial evaluation 1H-1, 2, 3-triazole- tethered ciprofloxacin and isatin conjugates. J. Heterocycl. Chem. 2017, 54, 3725–3729. [Google Scholar] [CrossRef]
- Karthik, R.; Vimaladevi, G.; Chen, S.M.; Elangovan, A.; Jeyaprabha, B.; Prakash, P. Corrosion inhibition and adsorption behavior of 4-amino acetophenone pyridine 2-aldehyde in 1M hydrochloric acid. Int. J. Electrochem. Sci. 2015, 10, 4666–4681. [Google Scholar]
- Ansari, K.R.; Quraishi, M.A. Experimental and quantum chemical evaluation of schiff bases of isatin as a new and green corrosion inhibitors for mild steel in 20% H2SO4. J. Taiwan Inst. Chem. Eng. 2015, 54, 145–154. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; El Shehry, M.F.; Badria, F.A. Design and synthesis of novel thiophenecarbohydrazide, thienopyrazole and thienopyrimidine derivatives as antioxidant and antitumor agents. Acta Pharm. 2010, 60, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.A.; Abu-Hashem, A.A. Synthesis, characterization, antioxidant and antitumor evaluation of some new thiazolidine and thiazolidinone. Arch. Pharm. Chem. Life Sci. 2011, 11, 170–177. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Youssef, M.M.; Hussein, H.A.R. Synthesis, antioxidant, antitumor activities of some new thiazolo pyrimidines, pyrrolothiazolopyrimidines and triazolopyrrolothiazolopyrimidines derivatives. J. Chin. Chem. Soc. 2011, 58, 41–48. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Aly, A.S. Synthesis of new pyrazole, triazole, thiazolidine,-pyrimido [4,5-b] quinoline derivatives with potential antitumor activity. Arch. Pharmacal Res. 2012, 35, 437–445. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Badria, F.A. Design, Synthesis of novel thiourea and pyrimidine derivatives as potential antitumor agents. J. Chin. Chem. Soc. 2015, 62, 506–512. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Hussein, H.A.R. Synthesis and antitumor activity of new pyrimidine and caffeine derivatives. Lett. Drug Des. Discov. 2015, 12, 471–478. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; El-Shazly, M. Synthesis of new quinoxaline, pyrimidine, and pyrazole furochromone derivatives as cytotoxic agents. Mon. Chem. 2017, 148, 1853–1863. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Aly, A.S. Chemistry of new dimethyl-benzo,-1, 3, 6-oxadiazepine and 1, 3, 5-triazepine derivatives as anticancer agents. Synth. Commun. 2017, 47, 2417–2425. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Gouda, M.A.; Badria, F.A. Design, synthesis and identification of novel substituted isothiochromene analogs as potential antiviral and cytotoxic agents. Med. Chem. Res. 2018, 27, 2297–2311. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Abu-Zied, K.M.; Zaki, M.E.A.; El-Shehry, M.F.; Awad, H.M.; Khedr, M.A. Design, synthesis, and anticancer potential of the enzyme (PARP-1) inhibitor with computational studies of new triazole, thiazolidinone,-thieno [2, 3-d] pyrimidinones. Lett. Drug Des. Discov. 2020, 17, 799–819. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A. Synthesis of new pyrazoles, oxadiazoles, triazoles, pyrrolotriazines and pyrrolotriazepines as potential cytotoxic agents. J. Heterocycl. Chem. 2021, 58, 805–821. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Al-Hussain, S.A.; Zaki, M.E.A. Design, synthesis and anticancer activity of new polycyclic: Imidazole, thiazine, oxathiine, pyrrolo-quinoxaline and thienotriazolopyrimidine derivatives. Molecules 2021, 26, 2031. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A. Synthesis and reaction of novel spiro-pyrimidine derivatives. J. Heterocycl. Chem. 2014, 51, 1020–1026. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; El-Shazly, M. Synthesis and antimicrobial evaluation of novel triazole, tetrazole, and spiropyrimidine- thiadiazole derivatives. Polycycl. Aromat. Compd. 2021, 41, 478. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Youssef, M.M. Synthesis of new visnagen and khellin furochromone pyrimidine derivatives and their anti-inflammatory and analgesic activity. Molecules 2011, 16, 1956–1972. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A. Synthesis of new furothiazolo pyrimido quinazolinones from visnagenone or khellinone and antimicrobial activity. Molecules 2018, 23, 2793. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A.; Al-Hussain, S.A.; Zaki, M.E.A. Synthesis of novel benzodifuranyl; 1, 3, 5-triazines; 1, 3, 5-oxadiazepines; and thiazolopyrimidines derived from visnaginone and khellinone as anti- inflammatory and analgesic agents. Molecules 2020, 25, 220. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Abu-Zied, K.M.; El-Shehry, M.F. Synthetic utility of bifunctional thiophene derivatives and antimicrobial evaluation of the newly synthesized agents. Mon. Chem. 2011, 142, 539–545. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Hussein, H.A.R.; Abu-zied, K.M. Synthesis of novel 1, 2, 4-triazolopyrimidines and their evaluation as antimicrobial agents. Med. Chem. Res. 2016, 26, 120–130. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Gouda, M.A. Synthesis and antimicrobial activity of some novel quinoline, chromene, pyrazole derivatives bearing triazolopyrimidine moiety. J. Heterocycl. Chem. 2017, 54, 850–858. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A. Synthesis and biological activity of pyrimidines, quinolines, thiazines and pyrazoles bearing a common thieno moiety. Acta Pol. Pharm. Drug Res. 2018, 75, 59–70. [Google Scholar]
- Abu-Hashem, A.A.; Faty, R.A.M. Synthesis, antimicrobial evaluation of some new 1, 3, 4-thiadiazoles and 1, 3, 4-thiadiazines. Curr. Org. Synth. 2018, 15, 1161–1170. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Fathy, U.; Gouda, M.A. Synthesis of 1, 2, 4-triazolopyridazines, isoxazolofuropyridazines, and Tetrazolo- pyridazines as antimicrobial agents. J. Heterocycl. Chem. 2020, 57, 3461–3474. [Google Scholar]
- Abu-Hashem, A.A.; Hussein, H.A.R.; Aly, A.S. Synthesis and antimicrobial activity of novel 1,2,4-triazolopyrimidofuro- quinazolinones from natural furochromones (visnagenone and khellinone). Med. Chem. 2021, 17, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A. Synthesis and antimicrobial activity of new 1, 2, 4-triazole, 1, 3, 4-oxadiazole, 1, 3, 4-thiadiazole, thiopyrane, thiazolidinone and azepine derivatives. J. Heterocycl. Chem. 2021, 58, 74–92. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Zaki, M.E.A. Direct amination and synthesis of fused N-substituted isothiochromene derivatives. J. Heterocycl. Chem. 2019, 56, 886–894. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; El-Shazly, M. Synthesis of new isoxazole-, pyridazine-, pyrimido- pyrazines and their anti-inflammatory and analgesic activity. Med. Chem. 2018, 14, 356–371. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Gouda, M.A.; Badria, F.A. Synthesis of some new pyrimido [2′, 1′: 2, 3] thiazolo [4, 5-b] quinoxaline derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2010, 45, 1976–1981. [Google Scholar] [CrossRef]
- El-Gazzar, A.B.A.; Youssef, A.M.S.; Youssef, M.M.; Abu-Hashem, A.A.; Badria, F.A. Design and synthesis of azolopyrimidoquinolines, pyrimidoquinazolines as anti-oxidant, anti-inflammatory and analgesic activities. Eur. J. Med. Chem. 2009, 44, 609–624. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Yogeeswari, P.; Sriram, D.; De Clercq, E.; Pannecouque, C.; Witvrouw, M. Synthesis and screening for anti-HIV activity of some N -Mannich bases of isatin derivatives. Chemotherapy 1999, 45, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, S.N.; Sriram, D.; Nath, G.; De Clercq, E. HIV evaluation of schiff and Mannich bases of isatin and its derivatives with triazole. Arzneimit-Telforschung 2000, 50, 55–59. [Google Scholar]
- Pandeya, S.N.; Sriram, D.; Nath, G.; Declercq, E. Synthesis, antibacterial, antifungal and anti-HIV activities of schiff and Mannich bases derived from isatin derivatives and N-[4-(4′-chlorophenyl)thiazol-2-yl]thiosemicarbazide. Eur. J. Pharm. Sci. 1999, 9, 25–31. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Lmmunological Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- De Paiva, R.E.F.; Vieira, E.G.; Da Silva, D.R.; Wegermann, C.A.; Ferreira, A.M.C. Anticancer compounds based on isatin-derivatives: Strategies to ameliorate selectivity and efficiency. Front. Mol. Biosci. 2021, 7, 627272. [Google Scholar] [CrossRef]




| Compounds | In Vitro Cytotoxicity IC50 (µM) ± SD | TI | |||
|---|---|---|---|---|---|
| MGC-803 a | MCF-7 a | CNE2 a | KB a | ||
| 1 | >50 | >50 | >50 | >50 | 2.1 |
| 2 | 35.7 ± 1.5 | 35.4 ± 1.2 | 35.3 ± 1.6 | 35.2 ± 1.4 | 1.9 |
| 3 | 27.8 ± 1.2 | 27.6 ± 1.4 | 27.4 ± 1.5 | 27.1 ± 1.2 | 1.8 |
| 4 | 20.9 ± 1.4 | 20.7 ± 1.5 | 20.5 ± 1.3 | 20.2 ± 1.1 | 1.7 |
| 5 | 11.8 ± 1.3 | 11.6 ± 1.4 | 11.5 ± 1.2 | 11.3 ± 1.3 | 1.4 |
| 6 | 10.9 ± 1.1 | 10.8 ± 1.3 | 10.7 ± 1.1 | 10.5 ± 1.5 | 1.3 |
| 7 | 10.5 ± 1.2 | 10.4 ± 1.1 | 10.2 ± 1.4 | 10.1 ± 1.2 | 1.1 |
| 8 | 12.9 ± 1.3 | 12.8 ± 1.5 | 12.6± 1.4 | 12.5 ± 1.6 | 1.6 |
| 9 | 9.9 ± 1.1 | 9.8 ± 1.2 | 9.7 ± 1.1 | 9.6 ± 1.2 | 0.6 |
| 10 | 9.8 ± 1.2 | 9.7 ± 1.1 | 9.6 ± 1.3 | 9.5 ± 1.1 | 0.5 |
| 11 | 14.5 ± 1.1 | 14.3 ± 1.3 | 14.2± 1.4 | 14.1 ± 1.2 | 1.4 |
| 12 | 13.8 ± 1.5 | 13.7 ± 1.2 | 13.5± 1.1 | 13.3 ± 1.4 | 1.3 |
| 13 | 10.7 ± 1.3 | 10.6 ± 1.5 | 10.4 ± 1.3 | 10.2 ± 1.1 | 1.2 |
| 14 | 12.6 ± 1.4 | 12.4 ± 1.6 | 12.3± 1.5 | 12.1 ± 1.2 | 1.5 |
| 15 | 10.3 ± 1.1 | 10.2 ± 1.2 | 10.1 ± 1.3 | 9.9 ± 1.4 | 0.9 |
| 16 | 10.1 ± 1.3 | 10.0 ± 1.4 | 9.9 ± 1.2 | 9.8 ± 1.3 | 0.8 |
| 17 | 9.7 ± 1.1 | 9.6 ± 1.2 | 9.5 ± 1.1 | 9.4 ± 1.2 | 0.4 |
| 5-Fluorouracil | 10.7 ± 1.2 | 10.5 ± 1.1 | 10.3 ± 1.3 | 10.1 ± 1.1 | 0.7 |
| Chemical Structures | In vitro Cytotoxicity IC50 (µM) ± SD | TI | |||
|---|---|---|---|---|---|
| MGC-803 a | MCF-7 a | CNE2 a | KB a | ||
| Functional Groups/Heterocyclic Moieties | |||||
 | 9.7 ± 1.1 | 9.6 ± 1.2 | 9.5 ± 1.1 | 9.4 ± 1.2 | 0.4 |
| Isatin, morpholine, pyridine, methyl, thioxo, imidazo [4,5-b] quinoxaline, carbonyl group, nitrogen, oxygen and sulfur atoms. | |||||
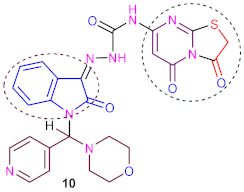 | 9.8 ± 1.2 | 9.7 ± 1.1 | 9.6 ± 1.3 | 9.5 ± 1.1 | 0.5 |
| Isatin, morpholine, pyridine, methyl, hydrazine, carboxamide, thiazolo [3,2-a] pyrimidine, four carbonyl groups, nitrogen, oxygen and sulfur atoms. | |||||
 | 9.9 ± 1.1 | 9.8 ± 1.2 | 9.7 ± 1.1 | 9.6± 1.2 | 0.6 |
| Isatin, morpholine, pyridine, methyl, hydrazine, carboxamide, pyrimidine ring, three carbonyl groups, nitrogen, oxygen and sulfur atoms. | |||||
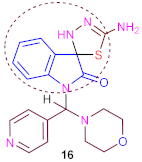 | 10.1 ± 1.3 | 10.0 ± 1.4 | 9.9 ± 1.2 | 9.8 ± 1.3 | 0.8 |
| Isatin, morpholine, pyridine, methyl, amino, spiro [indoline-1,3,4-thiadiazole], carbonyl group, nitrogen, oxygen and sulfur atoms. | |||||
 | 10.3 ± 1.1 | 10.2 ± 1.2 | 10.1 ± 1.3 | 9.9 ± 1.4 | 0.9 |
| Isatin, morpholine, pyridine, methyl, thioxo, spiro [indoline-1, 2, 4- triazolidinone], carbonyl group, nitrogen, oxygen and sulfur atoms. | |||||
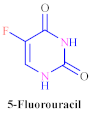 | 10.7 ± 1.2 | 10.5 ± 1.1 | 10.3 ± 1.3 | 10.1 ± 1.1 | 0.7 |
| Pyrimidine ring, two carbonyl groups, nitrogen, oxygen and fluorine atoms. | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Hashem, A.A.; Al-Hussain, S.A. Design, Synthesis of New 1,2,4-Triazole/1,3,4-Thiadiazole with Spiroindoline, Imidazo[4,5-b]quinoxaline and Thieno[2,3-d]pyrimidine from Isatin Derivatives as Anticancer Agents. Molecules 2022, 27, 835. https://doi.org/10.3390/molecules27030835
Abu-Hashem AA, Al-Hussain SA. Design, Synthesis of New 1,2,4-Triazole/1,3,4-Thiadiazole with Spiroindoline, Imidazo[4,5-b]quinoxaline and Thieno[2,3-d]pyrimidine from Isatin Derivatives as Anticancer Agents. Molecules. 2022; 27(3):835. https://doi.org/10.3390/molecules27030835
Chicago/Turabian StyleAbu-Hashem, Ameen Ali, and Sami A. Al-Hussain. 2022. "Design, Synthesis of New 1,2,4-Triazole/1,3,4-Thiadiazole with Spiroindoline, Imidazo[4,5-b]quinoxaline and Thieno[2,3-d]pyrimidine from Isatin Derivatives as Anticancer Agents" Molecules 27, no. 3: 835. https://doi.org/10.3390/molecules27030835
APA StyleAbu-Hashem, A. A., & Al-Hussain, S. A. (2022). Design, Synthesis of New 1,2,4-Triazole/1,3,4-Thiadiazole with Spiroindoline, Imidazo[4,5-b]quinoxaline and Thieno[2,3-d]pyrimidine from Isatin Derivatives as Anticancer Agents. Molecules, 27(3), 835. https://doi.org/10.3390/molecules27030835








