Abstract
Currently, the pharmaceutical industry is well-developed, and a large number of chemotherapeutics are being produced. These include antibacterial substances, which can be used in treating humans and animals suffering from bacterial infections, and as animal growth promoters in the agricultural industry. As a result of the excessive use of antibiotics and emerging resistance amongst bacteria, new antimicrobial drugs are needed. Due to the increasing trend of using natural, ecological, and safe products, there is a special need for novel phytocompounds. The compounds analysed in the present study include two triterpenoids ursolic acid (UA) and oleanolic acid (OA) and the flavonoid dihydromyricetin (DHM). All the compounds displayed antimicrobial activity against Gram-positive (Staphylococcus aureus ATCC 6538, Staphylococcus epidermidis ATCC 12228, and Listeria monocytogenes ATCC 19115) and Gram-negative bacteria (Escherichia coli ATCC 25922, Proteus hauseri ATCC 15442, and Campylobacter jejuni ATCC 33560) without adverse effects on eukaryotic cells. Both the triterpenoids showed the best antibacterial potential against the Gram-positive strains. They showed synergistic activity against all the tested microorganisms, and a bactericidal effect with the combination OA with UA against both Staphylococcus strains. In addition, the synergistic action of DHM, UA, and OA was reported for the first time in this study. Our results also showed that combination with triterpenoids enhanced the antimicrobial potential of DHM.
1. Introduction
Collectively, humans are gradually reverting to harnessing the benefits of nature, especially plants. To maintain and improve our health, we prefer natural products, including therapeutics. Unfortunately, the excessive and inappropriate use of chemotherapeutic agents has harmful effects on human health. Moreover, the increase in the number of bacterial strains resistant to many commercially available antibiotics has drawn the attention of researchers to search for alternative healing compounds. Many have been tested as new candidate antibacterial drugs, for example, antibacterial peptides produced by microorganisms [1], bacteriophages [2], and phytocompounds [3].
Plants are an abundant source of biologically active compounds, which can be potential therapeutics or precursors for the development of new drugs. Some plants may even produce phytocompounds with antimicrobial and antioxidant activities [4]. Selected substances can inhibit the growth of popular pathogens, such as Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa. Moreover, isolated components from various parts of plants showed antiviral, antitumor, anti-inflammatory, antiplatelet, and prostaglandin-inhibitory activities [5,6]. Furthermore, an increase in environmental pollution, especially from the use of antibiotics, has led to the search for safer medicinal products.
Among the main components of plant extracts that have been used in natural medicine for millennia are triterpenoids, which are the most abundant group of terpenoids found in dicotyledonous plants. Although they do not play a significant role in primary metabolism, they are involved in the adaptation of plants for survival. Some triterpenoids function as specific chemical weapons against competitive plants, pathogens, or herbivores. Previous studies have described the antioxidant, antimicrobial, antiallergic, antidiabetic [7], fungicidal [8], antiparasitic [9], and anticancer [10,11] potential of triterpenes. Other studies have also reported their anti-inflammatory [12], analgesic, hepatoprotective [13,14], cardiotonic, and sedative activity [15]. Triterpenoids are synthesised in plants via squalene cyclisation, which is a C30 hydrocarbon [16]. Based on their structural skeleton, triterpenes are classified into several groups: cucurbitanes, cycloartanes, dammaranes, euphanes, fiedelanes, holostanes, hopanes, isomalabaricanes, lanostanes, lupanes, oleananes, protostanes, tirucallanes, and ursanes [17]. The representative pentacyclic triterpenoids are oleanolic acid (OA) and ursolic acid (UA). They are commonly found in nature in free acid form or as an aglycone precursor for a triterpenoid called saponin [14]. The chemical structures of these phytocompounds are shown in Figure 1A–C. These pentacyclic triterpenoids affect the expression of bacterial genes involved in biofilm formation, peptidoglycan turnover, and cell autolysis [18]. In addition, OA and UA along with their derivatives have strong antimutagenic effects [19]. Owing to their similar structures, UA and OA often occur simultaneously. In addition, the activities of these triterpenes also include their potential to enhance bacterial susceptibility to other compounds [20].

Figure 1.
The chemical structures of (A) ursolic acid; (B) oleanoic acid; (C) dihydromyricetin.
Dihydromyricetin (DHM) belongs to the flavonoid family, whose chemical structure is shown in Figure 1C. It is a major secondary metabolite of the plant Ampelopsis grossedentata, which is widely distributed in the mountainous areas of China and has been used in herbal medicine for centuries [21]. Previous studies have shown many valuable properties of this compound, including antioxidant [21], anti-inflammatory [22], antibacterial [23,24,25,26], neuroprotective [27], anticancer [28], and metabolic regulation of glucose and lipids [29]. It is apparent that DHM has a wide range of prospects in the food industry as an antioxidant and antibacterial agent. However, the antimicrobial mechanism of this compound has not been adequately investigated [30]. Previous reports have confirmed that hydroxylation at positions 5 and 7 of their structure can play a key role in the antibacterial activity of flavanols, and that hydroxylation of the B and C rings can enhance it [31].
However, there are many contrasting data in the literature. The reason for this may be the test methods used, the purity of the phytocompound, and/or the bacterial strain used.
In addition to the possibility of using phytochemicals in medicine, their application in the food industry is also of interest, where food-borne diseases still pose a threat. In recent years, synthetic preservatives have been commonly used in food because of their low cost and high antimicrobial activity. However, increasing consumer demand for safe, unprocessed foods and prolonged storage time is mobilizing the food industry to introduce natural antimicrobial components as synthetic preservative replacements.
The aim of this study was to investigate the antibacterial activity of phytocompounds (UA, OA, and DHM) individually and in various combinations. Finally, the cytotoxic activity of the phytocompounds was determined in vitro using mammalian cells.
2. Results and Discussion
2.1. Antimicrobial Activity of Phytocompunds
Selected phytocompounds were tested for antimicrobial activity against six bacterial strains (S. aureus, S. epidermidis, L. monocytogenes, E. coli, P. hauseri, and C. jejuni) using the microdilution method. The antibacterial potential of UA, OA, and DHM is shown in Figure 2, Figure 3 and Figure 4 and Table S1.

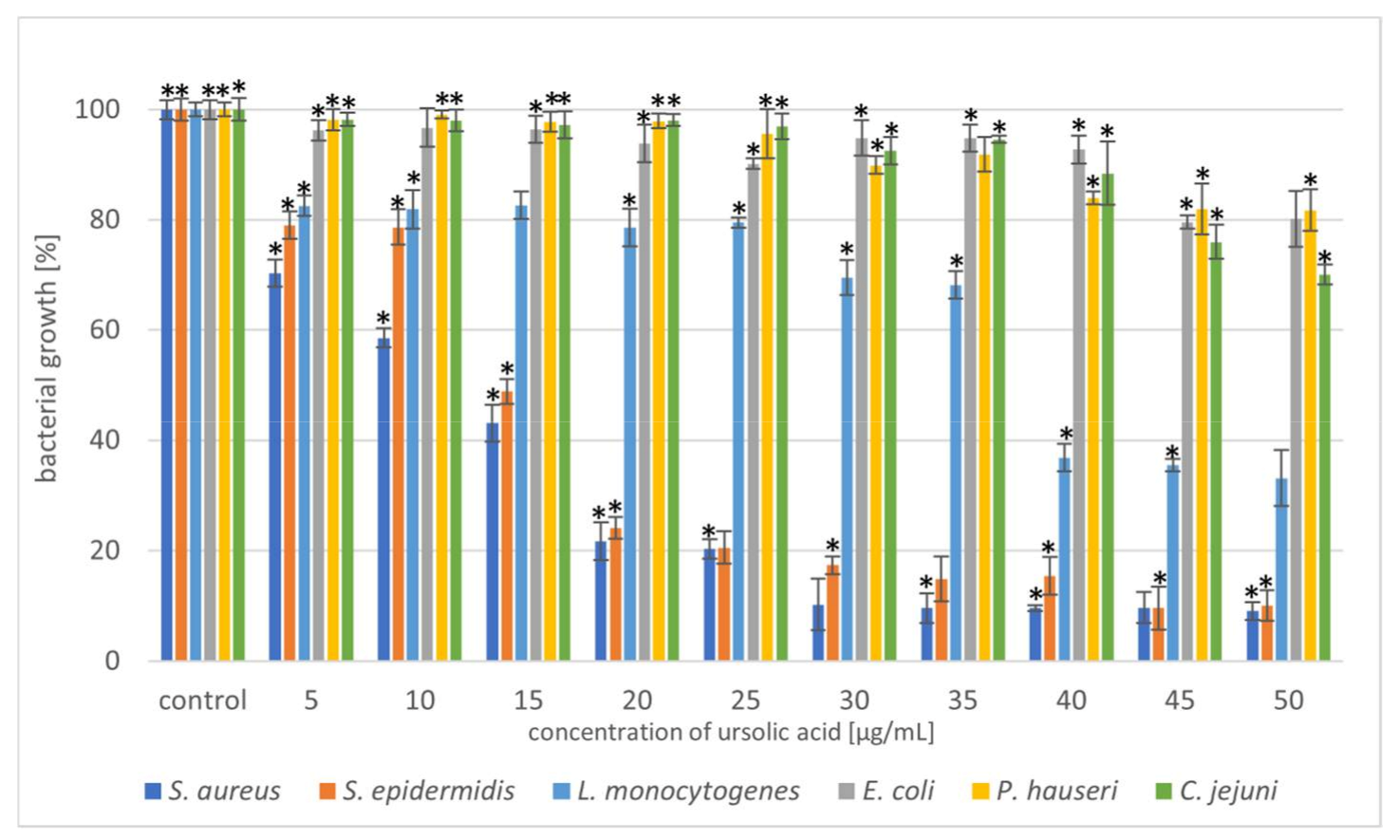
Figure 2.
Reduction in bacterial growth after ursolic acid treatment and incubation for 24 h. The data represent mean ± SD of the three different experiments performed in triplicate. p-values were determined by one-way analysis of variance (ANOVA), where (*) represents statistically significant results (p ≤ 0.05).

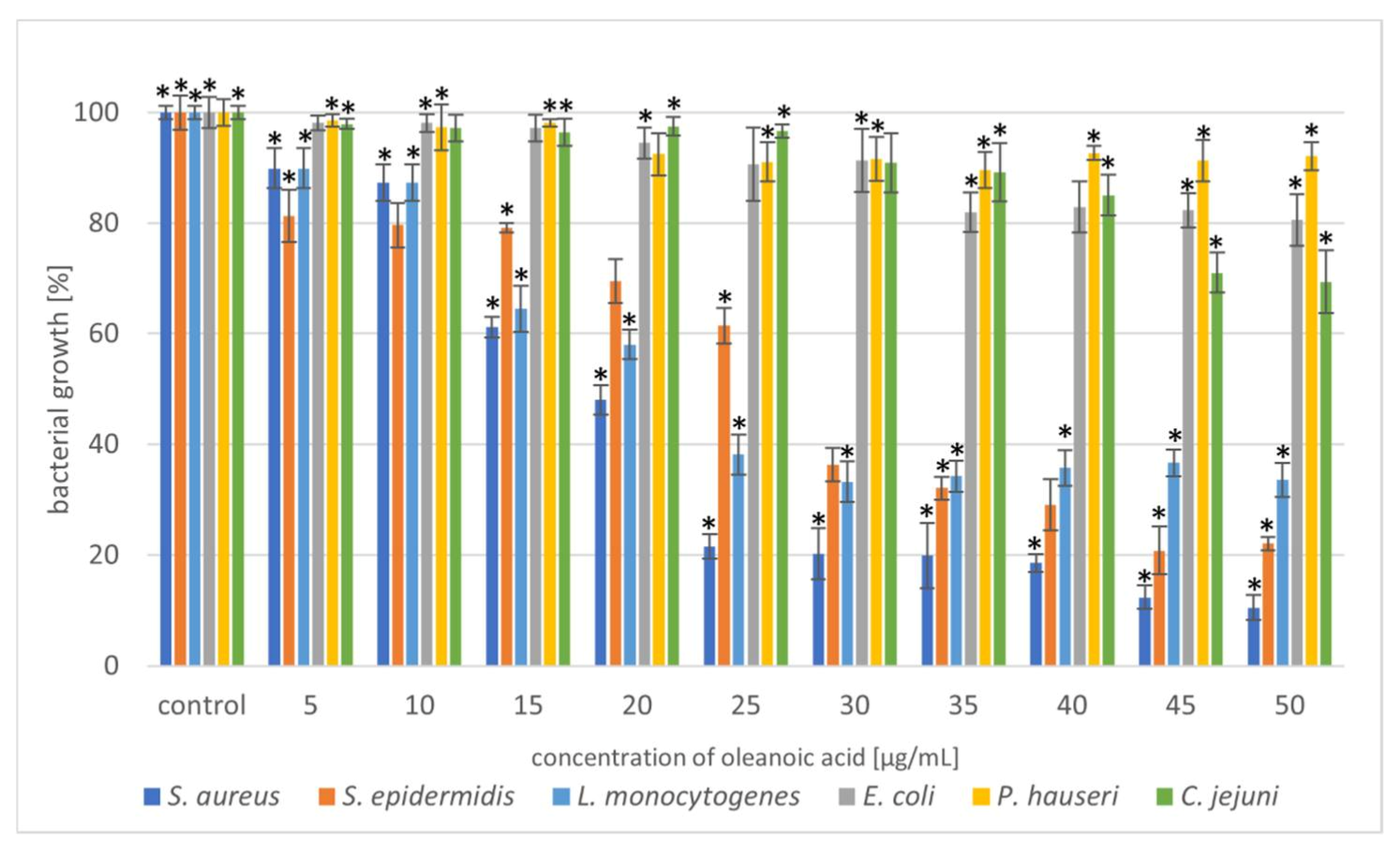
Figure 3.
Reduction in bacterial growth after oleanoic acid treatment and incubation for 24 h. The data represent mean ± SD of three different experiments performed in triplicate. p-values were determined by one-way analysis of variance (ANOVA), where (*) represents statistically significant results (p ≤ 0.05).

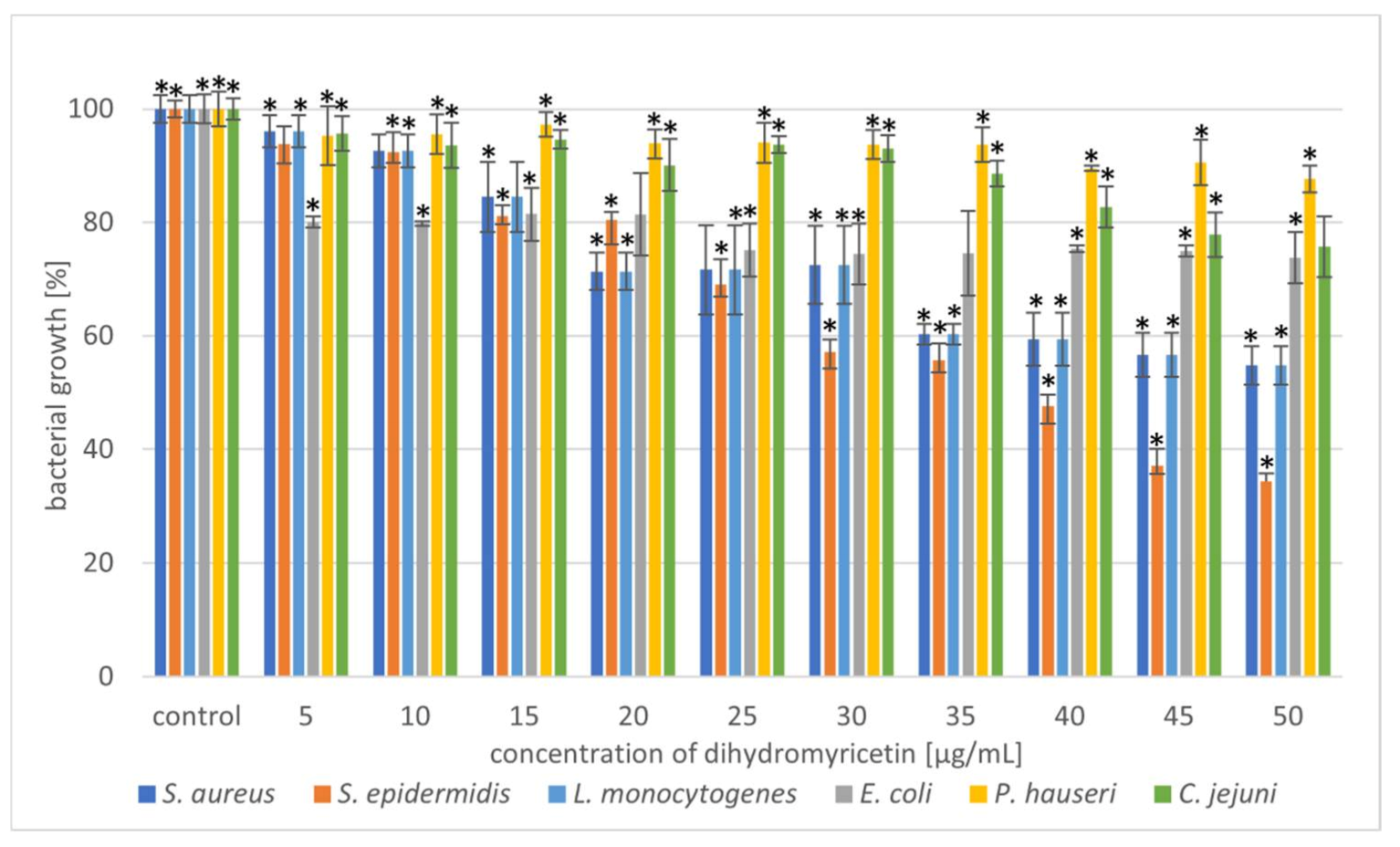
Figure 4.
Reduction in bacterial growth after dihydromyricetin treatment and incubation for 24 h. The data represent mean ± SD of three different experiments performed in triplicate. p-values were determined by ANOVA where (*) represents statistically significant results (p ≤ 0.05).
Triterpenoids showed great activity toward Gram-positive strains. The addition of UA (20 µg/mL) reduced the growth of the Gram-positive strains S. aureus and S. epidermidis by 80%. In the case of the Gram-negative strain, L. monocytogenes, a higher concentration (40 µg/mL) of the compound resulted in 60% growth inhibition (Figure 2). Moreover, we also noted that OA was more effective against Gram-positive bacteria (Figure 3). These similar effects of the tested triterpenoids may be due to their related structures. However, higher concentrations of the compound had to be added, compared to UA. Previous studies have demonstrated high antibacterial activity of UA [15,32,33] and its derivatives [34] against S. aureus. Methicillin-resistant S. aureus (MRSA) infections are a serious problem in hospitalised patients. Olean-27-carboxylic acid-type triterpenes possess antibacterial activity against various MRSA strains as well as quinolone-resistant S. aureus [35]. In addition, the antimicrobial potential of UA and OA against L. monocytogenes have also been confirmed [36,37]. However, Panizzi et al. [38] described the lack of OA (from Geum rivale) activity against S. aureus, E. coli, and P. aeruginosa. Similarly, Calis et al. [39] did not observe antibacterial activity of OA (from Cyclamen mirabile) against Gram-positive (S. aureus and E. faecalis) and Gram-negative (P. aeruginosa and E. coli) bacteria. The diversity of the antimicrobial properties of UA and OA has also been observed against Mycobacterium tuberculosis, which is the most common cause of deaths worldwide [40,41]. In addition, bacteria, such as E. faecalis [42], Streptococcus pneumoniae [33], E. faecium, Bacillus subtilis, and B. cereus [32,42,43] are sensitive to OA treatment. Cunha et al. [43] investigated the antimicrobial effect of OA against Streptococcus strains (S. mutans, S. sanguis, S. mitis, and S. salivarius) and determined that both the hydroxy and carboxy groups in triterpenes were responsible for OA antimicrobial activity. It has been proven that the tested pentacyclic triterpenoids affect peptidoglycan structure, gene expression, and biofilm formation [44,45] in bacteria. Another mechanism of action can be associated with the induction of stress response. Grudniak et al. [46] showed that E. coli treated with OA altered the synthesis of DnaK, thus inducing a heat-shock response in this species. When Zhou et al. [47] used TEM to analyse the morphological changes in MRSA cells after treating them with UA and oxacillin, they observed cell membrane disintegration, cell lysis, and cytoplasmic content release.
In the present study, the triterpenoids showed less activity toward Gram-negative bacteria. The addition of UA (50 µg/mL) limited the growth of E. coli, P. hauseri, and C. jejuni by 20–30%, whereas that of OA (50 µg/mL) inhibited the growth of the Gram-negative strains by 10–30%. Some studies have described poor UA activity against E. coli [48]. Mallavadhani et al. [49] showed moderate activity of UA and its lipophilic 3-O fatty acid ester chains (C12-C18) against Gram-negative strains (E. coli, S. typhi, and P. syringae). Interestingly, it has been reported that UA (50 mg/mL) could be a therapeutic agent for treating Helicobacter pylori infections [50].
Based on the results shown in Figure 2 and Figure 3, the triterpenoids were more active against Gram-positive bacteria than Gram-negative bacteria. This may be due to the structural differences in the cell walls of these bacterial classes. Cells of Gram-negative bacteria are surrounded by an additional outer membrane, which provides them with a hydrophilic surface that functions as a permeability barrier against many substances, including natural compounds [51,52]. Moreover, the intrinsic resistance in Gram-negative bacteria is supported by efflux pumps which pump many compounds, such as toxins and antibiotics from the periplasm to the outside of the cell [53,54].
The next part of our experiment investigated the antibacterial potential of DHM against the tested bacterial strains. The results showed that with an increase in DHM concentration, the inhibition level increased. The strain most sensitive to the action of DHM was S. epidermidis. At a concentration of 45–50 µg/mL, growth inhibition was 65%. Other Gram-positive bacteria showed moderate sensitivity to the phytocompounds, but only at higher concentrations (35–50 µg/mL). Wu et al. [23] has previously described the antibacterial potential of DHM against S. aureus. Moreover, we observed that the Gram-negative strains were weakly susceptible to DHM at each concentration range. Addition of DHM at a concentration of 50 µg/mL caused a 26%, 13%, and 24% growth reduction of E. coli, P. hauseri, and C. jejuni, respectively. Xiao et al. [55] demonstrated that DHM has great antibacterial activity against tested food-borne bacteria (S. aureus, B. subtilis, E. coli, S. paratyphi, and P. aeruginosa). Moreover, SEM analysis (E. coli and S. aureus) suggested that DHM induces aggregation, shrivelling, and adhesion of bacteria. Cui et al. [56] performed SEM analysis of E. coli treated with DHM and reported that the cell had a wrinkled surface and was lysed at both ends. Apart from disturbing the integrity of the cellular membrane, DHM can also inhibit the respiratory metabolism of bacteria [55].
2.2. Combined Antimicrobial Activity of Selected Phytocompounds
Given the issue of multidrug-resistant strains, new ways to eliminate these pathogens must be developed, such as combinatorial therapy using phytocompounds. Therefore, studying the synergistic effects of the compounds has become a key step in phytochemical studies [57,58].
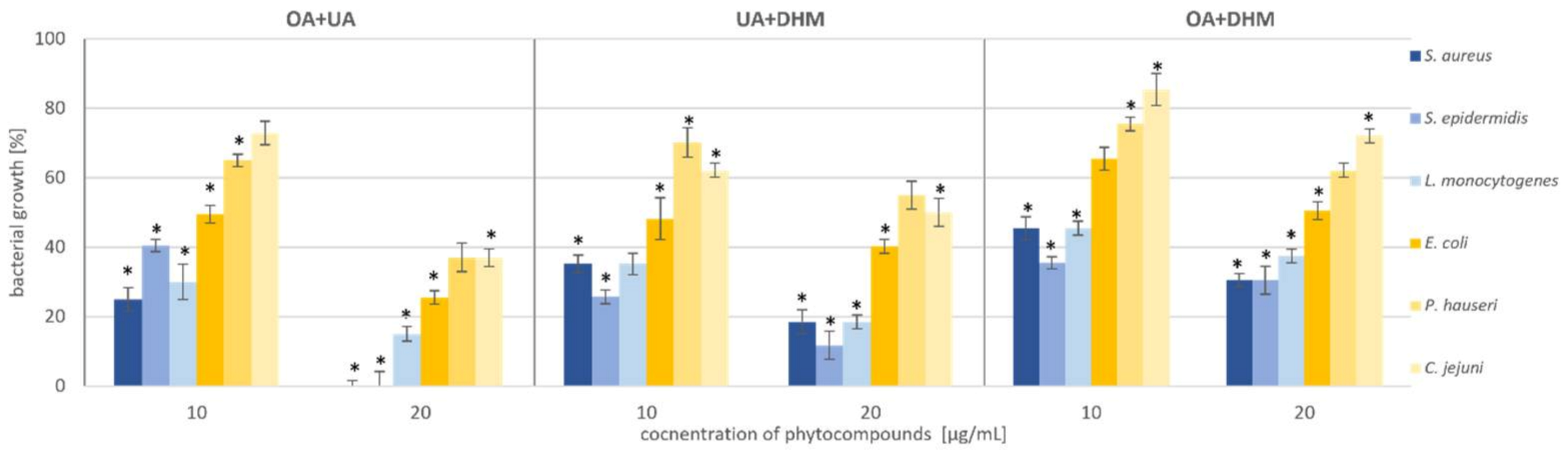
Therefore, we tested the antimicrobial synergy of the selected phytocompounds against the test strains. The results are presented in Figure 5. The most representative synergistic effect was observed by the treatment of 20 µg/mL OA in combination with 20 µg/mL UA against both the Staphylococcus strains, with bactericidal effect (Figure 5, Table 1, Table S2). Based on the previous experiment, we found that DHM had the weakest effect on the tested bacteria (Figure 4, Table 1). Our results showed that combination with triterpenoids (UA or OA) enhanced the antimicrobial potential of DHM. Coadministration of UA and DHM (20 µg/mL each) considerably reduced the growth of Gram-negative strains. The addition of both UA and DHM reduced bacterial growth by 60% for E. coli and approximately 45% for P. hauseri and C. jejuni (Figure 2 and Figure 4, Table 1). However, when UA and DHM were tested individually, bacterial growth was inhibited by only 7% and 6%, respectively (Figure 5, Table 1). In the case of Gram-negative strains, treatment with phytocompounds (10 and 20 µg/mL) alone did not inhibit bacterial growth. Thus, only a combination of these compounds yielded satisfactory results.

Figure 5.
Synergistic effect of phytocompounds (OA+UA; UA+DHM; OA+DHM) against Gram-positive (S. aureus, S. epidermidis, and L. monocytogenes) and Gram-negative (E. coli, P. hauseri, and C. jejuni) bacteria. The data represent mean ± SD of the three different experiments performed in triplicate. p-values were determined by ANOVA, where (*) represents statistically significant results (p ≤ 0.05).

Table 1.
Antibacterial activity of the tested phytocompounds: ursolic acid, oleanoic acid, dihydromyricetin against Staphylococcus aureus ATCC 6538 (S. a.), Staphylococcus epidermidis ATCC 12228 (S. e.), Listeria monocytogenes ATCC 19115 (L. m.), Escherichia coli ATCC 25922 (E. c.), Proteus hauseri ATCC 15442 (P. h.) and Campylobacter jejuni ATCC 33560 (C. j.) after phytocompound treatment and incubation for 24 h.
We concluded that the most promising results of synergistic effects were obtained in a combination of UA and OA. The data also indicated the possibility of reducing the dosage of the compounds.
2.3. Assessment of the Cytotoxic Activity of Selected Phytocompounds
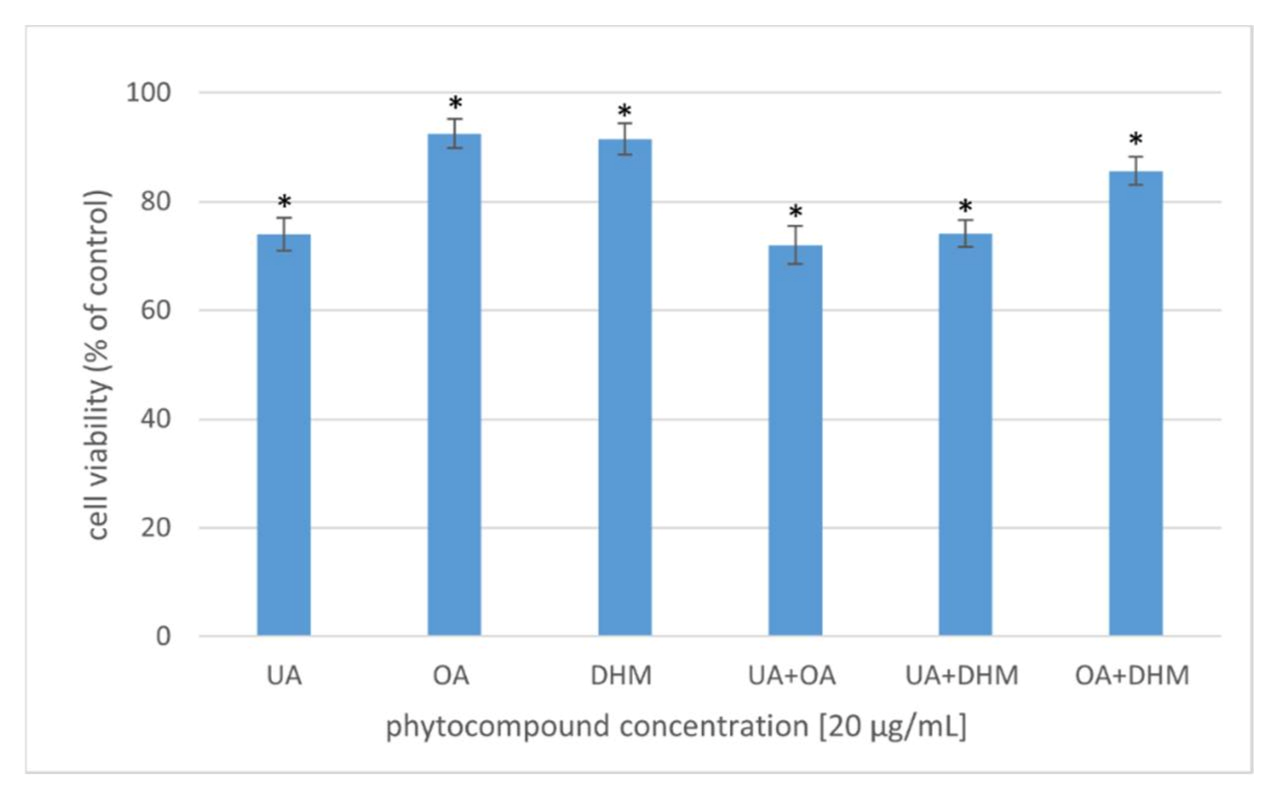
A safe antibacterial agent should be nontoxic to eukaryotic cells and show robust activity against microorganisms. The cytotoxic activity of the phytocompounds was studied in several variants at 20 µg/mL concentration (Figure 6). The effect of UA, OA, and DHM (individual or simultaneous addition to the bacterial culture) on the viability of human fibroblasts was assessed via the MTT assay. The percentage of viable cells was computed relative to that of the control (cells incubated without phytocompounds), the viability of which was considered 100%. After 24 h of incubation of the cells with the phytocompounds alone, cell viability slightly decreased to 90% after OA and DHM treatment and to 74% after UA treatment. Fibroblast viability reduced to 72%, 74%, and 85% in the cell cultures supplemented with two of the compounds (synergistic effect): UA+OA, UA+DHM, and OA+DHM, respectively. Wójciak-Kosior et al. [59] analysed the cytotoxic activity of UA and OA against human skin fibroblasts and reported higher cytotoxic activity of UA compared to that of OA. A similar relationship was observed in the present study. Zhang et al. [60], however, reported no cytotoxic effect of UA against HCT-8 and Bell-7402 cell lines. DHM has been shown to exhibit selective cytotoxicity against non-small-cell lung cancer cells (A549 and H1975) but not against normal cells (WI-38) [61] (Kao et al., 2017). DHM has also been shown to be noncytotoxic to normal hepatocytes [62,63], but it inhibited hepatocellular carcinoma (HCC) cell proliferation and triggered apoptosis in a p53-dependent manner [63].

Figure 6.
Viability of human fibroblasts after 24 h incubation with the phytocompounds [20 µg/mL]. The results were expressed as a percentage of viability of the untreated cells. The data represent mean ± SD of the three different experiments performed in triplicate. p-values were determined by ANOVA, where (*) represents statistically significant results (p ≤ 0.05).
3. Materials and Methods
3.1. Reagents
Phytocompounds (HPLC purity ≥ 98%), including UA, OA, and DHM, were purchased from Sigma-Aldrich (St. Louis, MO, USA). 3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (Darmstadt, Germany). The human fibroblast BJ (CRL-2522) cell line was purchased from the American Type Culture Collection (ATCC®, Manassas, VA, USA). Dulbecco’s Modified Eagle Medium (DMEM) and Fetal Bovine Serum (FBS) were obtained from BioWest (Nuaillé, France). DMSO was purchased from BioShop (Burlington, ON, Canada).
3.2. Determination of Antimicrobial Activity
The antimicrobial activity of UA, OA, and DHM was evaluated using the microdilution method for aerobic and anaerobic bacterial strains according to the CLSI documents M07 (11th Edition) [64] and M11 (9th Edition) [65], respectively. The antimicrobial activity of the phytocompounds was determined against aerobic bacteria (S. aureus ATCC 6538, S. epidermidis ATCC 12228, E. coli ATCC 25922, and P. hauseri ATCC 15442) and anaerobic bacteria (L. monocytogenes ATCC 19115 and C. jejuni ATCC 33560). The growth of the aerobic and anaerobic bacterial strains that were either treated with the phytocompounds or left untreated was evaluated in 96-well microtiter plates in Mueller–Hinton broth and Brucella broth supplemented with hemin, vitamin K1, and laked horse blood, respectively. The phytocompounds were supplemented in a range of 5–50 µg/mL, and their antimicrobial potential was determined. They were diluted in the appropriate growth medium before administration. An inoculum of bacteria grown in the Mueller–Hinton or Brucella broths was added to each well to achieve a final density of 5 × 105 CFU/mL and 1 × 106 CFU/mL for the aerobic and anaerobic strains, respectively. The microtiter plates were then incubated for 24 h at 37 °C (aerobic strains) and for 48 h at 37 °C (anaerobic strains). The plates inoculated with anaerobic strains were incubated in jars where anaerobic conditions were achieved using GasPak envelopes and monitored with a disposable BBL dry anaerobic indicator strip (Becton Dickinson). After incubation, the optical density was measured spectrophotometrically at 620 nm. Experiments for each type of phytocompound were performed in triplicate, leading to the analysis of six independent experiments. The antimicrobial activity of the tested compounds was calculated as the percentage of bacterial growth inhibition (SD) compared to that of the biotic control (bacteria incubated in the medium).
3.3. Determination of Cytotoxic Activity of Phytocompounds
The cytotoxic potential of phytocompounds against human cells was examined using the fibroblast BJ ATCC CRL-2522 cell line. Cells were suspended in Dulbecco’s modified Eagle medium (DMEM) containing foetal bovine serum (10%) and antibiotics (penicillin 100 IU/mL and streptomycin 100 μg/mL) at a final density of 1 × 105 cells/well. The final volume of the culture was 100 μL. The fibroblasts were incubated in 96-well microplates in a humidified atmosphere at 37 °C and 5% CO2 for 24 h. After incubation, exhausted DMEM was replaced with fresh medium and phytocompounds were supplemented at 10–20 μg/mL. Adequate control cultures that were untreated with the selected phytocompounds were also prepared and the plates were incubated under the same conditions. After 24 h of incubation, the media were removed from the cells and the wells were supplemented with 500 μg/mL MTT. Following a 2 h incubation under the same conditions, the solution was removed from the wells, which were then refilled with 100 μL of sterile DMSO to dissolve the formazan crystals. The viability of fibroblasts was calculated based on the spectrophotometric quantification of the cultures at λ = 550 nm using a SpectraMax i3x multimode microplate reader (Molecular Devices Ltd., Wokingham, Berkshire, UK). The results are presented as the mean values of the percentages of fibroblast viability in control cultures untreated with phytocompounds with the SD. The experiment, with n = 4, was performed in triplicate.
In addition, the combined effects of the tested compounds on the bacteria were investigated. This allowed for the selection of the best combination of two compounds, which could allow for reduction in dosage.
4. Conclusions
Nature is a rich source of plant resources that possess bioactive phytocompounds. As such, medicinal phytocompounds are one of the best sources for obtaining new therapeutics that would be clinically effective, biodegradable, and safe for human use. The present work enabled us to document the antibacterial activity of UA, OA, and DHM individually and in combination with each other to test for synergy. Triterpenoids (UA and OA) proved to be successful antibacterial agents, particularly in Gram-positive strains, and considering the cytotoxicity results, all the tested phytocompounds would be safe for use in the pharmaceutical industry.
Supplementary Materials
The following supporting information can be downloaded. Table S1: Antibacterial activity of the tested phytocompounds: ursolic acid, oleanoic acid, dihydromyricetin against Staphylococcus aureus ATCC 6538 (S. a.), Staphylococcus epidermidis ATCC 12228 (S. e.), Listeria monocytogenes ATCC 19115 (L. m.), Escherichia coli ATCC 25922 (E. c.), Proteus hauseri ATCC 15442 (P. h.) and Campylobacter jejuni ATCC 33560 (C. j.) after phytocompound treatment and incubation for 24 h. Table S2: Synergistic antibacterial activity of the tested phytocompounds: ursolic acid, oleanoic acid, dihydromyricetin against Staphylococcus aureus ATCC 6538 (S. a.), Staphylococcus epidermidis ATCC 12228 (S. e.), Listeria monocytogenes ATCC 19115 (L. m.), Escherichia coli ATCC 25922 (E. c.), Proteus hauseri ATCC 15442 (P. h.) and Campylobacter jejuni ATCC 33560 (C. j.) after phytocompound treatment and incubation for 24 h.
Author Contributions
Conceptualization, N.W. and K.L.; methodology, N.W. and K.Z.; investigation, M.S., N.W. and K.Z.; writing—original draft preparation, N.W.; writing—review and editing, K.L. and N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Lodz, B2111000000036.01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 2559–2580. [Google Scholar] [CrossRef] [PubMed]
- Romero-Calle, D.; Benevides, R.G.; Goes-Neto, A.; Billington, C. Bacteriophages as alternatives to antibiotics in clinical care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshshy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Nunes, C.; Barreto Arantes, M.; Menezes de Faria Pereira, S.; Leandro da Cruz, L.; de Souza Passos, M.; Pereira de Moraes, L.; Vieira, I.; Barros de Oliveira, D. Plants as sources of anti-inflammatory agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef]
- Beg, S.; Swain, S.; Hasan, H.; Barkat, M.A.; Hussain, M.S. Systematic review of herbals as potential anti-inflammatory agents: Recent advances, current clinical status and future perspectives. Pharmacogn. Rev. 2011, 5, 120–137. [Google Scholar] [CrossRef]
- Ortiz-Andrade, R.R.; García-Jiménez, S.; Castillo-España, P.; Ramírez-Ávila, G.; Villalobos-Molina, R.; Estrada-Soto, S. α-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: An anti-hyperglycemic agent. J. Ethnopharmacol. 2007, 109, 48–53. [Google Scholar] [CrossRef]
- Becker, H.; Scher, J.M.; Speakman, J.B.; Zapp, J. Bioactivity guided isolation of antimicrobial compounds from Lythrum salicaria. Fitotherapia 2005, 76, 580–584. [Google Scholar] [CrossRef]
- Cunha, W.R.; Martins, C.; Ferreira de Silva, D.; Crotti, A.E.; Lopez, N.P.; Albuqureque, S. In vitro trypanocidal activity of triterpenes from Miconia species. Planta Med. 2003, 69, 470–472. [Google Scholar] [CrossRef]
- Ghante, M.H.; Jamkhande, P.G. Role of pentacyclic triterpenoids in chemoprevention and anticancer treatment: An overview on targets and underling mechanisms. J. Pharmacopunct. 2019, 22, 55–67. [Google Scholar] [CrossRef]
- Hoenke, S.; Christoph, M.A.; Friedrich, F.; Heise, N.; Brandes, B.; Deigner, H.P.; Al-Harrasi, A.; Csuk, R. The presence of a cyclohexyldiamine moiety confers cytotoxicity to pentacyclic triterpenoides. Molecules 2021, 26, 2102. [Google Scholar] [CrossRef]
- Andre, C.M.; Greenwood, J.M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Evers, D.; Perry, N.B.; Laing, W.A. Anti-inflammatory procyanidins and triterpenes in 109 apple varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Li, D.; Ma, H.; Mu, Y.; Zheng, D.; Huang, X.; Li, L. Chemical characterization and hepatoprotective effects of a standardized triterpenoid-enriched guava leaf extract. J. Agric. Food Chem. 2021, 69, 3626–3637. [Google Scholar] [CrossRef]
- Udayama, M.; Ohkawa, M.; Yoshida, N.; Kinjo, J.; Nohara, T. Structures of three new oleanane glucuronides isolated from Lathyrus palustris var. pilosus and hepatoprotective activity. Chem. Pharm. Bull. 1998, 46, 1412–1415. [Google Scholar] [CrossRef][Green Version]
- Nascimento, P.G.G.; Lemos, T.L.G.; Bizerra, A.M.C.; Arriaga, A.M.C.; Ferreira, D.A.; Santiago, G.M.P.; Braz-Filho, R.; Costa, J.G.M. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial activity of oleanoic and ursolic acids: An update. Evid. Based Complementary Altern. 2015, 2015, 620472. [Google Scholar] [CrossRef]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2012, 29, 780–818. [Google Scholar] [CrossRef]
- Wolska, K.I.; Grudniak, A.M.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial activity of oleanoic and ursolic acid and their derivatives. Cent. Eur. J. Biol. 2010, 5, 543–553. [Google Scholar] [CrossRef]
- Lira, W.M.; dos Santos, F.V.; Sannomiya, M.; Rodrigues, C.M.; Vilegas, W.; Varanda, E.A. Modulatory effect of Byrsonima basiloba extracts on the mutagenicity of certain direct and indirect-acting mutagens in Salmonella typhimurium assays. J. Med. Food 2008, 11, 111–119. [Google Scholar] [CrossRef]
- Kurek, A.; Nadkowska, P.; Pliszka, S.; Wolska, K.I. Modulation of antibiotic resistance in bacterial pathogens by oleanoic acid and ursolic acid. Phytomedicine 2012, 19, 515–519. [Google Scholar] [CrossRef]
- Gao, Q.; Ma, R.; Chen, L.; Shi, S.; Cai, P.; Zhang, S.; Xiang, H. Antioxidant profiling of vine tea (Ampelopsis grossedentata): Off-line coupling heart-cutting HSCCC with HPLC-DAD-QTOF-MS/MS. Food Chem. 2016, 225, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.N.; Ma, J.; Wang, K.S.; Mi, C.L.; Lv, Y.; Piao, L.X.; Jin, X.J. Dihydromyricetin suppresses TNF-alpha-induced NF-kappa B activation and target gene expression. Mol. Cell Biochem. 2016, 422, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bai, J.; Zhong, K.; Huamg, Y.; Gao, H. A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R,3R-dihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem. 2017, 218, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Gosch, C.; Nagesh, K.M.; Thill, J.; Miosic, S.; Plaschil, S.; Milosevic, M. Isolation of dihydroflavonol 4-reductase cdna clones from angelonia x angustifolia and heterologous expression as gst fusion protein in Escherichia coli. PLoS ONE 2014, 9, e107755. [Google Scholar] [CrossRef]
- Ameen, F.; Alyahya, S.A.; Bakhrebah, M.A.; Nassar, M.S.; Aljuraifani, A. Flavonoid dihydromyricetin-mediated silver nanoparticles as potential nanomedicine for biomedical treatment of infections caused by opportunistic fungal pathogens. Res. Chem. Intermed. 2018, 44, 1–11. [Google Scholar] [CrossRef]
- Dalcin, A.J.F.; Santos, C.G.; Gϋndel, S.S.; Roggia, I.; Raffin, R.P.; Ourique, A.F.; Santos, R.C.V.; Gomes, P. Anti biofilm effect of dihydromyricetin-loaded nanocapsules on urinary catheter infected by Pseudomonas aeruginosa. Colloid Surf. B 2017, 156, 282–291. [Google Scholar] [CrossRef]
- Ren, Z.X.; Zhao, Y.F.; Cao, T.; Zhen, X.C. Dihydromyricetin protects neurons in an MPTP-induced model of Parkinson’s disease by suppressing glycogen synthase kinase-3 beta activity. Acta Pharmacol. Sin. 2017, 38, 1315–1324. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Zhang, H.Q.; Chen, S.Y.; Xu, Y.; Yao, A.J.; Liao, Q.; Zhang, X.H. Dihydromyricetin induces mitochondria-mediated apoptosis in HepG2 cells through down-regulation of the Akt/Bad pathway. Nutr. Res. 2017, 38, 27–33. [Google Scholar] [CrossRef]
- Williams, J.; Ensor, C.; Gardner, S.; Smith, R.; Lodder, R. Bsn723t prevents atherosclerosis and weight gain in apoE knockout mice fed a western diet. Webmedcentral 2015, 6, 5034. [Google Scholar]
- Huang, W.; Xie, J. Antibacterial effect of dihydromyricetin on specific spoilage organisms of hybrid grouper. J. Food Qual. 2021, 2021, 5569298. [Google Scholar] [CrossRef]
- Echeverria, J.; Opazo, J.; Mendoza, L.; Urzua, A.; Wilkens, M. Structure-activity and lipophilicity relationships of selected antibacterial natural flavanones of Chilean flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.M. Ursolic, oleanoic and betulic acids:antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef]
- Woldemichael, G.M.; Franzblau, S.G.; Zhang, F.; Wang, Y.; Timmermann, N. Inhibitory effect of sterols from Ruprechtia trifloral and diterpenes from Calceolaria pinnifolia on the growth of Mycobacterium tuberculosis. Planta Med. 2003, 69, 628–631. [Google Scholar] [CrossRef]
- Setzer, W.N.; Rozmus, G.F.; Setzer, M.C.; Schmidt, J.M.; Vogler, B.; Reeb, S.; Jackes, B.R.; Irvine, A.K. Bioactive principles in the bark of Philidiostigma tropicum. J. Mol. Model. 2006, 12, 703–711. [Google Scholar] [CrossRef]
- Zheng, C.J.; Sohn, M.J.; Kim, K.Y.; Yu, H.E.; Kim, W.G. Olean-27-carboxylic acid-type triterpenes with potent antibacterial activity from Aceriphyllum rossi. J. Agric. Food Chem. 2008, 56, 11752–11756. [Google Scholar] [CrossRef]
- Kurek, A.; Markowska, K.; Grudniak, A.M.; Janiszowska, W.; Wolska, K.I. The effect of oleanoic and ursolic acids on the hemolytic properties and biofilm formation of Listeria monocytogenes. Pol. J. Microbiol. 2014, 63, 21–25. [Google Scholar] [CrossRef]
- Kurek, A.; Grudniak, A.M.; Szwed, M.; Klicka, A.; Samluk, Ł.; Wolska, K.I.; Janiszowska, W.; Popowska, M. Oleanoic acid and ursolic acid effect peptidoglycan metabolism in Listeria monocytogenes. Anton. Leeuw. 2010, 97, 61–68. [Google Scholar] [CrossRef]
- Panizzi, L.; Catalano, S.; Miarelli, C.; Cioni, P.L.; Campeol, E. In vitro antimicrobial activity of extracts and isolated constituents of Geum rivale. Phytother. Res. 2000, 14, 561–563. [Google Scholar] [CrossRef]
- Calis, T.; Satana, M.E.; Yürüker, A.; Kelican, P.; Demirdamar, R.; Alacam, R.; Tanker, M.; Rüegger, H.; Sticher, O. Triterpene saponins from Cyclamen mirabile and their biological activities. J. Nat. Prod. 1997, 60, 315–318. [Google Scholar] [CrossRef]
- Jimenez-Arellanes, A.; Meckes, M.; Torres, J.; Luna-Herrera, J. Antimycobacterial triterpenoids from Lantana hispida (Verbenaceae). J. Ethnopharmacol. 2007, 111, 202–205. [Google Scholar] [CrossRef]
- Jimenez, A.; Meckes, M.; Alvarez, V.; Torres, J. Secondary metabolites from Chamaedora tepejilote (Palmae) are active against Mycobacterium tuberculosis. Phytother. Res. 2005, 19, 320–322. [Google Scholar] [CrossRef]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial activity of oleanoic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef]
- Cunha, W.R.; de Matos, G.X.; Souza, M.G.M.; Tozatti, M.G.; Andrade e Silva, M.L.; Martins, C.H.; da Silva, R.; da Silva Filho, A.A. Evaluation of the antibacterial activity of the methylene chloride extract of Miconia ligustroides, isolated triterpene acids, and ursolic acid derivatives. Pharm. Biol. 2010, 48, 166–169. [Google Scholar] [CrossRef]
- Huang, L.; Luo, H.; Li, Q.; Wang, D.; Zhang, J.; Hao, X.; Yang, X. Pentacyclic triterpene derivatives possessing polyhydroxyl ring A inhibit gram-positive bacteria growth by regulating metabolism and virulence genes expression. Eur. J. Med. Chem. 2015, 95, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Ahn, S.J.; Kook, J.K. Oleanoic acid and ursolic acid inhibit peptidoglycan biosynthesis in Streptococcus mutans UA159. Braz. J. Microbiol. 2015, 46, 613–617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grudniak, A.M.; Kurek, A.; Szarlak, J.; Wolska, K.I. Oleanolic and ursolic acids influence effect the expression of the cysteine regulon and the stress response in Escherichia coli. Curr. Microbiol. 2011, 4, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, Z.; Kang, O.-H.; Mun, S.-H.; Seo, Y.-S.; Kong, R.; Shin, D.-W.; Liu, X.-Q.; Kwon, D.-Y. Antimicrobial activity and synergism of ursolic acid 3-O-α-L-arabinopyranoside with oxacillin against methicillin-resistant Staphylococcus aureus. Int. J. Mol. Med. 2017, 40, 1285–1293. [Google Scholar] [CrossRef][Green Version]
- Chandramu, C.; Manohar, R.D.; Krupadanam, D.G.; Dashavantha, R.V. Isolation, characterization and biological activity of betulic acid and ursolic acid from Vitex negundo L. Phytother. Res. 2003, 17, 129–134. [Google Scholar] [CrossRef]
- Mallavadhani, U.V.; Mahapatra, A.; Jamil, K.; Reddy, P.S. Antimicrobial activity of some pentacyclic triterpenes and their synthesized 3-O-lipophilic chains. Biol. Pharm. Bull. 2004, 27, 1576–1579. [Google Scholar] [CrossRef]
- Kim, S.W.; Hwang, I.Y.; Lee, S.Y.; Jeong, C.S. Protective effects on gastric lesion of ursolic acid. J. Food Hyg. Saf. 2016, 31, 286–293. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Briers, Y.; Lavigne, R. Breaking barriers: Expansion of the use of endolysins as novel antibacterials against Gram-negative bacteria. Future Microbiol. 2015, 10, 377–390. [Google Scholar] [CrossRef]
- Opperman, T.J.; Nguyen, S.T. Recent advances toward a molecular mechanism of efflux pump inhibition. Front. Microbiol. 2015, 6, 421–437. [Google Scholar] [CrossRef]
- Venter, H.; Mowla, R.; Ohene-Agyei, T.; Ma, S. RND-type drug eux pumps from Gram-negative bacteria: Molecular mechanism and inhibition. Front. Microbiol. 2015, 28, 377–388. [Google Scholar] [CrossRef]
- Xiao, X.N.; Wang, F.; Yuan, Y.-T.; Liu, J.; Liu, Y.-Z.; Yi, X. Antibacterial activity and mode of action of dihydromyricetin from Ampelopsis grossedentata leaves against food-borne bacteria. Molecules 2019, 24, 2831. [Google Scholar] [CrossRef]
- Cui, S.M.; Li, T.; Liang, H.; He, K.-K.; Zheng, Y.M.; Tang, M.; Ke, C.; Song, L.Y. Antibacterial activities and mechanisms of vine tea extract and 2R,3R-Dihydromyricetin on Escherichia coli. LWT Food Sci. Technol. 2021, 146, 113393–113403. [Google Scholar] [CrossRef]
- Bessa, L.J.; Palmeira, A.; Gomes, A.S.; Vasconcelos, V.; Sousa, E.; Pinto, M.; da Costa, P.M. Synergistic effects between thioxanthones and axacillin against methicillin-resistant Staphylococcus aureus. Microb. Drug Resist. 2015, 21, 404–415. [Google Scholar] [CrossRef]
- Dos Santos, A.T.B.; da Silva Araújo, T.F.; da Silva, L.C.N.; da Silva, C.B.; de Oliveira, A.F.M.; Araújo, J.M.; dos Santos Correia, M.T.; de Menezes Lima, V.L. Organic extracts from Indigifera suffruticosa leaves have antimicrobial and synergistic actions with erythromycin against Staphylococcus aureus. Front. Microbiol. 2015, 6, 13. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M.; Paduch, R.; Matysik-Woźniak, A.; Niedziela, P.; Donica, H. The effect of ursolic and oleanoic acids on human skin fibroblast cells. Folia Histochem. Cytobiol. 2011, 49, 664–669. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, F.; Hu, J.; Liang, S.; Zhang, Y.; Du, G.; Zhang, C.; Cheng, Y. Antibacterial ligands and triterpenoids from Rostellularia procumbens. Planta Med. 2007, 73, 1596–1599. [Google Scholar] [CrossRef]
- Kao, S.-J.; Lee, W.-J.; Chang, J.-H.; Chow, J.-M.; Chung, C.-L.; Hung, W.-Y.; Chien, M.-H. Suppression of reactive oxygen species-mediated ERK and JNK activation sensitizes dihydromyricetin-induced mitochondrial apoptosis in human non-small cell lung cancer. Environ. Toxicol. 2017, 32, 1426–1438. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, Q.; Ren, H.; Ma, S.; Lu, C.; Liu, B.; Liu, J.; Liang, J.; Li, M.; Zhu, R. Dihydromyricetin enhances the chemo-sensitivity of nedaplatin via regulation of the p53/Bcl-2 pathway in hepatocellular carcinoma cells. PLoS ONE 2015, 10, e0124994. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shu, Y.; Zhang, Q.; Liu, B.; Xia, J.; Qiu, M.; Miao, H.; Li, M.; Zhu, R. Dihydromyricetin induces apoptosis and inhibits proliferation in hepatocellular carcinoma cells. Oncol. Lett. 2014, 8, 1645–1651. [Google Scholar] [CrossRef]
- Clinical & Laboratory Standards Institute: CLSI Guidelines. Available online: https://clsi.org/media/1928/m07ed11_sample.pdf (accessed on 21 January 2022).
- Clinical & Laboratory Standards Institute: CLSI Guidelines. Available online: https://clsi.org/media/2577/m11-ed9_sample.pdf (accessed on 21 January 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).