Metastable Kitaev Magnets
Abstract
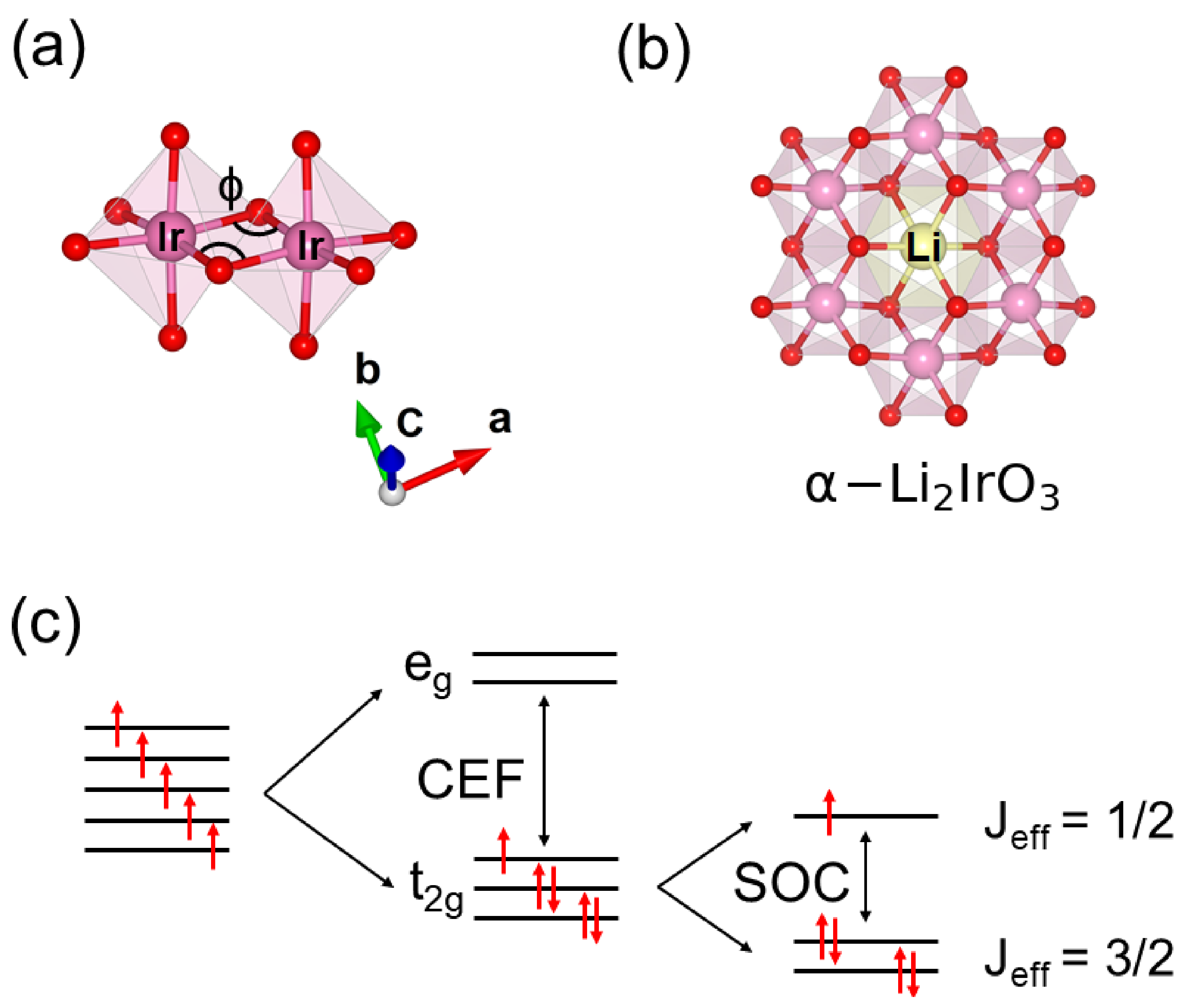
:1. Introduction
2. Topotactic Exchange Reactions
3. Synthesis Details
4. Stacking Faults
5. Tuning Magnetic Interactions with Topochemical Methods
6. Magnetic Characterization of Metastable Kitaev Materials
7. Challenges and Opportunities
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jackeli, G.; Khaliullin, G. Mott Insulators in the Strong Spin-Orbit Coupling Limit: From Heisenberg to a Quantum Compass and Kitaev Models. Phys. Rev. Lett. 2009, 102, 017205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, Y.; Manni, S.; Reuther, J.; Berlijn, T.; Thomale, R.; Ku, W.; Trebst, S.; Gegenwart, P. Relevance of the Heisenberg-Kitaev Model for the Honeycomb Lattice Iridates A2IrO3. Phys. Rev. Lett. 2012, 108, 127203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, H.; Takayama, T.; Jackeli, G.; Khaliullin, G.; Nagler, S.E. Concept and realization of Kitaev quantum spin liquids. Nat. Rev. Phys. 2019, 1, 264–280. [Google Scholar] [CrossRef]
- Plumb, K.W.; Clancy, J.P.; Sandilands, L.J.; Shankar, V.V.; Hu, Y.F.; Burch, K.S.; Kee, H.Y.; Kim, Y.J. α-RuCl3: A spin-orbit assisted Mott insulator on a honeycomb lattice. Phys. Rev. B 2014, 90, 041112. [Google Scholar] [CrossRef] [Green Version]
- Williams, S.C.; Johnson, R.D.; Freund, F.; Choi, S.; Jesche, A.; Kimchi, I.; Manni, S.; Bombardi, A.; Manuel, P.; Gegenwart, P.; et al. Incommensurate counterrotating magnetic order stabilized by Kitaev interactions in the layered honeycomb α-Li2IrO3. Phys. Rev. B 2016, 93, 195158. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Osterhoudt, G.B.; Tian, Y.; Lampen-Kelley, P.; Banerjee, A.; Goldstein, T.; Yan, J.; Knolle, J.; Ji, H.; Cava, R.J.; et al. The range of non-Kitaev terms and fractional particles in α-RuCl3. NPJ Quantum Mater. 2020, 5, 1–8. [Google Scholar] [CrossRef]
- Todorova, V.; Jansen, M. Synthesis, Structural Characterization and Physical Properties of a New Member of Ternary Lithium Layered Compounds—Li2RhO3. Z. Anorg. Allg. Chem. 2011, 637, 37–40. [Google Scholar] [CrossRef]
- Choi, S.; Manni, S.; Singleton, J.; Topping, C.V.; Lancaster, T.; Blundell, S.J.; Adroja, D.T.; Zapf, V.; Gegenwart, P.; Coldea, R. Spin dynamics and field-induced magnetic phase transition in the honeycomb Kitaev magnet α-Li2IrO3. Phys. Rev. B 2019, 99, 054426. [Google Scholar] [CrossRef] [Green Version]
- Kitaev, A. Anyons in an exactly solved model and beyond. Ann. Phys. 2006, 321, 2–111. [Google Scholar] [CrossRef] [Green Version]
- Knolle, J.; Moessner, R. A Field Guide to Spin Liquids. Annu. Rev. Condens. Matter Phys. 2019, 10, 451–472. [Google Scholar] [CrossRef] [Green Version]
- Chaloupka, J.; Jackeli, G.; Khaliullin, G. Kitaev-Heisenberg Model on a Honeycomb Lattice: Possible Exotic Phases in Iridium Oxides A2IrO3. Phys. Rev. Lett. 2010, 105, 027204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.J.; Jin, H.; Moon, S.J.; Kim, J.Y.; Park, B.G.; Leem, C.S.; Yu, J.; Noh, T.W.; Kim, C.; Oh, S.J.; et al. Novel Jeff=1/2 Mott State Induced by Relativistic Spin-Orbit Coupling in Sr2IrO4. Phys. Rev. Lett. 2008, 101, 076402. [Google Scholar] [CrossRef] [Green Version]
- Mehlawat, K.; Thamizhavel, A.; Singh, Y. Heat capacity evidence for proximity to the Kitaev quantum spin liquid in A2IrO3 (A = Na, Li). Phys. Rev. B 2017, 95, 144406. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.; Schoop, L.M.; Wurmbrand, D.; Nuss, J.; Seibel, E.M.; Tafti, F.F.; Ji, H.; Cava, R.J.; Dinnebier, R.E.; Lotsch, B.V. Trivalent Iridium Oxides: Layered Triangular Lattice Iridate K0.75Na0.25IrO2 and Oxyhydroxide IrOOH. Chem. Mater. 2017, 29, 8338–8345. [Google Scholar] [CrossRef]
- Merlino, S.; Labella, L.; Marchetti, F.; Toscani, S. Order-disorder transformation in RuBr3 and MoBr3: A two-dimensinal Ising model. Chem. Mater. 2004, 16, 3895–3903. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Lee, S.S.; Kim, Y.B. Spin—Orbital locking, emergent pseudo-spin and magnetic order in honeycomb lattice iridates. New J. Phys. 2012, 14, 073015. [Google Scholar] [CrossRef]
- Cao, G.; Schlottmann, P. The challenge of spin—Orbit-tuned ground states in iridates: A key issues review. Rep. Prog. Phys. 2018, 81, 042502. [Google Scholar] [CrossRef] [Green Version]
- Aykol, M.; Dwaraknath, S.S.; Sun, W.; Persson, K.A. Thermodynamic limit for synthesis of metastable inorganic materials. Sci. Adv. 2018, 4, eaaq0148. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, F.; Lafargue-Dit-Hauret, W.; Lebedev, O.I.; Movshovich, R.; Yang, H.Y.; Broido, D.; Rocquefelte, X.; Tafti, F. Thermodynamic Evidence of Proximity to a Kitaev Spin Liquid in Ag3LiIr2O6. Phys. Rev. Lett. 2019, 123, 237203. [Google Scholar] [CrossRef] [Green Version]
- Geirhos, K.; Lunkenheimer, P.; Blankenhorn, M.; Claus, R.; Matsumoto, Y.; Kitagawa, K.; Takayama, T.; Takagi, H.; Kézsmárki, I.; Loidl, A. Quantum paraelectricity in the Kitaev quantum spin liquid candidates H3LiIr2O6 and D3LiIr2O6. Phys. Rev. B 2020, 101, 184410. [Google Scholar] [CrossRef]
- Roudebush, J.H.; Ross, K.A.; Cava, R.J. Iridium containing honeycomb Delafossites by topotactic cation exchange. Dalton Trans. 2016, 45, 8783–8789. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, A.; Zager, B.; Bahrami, F.; DiScala, M.; Chamorro, J.R.; Upton, M.H.; Fabbris, G.; Haskel, D.; Casa, D.; McQueen, T.M.; et al. Enhanced hybridization in the electronic ground state of the intercalated honeycomb iridate Ag3LiIr2O6. Phys. Rev. B 2021, 104, L100416. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, W.; Imai, T.; Singer, P.M.; Bahrami, F.; Tafti, F. NMR investigation on the honeycomb iridate Ag3LiIr2O6. Phys. Rev. B 2021, 103, 214405. [Google Scholar] [CrossRef]
- Abramchuk, M.; Ozsoy-Keskinbora, C.; Krizan, J.W.; Metz, K.R.; Bell, D.C.; Tafti, F. Cu2IrO3: A New Magnetically Frustrated Honeycomb Iridate. J. Am. Chem. Soc. 2017, 139, 15371–15376. [Google Scholar] [CrossRef] [PubMed]
- Kenney, E.M.; Segre, C.U.; Lafargue-Dit-Hauret, W.; Lebedev, O.I.; Abramchuk, M.; Berlie, A.; Cottrell, S.P.; Simutis, G.; Bahrami, F.; Mordvinova, N.E.; et al. Coexistence of static and dynamic magnetism in the Kitaev spin liquid material Cu2IrO3. Phys. Rev. B 2019, 100, 094418. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Lee, C.; Lee, S.; Yoon, S.; Lee, W.J.; Park, J.; Ali, A.; Singh, Y.; Orain, J.C.; Kim, G.; et al. Exotic Low-Energy Excitations Emergent in the Random Kitaev Magnet Cu2IrO3. Phys. Rev. Lett. 2019, 122, 167202. [Google Scholar] [CrossRef] [Green Version]
- O’Malley, M.J.; Verweij, H.; Woodward, P.M. Structure and properties of ordered Li2IrO3 and Li2PtO3. J. Solid State Chem. 2008, 181, 1803–1809. [Google Scholar] [CrossRef]
- Bahrami, F.; Kenney, E.M.; Wang, C.; Berlie, A.; Lebedev, O.I.; Graf, M.J.; Tafti, F. Effect of structural disorder on the Kitaev magnet Ag3LiIr2O6. Phys. Rev. B 2021, 103, 094427. [Google Scholar] [CrossRef]
- Kitagawa, K.; Takayama, T.; Matsumoto, Y.; Kato, A.; Takano, R.; Kishimoto, Y.; Bette, S.; Dinnebier, R.; Jackeli, G.; Takagi, H. A spin–orbital-entangled quantum liquid on a honeycomb lattice. Nature 2018, 554, 341–345. [Google Scholar] [CrossRef]
- Tsirlin, A.A.; Gegenwart, P. Kitaev Magnetism through the Prism of Lithium Iridate. Phys. Status Solidi 2021. [Google Scholar] [CrossRef]
- Bette, S.; Takayama, T.; Kitagawa, K.; Takano, R.; Takagi, H.; Dinnebier, R.E. Solution of the heavily stacking faulted crystal structure of the honeycomb iridate H3LiIr2O6. Dalton Trans. 2017, 46, 15216–15227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzar, D. X-ray Diffraction Line Broadening: Modeling and Applications to High-Tc Superconductors. J. Res. Natl. Inst. Stand. Technol. 1993, 98, 321–353. [Google Scholar] [CrossRef] [PubMed]
- Abramchuk, M.; Lebedev, O.I.; Hellman, O.; Bahrami, F.; Mordvinova, N.E.; Krizan, J.W.; Metz, K.R.; Broido, D.; Tafti, F. Crystal Chemistry and Phonon Heat Capacity in Quaternary Honeycomb Delafossites: Cu[Li1/3Sn2/3]O2 and Cu[Na1/3Sn2/3]O2. Inorg. Chem. 2018, 57, 12709–12717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rau, J.G.; Lee, E.K.H.; Kee, H.Y. Spin-Orbit Physics Giving Rise to Novel Phases in Correlated Systems: Iridates and Related Materials. Annu. Rev. Condens. Matter Phys. 2016, 7, 195–221. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Kee, H.Y. Crystal structure and magnetism in α-RuCl3: An ab initio study. Phys. Rev. B 2016, 93, 155143. [Google Scholar] [CrossRef] [Green Version]
- Winter, S.M.; Li, Y.; Jeschke, H.O.; Valentí, R. Challenges in design of Kitaev materials: Magnetic interactions from competing energy scales. Phys. Rev. B 2016, 93, 214431. [Google Scholar] [CrossRef] [Green Version]
- Rau, J.G.; Lee, E.K.H.; Kee, H.Y. Generic Spin Model for the Honeycomb Iridates beyond the Kitaev Limit. Phys. Rev. Lett. 2014, 112, 077204. [Google Scholar] [CrossRef]
- Rusnacko, J.; Gotfryd, D.; Chaloupka, J. Kitaev-like honeycomb magnets: Global phase behavior and emergent effective models. Phys. Rev. B 2019, 99, 064425. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.K.; Coldea, R.; Kolmogorov, A.N.; Lancaster, T.; Mazin, I.I.; Blundell, S.J.; Radaelli, P.G.; Singh, Y.; Gegenwart, P.; Choi, K.R.; et al. Spin Waves and Revised Crystal Structure of Honeycomb Iridate Na2IrO3. Phys. Rev. Lett. 2012, 108, 127204. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.K.; Wang, J.; Arsenault, A.; Imai, T.; Abramchuk, M.; Tafti, F.; Singer, P.M. Spin Excitations of a Proximate Kitaev Quantum Spin Liquid Realized in Cu2IrO3. Phys. Rev. X 2019, 9, 031047. [Google Scholar] [CrossRef] [Green Version]
- Knolle, J.; Moessner, R.; Perkins, N.B. Bond-Disordered Spin Liquid and the Honeycomb Iridate H3LiIr2O6: Abundant Low-Energy Density of States from Random Majorana Hopping. Phys. Rev. Lett. 2019, 122, 047202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, W.H.; Knolle, J.; Halász, G.B.; Moessner, R.; Perkins, N.B. Vacancy-Induced Low-Energy Density of States in the Kitaev Spin Liquid. Phys. Rev. X 2021, 11, 011034. [Google Scholar] [CrossRef]
- Pal, S.; Seth, A.; Sakrikar, P.; Ali, A.; Bhattacharjee, S.; Muthu, D.V.S.; Singh, Y.; Sood, A.K. Probing signatures of fractionalization in the candidate quantum spin liquid Cu2IrO3 via anomalous Raman scattering. Phys. Rev. B 2021, 104, 184420. [Google Scholar] [CrossRef]
- Frolov, S. Quantum computing’s reproducibility crisis: Majorana fermions. Nature 2021, 592, 350–352. [Google Scholar] [CrossRef]
- Field, B.; Simula, T. Introduction to topological quantum computation with non-Abelian anyons. Quantum Sci. Technol. 2018, 3, 045004. [Google Scholar] [CrossRef] [Green Version]
- You, Y.Z.; Kimchi, I.; Vishwanath, A. Doping a spin-orbit Mott insulator: Topological superconductivity from the Kitaev-Heisenberg model and possible application to Na2/Li2IrO3. Phys. Rev. B 2012, 86, 085145. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahrami, F.; Abramchuk, M.; Lebedev, O.; Tafti, F. Metastable Kitaev Magnets. Molecules 2022, 27, 871. https://doi.org/10.3390/molecules27030871
Bahrami F, Abramchuk M, Lebedev O, Tafti F. Metastable Kitaev Magnets. Molecules. 2022; 27(3):871. https://doi.org/10.3390/molecules27030871
Chicago/Turabian StyleBahrami, Faranak, Mykola Abramchuk, Oleg Lebedev, and Fazel Tafti. 2022. "Metastable Kitaev Magnets" Molecules 27, no. 3: 871. https://doi.org/10.3390/molecules27030871





