Barrier Dispersion-Based Coatings Containing Natural and Paraffin Waxes
Abstract
:1. Introduction
2. Results
2.1. Dispersions: Composition and Properties
2.2. Properties of the Coated Papers
3. Discussion
3.1. Paraffin Dispersions (DP)
3.2. Beeswax Dispersions (DB)
4. Materials and Methods
4.1. Materials
4.2. Preparation of Coating Dispersions
4.3. Dynamic Viscosity
4.4. Basic Physicochemical Parameters
4.5. Evaluation of the Stability of the Dispersion in Static Conditions
4.6. Dispersion Droplet Size Determination
4.7. Zeta Potential of the Dispersions
4.8. Coating Process of the Paper
4.9. Paper Properties Analysis
- Breaking length (m);
- Width related force with break (N/m);
- Force at break index (Nm/g);
- Strain at break (%);
- Energy absorption (J/m2);
- Energy absorption index (J/g);
- Young’s modulus (Mpa).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera, M.A.; Mathew, A.P.; Oksman, K. Barrier and Mechanical Properties of Plasticized and Cross-Linked Nanocellulose Coatings for Paper Packaging Applications. Cellulose 2017, 24, 3969–3980. [Google Scholar] [CrossRef]
- Tobjörk, D.; Österbacka, R. Paper Electronics. Adv. Mater. 2011, 23, 1935–1961. [Google Scholar] [CrossRef] [PubMed]
- Samyn, P. Wetting and Hydrophobic Modification of Cellulose Surfaces for Paper Applications. J. Mater. Sci. 2013, 48, 6455–6498. [Google Scholar] [CrossRef]
- Alava, M.; Niskanen, K. The Physics of Paper. Rep. Prog. Phys. 2006, 69, 669–723. [Google Scholar] [CrossRef]
- Wulz, P.; Waldner, C.; Krainer, S.; Kontturi, E.; Hirn, U.; Spirk, S. Surface Hydrophobization of Pulp Fibers in Paper Sheets via Gas Phase Reactions. Int. J. Biol. Macromol. 2021, 180, 80–87. [Google Scholar] [CrossRef]
- Borch, J.; Lyne, M.; Mark, R.; Habeger, C. Handbook of Physical Testing of Paper; Marcel Dekker Inc.: New York, NY, USA, 2002. [Google Scholar]
- Samyn, P. Active Barrier Coating for Packaging Paper with Controlled Release of Sunflower Oils. Molecules 2021, 26, 3561. [Google Scholar] [CrossRef] [PubMed]
- Kontturi, E.; Spirk, S. Ultrathin Films of Cellulose: A Materials Perspective. Front. Chem. 2019, 7, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganicz, T.; Rozga-Wijas, K. Siloxane-Starch-Based Hydrophobic Coating for Multiple Recyclable Cellulosic Materials. Materials 2021, 14, 4977. [Google Scholar] [CrossRef]
- Khwaldia, K.; Arab-Tehrany, E.; Desobry, S. Biopolymer Coatings on Paper Packaging Materials. Compr. Rev. Food Sci. Food Saf. 2010, 9, 82–91. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Ramarao, B.V.; Ramaswamy, S. Transient Moisture Diffusion through Paperboard Materials. Colloids Surf. A Physicochem. Eng. Asp. 2002, 206, 455–467. [Google Scholar] [CrossRef]
- Kirwan, M. Paper and Paperboard Packaging. Food Packag. Technol. 2003, 241–281. [Google Scholar]
- Triantafillopoulos, N.; Koukoulas, A.A. The Future of Single-Use Paper Coffee Cups: Current Progress and Outlook. Bioresources 2020, 15, 7260–7287. [Google Scholar] [CrossRef]
- Ren, K.; Fei, T.; Metzger, K.; Wang, T. Coating Performance and Rheological Characteristics of Novel Soybean Oil-Based Wax Emulsions. Ind. Crops Prod. 2019, 140, 111654. [Google Scholar] [CrossRef]
- Frątczak, J.; de Paz Carmona, H.; Tišler, Z.; Hidalgo Herrador, J.M.; Gholami, Z. Hydrocracking of Heavy Fischer–Tropsch Wax Distillation Residues and Its Blends with Vacuum Gas Oil Using Phonolite-Based Catalysts. Molecules 2021, 26, 7172. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, J.B.; Bakar, A.B.; Mohamed, M. Review on Bee Products as Potential Protective and Therapeutic Agents in Male Reproductive Impairment. Molecules 2021, 26, 3421. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Hueckel, T.; Cavallaro, G.; Sacanna, S.; Lazzara, G. Pickering Emulsions Based on Wax and Halloysite Nanotubes: An Ecofriendly Protocol for the Treatment of Archeological Woods. ACS Appl. Mater. Interfaces 2021, 13, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Caruso, M.R.; Cavallaro, G.; Milioto, S.; Lazzara, G. Hydroxypropyl Cellulose Films Filled with Halloysite Nanotubes/Wax Hybrid Microspheres. Ind. Eng. Chem. Res. 2021, 60, 1656–1665. [Google Scholar] [CrossRef]
- Savolainen, A.; Kuusipalo, J.; Laiho, E.; Penttinen, T. Extrusion Coating and Product Applications. Pap. Paperboard Convert. 1998, 12, 108–166. [Google Scholar]
- Choi, J.O.; Jitsunari, F.; Asakawa, F.; Park, H.J.; Lee, D.S. Migration of Surrogate Contaminants in Paper and Paperboard into Water through Polyethylene Coating Layer. Null 2002, 19, 1200–1206. [Google Scholar] [CrossRef]
- Schuman, T.; Wikström, M.; Rigdahl, M. Coating of Surface-Modified Papers with Poly(Vinyl Alcohol). Surf. Coat. Technol. 2004, 183, 96–105. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-1-4398-6241-4. [Google Scholar]
- Begley, T.H.; White, K.; Honigfort, P.; Twaroski, M.L.; Neches, R.; Walker, R.A. Perfluorochemicals: Potential Sources of and Migration from Food Packaging. Food Addit. Contam. 2005, 22, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H. Bio-Nanocomposite Materials for Food Packaging Applications: Types of Biopolymer and Nano-Sized Filler. Agric. Agric. Sci. Procedia 2014, 2, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Wang, W.; Li, W.; Zhang, S. Reactive Water Vapor Barrier Coatings Derived from Cellulose Undecenoyl Esters for Paper Packaging. Coatings 2020, 10, 1032. [Google Scholar] [CrossRef]
- Hua, Q.; Liu, L.-Y.; Karaaslan, M.A.; Renneckar, S. Aqueous Dispersions of Esterified Lignin Particles for Hydrophobic Coatings. Front. Chem. 2019, 7, 515. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Kwon, S.; Oh, K.; Abhari, A.R.; Lee, H.L. Facile Fabrication of Hydrophobic Cellulosic Paper with Good Barrier Properties via PVA/AKD Dispersion Coating. Nord. Pulp Pap. Res. J. 2019, 34, 516–524. [Google Scholar] [CrossRef]
- Musikavanhu, B.; Hu, Z.; Dzapata, R.L.; Xu, Y.; Christie, P.; Guo, D.; Li, J. Facile Method for the Preparation of Superhydrophobic Cellulosic Paper. Appl. Surf. Sci. 2019, 496, 143648. [Google Scholar] [CrossRef]
- Rutter, T.; Hutton-Prager, B. Investigation of Hydrophobic Coatings on Cellulose-Fiber Substrates with in-Situ Polymerization of Silane/Siloxane Mixtures. Int. J. Adhes. Adhes. 2018, 86, 13–21. [Google Scholar] [CrossRef]
- Quan, C.; Werner, O.; Wågberg, L.; Turner, C. Generation of Superhydrophobic Paper Surfaces by a Rapidly Expanding Supercritical Carbon Dioxide–Alkyl Ketene Dimer Solution. J. Supercrit. Fluids 2009, 49, 117–124. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Wang, X. Fabrication of Superhydrophobic Cellulose-Based Materials through a Solution-Immersion Process. Langmuir 2008, 24, 5585–5590. [Google Scholar] [CrossRef]
- Yang, H.; Deng, Y. Preparation and Physical Properties of Superhydrophobic Papers. J. Colloid Interface Sci. 2008, 325, 588–593. [Google Scholar] [CrossRef]
- Krook, M.; Gällstedt, M.; Hedenqvist, M.S. A Study on Montmorillonite/Polyethylene Nanocomposite Extrusion-Coated Paperboard: Montmorillonite/Polyethylene-Coated Paperboard. Packag. Technol. Sci. 2005, 18, 11–20. [Google Scholar] [CrossRef]
- Rakotonirainy, A.M.; Padua, G.W. Effects of Lamination and Coating with Drying Oils on Tensile and Barrier Properties of Zein Films. J. Agric. Food Chem. 2001, 49, 2860–2863. [Google Scholar] [CrossRef] [PubMed]
- Vähä-Nissi, M.; Kervinen, K.; Savolainen, A.; Egolf, S.; Lau, W. Hydrophobic Polymers as Barrier Dispersion Coatings. J. Appl. Polym. Sci. 2006, 101, 1958–1962. [Google Scholar] [CrossRef]
- Samyn, P.; Deconinck, M.; Schoukens, G.; Stanssens, D.; Vonck, L.; Van den Abbeele, H. Modifications of Paper and Paperboard Surfaces with a Nanostructured Polymer Coating. Prog. Org. Coat. 2010, 69, 442–454. [Google Scholar] [CrossRef]
- Benkreira, H.; Patel, R. Direct Gravure Roll Coating. Chem. Eng. Sci. 1993, 48, 2329–2335. [Google Scholar] [CrossRef]
- Coyle, D.J. Roll Coating. Mod. Coat. Dry. Technol. 1992, 3, 63–115. [Google Scholar]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Review Article: Polymer-Matrix Nanocomposites, Processing, Manufacturing, and Application: An Overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Arora, A.; Padua, G.W. Review: Nanocomposites in Food Packaging. J. Food Sci. 2010, 75, R43–R49. [Google Scholar] [CrossRef]
- Tekin, E.; Smith, P.J.; Schubert, U.S. Inkjet Printing as a Deposition and Patterning Tool for Polymers and Inorganic Particles. Soft Matter 2008, 4, 703. [Google Scholar] [CrossRef]
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Zondervan-van den Beuken, E.; Tobback, P. Active and Intelligent Food Packaging: Legal Aspects and Safety Concerns. Trends Food Sci. Technol. 2008, 19, S103–S112. [Google Scholar] [CrossRef]
- Ibrahim, S.; El Saied, H.; Hasanin, M. Active Paper Packaging Material Based on Antimicrobial Conjugated Nano-Polymer/Amino Acid as Edible Coating. J. King Saud Univ.-Sci. 2019, 31, 1095–1102. [Google Scholar] [CrossRef]
- Abou-Zeid, R.E.; Diab, M.A.; Mohamed, S.A.A.; Salama, A.; Aljohani, H.A.; Shoueir, K.R. Surfactant-Assisted Poly(Lactic Acid)/Cellulose Nanocrystal Bionanocomposite for Potential Application in Paper Coating. J. Renew. Mater. 2018, 6, 394–401. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Assem, F.M.; Abd El-Salam, M.H. Novel Bionanocomposite Materials Used for Packaging Skimmed Milk Acid Coagulated Cheese (Karish). Int. J. Biol. Macromol. 2018, 115, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fraunhofer.de/Content/Dam/Zv/En/Press-Media/2021/May-2021/Igb-Ivv-Bioactive-Paper-Coatings-to-Replace-Plastic-for-Packaging-Foods.Pdf (accessed on 25 November 2021).
- Bucio, A.; Moreno-Tovar, R.; Bucio, L.; Espinosa-Dávila, J.; Anguebes-Franceschi, F. Characterization of Beeswax, Candelilla Wax and Paraffin Wax for Coating Cheeses. Coatings 2021, 11, 261. [Google Scholar] [CrossRef]
- Li, Y.; He, B.H. Study on Coating Surface Topography and Properties of Coated Paper Related to Pigment. Adv. Mater. Res. 2012, 466–467, 504–507. [Google Scholar] [CrossRef]
- Kamel, S.; El-Sakhawy, M.; Nada, A.M.A. Mechanical Properties of the Paper Sheets Treated with Different Polymers. Thermochim. Acta 2004, 421, 81–85. [Google Scholar] [CrossRef]
- Nada, A.M.A.; El-Sakhawy, M.; Kamel, S.; Eid, M.A.M.; Adel, A.M. Mechanical and Electrical Properties of Paper Sheets Treated with Chitosan and Its Derivatives. Carbohydr. Polym. 2006, 63, 113–121. [Google Scholar] [CrossRef]
- Bayer, I.S. Superhydrophobic Coatings from Ecofriendly Materials and Processes: A Review. Adv. Mater. Interfaces 2020, 7, 2000095. [Google Scholar] [CrossRef]
- Morrissette, J.M.; Carroll, P.J.; Bayer, I.S.; Qin, J.; Waldroup, D.; Megaridis, C.M. A Methodology to Produce Eco-Friendly Superhydrophobic Coatings Produced from All-Water-Processed Plant-Based Filler Materials. Green Chem. 2018, 20, 5169–5178. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, T.; Zhang, J. Superhydrophobic Coatings with High Repellency to Daily Consumed Liquid Foods Based on Food Grade Waxes. J. Colloid Interface Sci. 2018, 515, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhu, Y.; Wang, Y.; Fu, H.; Deng, X.; Yue, H.; Lu, H.; Jiang, W.; Liang, B. Preparation of Edible Superhydrophobic Fe Foil with Excellent Stability and Durability and Its Applications in Food Containers with Little Residue. New J. Chem. 2019, 43, 2908–2919. [Google Scholar] [CrossRef]
- Wu, M.; Xu, R.; Liu, C.; Li, B.; Long, Z. Amelioration of Physical Properties and Printability of Paper Coated with N-Methylated Chitosan. Sci. Rep. 2020, 10, 9936. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Sukyai, P.; Lv, D.; Zhang, F.; Wang, P.; Liu, C.; Li, B. Water and Humidity-Induced Shape Memory Cellulose Nanopaper with Quick Response, Excellent Wet Strength and Folding Resistance. Chem. Eng. J. 2020, 392, 123673. [Google Scholar] [CrossRef]
- Donigian, D.W. A New Mechanism for the Setting and Gloss Development of Offset Ink. J. Pulp Pap. Sci. 2006, 32, 163–177. [Google Scholar]
- Nechita, P. The Influence of Coating Composition on the Structural and Functional Properties of Coated Paper for Packaging Applications. Nord. Pulp Pap. Res. J. 2020, 35, 408–418. [Google Scholar] [CrossRef]
- Kapoor, S.G.; Wu, S.M.; Pandit, S.M. New Method for Evaluating the Printing Smoothness of Coated Papers. Tappi J. 1978, 61, 71–74. [Google Scholar]
- Hergert, W.; Wriedt, T. The Mie Theory: Basics and Applications (Springer Series in Optical Sciences); Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]

| Parameter | LTP 56/25 Paraffin | R-58 Paraffin | Sarawax SX70 | K60 Wax | K70 Wax | HT Wax | Beeswax |
|---|---|---|---|---|---|---|---|
| Congealing point, °C | n.s.* | n.s. | 68–72 | 60 | n.s. | 60–70 | n.s. |
| Freezing point, °C | 54–58 | 54–60 | n.s. | n.s. | 64–70 | n.s. | n.s. |
| Melting point, °C | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | approximately 65 °C |
| Needle penetration at 25 °C, 0.1 mm | max. 25 | 20 | 9–16 | 22–28 | 10–16 | n.s. | n.s. |
| Oil content, %wt | max. 1.1 | max. 0.8 | max. 0.5 | n.s. | max. 1.0 | n.s. | n.s. |
| Kinematic viscosity at 100 °C, cSt | n.s. | not specified | 5–8 | 5–7 | 5–7 | not specified | 8.9 [47] |
| Ingredient | DP 1 | DP 2 | DP 3 | DP 4 | DP 5 | DB 1 | DB 2 | DB 3 |
|---|---|---|---|---|---|---|---|---|
| Beeswax | - | - | - | - | - | 30.0 | 30.0 | 30.0 |
| LTP 56/25 paraffin | 17.0 | 17.0 | 17.0 | 17.2 | 17.0 | - | - | - |
| R-58 paraffin | - | - | - | - | 17.0 | - | - | - |
| Sarawax SX70 | 17.0 | - | - | - | - | - | - | - |
| K60 wax | - | - | 17.0 | - | - | - | - | - |
| K70 wax | - | 17.0 | - | - | - | - | - | - |
| HT wax | - | - | - | 17.0 | - | - | - | - |
| Hydroxides | ||||||||
| Sodium hydroxide | yes (constant quantity) | No | ||||||
| Potassium hydroxide | no | yes (constant quantity) | ||||||
| Additives | ||||||||
| Glycerol monostearate | yes (constant quantity) | yes (constant quantity) | ||||||
| Stearic acid | yes (constant quantity) | No | ||||||
| Emulsifier * | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 3 |
| Parameter | DP 1 | DP 2 | DP 3 | DP 4 | DP 5 | DB 1 | DB 2 | DB 3 |
|---|---|---|---|---|---|---|---|---|
| Dynamic viscosity, cP | 34.0 | 24.5 | 25.5 | 24.5 | 21.5 | 9.5 | 9.0 | 8.5 |
| pH | 9.96 | 9.20 | 9.70 | 9.70 | 9.80 | 8.58 | 8.71 | 8.79 |
| Dry matter, wt% | 41.8 | 40.8 | 41.9 | 41.0 | 41.8 | 32.5 | 32.5 | 32.8 |
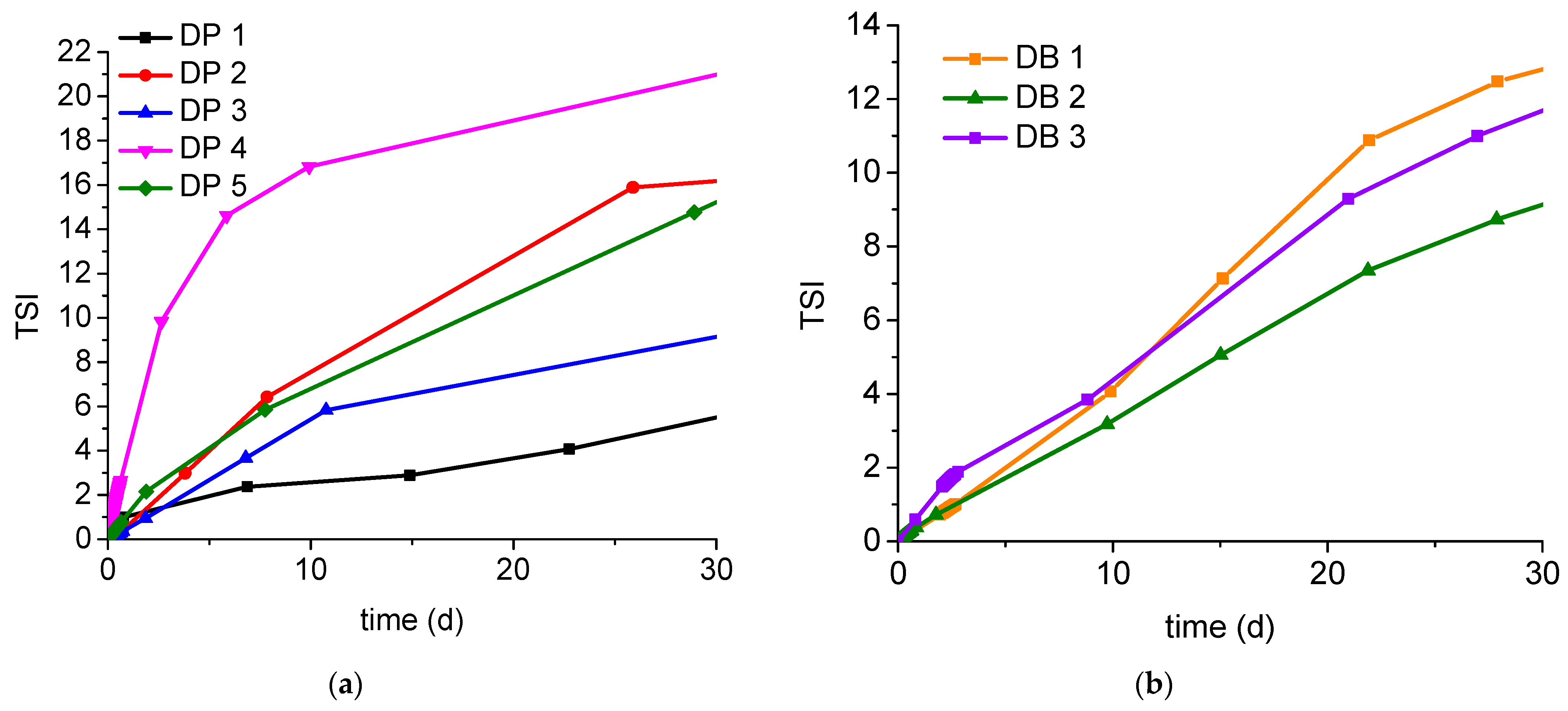
| Stability, TSI 30 days * | 5.5 | 16.1 | 9.1 | 21.0 | 15.2 | 12.9 | 9.1 | 11.7 |
| Mean particle size, nm | 437 | 401 | 422 | 359 | 358 | 358 | 363 | 375 |
| Polydispersity index | 0.260 | 0.257 | 0.264 | 0.245 | 0.231 | 0.274 | 0.252 | 0.288 |
| Zeta potential, mV | −42.4 | −29.8 | −34.3 | −26.0 | −28.4 | −49.5 | −47.1 | −47.6 |
| Sample | Front of Coated Sample | Reverse of Coated Sample |
|---|---|---|
| DP 1 |  |  |
| DP 2 |  |  |
| DP 3 |  |  |
| DP 4 |  |  |
| DP 5 |  |  |
| DB 1 |  |  |
| DB 2 |  |  |
| DB 3 |  |  |
| Sample | Images of Roughness Profiles | Sa, Sz Parameters, µm |
|---|---|---|
| DP 1 | 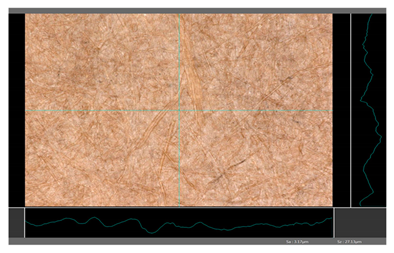 | Sa = 3.17 Sz = 27.13 |
| DP 2 | 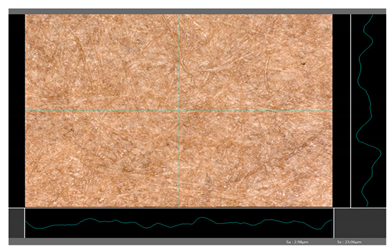 | Sa = 2.98 Sz = 23.06 |
| DP 3 | 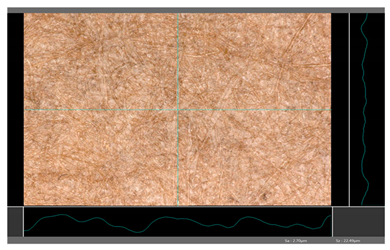 | Sa = 2.70 Sz = 22.49 |
| DP 4 |  | Sa = 2.60 Sz = 25.10 |
| DP 5 | 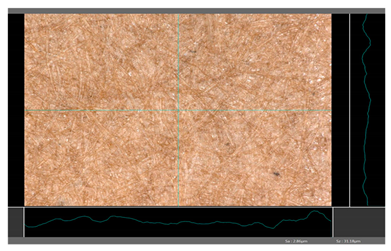 | Sa = 2.86 Sz = 31.18 |
| DB 1 |  | Sa = 40.81 Sz = 280.01 |
| DB 2 |  | Sa = 20.30 Sz = 167.89 |
| DB 3 |  | Sa = 11.64 Sz = 199.19 |
| Parameter | DP 1 | DP 2 | DP 3 | DP 4 | DP 5 | Mean Value | DB 1 | DB 2 | DB 3 | Mean Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Piercing | no | yes | yes | yes | yes | - | No | no | no | - |
| Air permeability, mL/min | 106 | 89 | 98 | 110 | 108 | 102.2 | 47 | 29 | 28 | 34.7 |
| Roughness, mL/min | 320 | 457 | 427 | 387 | 456 | 409.4 | 387 | 390 | 377 | 385 |
| Cobb60, g/m2 | 3.90 | 8.03 | 7.48 | 8.10 | 7.65 | 7.03 | 2.98 | 3.83 | 5.59 | 4.13 |
| Strength properties—machine direction | ||||||||||
| Breaking length, km | 12.32 | 11.54 | 11.47 | 11.93 | 11.07 | 11.67 | 11.78 | 12.14 | 11.89 | 11.94 |
| Tear in break, N | 159.1 | 148.9 | 150.8 | 152.4 | 146.0 | 151.4 | 147.5 | 153.0 | 152.1 | 150.9 |
| Width related force with break, N/m | 16,020 | 15,020 | 15,140 | 15,410 | 14,670 | 15,252 | 15,050 | 15,540 | 15,410 | 15,333 |
| Force at break index, Nm/g | 120.8 | 113.2 | 112.4 | 117.1 | 108.5 | 114.4 | 115.4 | 119.0 | 116.5 | 117.0 |
| Strain at break, % | 2.67 | 2.49 | 2.58 | 2.68 | 2.68 | 2.62 | 2.72 | 2.66 | 2.77 | 2.72 |
| Energy absorption, J/m2 | 261.9 | 226.1 | 236.0 | 254.6 | 235.4 | 242.8 | 252.2 | 257.4 | 264.6 | 258.1 |
| Energy absorption index, J/g | 1.98 | 1.70 | 1.75 | 1.93 | 1.74 | 1.82 | 1.94 | 1.97 | 2.00 | 1.97 |
| Young’s Modulus, Mpa | 11,170 | 10,890 | 10,630 | 10,870 | 10,261 | 10,764 | 10,410 | 11,030 | 10,660 | 10,700 |
| Strength properties—cross direction | ||||||||||
| Breaking length, km | 2.88 | 2.59 | 2.71 | 2.64 | 2.63 | 2.69 | 2.69 | 2.92 | 2.86 | 2.82 |
| Tear in break, N | 37.44 | 33.43 | 34.39 | 33.28 | 33.43 | 34.39 | 34.61 | 36.90 | 36.56 | 36.02 |
| Width related force with break, N/m | 3749 | 3373 | 3569 | 3470 | 3494 | 3531 | 3451 | 3733 | 3704 | 3629 |
| Force at break index, Nm/g | 28.29 | 25.37 | 26.54 | 25.93 | 25.85 | 26.40 | 26.44 | 25.58 | 28.04 | 26.69 |
| Strain at break, % | 4.47 | 5.13 | 4.97 | 5.67 | 5.54 | 5.16 | 5.69 | 5.77 | 5.24 | 5.57 |
| Energy absorption, J/m2 | 119.6 | 128.9 | 130.6 | 141.5 | 142.4 | 132.6 | 145.0 | 152.9 | 139.4 | 145.8 |
| Energy absorption index, J/g | 0.903 | 0.970 | 0.973 | 1.073 | 1.053 | 0.994 | 1.109 | 1.172 | 1.053 | 1.111 |
| Young’s Modulus, Mpa | 3400 | 3099 | 3274 | 3171 | 3188 | 3226 | 3190 | 3221 | 3173 | 3195 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woch, J.; Małachowska, E.; Korasiak, K.; Lipkiewicz, A.; Dubowik, M.; Chrobak, J.; Iłowska, J.; Przybysz, P. Barrier Dispersion-Based Coatings Containing Natural and Paraffin Waxes. Molecules 2022, 27, 930. https://doi.org/10.3390/molecules27030930
Woch J, Małachowska E, Korasiak K, Lipkiewicz A, Dubowik M, Chrobak J, Iłowska J, Przybysz P. Barrier Dispersion-Based Coatings Containing Natural and Paraffin Waxes. Molecules. 2022; 27(3):930. https://doi.org/10.3390/molecules27030930
Chicago/Turabian StyleWoch, Julia, Edyta Małachowska, Kamil Korasiak, Aneta Lipkiewicz, Marcin Dubowik, Justyna Chrobak, Jolanta Iłowska, and Piotr Przybysz. 2022. "Barrier Dispersion-Based Coatings Containing Natural and Paraffin Waxes" Molecules 27, no. 3: 930. https://doi.org/10.3390/molecules27030930
APA StyleWoch, J., Małachowska, E., Korasiak, K., Lipkiewicz, A., Dubowik, M., Chrobak, J., Iłowska, J., & Przybysz, P. (2022). Barrier Dispersion-Based Coatings Containing Natural and Paraffin Waxes. Molecules, 27(3), 930. https://doi.org/10.3390/molecules27030930





