Recent Developments of Liquid Chromatography Stationary Phases for Compound Separation: From Proteins to Small Organic Compounds
Abstract
:1. Introduction
2. The Principle of Separation of Compounds in Liquid Chromatography
3. Development of Stationary Phases for Selective Protein Capture and Purification
4. Development of Stationary Phases for Small Chiral Compounds
5. Development of Stationary Phases for PAHs Capture
6. Outlook
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Zeng, R.; Jin, B.-K.; Yang, Z.-H.; Guan, R.; Quan, C. Preparation of a modified crosslinked chitosan/polyvinyl alcohol blended affinity membrane for purification of His-tagged protein. J. Appl. Polym. Sci. 2019, 136, 47347. [Google Scholar] [CrossRef]
- Eldin, M.S.M.; Rahman, S.A.; El Fawal, G.F. Novel immobilized Cu2+-aminated poly (methyl methacrylate) grafted cellophane membranes for affinity separation of His-Tag chitinase. Polym. Bull. 2020, 77, 135–151. [Google Scholar] [CrossRef]
- Riguero, V.; Clifford, R.; Dawley, M.; Dickson, M.; Gastfriend, B.; Thompson, C.; Wang, S.-C.; O’Connor, E. Immobilized metal affinity chromatography optimization for poly-histidine tagged proteins. J. Chromatogr. A 2020, 1629, 461505. [Google Scholar] [CrossRef]
- Huberman, L.B.; Wu, V.W.; Kowbel, D.J.; Lee, J.; Daum, C.; Grigoriev, I.V.; O’Malley, R.C.; Glass, N.L. DNA affinity purification sequencing and transcriptional profiling reveal new aspects of nitrogen regulation in a filamentous fungus. Proc. Natl. Acad. Sci. USA 2021, 118, e2009501118. [Google Scholar] [CrossRef] [PubMed]
- Hovhannisyan, R.H.; Carstens, R.P. Affinity chromatography using 2′ fluoro-substituted RNAs for detection of RNA-protein interactions in RNase-rich or RNase-treated extracts. BioTechniques 2009, 46, 95–98. [Google Scholar] [CrossRef]
- Busby, K.N.; Fulzele, A.; Zhang, D.; Bennett, E.J.; Devaraj, N.K. Enzymatic RNA Biotinylation for Affinity Purification and Identification of RNA–Protein Interactions. ACS Chem. Biol. 2020, 15, 2247–2258. [Google Scholar] [CrossRef]
- Moravcová, D.; Planeta, J. Monolithic Silica Capillary Columns with Improved Retention and Selectivity for Amino Acids. Separations 2018, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Ye, M.; Xu, L.; Shi, Z.-G. A reversed-phase/hydrophilic interaction mixed-mode C18-Diol stationary phase for multiple applications. Anal. Chim. Acta 2015, 888, 182–190. [Google Scholar] [CrossRef]
- Cong, H.; Xing, J.; Ding, X.; Zhang, S.; Shen, Y.; Yu, B. Preparation of porous sulfonated poly(styrene-divinylbenzene) microspheres and its application in hydrophilic and chiral separation. Talanta 2020, 210, 120586. [Google Scholar] [CrossRef]
- Guo, D.; Lou, C.; Zhang, P.; Zhang, J.; Wang, N.; Wu, S.; Zhu, Y. Polystyrene-divinylbenzene-glycidyl methacrylate stationary phase grafted with poly (amidoamine) dendrimers for ion chromatography. J. Chromatogr. A 2016, 1456, 113–122. [Google Scholar] [CrossRef]
- Amran, M.B.; Aminah, S.; Rusli, H.; Buchari, B. Bentonite-based functional material as preconcentration system for determination of chromium species in water by flow injection analysis technique. Heliyon 2020, 6, e04051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Q.; Gao, Y.; Li, J.; Huang, Y.; Song, C.; Zhou, W.; Ma, G.; Su, Z. Hydrophilic modification gigaporous resins with poly(ethylenimine) for high-throughput proteins ion-exchange chromatography. J. Chromatogr. A 2014, 1343, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, E.S.; Koza, S.M. Advances in size-exclusion separations of proteins and polymers by UHPLC. TrAC Trends Anal. Chem. 2014, 63, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Sathitnaitham, S.; Suttangkakul, A.; Wonnapinij, P.; McQueen-Mason, S.J.; Vuttipongchaikij, S. Gel-permeation chromatography–enzyme-linked immunosorbent assay method for systematic mass distribution profiling of plant cell wall matrix polysaccharides. Plant J. 2021, 106, 1776–1790. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moral, N.; Plankeele, J.-M.; Domoney, C.; Warren, F.J. Ultra-high performance liquid chromatography-size exclusion chromatography (UPLC-SEC) as an efficient tool for the rapid and highly informative characterisation of biopolymers. Carbohydr. Polym. 2018, 196, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Kothencz, R.; Nagy, R.; Bartha, L.; Tóth, J.; Vágó, Á. Analysis of the interaction between polymer and surfactant in aqueous solutions for chemical-enhanced oil recovery. Part. Sci. Technol. 2018, 36, 887–890. [Google Scholar] [CrossRef]
- Janco, M.; Iv, J.N.A.; Bouvier, E.S.P.; Morrison, D. Ultra-high performance size-exclusion chromatography of synthetic polymers: Demonstration of capability. J. Sep. Sci. 2013, 36, 2718–2727. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, W.; Lei, X.; Chen, H.; Lin, C.; Wu, X. Rapid polymerization of polyhedral oligomeric siloxane-based zwitterionic sulfoalkylbetaine monolithic column in ionic liquid for hydrophilic interaction capillary electrochromatography. J. Chromatogr. A 2021, 1659, 462651. [Google Scholar] [CrossRef]
- Zong, R.; Wang, X.; Yin, H.; Li, Z.; Huang, C.; Xiang, Y.; Ye, N. Capillary coated with three-dimensional covalent organic frameworks for separation of fluoroquinolones by open-tubular capillary electrochromatography. J. Chromatogr. A 2021, 1656, 462549. [Google Scholar] [CrossRef]
- Ji, B.; Yi, G.; Gui, Y.; Zhang, J.; Long, W.; You, M.; Xia, Z.; Fu, Q. High-Efficiency and Versatile Approach To Fabricate Diverse Metal–Organic Framework Coatings on a Support Surface as Stationary Phases for Electrochromatographic Separation. ACS Appl. Mater. Interfaces 2021, 13, 41075–41083. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, S.; Li, H.; Shan, Y.; Dou, A.; Shi, X.; Xu, G. A novel surface-confined glucaminium-based ionic liquid stationary phase for hydrophilic interaction/anion-exchange mixed-mode chromatography. J. Chromatogr. A 2014, 1360, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, E.S.; Heydar, K.T. Preparation of two amide-bonded stationary phases and comparative evaluation under mixed-mode chromatography. J. Sep. Sci. 2021, 44, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Takafuji, M.; Ihara, H. Chromatographic evaluation of a newly designed peptide-silica stationary phase in reverse phase liquid chromatography and hydrophilic interaction liquid chromatography: Mixed mode behavior. J. Chromatogr. A 2012, 1266, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, E.S.; Heydar, K.T. Silica modification with 9-methylacridine and 9-undecylacridine as mixed-mode stationary phases in HPLC. Talanta 2021, 221, 121445. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Feng, J.; Luo, C.; Liu, X.; Jiang, S. Benzimidazole modified silica as a novel reversed-phase and anion-exchange mixed-mode stationary phase for HPLC. Talanta 2013, 105, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Li, X.; Dong, X.; Wei, J.; Guo, Z.; Liang, X. Glutathione-based zwitterionic stationary phase for hydrophilic interaction/cation-exchange mixed-mode chromatography. J. Chromatogr. A 2013, 1314, 63–69. [Google Scholar] [CrossRef]
- Wei, J.; Guo, Z.; Zhang, P.; Zhang, F.; Yang, B.; Liang, X. A new reversed-phase/strong anion-exchange mixed-mode stationary phase based on polar-copolymerized approach and its application in the enrichment of aristolochic acids. J. Chromatogr. A 2012, 1246, 129–136. [Google Scholar] [CrossRef]
- Bai, Q.; Liu, Y.; Wang, Y.; Zhao, K.; Yang, F.; Liu, J.; Shen, J.; Zhao, Q. Protein separation using a novel silica-based RPLC/IEC mixed-mode stationary phase modified with N-methylimidazolium ionic liquid. Talanta 2018, 185, 89–97. [Google Scholar] [CrossRef]
- Gao, J.; Luo, G.; Li, Z.; Li, H.; Zhao, L.; Qiu, H. A new strategy for the preparation of mixed-mode chromatographic stationary phases based on modified dialdehyde cellulose. J. Chromatogr. A 2020, 1618, 460885. [Google Scholar] [CrossRef]
- Hu, K.; Feng, S.; Wu, M.; Wang, S.; Zhao, W.; Jiang, Q.; Yu, A.; Zhang, S. Development of a V-shape bis(tetraoxacalix[2]arene[2]triazine) stationary phase for High performance liquid chromatography. Talanta 2014, 130, 63–70. [Google Scholar] [CrossRef]
- Wu, Q.; Hou, X.; Lv, H.; Li, H.; Zhao, L.; Qiu, H. Synthesis of octadecylamine-derived carbon dots and application in reversed phase/hydrophilic interaction liquid chromatography. J. Chromatogr. A 2021, 1656, 462548. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Zhang, L.; Zhang, W. Preparation and evaluation of maltose modified polymer-silica composite based on cross-linked poly glycidyl methacrylate as high performance liquid chromatography stationary phase. Anal. Chim. Acta 2018, 1036, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Hou, X.; Zhang, X.; Li, H.; Zhao, L.; Lv, H. Amphipathic carbon quantum dots-functionalized silica stationary phase for reversed phase/hydrophilic interaction chromatography. Talanta 2021, 226, 122148. [Google Scholar] [CrossRef] [PubMed]
- Bo, C.; Li, Y.; Liu, B.; Jia, Z.; Dai, X.; Gong, B. Grafting copolymer brushes on polyhedral oligomeric silsesquioxanes silsesquioxane-decorated silica stationary phase for hydrophilic interaction liquid chromatography. J. Chromatogr. A 2021, 1659, 462627. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Yuan, J.; Wang, Z.; Wang, S.; Yuan, C.; Wang, L. Preparation and evaluation of a hydrophilic interaction and cation-exchange chromatography stationary phase modified with 2-methacryloyloxyethyl phosphorylcholine. J. Chromatogr. A 2018, 1546, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wey, M.; Firooz, S.K.; Armstrong, D.W. Ionizable Cyclofructan 6-Based Stationary Phases for Hydrophilic Interaction Liquid Chromatography Using Superficially Porous Particles. Chromatographia 2021, 84, 821–832. [Google Scholar] [CrossRef]
- Aral, T.; Aral, H.; Ziyadanoğulları, B.; Ziyadanoğulları, R. Synthesis of a mixed-model stationary phase derived from glutamine for HPLC separation of structurally different biologically active compounds: HILIC and reversed-phase applications. Talanta 2015, 131, 64–73. [Google Scholar] [CrossRef]
- Mallik, A.K.; Noguchi, H.; Han, Y.; Kuwahara, Y.; Takafuji, M.; Ihara, H. Enhancement of Thermal Stability and Selectivity by Introducing Aminotriazine Comonomer to Poly(Octadecyl Acrylate)-Grafted Silica as Chromatography Matrix. Separations 2018, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Mallik, A.K.; Noguchi, H.; Rahman, M.M.; Takafuji, M.; Ihara, H. Facile preparation of an alternating copolymer-based high molecular shape-selective organic phase for reversed-phase liquid chromatography. J. Chromatogr. A 2018, 1555, 53–61. [Google Scholar] [CrossRef]
- Aral, H.; Aral, T.; Ziyadanoğulları, B.; Ziyadanoğulları, R. Development of a novel amide-silica stationary phase for the reversed-phase HPLC separation of different classes of phytohormones. Talanta 2013, 116, 155–163. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, C.; Yan, Z.; Zhou, Y.; Li, J.; Xie, Y.; Bai, L.; Jiang, L.; Li, F. Preparation, characterization, and performance evaluation of UiO-66 analogues as stationary phase in HPLC for the separation of substituted benzenes and polycyclic aromatic hydrocarbons. PLoS ONE 2017, 12, e0178513. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Zhang, F.; Geng, H.; Yang, B. A polymer-based zwitterionic stationary phase for hydrophilic interaction chromatography. Talanta 2020, 26, 120927. [Google Scholar] [CrossRef]
- Lin, H.; Ou, J.; Zhang, Z.; Dong, J.; Wu, M.; Zou, H. Facile Preparation of Zwitterionic Organic-Silica Hybrid Monolithic Capillary Column with an Improved “One-Pot” Approach for Hydrophilic-Interaction Liquid Chromatography (HILIC). Anal. Chem. 2012, 84, 2721–2728. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Ding, J.; Zhou, Y.; Xia, D.; Guo, Z.; Liang, X. Synthesis and chromatographic evaluation of pyrazinedicarboxylic anhydride bonded stationary phase. J. Chromatogr. A 2021, 1638, 461825. [Google Scholar] [CrossRef]
- Peng, K.; Wang, Q.; Chen, W.; Xia, D.; Zhou, Z.; Wang, Y.; Jiang, Z.; Wu, F. Phosphatidic acid-functionalized monolithic stationary phase for reversed-phase/cation-exchange mixed mode chromatography. RSC Adv. 2016, 6, 100891–100898. [Google Scholar] [CrossRef]
- Lu, H.-L.; Lin, D.-Q.; Zhang, Q.-L.; Yao, S.-J. Evaluation on adsorption selectivity of immunoglobulin G with 2-mercapto-1-methyl-imidazole-based hydrophobic charge-induction resins. Biochem. Eng. J. 2017, 119, 34–41. [Google Scholar] [CrossRef]
- Hou, Y.; Lu, J.; Wei, D.; Lv, Y.; He, H.; Wang, C.; He, L. Establishment of substance P modified affinity chromatography for specific detection and enrichment of Mas-related G protein–coupled receptor X2. J. Chromatogr. A 2021, 1659, 462633. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Liu, Y.; Zhao, T.; Zuo, L.; Ma, X.; Shan, G. Separation of chiral and achiral impurities in paroxetine hydrochloride in a single run using supercritical fluid chromatography with a polysaccharide stationary phase. J. Pharm. Biomed. Anal. 2022, 208, 114458. [Google Scholar] [CrossRef]
- Hroboňová, K.; Moravčík, J.; Lehotay, J.; Armstrong, D.W. Determination of methionine enantiomers by HPLC on the cyclofructan chiral stationary phase. Anal. Methods 2015, 7, 4577–4582. [Google Scholar] [CrossRef]
- Kawamura, I.; Mijiddorj, B.; Kayano, Y.; Matsuo, Y.; Ozawa, Y.; Ueda, K.; Sato, H. Separation of D-amino acid-containing peptide phenylseptin using 3,3′-phenyl-1,1′-binaphthyl-18-crown-6-ether columns. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2020, 1868, 140429. [Google Scholar] [CrossRef]
- Sun, X.; Li, J.; Xu, L. Synthesis of penetrable poly(methacrylic acid-co-ethylene glycol dimethacrylate) microsphere and its HPLC application in protein separation. Talanta 2018, 185, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.-L.; Hu, N.-J.; Juang, T.-Y.; Liu, Y.-C. Co-Immobilization of Xylanase and Scaffolding Protein onto an Immobilized Metal Ion Affinity Membrane. Catalysts 2020, 10, 1408. [Google Scholar] [CrossRef]
- Shen, S.S.; Yang, J.J.; Liu, C.X.; Bai, R.B. Immobilization of copper ions on chitosan/cellulose acetate blend hollow fiber membrane for protein adsorption. RSC Adv. 2017, 7, 10424–10431. [Google Scholar] [CrossRef] [Green Version]
- Morita, K.; Takeda, S.; Yunoki, A.; Tsuchii, T.; Tanaka, T.; Maruyama, T. Preparation of affinity membranes using polymer phase separation and azido-containing surfactants. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 611, 125802. [Google Scholar] [CrossRef]
- He, M.; Wang, C.; Wei, Y. Preparation of a novel Zr4+-immobilized metal affinity membrane for selective adsorption of phosphoprotein. J. Chromatogr. B 2016, 1029–1030, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Fu, Q.; Si, Y.; Liu, L.; Yin, X.; Ji, F.; Yu, J.; Ding, B. Electrospun regenerated cellulose nanofiber based metal-chelating affinity membranes for protein adsorption. Compos. Commun. 2018, 10, 168–174. [Google Scholar] [CrossRef]
- Chenette, H.C.S.; Welsh, J.M.; Husson, S.M. Affinity membrane adsorbers for binding arginine-rich proteins. Sep. Sci. Technol. 2017, 52, 276–286. [Google Scholar] [CrossRef]
- Liu, B.-L.; Ooi, C.W.; Ng, I.-S.; Show, P.L.; Lin, K.-J.; Chang, Y.-K. Effective purification of lysozyme from chicken egg white by tris(hydroxymethyl)aminomethane affinity nanofiber membrane. Food Chem. 2020, 327, 127038. [Google Scholar] [CrossRef]
- Lin, L.; Sun, H.; Zhang, K.; Zhong, Y.; Cheng, Q.; Bian, X.; Xin, Q.; Cheng, B.; Feng, X.; Zhang, Y. Novel affinity membranes with macrocyclic spacer arms synthesized via click chemistry for lysozyme binding. J. Hazard. Mater. 2017, 327, 97–107. [Google Scholar] [CrossRef]
- Fan, J.; Luo, J.; Wan, Y. Aquatic micro-pollutants removal with a biocatalytic membrane prepared by metal chelating affinity membrane chromatography. Chem. Eng. J. 2017, 327, 1011–1020. [Google Scholar] [CrossRef]
- Konovalova, V.; Guzikevich, K.; Burban, A.; Kujawski, W.; Jarzynka, K.; Kujawa, J. Enhanced starch hydrolysis using α-amylase immobilized on cellulose ultrafiltration affinity membrane. Carbohydr. Polym. 2016, 152, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Luo, J.; Wang, Z.; Khan, R.; Chen, X.; Wan, Y. Alginate dialdehyde meets nylon membrane: A versatile platform for facile and green fabrication of membrane adsorbers. J. Mater. Chem. B 2018, 6, 1640–1649. [Google Scholar] [CrossRef]
- Li, Y.; Lock, L.L.; Mills, J.; Ou, B.S.; Morrow, M.; Stern, D.; Wang, H.; Anderson, C.F.; Xu, X.; Ghose, S.; et al. Selective Capture and Recovery of Monoclonal Antibodies by Self-Assembling Supramolecular Polymers of High Affinity for Protein Binding. Nano Lett. 2020, 20, 6957–6965. [Google Scholar] [CrossRef]
- Hu, M.-X.; Li, X.; Li, J.-N.; Huang, J.-J.; Ren, G.-R. Multilayer affinity adsorption of albumin on polymer brushes modified membranes in a continuous-flow system. J. Chromatogr. A 2018, 1538, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Du, J.R.; Su, X.; Feng, X. Chitosan/sericin blend membranes for adsorption of bovine serum albumin. Can. J. Chem. Eng. 2017, 95, 954–960. [Google Scholar] [CrossRef]
- Mustafaoglu, N.; Kiziltepe, T.; Bilgicer, B. Antibody purification via affinity membrane chromatography method utilizing nucleotide binding site targeting with a small molecule. Anal. 2016, 141, 6571–6582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalli, E.; Sarti, G.C.; Boi, C. Effect of the spacer arm on non-specific binding in membrane affinity chromatography. MRS Commun. 2018, 8, 65–70. [Google Scholar] [CrossRef]
- Militano, F.; Poerio, T.; Mazzei, R.; Salerno, S.; De Bartolo, L.; Giorno, L. Development of biohybrid immuno-selective membranes for target antigen recognition. Biosens. Bioelectron. 2017, 92, 54–60. [Google Scholar] [CrossRef]
- Boi, C.; Castro, C.; Sarti, G.C. Plasminogen purification from serum through affinity membranes. J. Membr. Sci. 2015, 475, 71–79. [Google Scholar] [CrossRef]
- Fulton, A.; Lai, H.; Chen, Q.; Zhang, C. Purification of monoclonal antibody against Ebola GP1 protein expressed in Nicotiana benthamiana. J. Chromatogr. A 2015, 1389, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Yu, D.; Wu, Y.; Han, J.; Li, S.; Wu, Q.; Li, D.; Qi, J. An efficient large-scale refolding technique for recovering biologically active recombinant human FGF-21 from inclusion bodies. Int. J. Biol. Macromol. 2019, 135, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Gieseler, G.; Pepelanova, I.; Stuckenberg, L.; Villain, L.; Nölle, V.; Odenthal, U.; Beutel, S.; Rinas, U.; Scheper, T. Purification of bone morphogenetic protein-2 from refolding mixtures using mixed-mode membrane chromatography. Appl. Microbiol. Biotechnol. 2017, 101, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, G.; Kraft, H.P.; Fickentscher, K.; Köhler, F. Chromatographic separation of racemic thalidomide and teratogenic activity of its enantiomers (author’s transl). Arzneim.-Forsch. 1979, 29, 1640–1642. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. IJBS 2006, 2, 85. [Google Scholar] [PubMed]
- Soukupová, K.; Krafková, E.; Suchánková, J.; Tesařová, E. Comparison of zirconia- and silica-based reversed stationary phases for separation of enkephalins. J. Chromatogr. A 2005, 1087, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Maya, F.; Svec, F. A new approach to the preparation of large surface area poly(styrene-co-divinylbenzene) monoliths via knitting of loose chains using external crosslinkers and application of these monolithic columns for separation of small molecules. Polymer 2014, 55, 340–346. [Google Scholar] [CrossRef]
- Sanagi, M.M.; See, H.H. High Temperature Liquid Chromatography on a Poly(Styrene-Divinylbenzene) Stationary Phase. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 3065–3076. [Google Scholar] [CrossRef]
- Fu, Y.; Borrull, F.; Fontanals, N.; Marcé-Recasens, R.M. Comparison of polysaccharide-based and protein-based chiral liquid chromatography columns for enantioseparation of drugs. Chirality 2020, 32, 876–884. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, J.; Lei, S.; Guo, X.; Zhao, L. Simultaneous enantiomeric analysis of eight pesticides in soils and river sediments by chiral liquid chromatography-tandem mass spectrometry. Chemosphere 2018, 204, 210–219. [Google Scholar] [CrossRef]
- Horak, J.; Lämmerhofer, M. Stereoselective separation of underivatized and 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate derivatized amino acids using zwitterionic quinine and quinidine type stationary phases by liquid chromatography–High resolution mass spectrometry. J. Chromatogr. A 2019, 1596, 69–78. [Google Scholar] [CrossRef]
- Yu, J.; Armstrong, D.W.; Ryoo, J.J. Synthesis of new C3 symmetric amino acid- and aminoalcohol-containing chiral stationary phases and application to HPLC enantioseparations. Chirality 2018, 30, 74–84. [Google Scholar] [CrossRef]
- Naghdi, E.; Fakhari, A.R.; Baca, M.; De Malsche, W. Simultaneous enantioseparation of nonsteroidal anti-inflammatory drugs by a one-dimensional liquid chromatography technique using a dynamically coated chiral porous silicon pillar array column. J. Chromatogr. A 2020, 1615, 460752. [Google Scholar] [CrossRef] [PubMed]
- Pittler, E.; Grawatsch, N.; Paul, D.; Gübitz, G.; Schmid, M.G. Enantioseparation of amino acids, α-hydroxy acids, and dipeptides by ligand-exchange CEC using silica-based chiral stationary phases. Electrophoresis 2009, 30, 2897–2904. [Google Scholar] [CrossRef]
- Chen, J.; Xu, N.; Guo, P.; Wang, B.; Zhang, J.; Xie, S.; Yuan, L. A chiral metal-organic framework core-shell microspheres composite for high-performance liquid chromatography enantioseparation. J. Sep. Sci. 2021, 44, 3976–3985. [Google Scholar] [CrossRef] [PubMed]
- Wanga, C.; Zhanga, L.; Lia, X.; Yua, A.; Zhangb, S. Controlled fabrication of core-shell silica@chiral metal-organic framework for significant improvement chromatographic separation of enantiomers. Talanta 2020, 218, 121155. [Google Scholar] [CrossRef] [PubMed]
- He, S.; He, Y.; Cheng, L.; Wu, Y.; Ke, Y. Novel chiral ionic liquids stationary phases for the enantiomer separation of chiral acid by high-performance liquid chromatography. Chirality 2018, 30, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, H.; Qiu, R.; Huang, S. Synthesis of novel chiral imidazolium stationary phases and their enantioseparation evaluation by high-performance liquid chromatography. Anal. Chim. Acta 2016, 944, 70–77. [Google Scholar] [CrossRef]
- Zheng, Q.; He, Y.; Ma, W.; Wu, Y.; Chen, Z.; Wang, R.; Tong, W.; Lin, Z. Facile synthesis of spherical covalent organic frameworks as stationary phases for short-column liquid chromatography. Chem. Commun. 2021, 57, 7501–7504. [Google Scholar] [CrossRef]
- Hanai, T. Fundamental Properties of Packing Materials for Liquid Chromatography. Separations 2019, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Chu, X.; Sasaki, T.; Aono, A.; Kudo, Y.; Tanaka, K.; Fuse, Y. Thermal desorption gas chromatography-mass spectrometric analysis of polycyclic aromatic hydrocarbons in atmospheric fine particulate matter. J. Chromatogr. A 2021, 1655, 462494. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-F.; Chen, C.-W. Determination of Polycyclic Aromatic Hydrocarbons in Industrial Harbor Sediments by GC-MS. Int. J. Environ. Res. Public Health 2012, 9, 2175–2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penezić, A.; Gašparović, B.; Stipanicev, D.; Nelson, A. In-situ electrochemical method for detecting freely dissolved polycyclic aromatic hydrocarbons in water. Environ. Chem. 2014, 11, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Szopińska, M.; Szumińska, D.; Bialik, R.J.; Dymerski, T.; Rosenberg, E.; Polkowska, Ż. Determination of polycyclic aromatic hydrocarbons (PAHs) and other organic pollutants in freshwaters on the western shore of Admiralty Bay (King George Island, Maritime Antarctica). Environ. Sci. Pollut. Res. 2019, 26, 18143–18161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onopiuk, A.; Kołodziejczak, K.; Marcinkowska-Lesiak, M.; Poltorak, A. Determination of polycyclic aromatic hydrocarbons using different extraction methods and HPLC-FLD detection in smoked and grilled meat products. Food Chem. 2022, 373, 131506. [Google Scholar] [CrossRef]
- Wang, S.-W.; Hsu, K.-H.; Huang, S.-C.; Tseng, S.-H.; Wang, D.-Y.; Cheng, H.-F. Determination of polycyclic aromatic hydrocarbons (PAHs) in cosmetic products by gas chromatography-tandem mass spectrometry. J. Food Drug Anal. 2019, 27, 815–824. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Yadav, A.; Chopra, A.; Christopher, J.; Kapur, G.S. Comparison of five different HPLC columns with different particle sizes, lengths and make for the optimization of seven polycyclic aromatic hydrocarbons (PAH) analysis. SN Appl. Sci. 2019, 1, 313. [Google Scholar] [CrossRef] [Green Version]
- Sarrazin, L.; Diana, C.; Wafo, E.; Pichard-Lagadec, V.; Schembri, T.; Monod, J. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Marine, Brackish, and River Sediments by HPLC, Following Ultrasonic Extraction. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 69–85. [Google Scholar] [CrossRef]
- Lung, S.-C.C.; Liu, C.-H. Fast analysis of 29 polycyclic aromatic hydrocarbons (PAHs) and nitro-PAHs with ultra-high performance liquid chromatography-atmospheric pressure photoionization-tandem mass spectrometry. Sci. Rep. 2015, 5, 12992. [Google Scholar] [CrossRef]
- Fernández-Navarro, J.; Torres-Lapasió, J.R.; Ruiz-Angel, M.-J.; García-Álvarez-Coque, M. Silanol suppressing potency of alkyl-imidazolium ionic liquids on C18 stationary phases. J. Chromatogr. A 2012, 1232, 166–175. [Google Scholar] [CrossRef]
- Taskin, O.S.; Aksu, A.; Cetintasoglu, M.E.; Korkmaz, N.E.; Torlak, C.; Caglar, N.B. Preparation of analytical columns and HPLC silica-based C18 packing material synthesis from non-toxic silica source. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 583–587. [Google Scholar] [CrossRef]
- Mermat, N.; Ferroukhi, O.; Peulon-Agasse, V.; Bayle, J.P.; Guermouche, M.H.; Cardinael, P. Original Mesogenic Citronellol-Based Stationary Phase for Both Normal- and Reversed-Phase HPLC Modes: Properties and Applications. Chromatographia 2020, 83, 1495–1508. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, J.; You, J.; Duan, Y.; Li, Y.; Liu, C.; Liu, Z.; Yang, L.; Shen, Q.; Li, Z. A Sol–gel Method for Preparing Poly (N-Isopropyl Acrylamide) Hybrid Column and Its Application in Small Molecular Analysis Using Capillary Liquid Chromatography. Chromatographia 2020, 83, 985–992. [Google Scholar] [CrossRef]
- Stevenson, P.G.; Soliven, A.; Dennis, G.R.; Gritti, F.; Guiochon, G.; Shalliker, R.A. π-Selective stationary phases: (III) Influence of the propyl phenyl ligand density on the aromatic and methylene selectivity of aromatic compounds in reversed phase liquid chromatography. J. Chromatogr. A 2010, 1217, 5377–5383. [Google Scholar] [CrossRef]
- Yan, Z.; Zheng, J.; Chen, J.; Tong, P.; Lu, M.; Lin, Z.; Zhang, L. Preparation and evaluation of silica-UIO-66 composite as liquid chromatographic stationary phase for fast and efficient separation. J. Chromatogr. A 2014, 1366, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Demir, C.; Kip, Ç.; Tuncel, A. Alkanethiol-functionalized organosilicon monoliths for nano-reversed-phase liquid chromatography. Electrophoresis 2018, 39, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ou, J.; Liu, Z.; Wang, H.; Dong, J.; Zou, H. Facile construction of macroporous hybrid monoliths via thiol-methacrylate Michael addition click reaction for capillary liquid chromatography. J. Chromatogr. A 2015, 1379, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lei, F.-H.; Li, W.; Li, P.-F.; Wang, T.; Qin, L.-T.; Cheng, G.-G.; Sun, Y.; E, Y.-Y.; Xie, W.-B.; et al. Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids. e-Polymers 2019, 19, 290–296. [Google Scholar] [CrossRef]
- Yu, R.; Hu, W.; Lin, G.; Xiao, Q.; Zheng, J.; Lin, Z. One-pot synthesis of polymer monolithic column by combination of free radical polymerization and azide–alkyne cycloaddition “click” reaction and its application in capillary liquid chromatography. RSC Adv. 2015, 5, 9828–9836. [Google Scholar] [CrossRef]
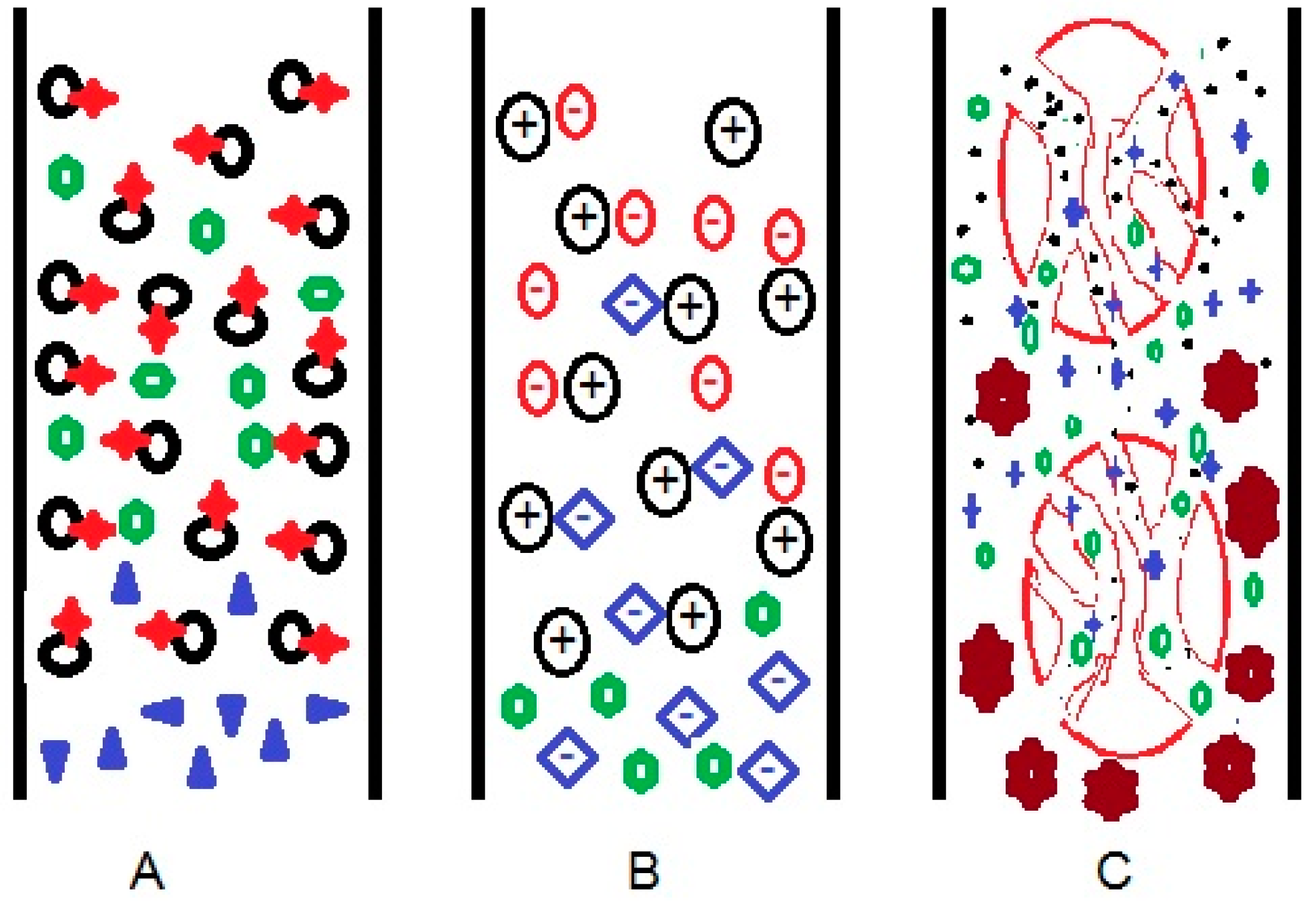

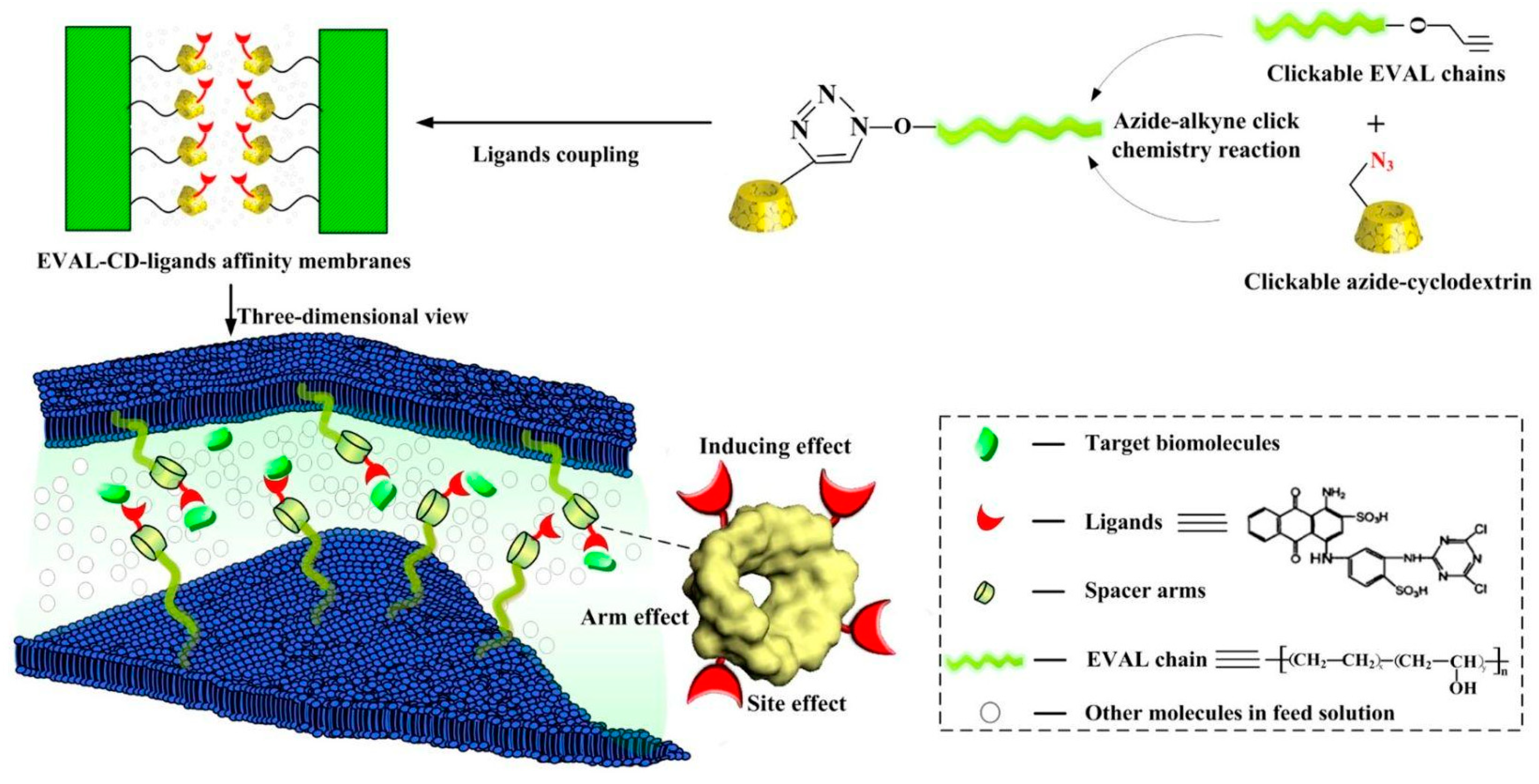





| Mode of Liquid Chromatography | Separation Principle | Stationary Phase | Analyte | Mobile Phase |
|---|---|---|---|---|
| Reverse-phase chromatography | Affinity | Silica modified with octadecyl acrylate and 2-vinyl-4,6-diamino-1,3,5-triazine [38] | PAHs | Methanol |
| Affinity | Silica modified with octadecyl acrylate and N-methylmaleimide [39] | PAHs and tocopherols | Mixed of methanol and water | |
| Affinity | Silica modified with N-Boc-phenylalanine and cyclohexylamine [40] | Phytohormones | Mixed of phosphate buffer and acetonitrile | |
| Affinity | Zr6O4(OH)4 MOF modified with 2-amino-terephthalic acid or 4,4′-biphenyl-dicarboxylic acid [41] | PAHs and aromatics compound | Mixed of methanol and water | |
| Hydrophilic interaction liquid chromatography | Ionic | Silica modified with (2-(methacryloyloxy)-ethyl)dimethyl-(3-sulfopropyl)ammonium hydroxide or 2-methacryloyloxyethyl phosphorylcholine [42] | Mixed of toluene, formamide, dimethylformamide, and thiourea | Mixed of water and acetonitrile |
| Affinity | Amino silica modified with polyhedral oligomeric silsesquioxane and acrylamide derivatives [34] | Nucleosides, organic acids, and β-agonists | Mixed of acetonitrile and ammonium formate solution | |
| Affinity | Silica modified with EGDMA and maltose [32] | Nucleobases and nucleotides | Mixed of water and acetonitrile | |
| Affinity | Silica modified with vinyl silsesquioxane and dithiothreitol [43] | |||
| Ionic and affinity | Silica modified with pyrazinedicarboxylic anhydrate [44] | Oligosaccharides, alkaloid, and organic acid groups | Mixed of acetonitrile and ammonium formate solution | |
| Mixed-mode chromatography | Ionic and affinity | Silica modified with 2-methacryloyloxyethyl phosphorylcholine [35] | Protein and lysozyme | Mixed of acetonitrile, ammonium formate solution, KH2PO4 solution, NaCl solution |
| Ionic and affinity | Silica modified with octadecyl and diol groups [8] | Aristolochic acid and derivatives | Mixed of formic acid and acetonitrile | |
| Ionic and affinity | Silica modified with glutathione [26] | Protein | Mixed of water, formic acid, acetonitrile | |
| Ionic and affinity | poly(12-methacryloyl dodecylphosphatidic acid-co- ethylene glycol dimethacrylate) [45] | Ketone aromatic, phenol and derivatives, small organic compounds | Mixed of ammonium formate solution and acetonitrile | |
| Affinity | Amino silica modified with octadecyl and carbon dots [31] | PAHs, nucleosides, and nucleobases | Mixed of water and methanol, acetonitrile, and ammonium acetate solution | |
| Affinity chromatography | Ionic and affinity | Agarose modified with 2-Mercapto-1-methylimidazole [46] | Protein | NaOH solution |
| Ionic and affinity | Sepharose modified with ligand complex [3] | Protein with histidine | Mixed of Tris buffer, sodium chloride, and imidazole | |
| Ionic and affinity | Silica modified with N-methylimidazolium ionic liquid [28] | Protein | Mixed of acetonitrile, trifluoroacetic acid, NaClO4 solution, KH2PO4 solution, and NaCl solution | |
| Ionic and affinity | Amino silica modified with glutaraldehyde [47] | Protein | Phosphate buffer | |
| Ionic chromatography | Ionic | Bentonite modified with chitosan and cetyltrimethylammonium bromide (CTAB) [11] | Cr(III) and Cr(VI) solution | Nitric acid solution for Cr(III) and ammonia solution for Cr(VI) |
| Ionic | Polystyrene-methacrylate derivatives modified with poly(amidoamine) [10] | Small anions like nitrate, sulfates, bromide, etc. | NaOH solution | |
| Chiral chromatography | Size and affinity | Polysaccharide modified with 3-chloro-4-methylphenylcarbamate [48] | Paroxetine hydrochloride groups | Mixed of supercritical CO2, methanol, and ammonium acetate solution |
| Affinity | Isopropylcarbamate cyclofructan 6 groups [49] | Methionine groups | Mixed of methanol, acetonitrile, acetic acid, and triethylamine | |
| Size | Silica modified with 3,3′- phenyl-1,1′-binaphthyl-18-crown-6-ether [50] | Amino acids and peptides | Mixed of perchloric acid solution, acetonitrile, and methanol | |
| Affinity | Poly(styrene-divinylbenzene) coated with chitosan [9] | Benzoin | Mixed of water and acetonitrile | |
| Electrochromatography | Size and ionic | Poly(POSS-co-META-co-DMMSA) [18] | Benzoic acid, nucleosides, bases, glycopeptides | Mixed of phosphate buffer, triethylamine, and acetonitrile |
| Affinity | Silica modified with a metal-organic framework (MOF) [20] | Benzenes and derivatives | Mixed of phosphate buffer and acetonitrile | |
| Size exclusion chromatography | Size | Poly(methacrylic acid-co-ethylene glycol dimethacrylate) [51] | Protein | Mixed of water and acetonitrile |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusli, H.; Putri, R.M.; Alni, A. Recent Developments of Liquid Chromatography Stationary Phases for Compound Separation: From Proteins to Small Organic Compounds. Molecules 2022, 27, 907. https://doi.org/10.3390/molecules27030907
Rusli H, Putri RM, Alni A. Recent Developments of Liquid Chromatography Stationary Phases for Compound Separation: From Proteins to Small Organic Compounds. Molecules. 2022; 27(3):907. https://doi.org/10.3390/molecules27030907
Chicago/Turabian StyleRusli, Handajaya, Rindia M. Putri, and Anita Alni. 2022. "Recent Developments of Liquid Chromatography Stationary Phases for Compound Separation: From Proteins to Small Organic Compounds" Molecules 27, no. 3: 907. https://doi.org/10.3390/molecules27030907
APA StyleRusli, H., Putri, R. M., & Alni, A. (2022). Recent Developments of Liquid Chromatography Stationary Phases for Compound Separation: From Proteins to Small Organic Compounds. Molecules, 27(3), 907. https://doi.org/10.3390/molecules27030907






