Bioactive Compounds of Ganoderma boninense Inhibited Methicillin-Resistant Staphylococcus aureus Growth by Affecting Their Cell Membrane Permeability and Integrity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of the Metabolites from EF
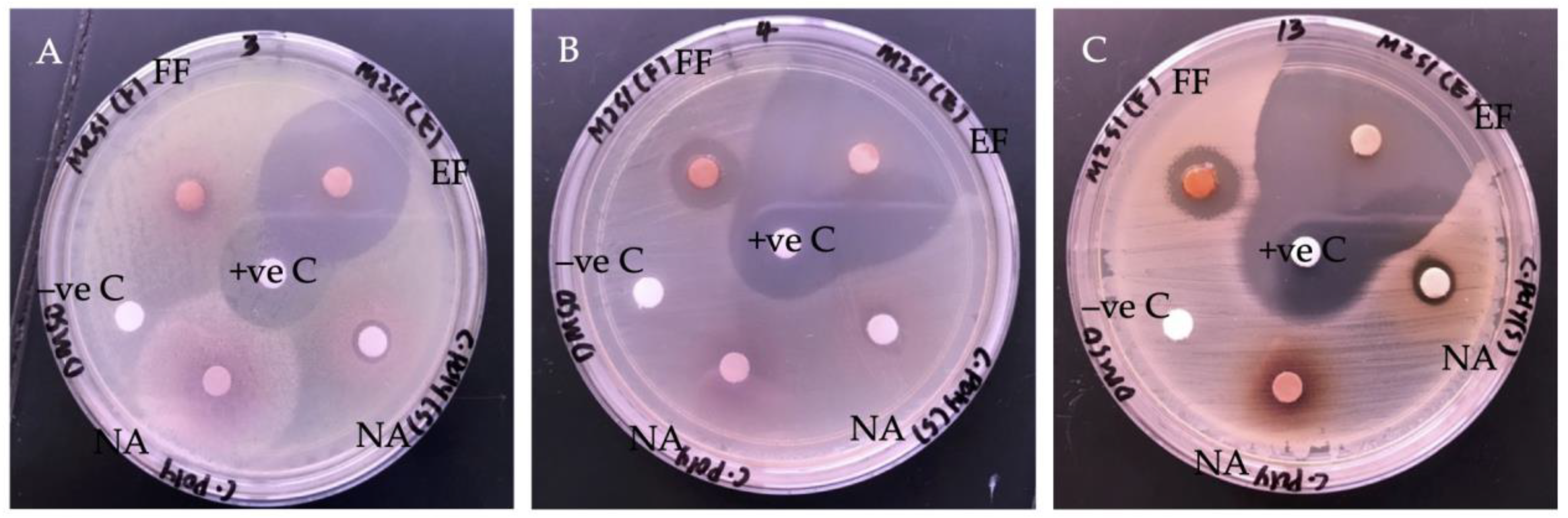
2.2. Antibacterial Activity of G. boninense SPE Extract
2.2.1. Bacteria Growth Inhibition
2.2.2. Determination of Minimum Inhibitory Concentration (MIC)
2.3. Antibacterial Mode of Action of EF against MRSA
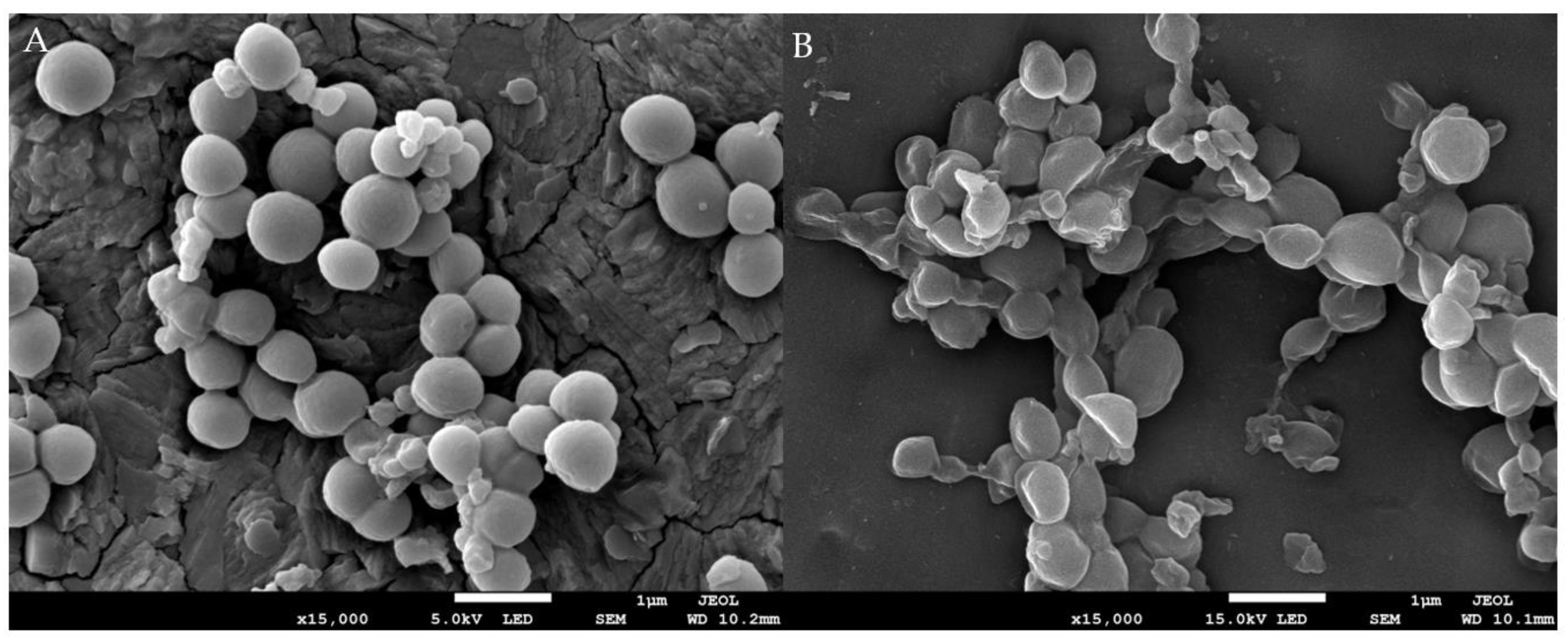
2.3.1. The Effect of EF on Bacteria Morphology
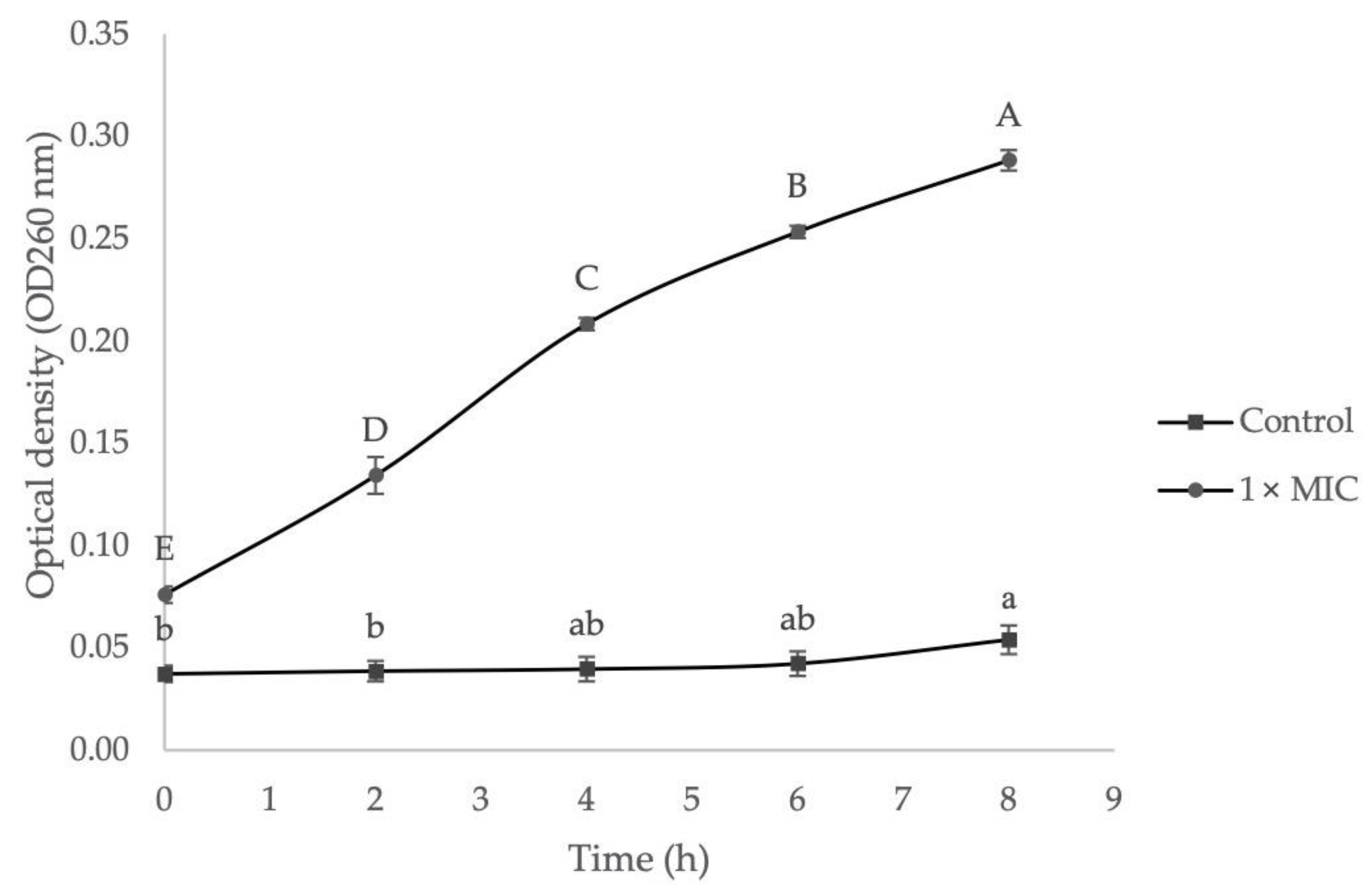
2.3.2. The Effect of EF on Bacteria Cell Membrane Permeability
2.3.3. The Effect of EF on Bacteria Cell Membrane Integrity
3. Materials and Methods
3.1. Preparation of G. boninense Crude Extract and Clean-Up
3.2. Chromatographic Separation and Mass Spectra Detection of G. boninense SPE Extract
3.3. Evaluation of Antibacterial Activity of G. boninense SPE Extract
3.3.1. Antibacterial Disc Diffusion Assay
3.3.2. Determination of Minimum Inhibition Concentration (MIC)
3.4. Mode of Action of G. boninense SPE Extract against Selected Pathogen
3.4.1. Observation of Morphological Changes under Scanning Electron Microscope (SEM)
3.4.2. Measurement of Cell Membrane Permeability
3.4.3. Measurement of Cell Membrane Integrity
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, L.; Li, J.Q.; Zhang, J.; Li, Z.M.; Liu, H.G.; Wang, Y.Z. Traditional uses, chemical components and pharmacological activities of the genus Ganoderma P. Karst.: A review. RSC Adv. 2020, 10, 42084–42097. [Google Scholar] [CrossRef]
- Miao, W.G.; Tang, C.; Ye, Y.; Quinn, R.J.; Feng, Y. Traditional Chinese medicine extraction method by ethanol delivers drug-like molecules. Chin. J. Nat. Med. 2019, 17, 713–720. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A. Ganoderma lucidum (Lingzhi or Reishi): A medicinal mushroom. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Watchtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 175–201. [Google Scholar]
- Huang, W.C.; Chang, M.S.; Huang, S.Y.; Tsai, C.J.; Kuo, P.H.; Chang, H.W.; Kao, M.C. Chinese herbal medicine Ganoderma tsugae displays potential anti-cancer efficacy on metastatic prostate cancer cells. Int. J. Mol. Sci. 2019, 20, 4418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhang, H.; Zuo, J.; Gong, X.; Yi, F.; Zhu, W.; Li, L. Advances in research on the active constituents and physiological effects of Ganoderma lucidum. Biomed. Dermatol. 2019, 3, 6–17. [Google Scholar] [CrossRef]
- Li, L.F.; Liu, H.B.; Zhang, Q.W.; Li, Z.P.; Wong, T.L.; Fung, H.Y.; Han, Q.B. Comprehensive comparison of polysaccharides from Ganoderma lucidum and G. sinense: Chemical, antitumor, immunomodulating and gut-microbiota modulatory properties. Sci. Rep. 2018, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Sharma, C.; Bhardwaj, N.; Sharma, A.; Tuli, H.S.; Batra, P.; Beniwal, V.; Sharma, A.K. Bioactive metabolites of Ganoderma lucidum: Factors, mechanism and broad spectrum therapeutic potential. J. Herb. Med. 2019, 17, 100268. [Google Scholar] [CrossRef]
- Lin, A.X.; Chan, G.; Hu, Y.; Ouyang, D.; Ung, C.O.L.; Shi, L.; Hu, H. Internationalization of traditional Chinese medicine: Current international market, internationalization challenges and prospective suggestions. Chin. Med. 2018, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Dong, C.; Wen, H.A.; Liu, X. Development of Ling-zhi industry in China emanated from the artificial cultivation in the Institute of Microbiology, Chinese Academy of Sciences (IMCAS). Mycology 2016, 7, 74–80. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.; Santos-Buelga, C.; Ferreira, I.C. Fruiting body, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: A comparative study of the antioxidant potential of phenolic and polysaccharide extracts. Food Res. Int. 2012, 46, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.W.; Su, K.Q.; Zhang, Y.M. Applied modern biotechnology for cultivation of Ganoderma and development of their products. Appl. Microbiol. Biotechnol. 2012, 93, 941–963. [Google Scholar] [CrossRef]
- Moradali, M.F.; Mostafavi, H.; Hejaroude, G.A.; Tehrani, A.S.; Abbasi, M.; Ghods, S. Investigation of potential antibacterial properties of methanol extracts from fungus Ganoderma applanatum. Chemotherapy 2006, 52, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Smania, E.D.F.A.; Delle Monache, F.; Yunes, R.A.; Paulert, R.; Smania, A., Jr. Antimicrobial activity of methyl australate from Ganoderma australe. Rev. Bras. Farmacogn. 2007, 17, 14–16. [Google Scholar] [CrossRef] [Green Version]
- Osinska-Jaroszuk, M.; Jaszek, M.; Mizerska-Dudka, M.; Blachowicz, A.; Rejczak, T.P.; Janusz, G.; Kandefer-Szerszen, M. Exopolysaccharide from Ganoderma applanatum as a promising bioactive compound with cytostatic and antibacterial properties. Biomed. Res. Int. 2014, 2014, 743812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, K.; Li, L.; Bao, L.; He, L.; Sun, C.; Zhou, B.; Liu, H. Six new 3, 4-seco-27-norlanostane triterpenes from the medicinal mushroom Ganoderma boninense and their antiplasmodial activity and agonistic activity to LXRβ. Tetrahedron 2015, 71, 1808–1814. [Google Scholar] [CrossRef]
- Ismail, K.; Abdullah, S.; Chong, K.P. Screening for potential antimicrobial compound from Ganoderma boninense against selected foodborne and skin disease pathogens. Int. J. Pharm. Pharm. Sci. 2014, 6, 771–774. [Google Scholar]
- Chan, Y.S.; Chong, K.P. Antimicrobial activity and metabolite analysis of Ganoderma boninense fruiting body. J. Pure Appl. Microbiol. 2020, 14, 114. [Google Scholar] [CrossRef]
- Chan, Y.S.; Chong, K.P. Phytochemical investigation and antioxidant activity of Ganoderma boninense. Malays. J. Biochem. Mol. Biol. 2020, 23, 138–149. [Google Scholar]
- Abdullah, S.; Oh, Y.S.; Kwak, M.K.; Chong, K.P. Biophysical characterization of antibacterial compounds derived from pathogenic fungi Ganoderma boninense. J. Microbiol. 2021, 59, 164–174. [Google Scholar] [CrossRef]
- Abdullah, S.; Jang, S.E.; Kwak, M.K.; Chong, K.P. Ganoderma boninense mycelia for phytochemicals and secondary metabolites with antibacterial activity. J. Microbiol. 2020, 58, 1054–1064. [Google Scholar] [CrossRef]
- Ramzi, A.B.; Me, M.L.C.; Ruslan, U.S.; Baharum, S.N.; Muhammad, N.A.N. Insight into plant cell wall degradation and pathogenesis of Ganoderma boninense via comparative genome analysis. PeerJ 2019, 7, e8065. [Google Scholar] [CrossRef] [Green Version]
- Govender, N.T.; Mahmood, M.; Seman, I.A.; Wong, M.Y. The phenylpropanoid pathway and lignin in defense against Ganoderma boninense colonized root tissues in oil palm (Elaeis guineensis Jacq.). Front. Plant Sci. 2017, 8, 1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniroh, M.S.; Nusaibah, S.A.; Vadamalai, G.; Siddique, Y. Proficiency of biocontrol agents as plant growth promoters and hydrolytic enzyme producers in Ganoderma boninense infected oil palm seedling. Curr. Plant Biol. 2019, 20, 100116. [Google Scholar] [CrossRef]
- Nusaibah, S.A.; Musa, H. A review report on the mechanism of Trichoderma spp. as biological control agent of basal stem root (BSR) disease of Elaeis guineensis. In Trichoderma—The Most Widely Used Fungicide, 1st ed.; Shah, M.M., Sharif, U., Buhari, T.R., Eds.; IntechOpen: London, UK, 2019; pp. 79–85. [Google Scholar]
- Alexander, A.; Sipaut, C.S.; Dayou, J.; Chong, K.P. Oil palm roots colonization by Ganoderma boninense: An insight study using scanning electron microscopy. J. Oil Palm Res. 2017, 29, 262–266. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in United States; Department of Health and Human Services: Atlanta, GA, USA, 2019; p. 3. [Google Scholar]
- World Health Organization (WHO). Available online: https://www.euro.who.int/en/health-topics/disease-prevention/antimicrobial-resistance/about-amr/global-trends-bacteria (accessed on 6 January 2022).
- Kali, A. Antibiotics and bioactive natural products in treatment of methicillin resistant Staphylococcus aureus: A brief review. Pharmacogn. Rev. 2015, 9, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 2167-0412. [Google Scholar]
- Faraji, M.; Yamini, Y.; Gholami, M. Recent advances and trends in applications of solid-phase extraction techniques in food and environmental analysis. Chromatographia 2019, 82, 1207–1249. [Google Scholar] [CrossRef]
- Otles, S.; Kartal, C. Solid-phase extraction (SPE): Principles and applications in food samples. Acta Sci. Pol. Technol. Aliment. 2016, 15, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartha, G.S.; Toth, G.; Horvath, P.; Kiss, E.; Papp, N.; Kerenyi, M. Analysis of aristolochlic acids and evaluation of antibacterial activity of Aristolochia clematitis L. Biol. Futur. 2019, 70, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Wolf, N.M.; Zhu, T.; Johnson, M.E.; Deng, J.; Cook, J.L.; Fung, L.W.M. Identification of Bacillus anthracis PurE inhibitors with antimicrobial activity. Bioorg. Med. Chem. 2015, 23, 1492–1499. [Google Scholar] [CrossRef]
- Qadir, M.A.; Ahmed, M.; Khaleeq, A. Synthesis, antibacterial and antifungal possession of amino acids containing sulfonamide moieties. Pak. J. Pharm. Sci. 2016, 29, 1609–1613. [Google Scholar]
- Stranix, B.R.; Lavallée, J.F.; Sévigny, G.; Yelle, J.; Perron, V.; LeBerre, N.; Wu, J.J. Lysine sulfonamides as novel HIV-protease inhibitors: Nε-acyl aromatic α-amino acids. Bioorg. Med. Chem. 2006, 16, 3459–3462. [Google Scholar] [CrossRef] [PubMed]
- Vince, R.; Daluge, S.; Brownell, J. Carbocyclic purmocyin: Synthesis and inhibition of protein biosynthesis. J. Med. Chem. 1986, 29, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, H.C.; Joffe, L.S.; Simon, K.S.; Castelli, R.F.; Reis, F.C.; Bryan, A.M.; Rodrigues, M.L. Fenbendazole controls in vitro growth, virulence potential, and animal infection in the Cryptococcus model. Antimicrob. Agents Chemother. 2020, 64, e00286-20. [Google Scholar] [CrossRef]
- Miro-Canturri, A.; Ayerbe-Algaba, R.; Smani, Y. Drug repurposing for the treatment of bacterial and fungal infections. Front. Microbial. 2019, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Ločárek, M.; Nováková, J.; Klouček, P.; Hošt’álková, A.; Kokoška, L.; Gábrlová, L.; Cahlíková, L. Antifungal and antibacterial activity of extracts and alkaloids of selected Amaryllidaceae species. Nat. Prod. Commun. 2015, 10, 1537–1540. [Google Scholar] [CrossRef] [Green Version]
- Livermore, D.M. Tigecycline: What is it, and where should it be used? J. Antimicrob. Chemother. 2005, 56, 611–614. [Google Scholar] [CrossRef] [Green Version]
- Flores, R.; Insel, P.A.; Nizet, V.; Corriden, R. Enhancement of neutrophil antimicrobial activity by the breast cancer drug tamoxifen. FASEB J. 2016, 30, 969–1014. [Google Scholar]
- Paulsen, J.E.; Sundby-Hall, K.; Endresen, L.; Rugstad, H.E.; Reichelt, K.L.; Elgjo, K. The peptide pyroGlu-Gln-Gly-Ser-Asn, isolated from mouse liver, inhibits growth of rat hepatoma cells in vitro. Carcinogenesis 1991, 12, 207–210. [Google Scholar] [CrossRef]
- Fukushima, T.; Kawai, Y.; Urasaki, Y.; Yoshida, A.; Ueda, T.; Nakamura, T. Influence of idarubicinol on the antileukemic effect of idarubicin. Leuk. Res. 1994, 18, 943–947. [Google Scholar] [CrossRef]
- Rimpelová, S.; Zimmermann, T.; Drašar, P.B.; Dolenský, B.; Bejček, J.; Kmoníčková, E.; Jurášek, M. Steroid glycosides hyrcanoside and deglucohyrcanoside: On Isolation, structural identification, and anticancer activity. Foods 2021, 10, 136. [Google Scholar] [CrossRef]
- Li, L.; Shen, Y.M.; Yang, X.S.; Zuo, G.Y.; Shen, Z.Q.; Chen, Z.H.; Hao, X.J. Antiplatelet aggregation activity of diterpene alkaloids from Spiraea japonica. Eur. J. Pharmacol. 2002, 449, 23–28. [Google Scholar] [CrossRef]
- Nakashima, H.; Watanabe, K.; Umegaki, H.; Suzuki, Y.; Kuzuya, M. Cilostazol for the prevention of pneumonia: A systematic review. Pneumonia 2018, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xiao, J.F.; Tuli, L.; Ressom, H.W. LC-MS based metabolomics. Mol. Biosyst. 2012, 8, 470–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2022. Available online: https://eucast.org/clinical_breakpoints/ (accessed on 6 January 2022).
- Chan, Y.S. Antibacterial, Antioxidant and Phytochemical Analysis of Ganoderma boninense. Master’s Thesis, Universiti Malaysia Sabah, Kota Kinabalu, Malaysia, 15 November 2021. [Google Scholar]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, J.M.; Richmond, G.E.; Piddock, L.J. Multidrug efflux pumps in gram-negative bacteria and their role in antibiotic resistance. Futur. Microbiol. 2014, 9, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- El-Fayoumy, E.A.; Shanab, S.M.; Gaballa, H.S.; Tantawy, M.A.; Shalaby, E.A. Evaluation of antioxidant and anticancer activity of crude extract and different fractions of Chlorella vulgaris axenic culture grown under various concentrations of copper ions. BMC Complement. Med. Ther. 2021, 21, 51. [Google Scholar] [CrossRef]
- Dugheri, S.; Marrubini, G.; Mucci, N.; Cappelli, G.; Bonari, A.; Pompilio, I.; Arcangeli, G. A review of micro-solid-phase extraction techniques and devices applied in sample pretreatment coupled with chromatographic analysis. Acta Chromatogr. 2020, 33, 99–111. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerda, V. Solid-phase extraction of organic compounds: A critical review part ii. TrAC Trend Anal. Chem. 2016, 80, 655–667. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, J.; Ma, F.; Tang, C.; Tang, Q.; Zhang, X. Investigation of lignocellulolytic enzymes during different growth phases of Ganoderma lucidum strain using genomic, transcriptomic and secretomic analyses. PLoS ONE 2018, 13, e0198404. [Google Scholar] [CrossRef] [Green Version]
- Wubshet, S.G.; Johansen, K.T.; Nyberg, N.T.; Jaroszewski, L.W. Direct 13C NMR detection in HPLC hyphenation mode: Analysis of Ganoderma lucidum terpenoids. J. Nat. Prod. 2012, 75, 876–882. [Google Scholar] [CrossRef]
- Quereshi, S.; Pandey, A.K.; Sandhu, S.S. Evaluation of antibacterial activity of different Ganoderma lucidum extracts. J. Sci. Res. 2010, 3, 9–13. [Google Scholar]
- Yoon, S.Y.; Eo, S.K.; Kim, Y.S.; Lee, C.K.; Han, S.S. Antimicrobial activity of Ganoderma lucidum extract alone and in combination with some antibiotics. Arch. Pharm. Res. 1994, 17, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.A.; Jansen, R.; Jülich, W.D.; Lindequist, U. Ganomycins A and B, new antimicrobial farnesyl hydroquinones from the basidiomycete Ganoderma pfeifferi. J. Nat. Prod. 2000, 63, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Etame, R.E.; Mouokeu, R.S.; Pouaha, C.L.C.; Kenfack, I.V.; Tchientcheu, R.; Assam, J.P.A.; Ngane, R.A.N. Effect of fractioning on antibacterial activity of Enantia chlorantha Oliver (Annonaceae) methanol extract and mode of action. Evid. Based Complement. Alternat. Med. 2018, 2018, 4831593. [Google Scholar]
- Silva, A.C.O.; Santana, E.F.; Saraiva, A.M.; Coutinho, F.N.; Castro, R.H.A.; Pisciottano, M.N.C.; Albuquerque, U.P. Which approach is more effective in the selection of plants with antimicrobial activity? Evid. Based Complement. Alternat. Med. 2013, 4, 308980. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 2020. Available online: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf (accessed on 6 January 2022).
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, J.R.; Warner, S.L.; Cates, R.G.; Gary, Y.D. Assessment of antimicrobial activity of fourteen essential oils when using dilution and diffusion methods. Pharm. Biol. 2005, 43, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Burman, S.; Bhattacharya, K.; Mukherjee, D.; Chandra, G. Antibacterial efficacy of leaf extracts of Combretum album Pers. against some pathogenic bacteria. BMC Complement. Altern. Med. 2018, 18, 118. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Gan, R.Y.; Zhang, D.; Farha, A.K.; Habimana, O.; Mayumengwana, V.; Corke, H. Large scale screening of 239 traditional Chinese medicinal plant extracts for their antibacterial activities against multidrug resistant Staphylococcus aureus and cytotoxic activities. Pathogens 2020, 9, 185. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, V.K.; Sharma, A.; Baek, K.H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Matijašević, D.; Pantić, M.; Rašković, B.; Pavlović, V.; Duvnjak, D.; Sknepnek, A.; Nikšić, M. The antibacterial activity of Coriolus versicolor methanol extract and its effect on ultrastructural changes of Staphylococcus aureus and Salmonella enteritidis. Front. Microbial. 2016, 7, 1226. [Google Scholar] [CrossRef]
- Younis, A.M.; Yosri, M.; Stewart, J.K. In vitro evaluation of pleiotropic properties of wild mushroom Laetiporus sulphureus. Ann. Agric. Sci. 2019, 64, 79–87. [Google Scholar] [CrossRef]
- Shang, X.; Muthu, M.; Keum, Y.S.; Chun, S.; Gopal, J. An agile, simplified and sonication mediated one-pot aqueous extraction and antibacterial assessment of predominant Korean mushrooms. RSC Adv. 2016, 6, 12143–12157. [Google Scholar] [CrossRef]
- Sadiq, M.B.; Tarning, J.; Aye Cho, T.Z.; Anal, A.K. Antibacterial activities and possible modes of action of Acacia nilotica (L.) Del. against multidrug-resistant Escherichia coli and Salmonella. Molecules 2017, 22, 47. [Google Scholar] [CrossRef] [PubMed]
- Sharafutdinoy, I.S.; Pavlova, A.S.; Akhatova, F.S.; Khabibrakhmanova, A.M.; Rozhina, E.V.; Romanva, Y.J.; Kayumov, A.R. Unraveling the molecular mechanism of selective antimicrobial activity of 2(5H)-furanone derivatives against Staphylococcus aureus. Int. J. Mol. Sci. 2019, 20, 694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Ye, K.P.; Zhang, X.; Pan, D.D.; Sun, Y.Y.; Cao, J.X. Antibacterial activity and mechanism of action of black pepper essential oil on meat-borne Escherichia coli. Front. Microbial. 2017, 7, 2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Liang, W.; Chen, W.; Li, S.; Cui, Y.; Qi, Q.; Zhang, L. Screening and analysis of the marker components in Ganoderma lucidum by HPLC and HPLC-MSn with the aid of chemometrics. Molecules 2017, 22, 584. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Testing: Twenty-First Informational Supplement; CLSI document M100-S21, Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011. [Google Scholar]
- Paula, C.C.; Martins, D.T.O.; Arunachalam, K.; Balogun, S.O.; Borges, Q.I.; Picone, M.G.; Barros, W.M.; Prado, R.M.S. Anti-microbial screening of medicinal plants popularly used Mato Grosso for treating infections: Advances on the evaluation of Conyza bonariensis (L.) Cronquist in vitro and in vivo antibacterial activity. Pharmacogn. J. 2018, 10, 152–166. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Baek, K.H. Antibacterial activity and action mechanism of the essential oil from Enteromorpha linza L. against foodborne pathogenic bacteria. Molecules 2016, 21, 388. [Google Scholar] [CrossRef] [Green Version]
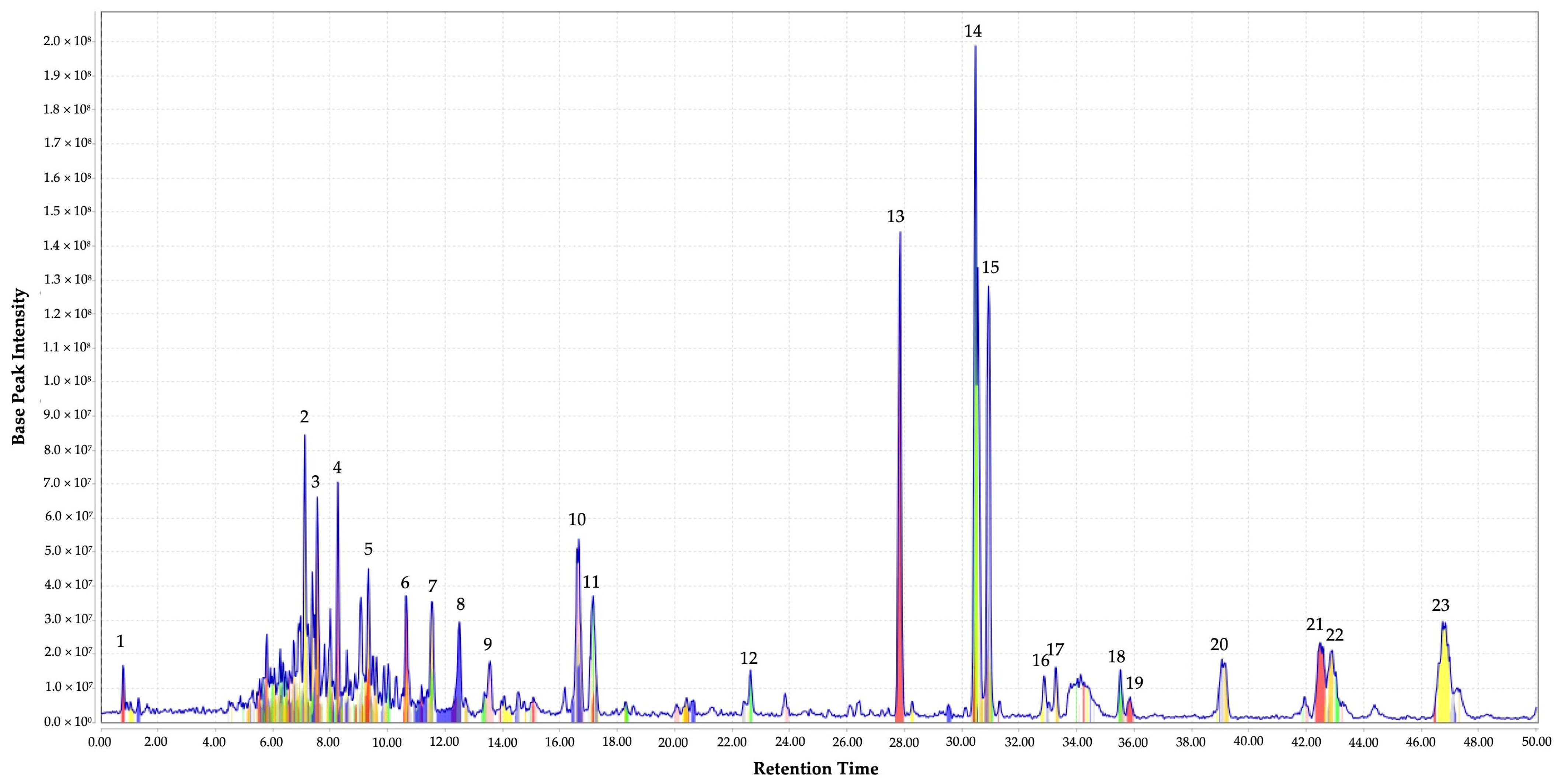

| Peak No | RT (Min) | Measured m/z | Putative Identity | Molecular Formula | Metabolite Class | Peak Height | References |
|---|---|---|---|---|---|---|---|
| 1 | 0.81 | 656.7744 | N-(3-acetamido-5-carbamoyl-2,4,6-triiodobenzoyl)glycine | C12H10I3N3O5 | Amino acid amide | 1.5 × 107 | NA |
| 2 | 7.11 | 515.1974 | Pyroglu-gln-gly-ser-asn | C19H29N7O10 | Peptide | 8.46 × 107 | NA |
| 3 | 7.54 | 499.1844 | Idarubicinol | C26H29NO9 | Glycoside | 6.63 × 107 | NA |
| 4 | 8.26 | 585.2618 | Carbanilic acid, diester with 2,2′-((2-butoxy-3-methoxyphenethyl)nitrilo)diethanol, hydrochloride | C31H40CIN3O6 | Heterocyclic compound | 7.07 × 107 | NA |
| 5 | 9.33 | 499.1782 | 5-O-(Indol-3-ylacetyl-myo-inositol) d-galactoside | C22H29NO12 | Glycoside | 4.53 × 107 | NA |
| 6 | 10.65 | 341.0627 | Aristolochic acid | C17H11NO7 | Isoquinoline alkaloid | 3.70 × 107 | [32] |
| 7 | 11.53 | 543.2351 | Ataralgin | C26H33N5O8 | Amine | 3.55 ×107 | NA |
| 8 | 12.50 | 483.1897 | But-2-enedioic acid; N-ethyl-N-[[4-fluoro-2-(4-fluorophenyl)phenyl]methoxy]-2-phenyl-ethanamine | C27H27F2NO5 | Benzhydryl compound | 2.96 × 107 | NA |
| 9 | 13.56 | 295.0576 | Aminoimidazole ribotide | C8H14N3O7P | Glycoside | 1.81 × 107 | [33] |
| 10 | 16.66 | 517.2613 | Lysine Sulfonamide 11v | C27H39N3O5S | Peptide hydrolases | 5.40 × 107 | [34,35] |
| 11 | 17.15 | 517.2434 | Deglucohyrcanoside | C28H38O9 | Glycoside | 3.72 × 107 | NA |
| 12 | 22.64 | 469.2465 | Carbocyclic puromycin | C23H31N7O4 | Glycoside | 1.56 × 107 | [36] |
| 13 | 27.85 | 279.0042 | Arylmercury | C6H5Hg | Organomercury compound | 1.44 × 108 | NA |
| 14 | 30.48 | 299.0668 | Fenbendazole | C15H13N3O2S | Heterocyclic compound | 1.99 × 108 | [37,38] |
| 15 | 30.93 | 281.05078 | 4,5-Methylenedioxy-6-hydroxyaurone | C16H10O5 | Flavonoid | 1.28 × 108 | NA |
| 16 | 32.86 | 313.1277 | Acetylcaranine | C18H19NO4 | Alkaloids | 1.37 × 107 | [39] |
| 17 | 33.26 | 585.2877 | Tigecycline | C29H39N5O8 | Polycyclic aromatic hydrocarbons | 1.62 × 107 | [40] |
| 18 | 35.52 | 383.0788 | Tetraphenylarsonium | C24H20As | Arsenicals | 1.56 × 107 | NA |
| 19 | 35.87 | 371.2174 | Tamoxifen | C26H29NO | Benzylidene compounds | 7.5 × 106 | [38,41] |
| 20 | 39.06 | 385.1992 | O-Methylandrocymbine | C22H27NO5 | Alkaloids | 1.86 × 107 | NA |
| 21 | 42.49 | 355.21083 | Spirasine I | C22H29NO3 | Terpenoid alkaloids | 2.35 × 107 | NA |
| 22 | 42.92 | 399.2483 | Spiramine A | C24H33NO4 | Terpenoid alkaloids | 2.11 × 107 | NA |
| 23 | 46.75 | 369.2216 | Cilostazol | C20H27N5O2 | Quinolinone | 3.0 × 107 | NA |
| Bacterial Strains | Inhibition Zones (mm) | ||
|---|---|---|---|
| Flush Fraction (FF) | Elute Fraction (EF) | Chloramphenicol | |
| S. pyogenes | 8.75 ± 0.04 c | 30.75 ± 0.03 a | 22.58 ± 0.05 b |
| CoNS | 20.42 ± 0.04 c | 47.25 ± 0.07 a | 30.33 ± 0.03 b |
| MRSA | 14.83 ± 0.01 c | 41.08 ± 0.04 a | 26.67 ± 0.03 b |
| Bacterial Strains | MIC | |
|---|---|---|
| EF (mg mL−1) | Chloramphenicol (µg mL−1) | |
| S. pyogenes | 0.156 | 7.50 |
| CoNS | 0.156 | 3.75 |
| MRSA | 0.078 | 3.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, Y.-S.; Chong, K.-P. Bioactive Compounds of Ganoderma boninense Inhibited Methicillin-Resistant Staphylococcus aureus Growth by Affecting Their Cell Membrane Permeability and Integrity. Molecules 2022, 27, 838. https://doi.org/10.3390/molecules27030838
Chan Y-S, Chong K-P. Bioactive Compounds of Ganoderma boninense Inhibited Methicillin-Resistant Staphylococcus aureus Growth by Affecting Their Cell Membrane Permeability and Integrity. Molecules. 2022; 27(3):838. https://doi.org/10.3390/molecules27030838
Chicago/Turabian StyleChan, Yow-San, and Khim-Phin Chong. 2022. "Bioactive Compounds of Ganoderma boninense Inhibited Methicillin-Resistant Staphylococcus aureus Growth by Affecting Their Cell Membrane Permeability and Integrity" Molecules 27, no. 3: 838. https://doi.org/10.3390/molecules27030838
APA StyleChan, Y.-S., & Chong, K.-P. (2022). Bioactive Compounds of Ganoderma boninense Inhibited Methicillin-Resistant Staphylococcus aureus Growth by Affecting Their Cell Membrane Permeability and Integrity. Molecules, 27(3), 838. https://doi.org/10.3390/molecules27030838






