Guggulsterone Mediated JAK/STAT and PPAR-Gamma Modulation Prevents Neurobehavioral and Neurochemical Abnormalities in Propionic Acid-Induced Experimental Model of Autism
Abstract
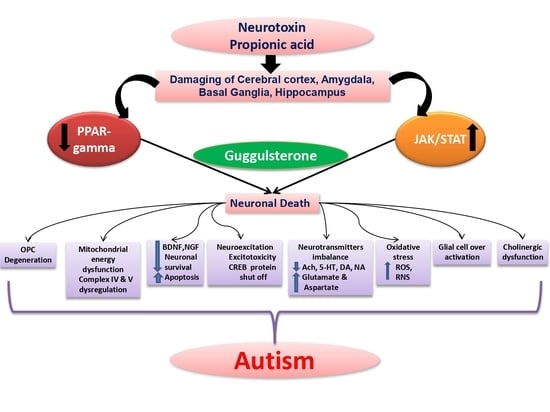
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Drugs and Chemicals
2.3. Experimental Grouping of Animals
2.4. Propionic Acid-Induced Experimental Animal Model of Autism
2.5. Parameters Assessed
2.5.1. Bodyweight Measurement
2.5.2. Measurement of Relative-Brain Body Weight
2.6. Behavioural Parameters
2.6.1. Beam Crossing Task (BCT)
2.6.2. Locomotor Activity Assessment
2.6.3. Forced Swim Test (FST)
2.6.4. Morris Water Maze Task (MWM)
2.7. Neurochemical Estimations
2.7.1. Brain Homogenate Preparation
2.7.2. Blood Plasma Collection and Separation
2.7.3. CSF Collection
2.8. Assessment of Molecular and Cellular Markers
2.8.1. Estimation of STAT3 and PPAR-Gamma Level
2.8.2. Estimation of MBP (Myelin Basic Protein) Level
2.9. Assessment of Apoptotic Markers
Estimation of Caspase-3, Bax, and Bcl-2 Level
2.10. Assessment of Neurotransmitter Levels
2.10.1. Estimation of Dopamine Level
2.10.2. Assessment of Brain Glutamate Level
2.10.3. Measurement of Acetylcholine (Ach) Level
2.10.4. Measurement of Serotonin Level
2.11. Assessment of Inflammatory Cytokines
2.11.1. Measurement of Tumor Necrotic Factor-Alpha (TNF-α) Level
2.11.2. Measurement of Interleukin-1 Beta (IL-1β) Level
2.12. Assessment of Oxidative Stress Markers
2.12.1. Estimation of Acetylcholinesterase (AChE) Level
2.12.2. Measurement of Superoxide Dismutase (SOD) Enzymatic Activity
2.12.3. Estimation of Lactate Dehydrogenase (LDH) Level
2.12.4. Estimation of Malondialdehyde (MDA) Level
2.12.5. Estimation of Reduced Glutathione Level
2.12.6. Assessment of Nitrite Level
2.13. Assessment of Gross Pathology and Morphology
2.14. Assessment of Histopathological Changes
2.15. Statistical Analysis
3. Results
3.1. Neuroprotective Potential of Guggulsterone in the Restoration of Weight Variations in Propionic Acid-Induced Experimental Model of Autism in Adult Rats
3.1.1. Improvement in Body Weight with Long-Term Guggulsterone Treatment
3.1.2. Improvement in the Relative Brain-Body Weight Ratio with Long Term Guggulsterone Treatment
3.2. Neuroprotective Potential of Guggulsterone in the Prevention of Neurobehavioral Impairments in Propionic Acid-Induced Experimental Model of Autism in Adult Rats
3.2.1. Improved Locomotor Activity with Long Term Guggulsterone Treatment
3.2.2. Improvement in Cognitive and Memory with Long Term Guggulsterone Treatment
3.2.3. Recovery of Muscle Coordination with Long Term Guggulsterone Treatment
3.2.4. Improvement in Depression-like Behaviour with Long Term Guggulsterone Treatment
3.3. Neuroprotective Potential of Guggulsterone on Neurochemical Alterations in Propionic Acid-Induced Experimental Model of Autism in Adult Rats
3.3.1. Decreased STAT3 Level with Long-Term Guggulsterone Treatment
3.3.2. Increased PPAR-Gamma Level with Long Term Guggulsterone Treatment
3.3.3. Increased Myelin Basic Protein Level with Long Term Guggulsterone Treatment
3.3.4. Decreased Level of Caspase-3, Bax, and Increased Level of Bcl-2 with Long Term Guggulsterone Treatment
3.3.5. Restoration of Neurotransmitter Levels after Long Term Treatment with Guggulsterone
3.3.6. Reduction in Neuroinflammatory Cytokines after Long-Term Treatment with Guggulsterone
3.3.7. Restoration of the Anti-Oxidant Level after Long-Term Treatment with Guggulsterone
3.4. Neuroprotective Potential of Guggulsterone in the Restoration of Gross Pathological Abnormalities in Propionic Acid-Induced Experimental Model of Autism in Adult Rats
3.4.1. Improvement in Whole-Brain Alterations after Long Term Treatment with Guggulsterone
3.4.2. Reduced Pathological Abnormalities in Brain Sections after Long Term Administration of Guggulsterone
3.4.3. Reduced Demyelination Volume after Long Term Administration with Guggulsterone
3.5. Neuroprotective Effect of Guggulsterone in Propionic Acid-Induced Histopathological Changes
4. Discussion
5. Conclusions
6. Chemical Compounds Studied in This Article
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| 15d-PGJ2 | 15-deoxy-Δ12,14-prostaglandin J2 |
| Ach | Acetylcholine |
| AchE | Acetylcholinesterase |
| AP | Anterior/posterior |
| ASD | Autism spectrum disorder |
| BCT | Beam crossing task |
| BNDF | Brain-derived neurotrophic factor |
| BTBR | BTBR T+tf/J |
| CREB | Cyclic AMP response element binding protein |
| CSF | Cerebrospinal fluid |
| DV | Dorsal/ventral |
| ELT | Escape latency time |
| FST | Forced swim test |
| FXR | FarnesoidX receptor |
| GST | Guggulsterone |
| IAEC | Institutional Animal Ethics Committee |
| ICV-PPA | Intracerebroventricular-propionic acid |
| IL-1β | Interleukin 1 beta |
| JAK | Janus kinase |
| LDH | Lactate dehydrogenase |
| MBP | Myelin basic protein |
| MDA | Malondialdehyde |
| ML | Medial/lateral |
| MWM | Morris water maze |
| NO | Nitric oxide |
| PPAR | Peroxisome proliferator-activated receptor |
| SHP-1 | Srchomology 2 domain-containing protein tyrosine |
| SOD | Superoxide dismutase |
| STAT3 | Signal transducer and activator of transcription 3 |
| TNF-α | Tumornecrotic factor-alpha |
| TSTQ | Time spent in target quadrant |
References
- Vaquerizo-Serrano, J.; de Pablo, G.S.; Singh, J.; Santosh, P. Autism Spectrum Disorder and Clinical High Risk for Psychosis: A Systematic Review and Meta-analysis. J. Autism Dev. Disord. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yates, K.; Le Couteur, A. Diagnosing autism/autism spectrum disorders. Paediatr. Child Health 2016, 26, 513–518. [Google Scholar] [CrossRef]
- Meral, B.F. Parental Views of Families of Children with Autism Spectrum Disorder and Developmental Disorders During the COVID-19 Pandemic. J. Autism Dev. Disord. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, W.E. Neonatal and regressive forms of autism: Diseases with similar symptoms but a different etiology. Med. Hypotheses 2017, 109, 46–52. [Google Scholar] [CrossRef]
- Nicolas, C.S.; Amici, M.; Bortolotto, Z.A.; Doherty, A.; Csaba, Z.; Fafouri, A.; Dournaud, P.; Gressens, P.; Collingridge, G.L.; Peineau, S. The role of JAK-STAT signaling within the CNS. JAKSTAT 2013, 2, e22925. [Google Scholar] [CrossRef] [Green Version]
- Copf, T.; Goguel, V.; Lampin-Saint-Amaux, A.; Scaplehorn, N.; Preat, T. Cytokine signaling through the JAK/STAT pathway is required for long-term memory in Drosophila. Proc. Natl. Acad. Sci. USA 2011, 108, 8059–8064. [Google Scholar] [CrossRef] [Green Version]
- Benveniste, E.N.; Liu, Y.; Mc Farland, B.C.; Qin, H. Involvement of the Janus Kinase/Signal Transducer and Activator of Transcription Signaling Pathway in Multiple Sclerosis and the Animal Model of Experimental Autoimmune Encephalomyelitis. J. Interf. Cytokine Res. 2014, 34, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Sharma, N.; Mehan, S. Connection between JAK/STAT and PPARγ Signaling During the Progression of Multiple Sclerosis: Insights into the Modulation of T-Cells and Immune Responses in the Brain. Curr. Mol. Pharmacol. 2021, 14, 823–837. [Google Scholar] [CrossRef]
- Hixson, K.; Cogswell, M.; Brooks-Kayal, A.R.; Russek, S.J. Evidence for a non-canonical JAK/STAT signaling pathway in the synthesis of the brain’s major ion channels and neurotransmitter receptors. BMC Genom. 2019, 20, 677. [Google Scholar] [CrossRef]
- Ben Haim, L.; Ceyzériat, K.; Carrillo-de Sauvage, M.A.; Aubry, F.; Auregan, G.; Guillermier, M.; Ruiz, M.; Petit, F.; Houitte, D.; Faivre, E.; et al. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. J. Neurosci. 2015, 35, 2817–2829. [Google Scholar] [CrossRef]
- Hurtado-Guerrero, I.; Pinto-Medel, M.J.; Urbaneja, P.; Bada, J.L.R.; León, A.; Guerrero, M.; Fernández, O.; Leyva, L.; Oliver-Martos, B. Activation of the JAK-STAT Signaling Pathway after In Vitro Stimulation with IFNß in Multiple Sclerosis Patients According to the Therapeutic Response to IFNß. PLoS ONE 2017, 12, e0170031. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Gibson, S.A.; Buckley, J.A.; Qin, H.; Benveniste, E.N. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin. Immunol. 2016, 189, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Alshammari, M.A.; Khan, M.R.; Alsaad, A.M.; Attia, S.M. S3I-201, a selective Stat3 inhibitor, restores neuroimmune function through upregulation of Treg signaling in autistic BTBR T+ Itpr3tf/J mice. Cell. Signal. 2018, 52, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Alanazi, A.Z.; Alsanea, S.; Sobeai, H.M.A.; Almutairi, M.M.; Mahmood, H.M.; Attia, S.M. The Stat3 inhibitor, S3I-201, downregulates lymphocyte activation markers, chemokine receptors, and inflammatory cytokines in the BTBR T+ Itpr3tf/J mouse model of autism. Brain Res. Bull. 2019, 152, 27–34. [Google Scholar] [CrossRef]
- Jun, T.; Luo, D.; Shytle, R.D. Luteolin and Diosmin/Diosmetin as Novel STAT3 Inhibitors for Treating. Autism. Patent Number US 8,778,894 B2, 15 July 2014. [Google Scholar]
- Zhang, X.; Jin, J.; Peng, X.; Ramgolam, V.S.; Markovic-Plese, S. Simvastatin Inhibits IL-17 Secretion by Targeting Multiple IL-17-Regulatory Cytokines and by Inhibiting the Expression of IL-17 Transcription Factor RORC in CD4+ Lymphocytes. J. Immunol. 2008, 180, 6988–6996. [Google Scholar] [CrossRef] [Green Version]
- Taler, M.; Gil-Ad, I.; Lomnitski, L.; Korov, I.; Baharav, E.; Bar, M.; Zolokov, A.; Weizman, A. Immunomodulatory effect of selective serotonin reuptake inhibitors (SSRIs) on human T lymphocyte function and gene expression. Eur. Neuropsychopharmacol. 2007, 17, 774–780. [Google Scholar] [CrossRef]
- Jensen, K.V.; Cseh, O.; Aman, A.; Weiss, S.; Luchman, H.A. The JAK2/STAT3 inhibitor pacritinib effectively inhibits patient-derived GBM brain tumor initiating cells in vitro and when used in combination with temozolomide increases survival in an orthotopic xenograft model. PLoS ONE 2017, 12, e0189670. [Google Scholar]
- Qin, H.; Buckley, J.A.; Li, X.; Liu, Y.; Fox, T.H.; Meares, G.; Yu, H.; Yan, Z.; Harms, A.; Li, Y.; et al. Inhibition of the JAK/STAT pathway protects against α-synuclein-induced neuroinflammation and dopaminergic neurodegeneration. J. Neurosci. 2016, 36, 5144–5159. [Google Scholar] [CrossRef]
- Choi, M.; Kim, H.; Yang, E.J.; Kim, H.S. Inhibition of STAT3 phosphorylation attenuates impairments in learning and memory in 5XFAD mice, an animal model of Alzheimer’s disease. J. Pharmacol. Sci. 2020, 143, 290–299. [Google Scholar] [CrossRef]
- Yu, J.H.; Kim, K.H.; Kim, H. SOCS 3 and PPAR-γ ligands inhibit the expression of IL-6 and TGF-β1 by regulating JAK2/STAT3 signaling in pancreas. Int. J. Biochem. Cell Biol. 2008, 40, 677–688. [Google Scholar] [CrossRef]
- Wafer, R.; Tandon, P.; Minchin, J.E.N. The Role of Peroxisome Proliferator-Activated Receptor Gamma (PPARG) in Adipogenesis: Applying Knowledge from the Fish Aquaculture Industry to Biomedical Research. Front. Endocrinol. 2017, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heming, M.; Gran, S.; Jauch, S.L.; Fischer-Riepe, L.; Russo, A.; Klotz, L.; Hermann, S.; Schäfers, M.; Roth, J.; Barczyk-Kahlert, K. Peroxisome Proliferator-Activated Receptor-γ Modulates the Response of Macrophages to Lipopolysaccharide and Glucocorticoids. Front. Immunol. 2018, 9, 893. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.-F.; Chen, G.-B.; Xu, M.-G.; Long, R.-T.; Wang, H.; Wang, X.-Y.; Jiang, C.-N.; Yi, X.-N. Upregulation of PPAR-gamma activity inhibits cyclooxygenase 2 expression in cortical neurons with N-methyl-d-aspartic acid induced excitatory neurotoxicity. Biotechnol. Biotechnol. Equip. 2019, 33, 1018–1023. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Ubaldi, M. Nuclear peroxisome proliferator activated receptor-gamma (PPARγ) as a therapeutic target to treat neurodegeneration and dependence elicited by drugs of abuse. Neural Regen. Res. 2021, 16, 984–985. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Gil, P.; Joglar, B.; Rodriguez-Perez, A.I.; Guerra, M.J.; Labandeira-Garcia, J.L. Involvement of PPAR-γ in the neuroprotective and anti-inflammatory effects of angiotensin type 1 receptor inhibition: Effects of the receptor antagonist telmisartan and receptor deletion in a mouse MPTP model of Parkinson’s disease. J. Neuroinflamm. 2012, 9, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corona, J.C.; Duchen, M.R. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic. Biol. Med. 2016, 100, 153–163. [Google Scholar] [CrossRef] [Green Version]
- García-Bueno, B.; Madrigal, J.L.; Lizasoain, I.; Moro, M.A.; Lorenzo, P.; Leza, J.C. Peroxisome proliferator-activated receptor gamma activation decreases neuroinflammation in brain after stress in rats. Biol. Psychiatry 2005, 57, 885–894. [Google Scholar] [CrossRef]
- Jahrling, J.B.; Hernandez, C.M.; Denner, L.; Dineley, K.T. PPARγ recruitment to active ERK during memory consolidation is required for Alzheimer’s disease-related cognitive enhancement. J. Neurosci. 2014, 34, 4054–4063. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.; Morgese, M.G.; Pisanu, A.; Macheda, T.; Paquette, M.; Seillier, A.; Cassano, T.; Carta, A.; Giuffrida, A. Activation of PPAR gamma receptors reduces levodopa-induced dyskinesias in 6-OHDA-lesioned rats. Neurobiol. Dis. 2014, 74, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Mirza, R.; Sharma, B. A selective peroxisome proliferator-activated receptor-γ agonist benefited propionic acid induced autism-like behavioral phenotypes in rats by attenuation of neuroinflammation and oxidative stress. Chem. Interact. 2019, 311, 108758. [Google Scholar] [CrossRef]
- Seok, H.; Lee, M.; Shin, E.; Yun, M.R.; Lee, Y.-H.; Moon, J.H.; Kim, E.; Lee, P.H.; Lee, B.-W.; Kang, E.S.; et al. Low-dose pioglitazone can ameliorate learning and memory impairment in a mouse model of dementia by increasing LRP1 expression in the hippocampus. Sci. Rep. 2019, 9, 4414. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-J.; Sun, X.-H.; Wang, S.-W.; Chen, J.-L.; Bi, Y.-H.; Jiang, D.-X. Mifepristone alleviates cerebral ischemia-reperfusion injury in rats by stimulating PPAR γ. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5688–5696. [Google Scholar] [PubMed]
- Ding, Y.; Kang, J.; Liu, S.; Xu, Y.; Shao, B. The Protective Effects of Peroxisome Proliferator-Activated Receptor Gamma in Cerebral Ischemia-Reperfusion Injury. Front. Neurol. 2020, 11, 1469. [Google Scholar] [CrossRef] [PubMed]
- Song, E.A.; Lim, J.W.; Kim, H. Docosahexaenoic acid inhibits IL-6 expression via PPARγ-mediated expression of catalase in cerulein-stimulated pancreatic acinar cells. Int. J. Biochem. Cell Biol. 2017, 88, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Park, S.Y.; Joe, E.-H.; Jou, I. 15d-PGJ2 and Rosiglitazone Suppress Janus Kinase-STAT Inflammatory Signaling through Induction of Suppressor of Cytokine Signaling 1 (SOCS1) and SOCS3 in Glia. J. Biol. Chem. 2003, 278, 14747–14752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panzer, U.; Zahner, G.; Wienberg, U.; Steinmetz, O.M.; Peters, A.; Turner, J.E.; Paust, H.J.; Wolf, G.; Stahl, R.A.; Schneider, A. 15-Deoxy-Δ12, 14-prostaglandin J2 inhibits INF-γ-induced JAK/STAT1 signalling pathway activation and IP-10/CXCL10 expression in mesangial cells. Nephrol. Dial. Transpl. 2008, 23, 3776–3785. [Google Scholar] [CrossRef] [Green Version]
- Saxena, G.; Singh, S.P.; Pal, R.; Singh, S.; Pratap, R.; Nath, C. Gugulipid, an extract of Commiphora whighitii with lipid-lowering properties, has protective effects against streptozotocin-induced memory deficits in mice. Pharmacol. Biochem. Behav. 2007, 86, 797–805. [Google Scholar] [CrossRef]
- Liu, F.G.; Hu, W.F.; Wang, J.L.; Wang, P.; Gong, Y.; Tong, L.J.; Jiang, B.; Zhang, W.; Qin, Y.B.; Chen, Z.; et al. Z-guggulsterone produces antidepressant-like effects in mice through activation of the BDNF signaling pathway. Internat. J. Neuropsychopharmacol. 2017, 20, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Huang, C.; Ding, W. Z-Guggulsterone Improves the Scopolamine-Induced Memory Impairments Through Enhancement of the BDNF Signal in C57BL/6J Mice. Neurochem. Res. 2016, 41, 3322–3332. [Google Scholar] [CrossRef]
- Zhang, J.H.; Shangguan, Z.S.; Chen, C.; Zhang, H.J.; Lin, Y. Anti-inflammatory effects of guggulsterone on murine macrophage by inhibiting LPS-induced inflammatory cytokines in NF-κBsignaling pathway. Drug Des. Dev. Ther. 2016, 10, 1829. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Sharma, N.; Khera, R.; Gupta, R.; Mehan, S. Guggulsterone ameliorates ethidium bromide-induced experimental model of multiple sclerosis via restoration of behavioral, molecular, neurochemical and morphological alterations in rat brain. Metab. Brain Dis. 2021, 36, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Zhu, M.; Chen, K.; Xie, H.; Bai, H.; Chen, Q. Z-Guggulsterone Induces Apoptosis in Gastric Cancer Cells through the Intrinsic Mitochondria-Dependent Pathway. Sci. World J. 2021, 2021, 3152304. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, B.; Karuna, R.; Sreenivasa, R.S.; Haritha, K.; Sai, M.D.; Sasis, B.R.B.; Saralakumari, D. Effect of Commiphora mukul gum resin on hepatic marker enzymes, lipid peroxidation and antioxidants status in pancreas and heart of streptozotocin induced diabetic rats. Asian Pac. J. Trop. Biomed. 2012, 2, 895–900. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Xia, C.; Meier, J.; Li, S.; Hu, X.; Lala, D.S. The hypolipidemic natural product guggulsterone acts as an antagonist of the bile acid receptor. Mol. Endocrinol. 2002, 16, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Thappa, D.M.; Dogra, J. Nodulocystic Acne: Oral Gugulipid versus Tetracycline. J. Dermatol. 1994, 21, 729–731. [Google Scholar] [CrossRef]
- Shah, R.; Gulati, V.; Palombo, E.A. Pharmacological properties of guggulsterones, the major active components of gum guggul. Phytother. Res. 2012, 26, 1594–1605. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Sung, B.; Goel, A.; Ralhan, R.; Aggarwal, B.B. Guggulsterone, a Farnesoid X Receptor Antagonist, Inhibits Constitutive and Inducible STAT3 Activation through Induction of a Protein Tyrosine Phosphatase SHP-1. Cancer Res. 2008, 68, 4406–4415. [Google Scholar] [CrossRef] [Green Version]
- Suiqing, C.; Min, Z.; Lirong, C. Overexpression of Phosphorylated-STAT3 Correlated with the Invasion and Metastasis of Cutaneous Squamous Cell Carcinoma. J. Dermatol. 2005, 32, 354–360. [Google Scholar] [CrossRef]
- Cheon, J.H.; Kim, E.S.; Hong, S.Y.; Lee, H.K.; Kim, S.W.; An, M.J.; Kim, T.I.; Lee, K.R.; Kim, W.H. Guggulsterone inhibits angiogenesis by blocking STAT3 and VEGF expression in colon cancer cells. Oncol. Rep. 1994, 20, 1321–1327. [Google Scholar] [CrossRef] [Green Version]
- Cornick, C.L.; Strongitharm, B.H.; Sassano, G.; Rawlins, C.; Mayes, A.E.; Joseph, A.N.; O’Dowd, J.; Stocker, C.; Wargent, E.; Cawthorne, M.A.; et al. Identification of a novel agonist of peroxisome proliferator-activated receptors α and γ that may contribute to the anti-diabetic activity of guggulipid in Lepob/Lepob mice. J. Nutr. Biochem. 2009, 20, 806–815. [Google Scholar] [CrossRef]
- Saikumar, I.; Rasalkar, A.A.; Shivakumar, B.M.; Reddy, D.N.; Malempati, R. Effect of Guggulsterone on the Expression of Adiponectin in 3T3-L1 Cells. Nat. Prod. Commun. 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.R.; Kim, J.; Lee, J.E.; Lee, E.J.; Yoon, J.S. Therapeutic Effect of Guggulsterone in Primary Cultured Orbital Fibroblasts Obtained from Patients with Graves’ Orbitopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, A.; Khera, R.; Rahi, S.; Mehan, S.; Makeen, H.; Khormi, Y.; Rehman, M.; Khan, A. Neuroprotective Effect of α-Mangostin in Ameliorating Propionic Acid-Induced Experimental Model of Autism in Wistar Rats. Brain Sci. 2021, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- González-Fraguela, M.E.; Hung, M.-L.D.; Vera, H.; Maragoto, C.; Noris, E.; Blanco, L.; Galvizu, R.; Robinson, M. Oxidative Stress Markers in Children with Autism Spectrum Disorders. Br. J. Med. Med. Res. 2013, 3, 307–317. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Sharma, R.; Rahi, S.; Mehan, S. Neuroprotective potential of solanesol in intracerebroventricular propionic acid induced experimental model of autism: Insights from behavioral and biochemical evidence. Toxicol. Rep. 2019, 6, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Gopi, C.; Sastry, V.G.; Dhanaraju, M.D. Effect of novel phenothiazine derivatives on brain dopamine in Wistar rats. Beni-Suef Univ. J. Basic Appl. Sci. 2019, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Rajdev, K.; Siddiqui, E.M.; Jadaun, K.S.; Mehan, S. Neuroprotective potential of solanesol in a combined model of intracerebral and intraventricular hemorrhage in rats. IBRO Rep. 2020, 8, 101–114. [Google Scholar] [CrossRef]
- Mehan, S.; Monga, V.; Rani, M.; Dudi, R.; Ghimire, K. Neuroprotective effect of solanesol against 3-nitropropionic acid-induced Huntington’s disease-like behavioral, biochemical, and cellular alterations: Restoration of coenzyme-Q10-mediated mitochondrial dysfunction. Indian J. Pharmacol. 2018, 50, 309. [Google Scholar] [CrossRef]
- Minj, E.; Upadhayay, S.; Mehan, S. Nrf2/HO-1 Signaling Activator Acetyl-11-keto-beta Boswellic Acid (AKBA)-Mediated Neuroprotection in Methyl Mercury-Induced Experimental Model of ALS. Neurochem. Res. 2021, 1–18. [Google Scholar] [CrossRef]
- Kaur, R.; Parveen, S.; Mehan, S.; Khanna, D.; Kalra, S. Neuroprotective effect of ellagic acid against chronically scopolamine induced Alzheimer’s type memory and cognitive dysfunctions: Possible behavioural and biochemical evidences. Int. J. Preven. Med. Res. 2015, 1, 45–64. [Google Scholar]
- Sharma, N.; Upadhayay, S.; Shandilya, A.; Sahu, R.; Singh, A.; Rajkhowa, B.; Mehan, S. Neuroprotection by solanesol against ethidium bromide-induced multiple sclerosis-like neurobehavioral, molecular, and neurochemical alterations in experimental rats. Phytomed. Plus 2021, 1, 100051. [Google Scholar] [CrossRef]
- Hayami, S.; Yaita, M.; Ogiri, Y.; Sun, F.; Nakata, R.; Kojo, S. Change in caspase-3-like protease in the liver and plasma during rat liver regeneration following partial hepatectomy. Biochem. Pharmacol. 2000, 60, 1883–1886. [Google Scholar] [CrossRef]
- Rosenling, A.T.I. Proteomic screening of cerebrospinal fluid: Candidate proteomic biomarkers for sample stability and experimental autoimmune encephalomyelitis. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 20 December 2010. [Google Scholar]
- Gao, D.; Jin, N.; Fu, Y.; Zhu, Y.; Wang, Y.; Wang, T.; Chen, Y.; Zhang, M.; Xiao, Q.; Huang, M.; et al. Rational drug design of benzothiazole-based derivatives as potent signal transducer and activator of transcription 3 (STAT3) signaling pathway inhibitors. Eur. J. Med. Chem. 2021, 216, 113333. [Google Scholar] [CrossRef] [PubMed]
- Duelsner, A.; Gatzke, N.; Hillmeister, P.; Glaser, J.; Zietzer, A.; Nagorka, S.; Janke, D.; Pfitzner, J.; Stawowy, P.; Meyborg, H.; et al. PPAR γ activation inhibits cerebral arteriogenesis in the hypoperfused rat brain. ACTA Physiol. 2014, 210, 354–368. [Google Scholar] [CrossRef]
- Rahi, S.; Gupta, R.; Sharma, A.; Mehan, S. Smo-Shh signaling activator purmorphamine ameliorates neurobehavioral, molecular, and morphological alterations in an intracerebroventricular propionic acid-induced experimental model of autism. Hum. Exp. Toxicol. 2021, 40, 1880–1898. [Google Scholar] [CrossRef]
- Omezzine, A.; Mauduit, C.; Tabone, E.; Nabli, N.; Bouslama, A.; Benahmed, M. Caspase-3 and-6 expression and activation are targeted by hormone action in the rat ventral prostate during the apoptotic cell death process. Biol. Reprod. 2003, 69, 752–760. [Google Scholar] [CrossRef]
- He, X.; Sun, J.; Huang, X. Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Exp. Ther. Med. 2017, 15, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Li, G. Tanshinone modulates the expression of Bcl-2 and Bax in cardiomyocytes and has a protective effect in a rat model of myocardial ischemia-reperfusion. Hell. J. Cardiol. 2018, 59, 323–328. [Google Scholar] [CrossRef]
- Nwangwu, S.; Josiah, S.J.; Urom-Ndubuisi, C.U.; Owoeye, S.; Osagiede, P.; Oyeyemi, A.W.; Omotosho, D.R. Plasma Interleukin-1β (IL-1β) Concentrations at the Different Phases of the Oestrus Cycle in Clomiphene Treated Female Rats. Niger. J. Physiol. Sci. 2017, 32, 33–36. [Google Scholar]
- Deshmukh, R.; Sharma, V.; Mehan, S.; Sharma, N.; Bedi, K.L. Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine—A PDE1 inhibitor. Eur. J. Pharmacol. 2009, 620, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mehan, S.; Parveen, S.; Kalra, S. Adenyl cyclase activator forskolin protects against Huntington’s disease-like neurodegenerative disorders. Neural Regen. Rese. 2017, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Khanna, D.; Mehan, S.; Kalra, S. Experimental evidence for the potential of lycopene in the management of scopolamine induced amnesia. RSC Adv. 2015, 5, 72881–72892. [Google Scholar] [CrossRef]
- Mehan, S.; Rahi, S.; Tiwari, A.; Kapoor, T.; Rajdev, K.; Sharma, R.; Khera, H.; Kosey, S.; Kukkar, U.; Dudi, R. Adenylate cyclase activator forskolin alleviates intracerebroventricular propionic acid-induced mitochondrial dysfunction of autistic rats. Neural Regen. Res. 2020, 15, 1140–1149. [Google Scholar] [CrossRef]
- Milner, V.; McIntosh, H.; Colvert, E.; Happé, F. A qualitative exploration of the female experience of autism spectrum disorder (ASD). J. Autism Dev. Disord. 2019, 49, 2389–2402. [Google Scholar] [CrossRef] [Green Version]
- Mirza, R.; Sharma, B. Selective modulator of peroxisome proliferator-activated receptor-α protects propionic acid induced autism-like phenotypes in rats. Life Sci. 2018, 214, 106–117. [Google Scholar] [CrossRef]
- Paudel, R.; Raj, K.; Gupta, Y.; Singh, S. Oxiracetam and Zinc Ameliorates Autism-Like Symptoms in Propionic Acid Model of Rats. Neurotox. Res. 2020, 37, 815–826. [Google Scholar] [CrossRef]
- MacFabe, D.F.; Cain, N.E.; Boon, F.; Ossenkopp, K.P.; Cain, D.P. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder. Behav. Brain Res. 2011, 217, 47–54. [Google Scholar] [CrossRef]
- MacFabe, D.F.; Cain, D.P.; Rodriguez-Capote, K.; Franklin, A.E.; Hoffman, J.E.; Boon, F.; Taylor, A.R.; Kavaliers, M.; Ossenkopp, K.P. Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 2007, 176, 149–169. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.; Won, J.; Jin, Y.; Hong, Y.; Hur, T.Y.; Kim, J.H.; Lee, S.R.; Hong, Y. Pathophysiological and neurobehavioral characteristics of a propionic acid-mediated autism-like rat model. PLoS ONE 2018, 13, e0192925. [Google Scholar]
- Heimann, E.; Nyman, M.; Pålbrink, A.-K.; Lindkvist, K.; Degerman, E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Nankova, B.B.; Agarwal, R.; MacFabe, D.F.; La Gamma, E.F. Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells-possible relevance to autism spectrum disorders. PLoS ONE 2014, 9, e103740. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Kuhad, A. Resveratrol suppresses neuroinflammation in the experimental paradigm of autism spectrum disorders. Neurochem. Int. 2017, 103, 8–23. [Google Scholar] [CrossRef]
- Kong, E.; Sucic, S.; Monje, F.J.; Reisinger, S.N.; Savalli, G.; Diao, W.; Khan, D.; Ronovsky, M.; Cabatic, M.; Koban, F.; et al. STAT3 controls IL6-dependent regulation of serotonin transporter function and depression-like behavior. Sci. Rep. 2015, 5, 9009. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Wang, Q.; Liu, M.; Sun, A.; Li, X. Possible Involvement of the IL-6/JAK2/STAT3 Pathway in the Hypothalamus in Depressive-Like Behavior of Rats Exposed to Chronic Mild Stress. Neuropsychobiology 2021, 80, 279–287. [Google Scholar] [CrossRef]
- Rossi, S.; Studer, V.; Motta, C.; Polidoro, S.; Perugini, J.; Macchiarulo, G.; Giovannetti, A.M.; Pareja-Gutierrez, L.; Calò, A.; Colonna, I.; et al. Neuroinflammation drives anxiety and depression in relapsing-remitting multiple sclerosis. Neurology 2017, 89, 1338–1347. [Google Scholar] [CrossRef]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef]
- Holbrook, S. The effects of Single Acute and Repeated Intracerebroventricular Infusions of Propionic Acid on Locomotor Activity and Neuroinflammation in Rats. Master’s Thesis, The University of Western Ontario, London, ON, Canada, 26 June 2013. [Google Scholar]
- Mepham, J.R.; MacFabe, D.F.; Boon, F.H.; Foley, K.A.; Cain, D.P.; Ossenkopp, K.P. Examining the non-spatial pretraining effect on a water maze spatial learning task in rats treated with multiple intracerebroventricular (ICV) infusions of propionic acid: Contributions to a rodent model of ASD. Behav. Brain Res. 2021, 403, 113140. [Google Scholar] [CrossRef]
- Parker-Athill, E.; Luo, D.; Bailey, A.; Giunta, B.; Tian, J.; Shytle, R.D.; Murphy, T.; Legradi, G.; Tan, J. Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism. J. Neuroimmunol. 2009, 217, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Vallée, A.; Vallée, J.-N.; Lecarpentier, Y. PPARγ agonists: Potential treatment for autism spectrum disorder by inhibiting the canonical WNT/β-catenin pathway. Mol. Psychiatry 2018, 24, 643–652. [Google Scholar] [CrossRef]
- Boris, M.; Kaiser, C.C.; Goldblatt, A.; Elice, M.W.; Edelson, S.M.; Adams, J.B.; Feinstein, D.L. Effect of pioglitazone treatment on behavioral symptoms in autistic children. J. Neuroinflam. 2007, 4, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzunova, G.; Pallanti, S.; Hollander, E. Excitatory/inhibitory imbalance in autism spectrum disorders: Implications for interventions and therapeutics. World J. Biol. Psychiatry 2015, 17, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Penzes, P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr. Mol. Med. 2015, 15, 146–167. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.J.; Angoa-Peréz, M.; Briggs, D.I.; Sykes, C.E.; Francescutti, D.M.; Rosenberg, D.R.; Kuhn, D.M. Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: Possible relevance to autism. PLoS ONE 2012, 7, e48975. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, D.A.; Oliver, B.; Pahua, A.; Hitchcock, K.; Bykowski, A.; Tice, D.; Musleh, A.; Ryan, B.C. Serotonin 6 receptor blockade reduces repetitive behavior in the BTBR mouse model of autism spectrum disorder. Pharmacol. Biochem. Behav. 2020, 200, 173076. [Google Scholar] [CrossRef] [PubMed]
- Dicarlo, G.E.; Aguilar, J.I.; Matthies, H.; Harrison, F.E.; Bundschuh, K.E.; West, A.; Hashemi, P.; Herborg, F.; Rickhag, M.; Chen, H.; et al. Autism-linked dopamine transporter mutation alters striatal dopamine neurotransmission and dopamine-dependent behaviors. J. Clin. Investig. 2019, 129, 3407–3419. [Google Scholar] [CrossRef]
- Karvat, G.; Kimchi, T. Acetylcholine Elevation Relieves Cognitive Rigidity and Social Deficiency in a Mouse Model of Autism. Neuropsychopharmacology 2013, 39, 831–840. [Google Scholar] [CrossRef]
- Drenthen, G.S.; Barendse, E.M.; Aldenkamp, A.P.; van Veenendaal, T.M.; Puts, N.A.; Edden, R.A.; Zinger, S.; Thoonen, G.; Hendriks, M.P.; Kessels, R.P.; et al. Altered neurotransmitter metabolism in adolescents with high-functioning autism. Psychiatry Res. Neuroimaging 2016, 256, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Dong, D.; Zielke, H.R.; Yeh, D.; Yang, P. Cellular stress and apoptosis contribute to the pathogenesis of autism spectrum disorder. Autism Res. 2018, 11, 1076–1090. [Google Scholar] [CrossRef]
- Khemakhem, A.M.; Frye, R.E.; El-Ansary, A.; Al-Ayadhi, L.; Ben Bacha, A. Novel biomarkers of metabolic dysfunction is autism spectrum disorder: Potential for biological diagnostic markers. Metab. Brain Dis. 2017, 32, 1983–1997. [Google Scholar] [CrossRef]
- Khalil, S.R.; Abd-Elhakim, Y.M.; Selim, M.E.; Al-Ayadhi, L.Y. Apitoxin protects rat pups brain from propionic acid-induced oxidative stress: The expression pattern of Bcl-2 and Caspase-3 apoptotic genes. Neurotoxicology 2015, 49, 121–131. [Google Scholar] [CrossRef]
- Burke, S.L.; Cobb, J.; Agarwal, R.; Maddux, M.; Cooke, M.S. How Robust is the Evidence for a Role of Oxidative Stress in Autism Spectrum Disorders and Intellectual Disabilities? J. Autism Dev. Disord. 2020, 51, 1428–1445. [Google Scholar] [CrossRef]
- Morimoto, M.; Hashimoto, T.; Tsuda, Y.; Nakatsu, T.; Kitaoka, T.; Kyotani, S. Assessment of oxidative stress in autism spectrum disorder using reactive oxygen metabolites and biological antioxidant potential. PLoS ONE 2020, 15, e0233550. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, P.M.; Matté, A.; Bolotta, A.; Federti, E.; Ghezzo, A.; Guarnieri, T.; Marini, M.; Posar, A.; Siciliano, A.; De Franceschi, L.; et al. Plasma peroxiredoxin changes and inflammatory cytokines support the involvement of neuro-inflammation and oxidative stress in Autism Spectrum Disorder. J. Transl. Med. 2019, 17, 332. [Google Scholar] [CrossRef] [PubMed]
- Guloksuz, S.A.; Abali, O.; Cetin, E.A.; Gazioglu, S.B.; Deniz, G.; Yildirim, A.; Kawikova, I.; Guloksuz, S.; Leckman, J.F. Elevated plasma concentrations of S100 calcium-binding protein B and tumor necrosis factor alpha in children with autism spectrum disorders. Rev. Bras. Psiquiatr. 2017, 39, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chez, M.G.; Dowling, T.; Patel, P.B.; Khanna, P.; Kominsky, M. Elevation of Tumor Necrosis Factor-Alpha in Cerebrospinal Fluid of Autistic Children. Pediatr. Neurol. 2007, 36, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.K.; Keehn, B.; Lincoln, A.J.; Müller, R.-A. White Matter Compromise of Callosal and Subcortical Fiber Tracts in Children With Autism Spectrum Disorder: A Diffusion Tensor Imaging Study. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 1269–1278.e2. [Google Scholar]
- Catani, M.; Jones, D.K.; Daly, E.; Embiricos, N.; Deeley, Q.; Pugliese, L.; Curran, S.; Robertson, D.; Murphy, D. Altered cerebellar feedback projections in Asperger syndrome. NeuroImage 2008, 41, 1184–1191. [Google Scholar] [CrossRef]
- Stoner, R.; Chow, M.L.; Boyle, M.P.; Sunkin, S.M.; Mouton, P.R.; Roy, S.; Wynshaw-Boris, A.; Colamarino, S.A.; Lein, E.S.; Courchesne, E. Patches of Disorganization in the Neocortex of Children with Autism. N. Engl. J. Med. 2014, 370, 1209–1219. [Google Scholar] [CrossRef] [Green Version]
- Jacot-Descombes, S.; Uppal, N.; Wicinski, B.; Santos, M.; Schmeidler, J.; Giannakopoulos, P.; Heinsein, H.; Schmitz, C.; Hof, P.R. Decreased pyramidal neuron size in Brodmann areas 44 and 45 in patients with autism. ACTA Neuropathol. 2012, 124, 67–79. [Google Scholar] [CrossRef]
- Hayes, T.L.; Lewis, D.A. Hemispheric Differences in Layer III Pyramidal Neurons of the Anterior Language Area. Arch. Neurol. 1993, 50, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, J.; Kuchna, I.; Nowicki, K.; Imaki, H.; Wegiel, J.; Marchi, E.; Ma, S.Y.; Chauhan, A.; Chauhan, V.; Bobrowicz, T.W.; et al. The neuropathology of autism: Defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 2010, 119, 755–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcone, C.; Mevises, N.-Y.; Hong, T.; Dufour, B.; Chen, X.; Noctor, S.C.; Cerdeño, V.M. Neuronal and glial cell number is altered in a cortical layer-specific manner in autism. Autism 2021, 25, 2238–2253. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of Oligodendrocyte and Myelin in the Mammalian Central Nervous System. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef]
- Ludwig, P.E.; Das, J.M. Histology, glial cells. In StatPearls; StatPearls Publishing: Omaha, NE, USA, 10 May 2021. [Google Scholar]
- Castelijns, B.; Baak, M.L.; Timpanaro, I.S.; Wiggers, C.; Vermunt, M.W.; Shang, P.; Kondova, I.; Geeven, G.; Bianchi, V.; De Laat, W.; et al. Hominin-specific regulatory elements selectively emerged in oligodendrocytes and are disrupted in autism patients. Nat. Commun. 2020, 11, 301. [Google Scholar] [CrossRef]
- Kawamura, A.; Katayama, Y.; Nishiyama, M.; Shoji, H.; Tokuoka, K.; Ueta, Y.; Miyata, M.; Isa, T.; Miyakawa, T.; Hayashi-Takagi, A.; et al. Oligodendrocyte dysfunction due to Chd8 mutation gives rise to behavioral deficits in mice. Hum. Mol. Genet. 2020, 29, 1274–1291. [Google Scholar] [CrossRef]
- DiStasio, M.M.; Nagakura, I.; Nadler, M.J.; Anderson, M.P. T lymphocytes and cytotoxic astrocyte blebs correlate across autism brains. Ann. Neurol. 2019, 86, 885–898. [Google Scholar] [CrossRef]











| S. no. | Groups | STAT3 | PPAR-Gamma | Myelin Basic Protein | ||
|---|---|---|---|---|---|---|
| Brain Homogenate (nM/µg Protein) | CSF (ng/mL) | Brain Homogenate (nM/µg Protein) | CSF (ng/mL) | Brain Homogenate (µg/mg Protein) | ||
| 1. | Vehicle Control | 1.72 ± 0.038 | 0.29 ± 0.004 | 6.173 ± 0.041 | 2.05 ± 0.042 | 106.3 ± 0.325 |
| 2. | Sham Control | 1.73 ± 0.052 | 0.29 ± 0.006 | 6.293 ± 0.036 | 2.06 ± 0.049 | 106.2 ± 0.422 |
| 3. | GST60 Perse | 1.76 ± 0.050 | 0.31 ± 0.005 | 6.397 ± 0.069 | 2.10 ± 0.051 | 107.1 ± 0.146 |
| 4. | PPA | 6.33 ± 0.057 * | 0.69 ± 0.007 * | 2.388 ± 0.109 * | 0.83 ± 0.030 * | 61.28 ± 0.313 * |
| 5. | PPA + GST30 | 4.83 ± 0.045 @ | 0.59 ± 0.003 @ | 3.333 ± 0.037 @ | 1.35 ± 0.022 @ | 77.44 ± 0.252 @ |
| 6. | PPA + GST60 | 3.18 ± 0.029 @# | 0.50 ± 0.003 @# | 4.482 ± 0.088 @# | 1.73 ± 0.021 @# | 92.55 ± 0.157 @# |
| S. no. | Groups | Caspase-3 | Bax | Bcl-2 | |||
|---|---|---|---|---|---|---|---|
| Brain Homogenate (nM/mg Protein) | Blood Plasma (ng/mL) | Brain Homogenate (ng/mg Protein) | Blood Plasma (ng/mL) | Brain Homogenate (ng/mg Protein) | Blood Plasma (ng/mL) | ||
| 1. | Vehicle Control | 106.10 ± 1.103 | 1.94 ± 0.014 | 5.35 ± 0.069 | 1.06 ± 0.013 | 30.23 ± 0.366 | 8.84 ± 0.016 |
| 2. | Sham Control | 105.30 ± 0.454 | 1.94 ± 0.014 | 5.23 ± 0.056 | 1.05 ± 0.014 | 30.53 ± 0.359 | 8.83 ± 0.012 |
| 3. | GST60 Perse | 105.70 ± 0.355 | 1.95 ± 0.011 | 5.31 ± 0.074 | 1.06 ± 0.014 | 29.86 ± 0.501 | 8.83 ± 0.011 |
| 4. | PPA | 159.90 ± 0.549 * | 6.73 ± 0.022 * | 12.14 ± 0.308 * | 5.83 ± 0.012 * | 20.24 ± 0.299 * | 2.58 ± 0.009 * |
| 5. | PPA + GST30 | 145.92 ± 0.436 @ | 4.66 ± 0.101 @ | 9.54 ± 0.126 @ | 4.46 ± 0.016 @ | 24.13 ± 0.256 @ | 4.66 ± 0.029 @ |
| 6. | PPA + GST60 | 134.60 ± 1.073 @# | 3.68 ± 0.033 @# | 7.31 ± 0.118 @# | 3.27 ± 0.025 @# | 26.60 ± 0.168 @# | 6.97 ± 0.017 @# |
| S. no. | Groups | Serotonin (ng/mg Protein) | Glutamate (ng/mg Protein) | Dopamine (ng/mg Protein) | Ach (ng/mg Protein) |
|---|---|---|---|---|---|
| 1. | Vehicle Control | 39.15 ± 0.542 | 111.80 ± 0.400 | 90.84 ± 0.566 | 11.62 ± 0.177 |
| 2. | Sham Control | 40.84 ± 0.478 | 111.60 ± 0.409 | 90.93 ± 0.594 | 11.68 ± 0.107 |
| 3. | GST60 Perse | 39.55 ± 0.607 | 111.50 ± 0.549 | 91.07 ± 0.475 | 11.79 ± 0.181 |
| 4. | PPA | 14.12 ± 0.133 * | 308.60 ± 2.029 * | 31.70 ± 0.527 * | 5.81 ± 0.127 * |
| 5. | PPA + GST30 | 17.17 ± 0.256 @ | 214.50 ± 2.314 @ | 49.23 ± 0.335 @ | 7.28 ± 0.102 @ |
| 6. | PPA + GST60 | 24.65 ± 0.373 @# | 184.20 ± 1.937 @# | 54.12 ± 1.063 @# | 9.46 ± 0.125 @# |
| S. no. | Groups | TNF-α | IL-1β | ||
|---|---|---|---|---|---|
| Brain Homogenate (pg/mg protein) | Blood Plasma (pg/mL) | Brain Homogenate (pg/mg Protein) | Blood Plasma (pg/mL) | ||
| 1. | Vehicle Control | 31.04 ± 0.379 | 30.73 ± 0.440 | 13.60 ± 0.223 | 16.58 ± 0.147 |
| 2. | Sham Control | 31.33 ± 0.430 | 30.82 ± 0.481 | 13.48 ± 0.175 | 16.33 ± 0.143 |
| 3. | GST60 Perse | 30.69 ± 0.368 | 31.69 ± 0.305 | 14.02 ± 0.274 | 16.66 ± 0.108 |
| 4. | PPA | 71.59 ± 0.371 * | 102.00 ± 0.389 * | 24.51 ± 0.207 * | 92.29 ± 0.120 * |
| 5. | PPA + GST30 | 62.34 ± 0.132 @ | 81.54 ± 0.397 @ | 20.32 ± 0.364 @ | 76.47 ± 0.110 @ |
| 6. | PPA + GST60 | 51.34 ± 0.610 @# | 55.47 ± 0.378 @# | 18.41 ± 0.097 @# | 52.46 ± 0.159 @# |
| S.no. | Groups | AchE μM/mg protein) | LDH (Unit/mg protein) | SOD (µM/mg protein) | GSH (µM/mg protein) | Nitrite (µM/mg protein) | MDA (nM/mg protein) |
|---|---|---|---|---|---|---|---|
| 1. | Vehicle Control | 17.74 ± 0.215 | 111.20 ± 0.520 | 469.30 ± 0.756 | 32.43 ± 0.189 | 7.07 ± 0.034 | 31.24 ± 0.964 |
| 2. | Sham Control | 18.42 ± 0.277 | 111.50 ± 1.050 | 468.80 ± 0.441 | 32.65 ± 0.326 | 7.05 ± 0.061 | 30.82 ± 0.476 |
| 3. | GST60 Perse | 17.67 ± 0.214 | 112.1 ± 1.120 | 470.70 ± 1.602 | 32.13 ± 0.402 | 7.08 ± 0.041 | 31.44 ± 0.500 |
| 4. | PPA | 54.11 ± 0.419 * | 401.20 ± 2.100 * | 320.90 ± 0.540 * | 11.42 ± 0.153 * | 9.03 ± 0.088 * | 72.07 ± 0.869 * |
| 5. | PPA + GST30 | 38.83 ± 0.514 @ | 307.30 ± 4.054 @ | 366.10 ± 0.621 @ | 18.40 ± 0.171 @ | 8.11 ± 0.045 @ | 65.74 ± 0.526 @ |
| 6. | PPA + GST60 | 31.18 ± 0.357 @# | 282.40 ± 3.630 @# | 384.70 ± 2.096 @# | 23.08 ± 0.167 @# | 7.55 ± 0.058 @# | 56.52 ± 0.832 @# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khera, R.; Mehan, S.; Bhalla, S.; Kumar, S.; Alshammari, A.; Alharbi, M.; Sadhu, S.S. Guggulsterone Mediated JAK/STAT and PPAR-Gamma Modulation Prevents Neurobehavioral and Neurochemical Abnormalities in Propionic Acid-Induced Experimental Model of Autism. Molecules 2022, 27, 889. https://doi.org/10.3390/molecules27030889
Khera R, Mehan S, Bhalla S, Kumar S, Alshammari A, Alharbi M, Sadhu SS. Guggulsterone Mediated JAK/STAT and PPAR-Gamma Modulation Prevents Neurobehavioral and Neurochemical Abnormalities in Propionic Acid-Induced Experimental Model of Autism. Molecules. 2022; 27(3):889. https://doi.org/10.3390/molecules27030889
Chicago/Turabian StyleKhera, Rishabh, Sidharth Mehan, Sonalika Bhalla, Sumit Kumar, Abdulrahman Alshammari, Metab Alharbi, and Satya Sai Sadhu. 2022. "Guggulsterone Mediated JAK/STAT and PPAR-Gamma Modulation Prevents Neurobehavioral and Neurochemical Abnormalities in Propionic Acid-Induced Experimental Model of Autism" Molecules 27, no. 3: 889. https://doi.org/10.3390/molecules27030889
APA StyleKhera, R., Mehan, S., Bhalla, S., Kumar, S., Alshammari, A., Alharbi, M., & Sadhu, S. S. (2022). Guggulsterone Mediated JAK/STAT and PPAR-Gamma Modulation Prevents Neurobehavioral and Neurochemical Abnormalities in Propionic Acid-Induced Experimental Model of Autism. Molecules, 27(3), 889. https://doi.org/10.3390/molecules27030889