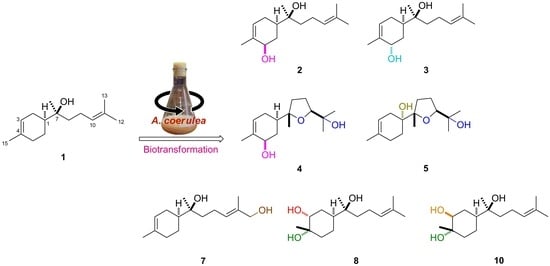
Biotransformation of (−)-α-Bisabolol by Absidia coerulea
Abstract
:1. Introduction
2. Results and Discussion
| Position | Compound | ||||||
|---|---|---|---|---|---|---|---|
| 2 a | 3 a | 4 b | 5 b | 7 b | 8 b | 10 b | |
| 1 | 1.72, 1H, m | 1.79, 1H, m | 1.77, 1H, m | - | 1.57, 1H, m | 1.72, 1H, m | 1.53, 1H, tt (11.8, 2.8) |
| 2 | 1.98, 1H, m | 2.06, 1H, m | 1.97, 2H, m | 2.14, 1H, m | 1.98, 1H, m | 1.78, 1H, d (3.0) | 1.89, 1H, d (11.8) |
| 1.86, 1H, m | 1.77, 1H, m | 1.92, 1H, m | 1.79, 1H, m | 1.73 1H, dt (3.0, 13.7) | 1.18, 1H, m | ||
| 3 | 5.47, 1H, m | 5.56, 1H, dt (5.4, 1.5) | 5.47, 1H, m | 5.30, 1H, m | 5.37, 1H, m | 3.63, 1H, br s | 3.53, 1H, dd (11.6, 4.0) |
| 5 | 4.17, 1H, brs | 4.05, 1H, brs | 4.19, 1H, m | 2.21, 2H, m | 1.99, 2H, m | 1.74, 1H, m 1.55, 1H, m | 1.80, 1H, dt (3.1, 12.9) 1.42, 1H, td (12.9, 3.1) |
| 6 | 2.25, 1H, ddt (12.0, 6.0, 2.3) | 2.03, 1H, m | 2.26, 1H, dd (11.8, 5.6) | 1.65, 1H, dd (5.8, 12.8) | 1.91, 1H, m | 1.60, 1H, m | 1.72, 2H, m |
| 1.35, 1H, td (12.0, 10.0) | 1.44, 1H td (13.0, 3.8) | 1.28, 1H, m | 1.76, 1H, ddt (12.8, 5.8, 2.0) | 1.29, 1H, m | 1.42, 1H, m | ||
| 8 | 1.51, 2H, m | 1.52, 2H, m | 1.85, 1H, m 1.62, 1H, m | 2.29, 1H, dt (12.2, 7.5), 1.57, 1H, dd (12.2, 7.5) | 1.52, 2H, m | 1.50, 2H, m | 1.49, 2H, m |
| 9 | 2.04, 2H, m | 2.07, 2H, m | 1.83, 2H, m 1.78, 2H, m | 1.90, 2H, m 1.85, 2H, m | 2.12, 2H, ddd (7.8) | 2.05, 2H, m | 2.04, 2H, m |
| 10 | 5.12, 1H, tq (7.0, 1.4) | 5.13, 1H, tt (7.3, 1.3) | 3.68, 1H, dd (9.2, 5.7) | 3.76, 1H, dd (10.6, 5.4) | 5.42, 1H, tq (7.2, 1.6) | 5.13, 1H, t (7.0) | 5.12, 1H, t (6.6) |
| 12 | 1.69, 3H, s | 1.69, 3H, s | 1.11, 3H, s | 1.13, 3H, s | 4.00, 3H, s | 1.69, 3H, s | 1.69, 3H, s |
| 13 | 1.62, 3H, s | 1.62, 3H, s | 1.21, 3H, s | 1.23, 3H, s | 1.68, 3H, s | 1.63, 3H, s | 1.62, 3H, s |
| 14 | 1.13, 3H, s | 1.12, 3H, s | 1.13, 3H, s | 1.16, 3H, s | 1.12, 3H, s | 1.14, 3H, s | 1.14, 3H, s |
| 15 | 1.76, 3H, s | 1.79, 3H, s | 1.75, 3H, s | 1.70, 3H, s | 1.65, 3H, s | 1.26, 3H, s | 1.18, 3H, s |
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Materials and Microorganisms
3.3. Screening Procedures
3.4. Extraction and Isolation of Metabolites
3.5. Spectroscopic Data of Metabolites
(1R,5R,7S)-5-hydroxy-α-bisabolol (2)
(1R,5S,7S)-5-hydroxy-α-bisabolol (3)
(1R,5R,7S,10S)-5-hydroxybisabolol oxide B (4)
(1R,7S,10S)-1-hydroxybisabolol oxide B (5)
12-hydroxy-α-bisabolol (7)
(1S,3R,4S,7S)-3,4-dihydroxy-α-bisabolol (8)
(1S,3S,4S,7S)-3,4-dihydroxy-α-bisabolol (10)
3.6. Determination of Solubility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ma, Y.; Li, W.; Mai, J.; Wang, J.; Wei, Y.; Ledesma-Amaro, R.; Ji, X.J. Engineering Yarrowia lipolytica for sustainable production of the chamomile sesquoterpene (−)-α-bisabolol. Green Chem. 2021, 23, 780–787. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, C.; Ou, Z.; Liang, X.; Shi, Y.; Chi, L.; Zhang, Z.; Zheng, X.; Li, C.; Xiang, H. Chemical profiling and bioactivity of essential oils from Alpinia officinarum Hance from ten localities in China. Ind. Crops Prod. 2020, 153, 112583. [Google Scholar] [CrossRef]
- Han, G.H.; Kim, S.K.; Yoon, P.K.S.; Kang, Y.; Kim, B.S.; Fu, Y.; Sung, B.H.; Jung, H.C.; Lee, D.H.; Kim, S.W.; et al. Fermentative production and direct extraction of (−)-α-bisabolol in metabolically engineered Escherichia coli. Microb. Cell Fact. 2016, 15, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamatou, G.P.P.; Viljoen, A.M. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J. Am. Oil Chem. Soc. 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Kim, S.; Jung, E.; Kim, J.H.; Park, Y.H.; Lee, J.; Park, D. Inhibitory effects of (−)-α-bisabolol on LPS-induced inflammatory response in RAW264.7 macrophages. Food Chem. Toxicol. 2011, 49, 2580–2585. [Google Scholar] [CrossRef] [PubMed]
- Mautya, A.K.; Singh, M.; Dubey, V.; Srivastava, S.; Luqman, S.; Bawankule, D.U. (−)-α-Bisabolol reduces pro-inflammatory cytokine production and ameliorates skin inflammation. Curr. Pharm. Biotechnol. 2014, 15, 173–181. [Google Scholar]
- Rottini, M.M.; Amaral, A.C.F.; Ferreira, J.L.P.; Silva, J.R.A.; Taniwaki, N.N.; de Souza, C.S.F.; d’Escoffier, L.N.; Almeida-Souza, F.; Hardoim, D.J.; da Costa, S.C.G.; et al. In vitro evaluation of (−)-α-bisabolol as a promising agent against Leishmania amazonensis. Exp. Parasitol. 2015, 148, 66–72. [Google Scholar] [CrossRef]
- Corpas-López, V.; Merino-Espinosa, G.; López-Viota, M.; Gijón-Robles, P.; Morillas-Mancilla, M.J.; López-Viota, J.; Díaz-Sáez, V.; Morillas-Márquez, F.; Moll, M.C.N.; Martín-Sábchez, J. Topical treatment of Leishmania tropica infection using (−)-α-bisabolol ointment in a hamster model: Effectiveness and safety assessment. J. Nat. Prod. 2016, 79, 2403–2407. [Google Scholar] [CrossRef]
- Rodrigues, F.F.G.; Colares, A.V.; Nonato, C.F.A.; Galvāo-Rodrigues, F.F.; Mota, M.L.; Braga, M.F.B.M.; Costa, J.G.M. In vitro antimicrobial activity of the essrntial oil from Vanillosmopsis arborea Barker (Asteraceae) and its major constituent, α-bisabolol. Microb. Pathog. 2018, 125, 144–149. [Google Scholar] [CrossRef]
- Teixeira, G.F.D.; Costa, F.N.; Campos, A.R. Corneal antinociceptive effect of (−)-α-bisabolol. Pharm. Biol. 2017, 55, 1089–1092. [Google Scholar] [CrossRef]
- Terroso, T.F.; Condotta, K.B.; Fonseca, F.N.; Jornada, D.S.; Ferreira, G.O.; Ellwanger, J.H.; Schmidt, J.A.; Pohlmann, A.R.; Guterres, S.S. In vivo prophylactic gastroprotection using α-bisabolol encapsulated in lipid-core nanocapsules and in cocoa-theospheres. J. Drug Deliv. Sci. Technol. 2016, 36, 99–109. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Azimullah, S.; Laham, F.; Tariq, S.; Goyal, S.N.; Adeghate, E.; Ojha, S. α-Bisabolol protects against β-adrenergic agonist-induced myocardial infarction in rats by attenuating inflammation, lysosomal dysfunction, NLRP3 inflammasome activation and modulating autophagic flux. Food Funct. 2020, 11, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Peng, L.; Sang, H.; Li, Q.P.; Cheng, S. Anticancer effects of α-bisabolol in human non-small cell lung carcinoma cells are mediated via apoptosis induction, cell cycle arrest, inhibition of cell migration and invasion and upregulation of P13K/AKT signalling pathway. J. BUON 2018, 23, 1407–1412. [Google Scholar] [PubMed]
- Fırat, Z.; Demirci, F.; Demirci, B. Antioxidant activity of chamomile essential oil and main components. Nat. Volatiles Essent. Oils 2018, 5, 11–16. [Google Scholar]
- Tomić, M.; Popović, V.; Petrović, S.; Stepanović-Petrović, R.; Micov, A.; Pavlović-Drobac, M.; Couladis, M. Antihyperalgesic and antiedematous activities of bisabolol-oxides-rich matricaria oil in a rat model of inflammation. Phytother. Res. 2014, 28, 759–766. [Google Scholar] [CrossRef]
- Kazemi, M. Chemical composition and antimicrobial activity of essential oil of Matricaria recutita. Int. J. Food Prop. 2015, 18, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, M.; Nankai, H.; Kameoka, H. Biotransformation of (−)-α-bisabolol by plant pathogenic fungus, Glomerella cingulata. Phytochemistry 1995, 39, 1077–1080. [Google Scholar] [CrossRef]
- Limberger, R.P.; Ferreira, L.; Castilhos, T.; Aleixo, A.M.; Petersen, R.Z.; Germani, J.C.; Zuanazzi, J.A.; Fett-Neto, A.G.; Henriques, A.T. The ability of Bipolaris sorokiniana to modify geraniol and (−)-alpha-bisabolol as exogenous substrates. Appl. Microbiol. Biotechnol. 2003, 61, 552–555. [Google Scholar] [CrossRef]
- Firat, Z.; Demirci, F.; Demirci, B.; Kırmızıbekmez, H.; Başer, K.H.C. Microbial transformation of (−)-α-bisabolol towards bioactive metabolites. Rec. Nat. Prod. 2021, 15, 593–601. [Google Scholar] [CrossRef]
- Han, F.; Xiao, Y.; Lee, I.-S. Microbial transformation of galangin derivatives and cytotoxicity evaluation of their metabolites. Catalysts 2021, 11, 1020. [Google Scholar] [CrossRef]
- Cano-Flores, A.; Gómez, J.; Escalona-Torres, I.S.; Velasco-Bejarano, B. Microorganisms as biocatalysts and enzyme sources. In Microorganisms; Blumenberg, M., Shaaban, M., Elgaml, A., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Sultana, N.; Saify, Z.S. Enzymatic biotransformation of terpenes as bioactive agents. J. Enzym. Inhib. Med. Chem. 2013, 28, 1113–1128. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, H.N.; Zubair, M.; Rasool, N.; Hassan, Z.; Ahmad, V.U. Microbial transformation of sesquiterpenoids. Nat. Prod. Commun. 2009, 4, 1155–1168. [Google Scholar] [CrossRef] [Green Version]
- Gliszczyńska, A.; Łysek, A.; Janeczko, T.; Świtalska, M.; Wietrzyk, J.; Wawrzeńczyk, C. Microbial transformation of (+)-nootkatone and the antiproliferative activity of its metabolites. Bioorg. Med. Chem. 2011, 19, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Xiao, Y.; Lee, I.-S. Microbial conjugation studies of licochalcones and xanthohumol. Int. J. Mol. Sci. 2021, 22, 6893. [Google Scholar] [CrossRef] [PubMed]
- Brzonova, I.; Asina, F.; Andrianova, A.A.; Kubátová, A.; Smoliakova, I.P.; Kozliak, E.I.; Ji, Y. Fungal biotransformation of insoluble kraft lignin into a water soluble polymer. Ind. Eng. Chem. Res. 2017, 56, 6103–6113. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Tan, H.L.; Sultan, S.; Faridz, M.A.B.M.; Shah, M.A.B.M.; Nurfazilah, S.; Hussain, M. Microbial-catalyzed biotransformation of multifunctional triterpenoids derived from phytonutrients. Int. J. Mol. Sci. 2014, 15, 12027–12060. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, A.P.; de Oliveira, M.T.P.; de Souza, É.T.; Coutinho, D.S.; Ciambarella, B.T.; Gomes, C.R.; Terroso, T.; Guterres, S.S.; Pohlmann, A.R.; Silva, P.M.R.; et al. α-Bisabolol-loaded lipid-core nanocapsules reduce lipopolysaccharide-induced pulmonary inflammation in mice. Int. J. Nanomed. 2017, 12, 4479–4491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K.; Kato, M. Synthesis of (6S,1′S)-(+)-hernandulcin, a sweetner, and its stereoisomers. Tetrahedron 1986, 42, 5895–5900. [Google Scholar] [CrossRef]
- Matos, M.E.O.; De Sousa, M.P.; Matos, F.J.A.; Craveiro, A.A. Sesquiterpenes from Vanillosmopsis arborea. J. Nat. Prod. 1988, 51, 780–782. [Google Scholar] [CrossRef]
- Ding, L.; Pfoh, R.; Ruhl, S.; Qin, S.; Laatsch, H. T-muurolol sesquiterpenes from the marine Streptomyces sp. M491 and revision of the configuration of previously reported amorphanes. J. Nat. Prod. 2009, 72, 99–101. [Google Scholar] [CrossRef]
- Campagnuolo, C.; Fattorusso, E.; Petrucci, F.; Taglialatela-Scafati, O.; Appendino, G.; Marquez, N.; Muñoz, E. A prenylbisabolane with NF-κB inhibiting properties from Cascarilla (Croton eluteria). Bioorg. Med. Chem. 2005, 13, 4238–4242. [Google Scholar] [CrossRef] [PubMed]
- Smitt, O.; Högberg, H. Syntheses of a prenylbisabolane diterpene, a natural insecticide from Corton linearis, and the biabolane sesquiterpenes (−)-delobanone and (−)-epi-delobanone. Tetrahedron 2002, 58, 7691–7700. [Google Scholar] [CrossRef]
- Flaskamp, E.; Nonnenmacher, G.; Zimmermann, G.; Isaac, O. On the stereochemistry of the bisaboloids from Matricaria chamomilla L. Z. Naturforsch. 1981, 36b, 1023–1030. [Google Scholar] [CrossRef]
- Yin, X.; Feng, T.; Li, Z.H.; Su, J.; Li, Y.; Tan, N.H.; Liu, J.K. Chemical investigation on the cultures of the fungus Xylaria carpophila. Nat. Prod. Bioprospect. 2011, 1, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Ohloff, G.; Giersch, W.; Näf, R.; Delay, F. The absolute configuration of β-bisabolol. Helv. Chim. Acta 1986, 69, 698–703. [Google Scholar] [CrossRef]
- Fráter, G.; Müller, U. Synthesis of (+)-(4S,8R)-8-epi- and (−)-(4R,8S)-4-epi-β-bisabolol. Helv. Chim. Acta 1989, 72, 653–658. [Google Scholar] [CrossRef]
- Alpha, T.; Raharivelomanana, P.; Bianchini, J.; Faure, R.; Cambon, A. Bisabolane sesquiterpenoids from Santalum austrocaledonicum. Phytochemistry 1997, 44, 1519–1522. [Google Scholar] [CrossRef]
- Matsumura, T.; Ishikawa, T.; Kitajima, J. Water-soluble constituents of caraway: Carvone derivatives and their glucosides. Chem. Pharm. Bull. 2002, 50, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Chellan, P.; Land, K.M.; Shoker, A.; Au, A.; An, S.H.; Taylor, D.; Simth, P.J.; Riedel, T.; Dyson, P.J.; Chibale, K.; et al. Synthesis and evaluation of new polynuclear organometallic Ru(II), Rh(III) and Ir(III) pyridyl ester complexes as in vitro antiparasitic and antitumor agents. Dalton Trans. 2014, 43, 513–526. [Google Scholar] [CrossRef] [Green Version]
- Nagai, Y.; Kawano, S.; Motoda, K.; Tomida, M.; Tatebe, C.; Sato, K.; Akiyama, H. Solubility testing of sucrose esters of fatty acids in international food additive specifications. Biol. Pharm. Bull. 2017, 40, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Chawla, R.C.; Doura, K.F.; McKay, D. Effect of alcohol cosolvents on the aqueous solubility of trichloroethylene. In Proceedings of the 2001 Conference on Environmental Research, Manhattan, KA, USA, 21–24 May 2001; pp. 52–66. [Google Scholar]
- Blair, M.; Tuck, K.L. A new diastereoselective entry to the (1S,4R)- and (1S,4S)-isomers of 4-isopropyl-1-methyl-2-cyclohexen-1-ol, aggregation pheromones of the ambrosia beetle Platypus quercivorus. Tetrahedron Asymmetry 2009, 20, 2149–2153. [Google Scholar] [CrossRef]
- Liu, Q.; Beyraghdar Kashkooli, A.; Manzano, D.; Pateraki, I.; Richard, L.; Kolkman, P.; Lucas, M.F.; Guallar, V.; de Vos, R.C.H.; Franssen, M.C.R.; et al. Kauinolide synthase is P450 with unusaual hydroxylation and cyclization-elimination activity. Nat. Commun. 2018, 9, 4657. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Hashemi, E. Biotransformation of myrcene by Pseudomonas aeruginosa. Chem. Cent. J. 2011, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, F.; Lee, I.-S. Microbial transformation of the antimalarial sesquiterpene endoperoxide dihydroartemisinin. Nat. Prod. Res. 2017, 31, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Lee, I.-S. Microbial transformation of bavachin by Absidia coerulea. Phytochem. Lett. 2016, 18, 136–139. [Google Scholar] [CrossRef]
- Hoelke, B.; Gieringer, B.; Arlt, M.; Saal, C. Comparison of nephelometric, UV-spectroscopic, and HPLC methods for high-throughput determination of aqueous drug solubility in microtiter plates. Anal. Chem. 2009, 81, 3165–3172. [Google Scholar] [CrossRef]






| Position | Compound | ||||||
|---|---|---|---|---|---|---|---|
| 2 a | 3 a | 4 b | 5 b | 7 b | 8 b | 10 b | |
| 1 | 42.1 | 36.8 | 43.5 | 73.6 | 43.0 | 39.3 | 45.6 |
| 2 | 27.1 | 27.1 | 27.3 | 32.3 | 27.0 | 29.9 | 32.1 |
| 3 | 123.7 | 125.3 | 123.8 | 117.9 | 120.5 | 74.0 | 77.4 |
| 4 | 136.6 | 134.4 | 136.6 | 134.0 | 134.2 | 70.9 | 74.0 |
| 5 | 71.0 | 68.6 | 71.1 | 26.7 | 31.0 | 33.6 | 38.4 |
| 6 | 33.8 | 32.0 | 34.9 | 28.5 | 23.3 | 21.3 | 23.6 |
| 7 | 73.9 | 73.8 | 84.4 | 88.0 | 74.3 | 74.3 | 73.9 |
| 8 | 39.9 | 40.2 | 35.5 | 32.2 | 39.8 | 39.5 | 39.8 |
| 9 | 22.1 | 22.1 | 26.4 | 26.3 | 21.7 | 22.2 | 22.2 |
| 10 | 124.3 | 124.4 | 86.1 | 87.9 | 126.2 | 124.5 | 124.2 |
| 11 | 131.9 | 131.8 | 70.5 | 70.4 | 134.9 | 131.9 | 132.1 |
| 12 | 25.7 | 25.7 | 24.0 | 24.0 | 68.9 | 25.7 | 25.8 |
| 13 | 17.7 | 17.7 | 27.7 | 27.8 | 13.7 | 17.7 | 17.7 |
| 14 | 23.4 | 23.3 | 23.5 | 22.9 | 23.2 | 24.2 | 24.1 |
| 15 | 18.8 | 20.8 | 18.8 | 23.5 | 23.4 | 27.5 | 18.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Han, F.; Lee, I.-S. Biotransformation of (−)-α-Bisabolol by Absidia coerulea. Molecules 2022, 27, 881. https://doi.org/10.3390/molecules27030881
Park J, Han F, Lee I-S. Biotransformation of (−)-α-Bisabolol by Absidia coerulea. Molecules. 2022; 27(3):881. https://doi.org/10.3390/molecules27030881
Chicago/Turabian StylePark, Jisu, Fubo Han, and Ik-Soo Lee. 2022. "Biotransformation of (−)-α-Bisabolol by Absidia coerulea" Molecules 27, no. 3: 881. https://doi.org/10.3390/molecules27030881
APA StylePark, J., Han, F., & Lee, I.-S. (2022). Biotransformation of (−)-α-Bisabolol by Absidia coerulea. Molecules, 27(3), 881. https://doi.org/10.3390/molecules27030881