Abstract
The cause of liver damage by using black cohosh preparation has been concerned but remains unclear. After a preliminary investigation, the black cohosh medicinal materials sold in the market were adulterated with Asian cohosh (Cimicifuga) without removing the fibrous roots. The safety of Cimicifuga rhizome and fibrous roots is unknown and has not been reported. Therefore, in this paper, the rhizome and fibrous roots of Cimicifuga dahurica (Turcz.) Maxim (C. dahurica) were completely separated, extracted with 70% ethanol, and freeze-dried to obtain crude rhizome extract (RC) and fibrous roots extract (FRC). UHPLC-Q-TOF-MS was used to identify 39 compounds in the rhizome and fibrous roots of Cimicifuga, mainly saponins and phenolic acids. In the L-02 cytotoxicity experiment, the IC50 of fibrous roots (1.26 mg/mL) was slightly lower than that of rhizomes (1.417 mg/mL). In the 90-day sub-chronic toxicity study, the FRC group significantly increased the level of white blood cells, ALP, ALT, AST, BILI and CHOL (p < 0.05); large area of granular degeneration and balloon degeneration occurred in liver tissue; and the expression of p-NF-kB in the nucleus increased in a dose-dependent manner. Overall, Fibrous roots of Cimicifuga are at risk of hepatotoxicity and should be strictly controlled and removed during the processing.
1. Introduction
Black cohosh extract can produce estrogen-like effects and regulate endocrine balance, so it is widely used as a hormone replacement and an anti-inflammatory agent for the treatment of menopausal syndrome. Since around 2000, clinical cases of liver injury caused by taking black cohosh extract appeared successively in Australia, the United States and European countries [1,2,3,4]. However, according to the current study, the cause of liver injury caused by black cohosh preparation has not been found [5,6,7,8]. With the shortage of black cohosh resources, some herbs similar to black cohosh have been adulterated into the medicinal material market. Many Asian species of cohosh (Cimicifuga foetida, C. dahurica and C. heracleifolia) are sold as black cohosh [9].
The Asian species of cohosh-cimicifuga has a long history of medicinal use in Asian countries such as China, Japan and Korea [10]. The main chemical constituents of Cimicifuga are triterpenoid glycosides, Phenylpropanes, nitrogenous compounds and chromones [11]. Among them, saponins have the functions of regulating immunity, protecting the brain and heart, delaying aging, antitumor, expanding cerebral vessels and increasing blood flow [12,13]. Phenylpropanoids have antitumor, anti-HIV, antioxidant, anti-inflammatory, anti-microbial, anticoagulant and other biological activities. Some phenylpropanoids also have the effects of lowering blood lipid, blood pressure, blood glucose, antithrombotic, antimutagenic, analgesia and sedation [14]. Alkaloids have anti-inflammatory, antibacterial, vasodilator, cardiotonic, anti-asthmatic, anticancer and other effects [15]. Similar to black cohosh, it is also used to relieve menopausal symptoms [16].
Cimicifuga has been used in China for thousands of years, and no clinical cases of liver injury have been reported so far. We found that the processing method of Cimicifuga in the Chinese Pharmacopoeia clearly stipulates “removal of soil, sun-drying, singe or remove the fibrous roots”. While in the west, there was no such operation in the preparation of black cohosh. In addition, our previous studies found that the chemical composition and content of Cimicifuga rhizome and fibrous roots were different, and cimicifugone A and B isolated from fibrous roots of cimicifuga may have hepatotoxicity.
Therefore, it is necessary to comprehensively evaluate the toxicity of the rhizome and fibrous roots of cimicifuga. In this study, UHPLC-Q-TOF-MS was used to identify the main chemical components, and the hepatotoxicity was evaluated by the cytotoxicity test on normal human hepatocytes (L-02) in vitro and the 90-day sub-chronic toxicity test in vivo.
2. Results and Discussion
2.1. Identification of the Chemical Compositions by UHPLC-Q-TOF-MS
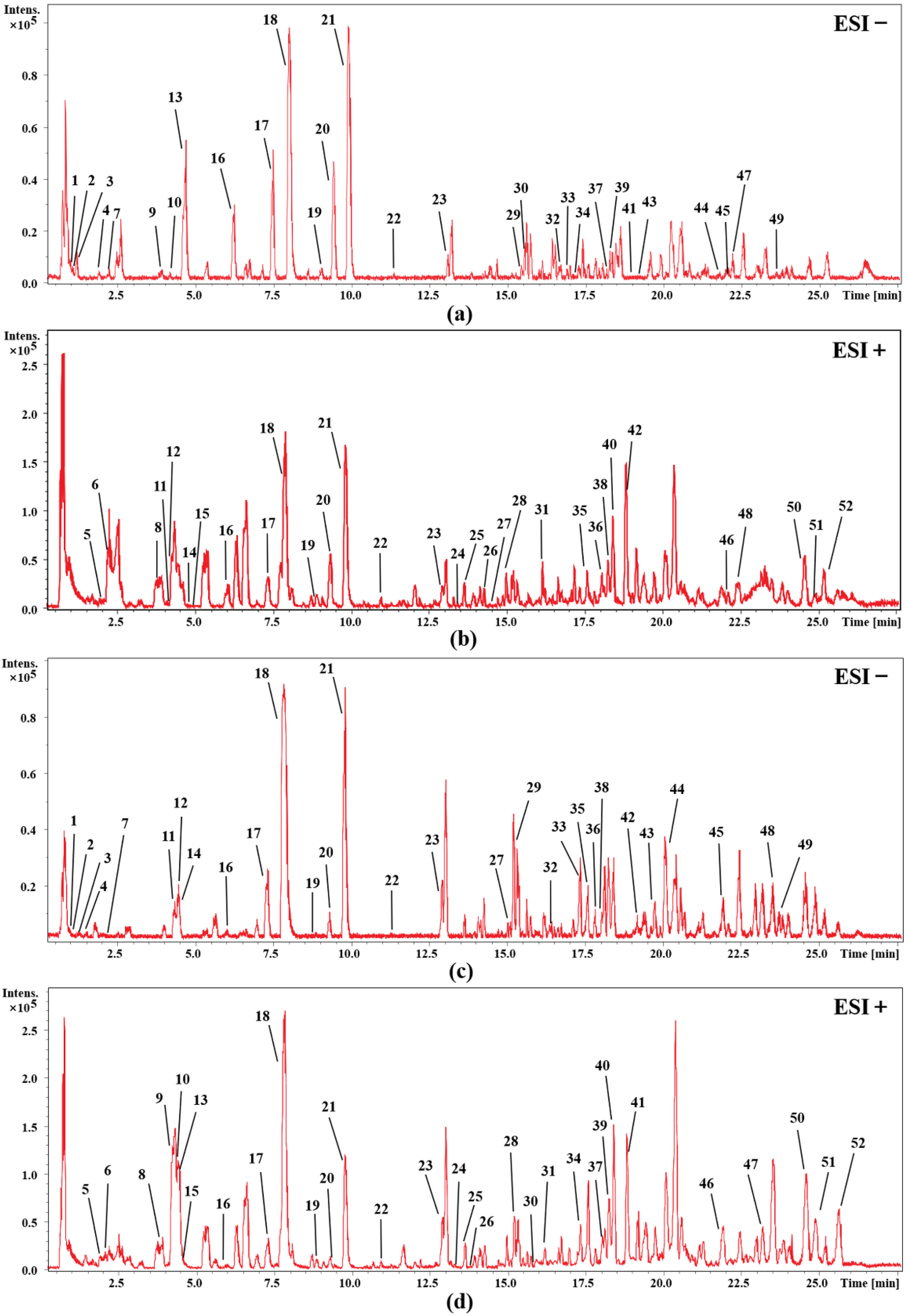
In order to identify the main chemical components of cohosh, firstly, a comprehensive search and classification of the compounds of cimicifuga species was carried out in the Scifinder database, and a potential database was initially established. Then, by comparing the retention time and the accurate relative molecular mass, the structure of the detected chemical composition is determined. In addition, we combined our laboratory’s previous experience in the isolation and identification of cimicifuga and summarized the fragmentation regularities of these compounds by mass spectrometry [10,17,18]; some compounds were determined by comparison with the mass spectrometry data of the reference substance. Figure 1 shows the UHPLC-Q-TOF-MS base peak intensity chromatograms of the rhizomes and fibrous roots of C. dahurica in positive and negative electrospray (ESI) modes. Finally, 52 compounds in the rhizome extract (RC) and fibrous roots extract (FRC) of C. dahurica were analyzed. The corresponding mass spectra data are listed in Table 1 and Table 2. The analysis results showed that the main chemical components of the rhizomes and fibrous roots of C. dahurica are all saponin and phenolic acid compounds, in addition to a small number of alkaloids.
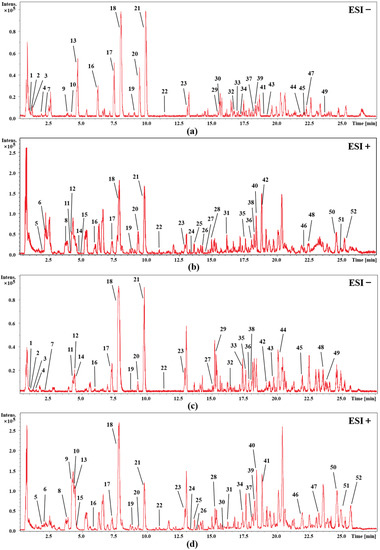
Figure 1.
UHPLC-Q-TOF-MS base peak intensity chromatograms of C. dahurica. (a) rhizome of C. dahurica in negative mode; (b) rhizome of C. dahurica in positive mode; (c) fibrous roots of C. dahurica in negative mode; (d) fibrous roots of C. dahurica in positive mode.

Table 1.
Characterization of compounds identified from rhizome of C. dahurica by UHPLC-Q-TOF-MS.

Table 2.
Characterization of compounds identified from fibrous roots of C. dahurica by UHPLC-Q-TOF-MS.
2.2. Cytotoxicity Results of L-02 Cells
In the in vitro cytotoxicity test, the MTT method was used to detect the effects of the RC and FRC of C. dahurica on the viability of normal human liver cells L-02. RC and FRC have no obvious inhibitory effect on the proliferation of L-02 cells, but it is worth noting that the IC50 of the FRC (1260 μg/mL) is slightly lower than the IC50 value of the RC (1417 μg/mL), suggesting that the fibrous roots may have a higher potential for hepatotoxicity than the rhizome.
2.3. General Observation and Body Weight
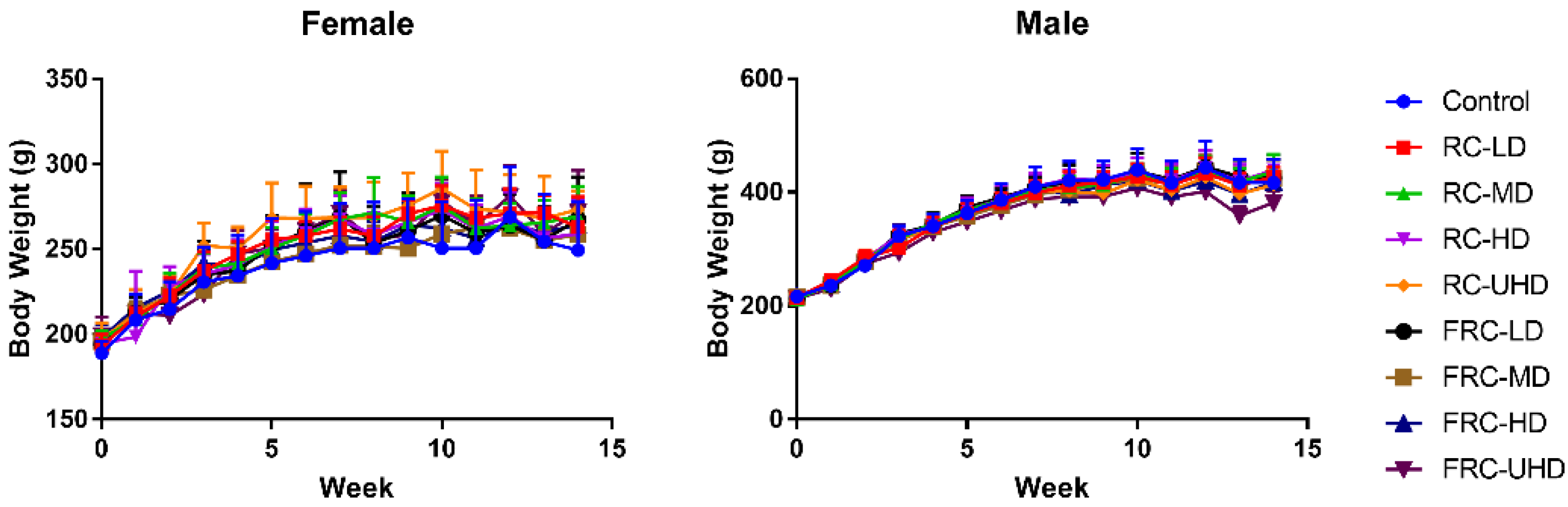
During the whole experiment period, the weight of female and male rats changed normally, and there was no significant difference between the treatment groups and the control group (Figure 2). The diet and water intake of each group were normal. However, it is worth noting that a total of six rats died during the experiment, of which the mortality rate of the fibrous roots group and rhizome group was 5:1, while the death ratio of female and male rats was 2:1. These results suggested that the toxicity risk of fibrous roots was significantly higher than rhizome, and the females were more easily damaged than males.
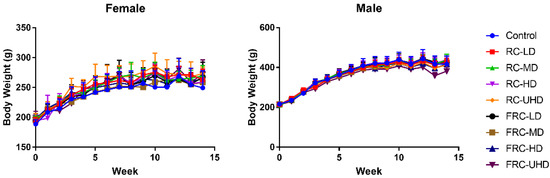
Figure 2.
Effects of rhizome and fibrous roots of C. dahurica on the body weight changes after oral administration in male and female rats for 90 days: 0.585 g/kg (LD), 1.755 g/kg (MD), 5.265 g/kg (HD) and 15.795 g/kg (UHD). Data expressed as means ± SD.
2.4. Urinalysis, Hematology and Biochemical Analysis
The results of urinalysis are shown in Table 3. No dose-related adverse reactions were observed in any administration group of female and male rats. There are some slight gaps in various test indicators, but these changes are sporadic and within the acceptable range of normal physiology.

Table 3.
Urinalysis of rats with 90-day repeated administration of rhizome and fibrous roots of C. dahurica.
The results of the hematology test are shown in Table 4. For female rats, compared with the control group, the white blood cell (WBC) level of each dose group of FRC was significantly increased (p < 0.05), but there was no significant difference in the RC group. For male rats, only the FRC-UHD group had a significant increase in WBC (p < 0.01). As the “guard” of the human body’s fight against diseases, WBC can deform and pass through the capillary wall to concentrate on the invasion site of the disease, enveloping and swallowing the disease [19,20]. The number of WBC in the body is higher than the normal value, so it is likely that the body has inflammation. Hematological test results show that long-term use of fibrous roots is likely to cause inflammation, and females are more sensitive than males.

Table 4.
Hematological analysis of rats with 90-day repeated administration of rhizome and fibrous roots of C. dahurica.
The results of serum biochemical analysis are shown in Table 5. Compared with the control group, there was no significant difference in the biochemical indicators of the RC groups (p > 0.05). Yun [21] selected the crude extract of the rhizome of Cimicifuga heracleifolia Kom. for 13 weeks of safety evaluation, and the biochemical test results showed that the changes in various indicators are sporadic and within physiologically acceptable ranges. This is consistent with the results of the rhizome of C. dahurica in this study, suggesting that the rhizome part of Cimicifuga is safe and will not cause physical lesions under normal clinical doses.

Table 5.
Biochemical analysis of rats with 90-day repeated administration of rhizome and fibrous roots of C. dahurica.
The FRC groups had a significant increase in Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in female rats (p < 0.05), and the content of ALT increased significantly (p < 0.001) in the FRC-UHD group. ALT and AST are important clinical indicators of liver function tests to determine whether the liver is damaged [22,23]. It can be seen from the test results that long-term use of FRC extract may cause liver damage, thereby increasing the content of AST in the blood. At the same time, it may be accompanied by a biliary obstruction, leading to ALT excretion obstacles and backflow into the blood, making the serum ALT content significantly increased. At the same time, for male rats, the FRC-UHD group also showed a significant increase in Alkaline phosphatase (ALP) and ALT levels (p < 0.001). ALP is excreted through the hepatobiliary system. When the liver is damaged or impaired, liver cells over-produce ALP and enter the blood through the lymphatic tract and sinusoids, causing high levels of ALP in serum [24,25].
Most of the body’s bilirubin (TBIL) comes from hemoglobin released by the lysis of senescent red blood cells, including indirect and direct bilirubin. Total gallbladder = direct bilirubin + indirect bilirubin. Indirect bilirubin is transported to the liver through the blood, and direct bilirubin is produced through the action of hepatocytes [26]. The total bilirubin content in the blood of male rats after administration of fibrous roots was generally higher than that in the control group, and the FRC-UHD group was statistically significant (p < 0.05). It is suggested that the liver of rats after taking fibrous roots is damaged, and the process of direct bilirubin transport through the blood to the liver will be correspondingly inhibited, thereby increasing the total bilirubin content in the blood. Total cholesterol (CHOL) includes free cholesterol and cholesterol esters. The liver is the main organ for cholesterol synthesis and storage, and its serum concentration can be used as an indicator of lipid metabolism [27]. The total cholesterol content of the FRC-UHD group was significantly higher than that of the blank group, and the female rats were significantly (p < 0.01) higher than that of the male rats (p < 0.05). It is suggested that excessively high doses of fibrous roots are likely to cause damage to the liver of rats, leading to obstruction of bile excretion, increasing the synthesis of blood lipoproteins and cholesterol in the liver, and increasing free cholesterol, leading to high total cholesterol.
In addition, for male rats, the urea nitrogen (UREA) and creatinine (CREA) of the fibrous roots administration group increased to varying degrees but did not show a dose dependence. When the kidney’s ability to eliminate and excrete decreases, it will also lead to an increase in blood urea nitrogen, which is often accompanied by an increase in blood creatinine [28]. This suggests that the fibrous roots of C. dahurica may also cause slight damage to the kidneys.
2.5. Organ Weights and Histopathological Changes
The organ weights and organ coefficients are shown in Table 6. By comparing the absolute and relative organ weights of the main organs of rats in each dose group of RC and FRC, it was found that there was no significant difference compared with the control group (p > 0.05). Visual observation of the organs showed that the RC group and the FRC group were similar in volume, color and shape to the control group, and there was no obvious damage or pathology.

Table 6.
Organ weights and Organ index of rats with 90-day repeated administration of rhizome and fibrous roots of C. dahurica.
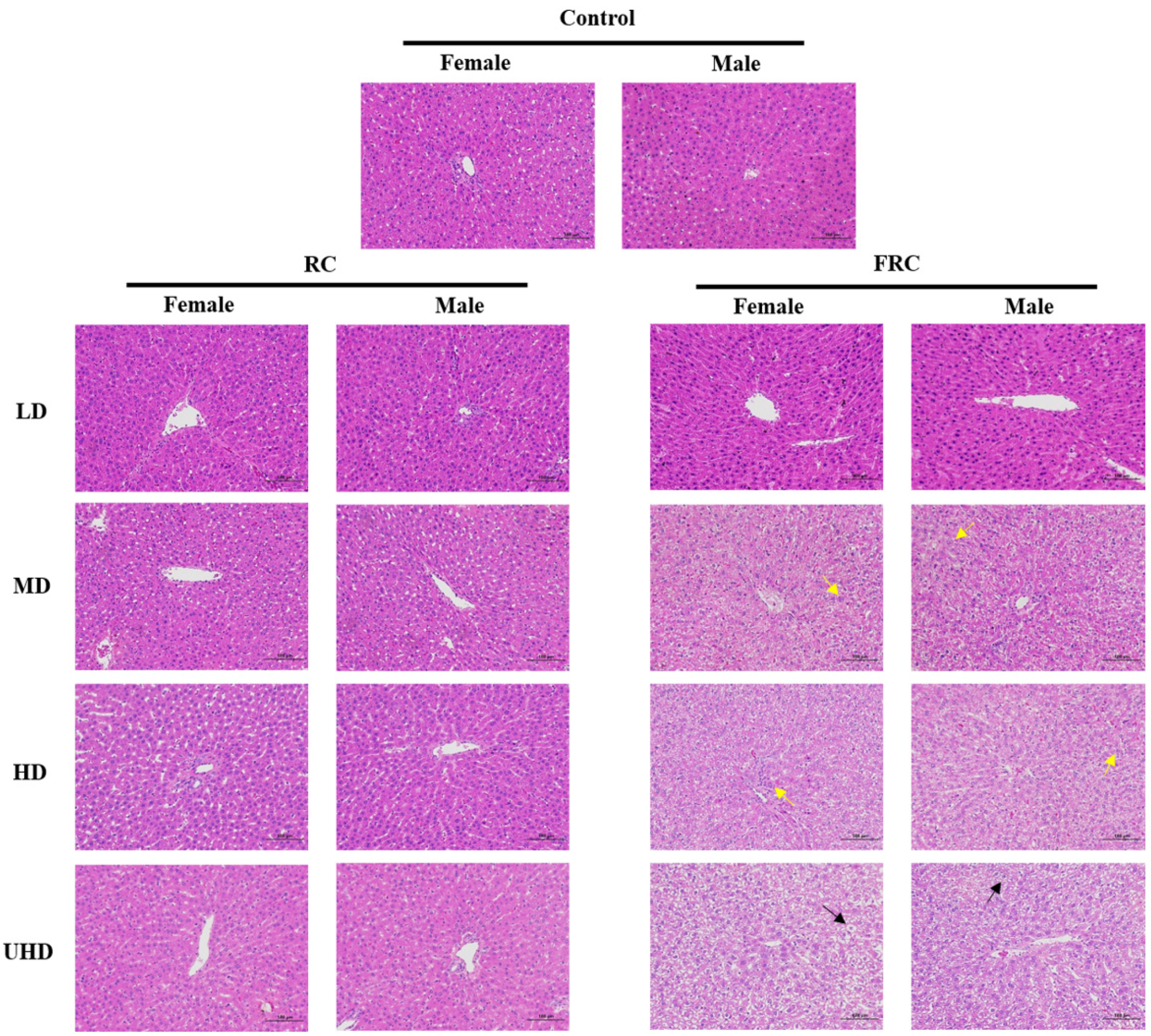
Histopathological examination of the liver (Figure 3) found that the liver tissue sections of the female and male rats in the control group were basically normal. The liver tissue envelope was composed of dense connective tissue rich in elastic fibers. The liver lobules were clearly demarcated and arranged regularly. The central part of the hepatic lobule is the central vein, surrounded by roughly radially arranged liver cells and hepatic sinusoids. The liver cells are round and full, the liver plates are arranged regularly and neatly and the sinusoids are not significantly expanded or squeezed. The portal area between adjacent liver lobules has no obvious abnormalities and no obvious inflammatory changes. Compared with the control group, the liver tissue sections of each dose of the RC administration group showed no significant difference, and this is consistent with the research report of Mazzanti [7]. Mazzanti conducted morphological tests on liver samples after oral administration of black cohosh extract to Wistar rats for 30 days, and the results showed that black cohosh extract had no significant effect on the liver morphology of rats. However, in the liver tissues of the FRC-MD and FRC-HD groups, a large number of granular degeneration of hepatocytes, swelling of the cells, loose cytoplasm and a fine granular shape were seen (yellow arrows). In the FRC-UHD group, a large number of balloon-like degeneration of hepatocytes, swelling of the cells, centered nucleus and vacuolation of the cytoplasm were seen in the tissues of the FRC-UHD group (Black arrows). It is suggested that fibrous roots of C. dahurica caused liver tissue lesions.
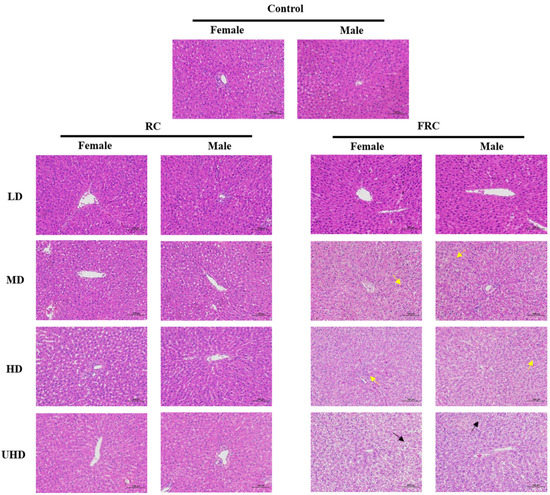
Figure 3.
Effects of rhizome and fibrous roots of C. dahurica on histopathological changes after oral administration in male and female rats for 90 days. Hematoxylin–Eosin stain, 200×.
2.6. Effect on Protein Expression of p-NF-κB
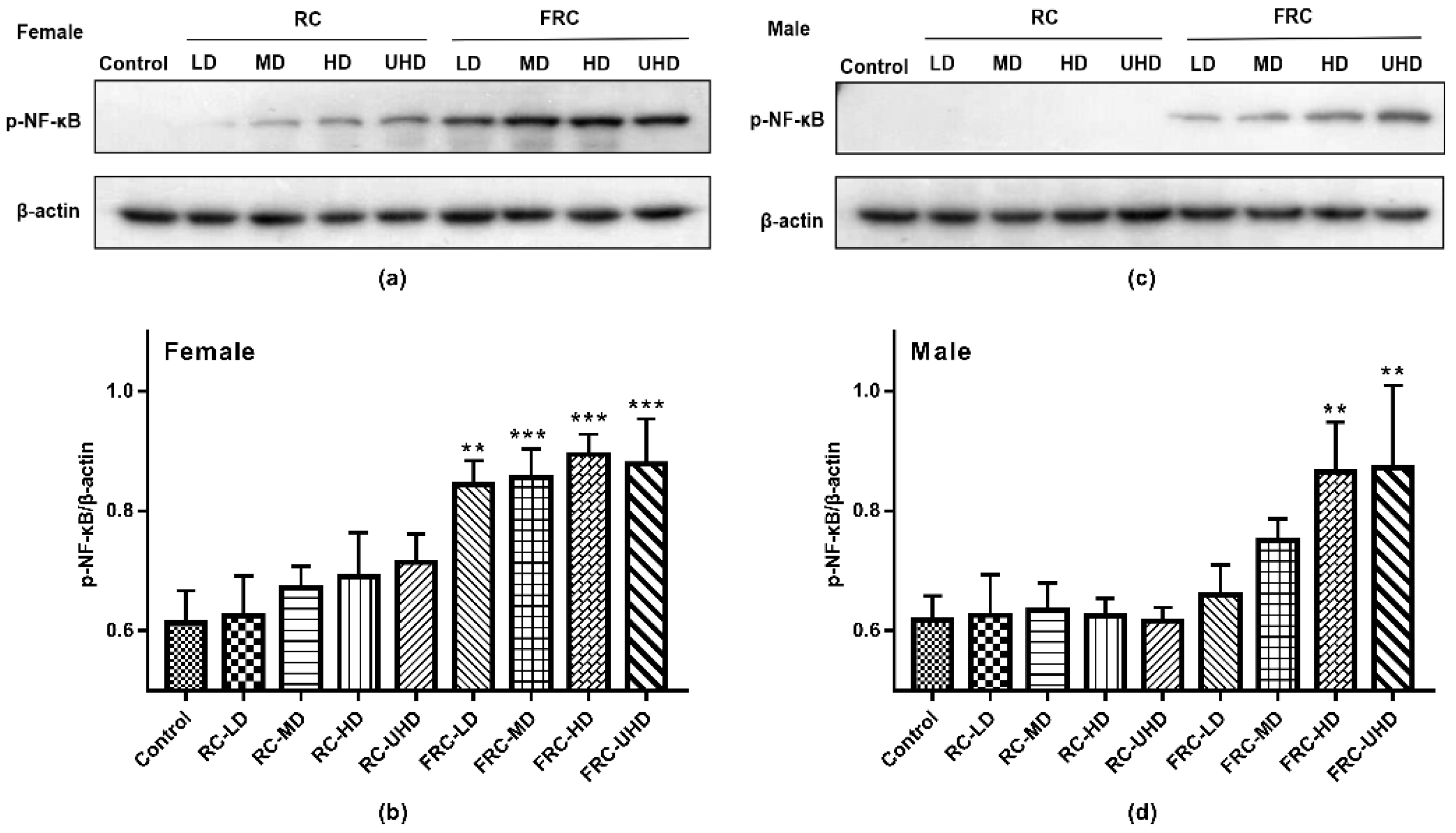
This study explored the molecular mechanism of drug-induced liver injury by detecting the expression of p-NF-κB in the nucleus of rat liver tissue. Western blot results are shown in Figure 4. In the control group, p-NF-κB was almost not expressed. Compared with the control group, there was no statistical difference in the expression of p-NF-κB in the RC group at the dose of this experiment (p > 0.05), but there was still a small amount of protein expression in the liver cells of female rats, suggesting that long time exposure or an increased dose may lead to increased risk of hepatotoxicity to female. In accordance with the above experimental results, the expression of p-NF-κB in the hepatic nuclei of both male and female rats was significantly increased in the FRC group (p < 0.01) in a dose-dependent manner. In addition, the expression of p-NF -κB in female rats was significantly higher than that in male rats. NF-κB is a heterodimer combined with inactivated inhibitory protein (IκB). It is located in the cytoplasm and is an important transcription factor in the process of inflammation. Long-term administration of cohosh root causes liver damage and inflammation in rats, which activates the phosphorylation of NF-κB and transfers to the nucleus, increasing the expression of p-NF -κB in the nucleus of rats. The p-NF -κB activates specific target genes through transcription, e.g., inflammatory mediators such as cytokines, chemokines, cell adhesion molecules, etc. [29,30]. This translocation is the mechanism by which cells respond to oxidants or inflammatory and immune stimuli. It suggests that the fibrous roots of C. dahurica can cause liver tissue damage in rats and trigger inflammation.
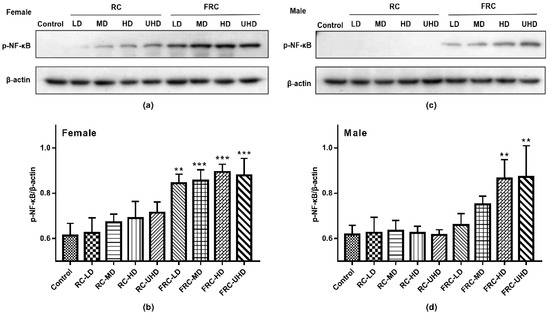
Figure 4.
Effect of rhizome and fibrous roots of C. dahurica on the expression of p-NF-κB Protein in the liver after oral administration in male and female rats for 90 days. (a) p-NF-κB and β-actin protein electrophoresis images of female rats. (b) p-NF-κB/β-actin column charts of female rats. (c) p-NF-κB and β-actin protein electrophoresis images of male rats. (d) p-NF-κB/β-actin column charts of male rats. Data were expressed as means ± SD (n = 3). ** p < 0.01; *** p < 0.001.
3. Materials and Methods
3.1. Plant Material and Animals
C.dahurica was purchased from the Shenyang medicine market in China and authenticated by Prof. Jin-cai Lu (School of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University). The medicinal material specimens are stored in the Department of Traditional Chinese Medicine Resources of Shenyang Pharmaceutical University (No. 2019102301). We artificially separate the rhizomes and fibrous roots when C. dahurica is fresh and cut them into small pieces after sun-drying. Then, they were extracted with 70% ethanol at a factory, concentrated and freeze-dried. (The extraction yield of the alcohol extraction of RC was 0.32 g/g, and the extraction yield of the alcohol extraction of FRC was 0.29 g/g).
3.2. Identification of Chemical Compositions in Crude Extract
Using Compact™ QTOF mass spectrometry (Bruker Daltonik GmbH, Bremen, Germany) and elution autosampler UHPLC system tandem technology (Bruker Daltonik GmbH, Bremen, Germany), the crude extracts of rhizomes and fibrous roots of C. dahurica were qualitatively analyzed in positive and negative electrospray (ESI) mode at m/z 50–1300. A Waters Acquity BEH C18 column (2.1 × 100 mm, 1.7 μm) (Waters Corp., Milford, MA, USA) was used. The mobile phase consisted of water-formic acid (100:0.1, v/v) (A) and acetonitrile (B). The injection volume was 1 μL, and the flow rate was 0.3 mL/min. The column temperature was maintained at 35 °C. The optimized gradient elution conditions for UHPLC are: 15~25% B in 0~10 min, 25~40% B in 10~15 min, 40~47% B in 15~25 min and 25~26 min within 47~15% B. The instrument parameters used for mass spectrometry are as follows: At 1.8 Bar, 220 °C and a flow rate of 8.0 L/min, and nitrogen is used as the nebulizer gas. Collision energy values: 100 m/z, 20 eV; 500 m/z, 30 eV; 1000 m/z, 50 eV; and m/z 1300, 55 eV. All data were collected and processed using Bruker Compass data analysis 5.1 software (Bruker Daltonik GmbH, Bremen, Germany).
3.3. In Vitro Hepatocyte Toxicity Test
After resuscitation, human normal hepatocytes L-02 were cultured in a DMEM medium containing 10% fetal bovine serum and 1% double antibody. As for the cells, they were cultured in an incubator at 37 °C and 5% CO2 at saturated humidity. The cell density grew. When it reaches 70–80%, trypsin was added to digest and pass it down to a new cell culture dish. The L-02 cells in the logarithmic growth phase were seeded in a 96-well plate at 5 × 103 cells/well, with 100 μL of cell culture medium per well. The 96-well plate was placed in an incubator for about 12 h to adhere to the cell wall and then starved for another 12 h. Then, the serum-free culture medium replaced with different concentrations of drugs (100, 200, 400, 800, 1600 μg/mL). For the medium of drug RC or FRC and 2% serum, the control group was replaced with an equal volume of 2% serum-containing culture medium and returned to the incubator to continue culturing for 72 h. After the culture plate was removed, we added μL of MTT working solution to each well and incubated it in the incubator for 4 h. Then, all the liquid in the wells was aspirated; 150 μL of DMSO was added to each well to dissolve the crystals; the culture plate was placed on a shaker and shook for 10 min to fully dissolve the crystals. We used a multifunctional microplate reader to detect the absorbance (OD) value at a 492 nm wavelength. The experiment was repeated three times [30]. Finally, we calculated the cell survival rate according to the following formula and used SPSS 19.0 software to calculate the IC50 value of the drug. Calculated as follows:
Inhibition rate of cell proliferation (%) = (1 − OD administration group/OD control group) × 100%.
3.4. Experimental Design for the Oral Toxicity Study
One hundred and eight Special pathogen-free (SPF) Sprague Dawley rats (aged 4~8 weeks, weight 165 ± 15 g) were provided by the Experimental Animal Center of Shenyang Pharmaceutical University (Shenyang, China), and they were used after a week of quarantine and acclimatization. Animals were housed in an SPF laboratory under controlled temperatures (25 ± 1 °C), humidity (60 ± 10%) conditions and a 12 h light/dark cycle, and they were allowed free access to a balanced murine diet and water during the experiment (Lab Diet 5002 Certified Rodent Diet). All of the animal experiments were approved by the Institutional Animal Care and Use Committee of the Biomedical Research Institute at the Shenyang Pharmaceutical University and complied with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). Combined with the previous acute toxicity experiment and the clinical dosage of cimicifuga, the dosage for the 90-day repeated administration toxicity experiment was determined. The sample of RC and FRC was Orally administered (10 mL/kg) to rats (6/sex/group) at doses of 0.585 g/kg (LD), 1.755 g/kg (MD), 5.265 g/kg (HD) and 15.795 g/kg (UHD), and the normal control group was given vehicle (0.5% CMC-Na solution) once a day. During the experiment, we observed the test animals’ general clinical symptoms, food and water consumption and mortality every day. Additionally, we measured their body weight once a week.
3.5. Urinalysis, Hematology and Biochemistry Analysis
In the last week of treatment, fresh urine samples of 10 rats (5 males and 5 females) were taken from each group and analyzed with a urine analyzer (URIT-500B, Guangxi, China) to evaluate the following parameters: WBC, KET, NIT, URO, BIL, PRO, GLU, SG, BLD, pH and Vc.
The rats were anesthetized with isoflurane after the final gavage. Blood samples are collected through the posterior vena cava. Collect the whole blood sample into the EDTA blood collection tube. Use the automatic hematology analyzer ADVIA 2120i hematology counter (Siemens, Germany) to perform hematology determination of the following parameters: WBC, RBC, HGB, HCT, PLT, MCV, MCH, MCHC, neutrophils, eosinophils, basophils, lymphocytes, monocytes and reticulocytes.
For the biochemical analysis, whole blood was separated at 3000 rpm for 15 min, and serum was separated immediately. Measured with automatic biochemical analyzer 7020 (Hitachi, Tokyo, Japan): ALP, ALT, AST, BUN, CREA, TBIL, GLU, TC, TG, TP, ALB.
3.6. Gross Findings, Organ Weights, and Histopathological Assessments
During necropsy, the organs and tissues were observed macroscopically, and the absolute weight of the heart, liver, spleen, lung, kidney, stomach, brain, adrenal gland, thymus and genitalia were recorded, and the relative weight (organ-to-body weight ratios) was calculated.
Part of the liver tissue (2.0 cm × 2.0 cm × 0.3 cm) was taken and fixed in 4% paraformaldehyde solution, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Then, use an optical microscope for pathological examination to analyze whether there are histological changes in the liver of each group of rats.
3.7. Western Blot Analysis
We collected 60mg liver samples of rats in each experimental group and extracted nuclear protein according to the instructions of the nuclear protein extraction kit. The protein concentration was determined by the bicinchoninic acid (BCA) method. Equal protein samples were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (Bio-Rad, Hercules, CA, USA) and electro transferred onto polyvinylidene difluoride (PVDF) membranes for 2 h. The membranes were incubated for 1 h with 5% skim milk in the Tris-buffered saline containing Tween 20 to block nonspecific binding. After incubating at room temperature for 1 h, the blocking solution was discarded, and the primary antibodies of Phospho-NF-κB p65 (3033, 1:1000) and β-actin (4970, 1:1000) (Cell Signaling Technology, Danvers, MA, USA) were added to the membranes, which were incubated at 4 °C overnight and then washed with Tris buffered saline (TBS) containing 0.1% Tween-20 (TBST) buffer for 3 × 10 min. The secondary antibodies Anti-rabbit IgG (7074, 1:1000) (Cell Signaling Technology, Danvers, MA, USA) were added and incubated for 2 h at room temperature, after washing three times with TBST, and developed with enhanced chemiluminescence (ECL) developer and exposed to X-ray film. The expression levels of β-actin were used as control.
3.8. Statistical Analysis
All of the values are expressed as mean ± SD. The statistical analysis was performed using a one-way ANOVA, followed by a multiple comparison procedure with a Tukey/Duncan test using SPSS software version 19.0 (IBM corporation, Armonk, NY, USA). p values of less than 0.05 were considered to be statistically significant. The figures were conducted using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA).
4. Conclusions
This study found that the rhizome of C. dahurica was safe to take for a long time under the dose of 15.795 g/kg crude drug a day in rats. (It is equivalent to 177 g/d of clinical intake for adults with a weight of about 70 kg.) Additionally, there were no obvious toxic and side effects. However, there is a risk of liver damage in the long-term high-dose administration of C. dahurica fibrous roots since with an increase in the dose, the number of white blood cells in the blood increases significantly, and the biochemical test shows ALP, ALT, AST, BILI and CHOL all have a significant increase, and the expression of p-NF-κB is also significantly increased, which are all landmark indicators of liver damage. This result explains the rationality and necessity of removing fibrous roots in the processing method of cimicifuga. In addition, the material basis of hepatotoxicity of Cimicifuga fibrous roots remains to be further studied.
Author Contributions
Conceptualization, Y.Y., C.L. and J.L.; data curation, Y.Y.; formal analysis, Y.Y. and J.N.; funding acquisition, J.L.; investigation, J.N.; methodology, Y.Y.; project administration, J.L.; software, Y.Y. and J.T.; supervision, C.L. and J.L.; validation, J.T.; writing—original draft, Y.Y.; writing—review and editing, C.L. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Research Fund of Liaoning Provincial Education Department of China (No. 2017LQN09) and the National Natural Science Foundation of China (Grant No. U1508220).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of the Biomedical Research Institute at the Shenyang Pharmaceutical University (protocol code: SYPU-IACUC-S2018-04.09-201, and the date of approval was 9 April 2018). The HL-7702[L-02] cell line was originally thought to originate from a normal fetal liver. and the L-02 cells used in our experiments were provided by FuHeng Bio Co., Ltd. (product ID: FH0109).
Informed Consent Statement
Not applicable.
Data Availability Statement
This article has fully reflected all the data of this study.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of cimicifuga rhizome and fibrous roots crude extracts are available from the authors.
References
- Enbom, E.T.; Le, M.D.; Oesterich, L.; Rutgers, J.; French, S.W. Mechanism of hepatotoxicity due to black cohosh (Cimicifuga racemosa): Histological, immunohistochemical and electron microscopy analysis of two liver biopsies with clinical correlation. Exp. Mol. Pathol. 2014, 96, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Schwarzenboeck, A. Suspected hepatotoxicity by Cimicifugae racemosae rhizoma (black cohosh, root): Critical analysis and structured causality assessment. Phytomedicine 2009, 16, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Fabio, F.; Luigi, G.; Paolo, R.D.S. Black Cohosh Hepatic Safety: Follow-Up of 107 Patients Consuming a Special Cimicifuga racemosa rhizome Herbal Extract and Review of Literature. Evid.-Based Compl. Alt. 2011, 2011, 821392. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.Y.; Considine, A.; Quaglia, A.; Shawcross, D.L. Subacute liver failure secondary to black cohosh leading to liver transplantation. BMJ Case Rep. 2013, 2013, bcr2013009325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.C.; Dai, Y.Q.; Hui, R.R.; Hua, J.; Chen, H.J.; Luo, Q.Y.; Li, J.X. NMR-based metabonomic approach on the toxicological effects of a Cimicifuga triterpenoid. J. Appl. Toxicol. 2012, 32, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Lude, S.; Torok, M.; Dieterle, S.; Knapp, A.C.; Kaeufeler, R.; Jaggi, R.; Spornitz, U.; Krahenbuhl, S. Hepatic effects of Cimicifuga racemosa extract in vivo and in vitro. Cell. Mol. Life Sci. 2007, 64, 2848–2857. [Google Scholar] [CrossRef]
- Mazzanti, G.; Di Sotto, A.; Franchitto, A.; Mastrangelo, S.; Pezzella, M.; Vitalone, A.; Mammola, C.L. Effects of Cimicifuga racemosa extract on liver morphology and hepatic function indices. Phytomedicine 2008, 15, 1021–1024. [Google Scholar] [CrossRef]
- Naser, B.; Schnitker, J.; Minkin, M.J.; de Arriba, S.G.; Nolte, K.U.; Osmers, R. Suspected black cohosh hepatotoxicity: No evidence by meta-analysis of randomized controlled clinical trials for isopropanolic black cohosh extract. Menopause 2011, 18, 366–375. [Google Scholar] [CrossRef]
- Mahady, G.B.; Low Dog, T.; Barrett, M.L.; Chavez, M.L.; Gardiner, P.; Ko, R.; Marles, R.J.; Pellicore, L.S.; Giancaspro, G.I.; Sarma, D.N. United States Pharmacopeia review of the black cohosh case reports of hepatotoxicity. Menopause 2008, 15 Pt 1, 628–638. [Google Scholar] [CrossRef]
- Qin, R.-L.; Lv, C.-N.; Zhao, Y.; Zhao, Y.-D.; Yu, Y.; Lu, J.-C. Assessment of phenolics contents and antioxidant properties in Cimicifuga dahurica (Turcz.) Maxim during drying process. Ind. Crops Prod. 2017, 107, 288–296. [Google Scholar] [CrossRef]
- Guo, Y.; Yin, T.; Wang, X.; Zhang, F.; Pan, G.; Lv, H.; Wang, X.; Owoicho Orgah, J.; Zhu, Y.; Wu, H. Traditional uses, phytochemistry, pharmacology and toxicology of the genus Cimicifuga: A review. J. Ethnopharmacol. 2017, 209, 264–282. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, X.; Liu, W.; Qin, X.; Hu, B.; Ma, Q.; Lv, C.; Lu, J. Cimicifuga dahurica extract inhibits the proliferation, migration and invasion of breast cancer cells MDA-MB-231 and MCF-7 in vitro and in vivo. J. Ethnopharmacol. 2021, 277, 114057. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, Z.-L.; Su, Y.; Wang, Q.-H.; Kuang, H.-X. Cycloartenol triterpenoid saponins from Cimicifuga simplex (Ranunculaceae) and their biological effects. Chin. J. Nat. Med. 2015, 13, 81–89. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, W.-Y.; Pan, D.-B.; Shi, D.-F.; Pang, Q.-Q.; Li, H.-B.; Yao, X.-J.; Yao, Z.-H.; Yu, Y.; Yao, X.-S. Phenolic acids and their glycosides from the rhizomes of Cimicifuga dahurica. Fitoterapia 2019, 134, 485–492. [Google Scholar] [CrossRef]
- Lourenço, A.M.; Máximo, P.; Ferreira, L.M.; Pereira, M.M.A. Indolizidine and quinolizidine alkaloids structure and bioactivity. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 27, pp. 233–298. [Google Scholar]
- Miao, L.-Y.; Chu, T.T.H.; Li, P.; Jiang, Y.; Li, H.-J. Cimicifuga heracleifolia is therapeutically similar to black cohosh in relieving menopausal symptoms: Evidence from pharmacological and metabolomics studies. Chin. J. Nat. Med. 2019, 17, 435–445. [Google Scholar] [CrossRef]
- Fan, M.; Qin, K.; Ding, F.; Huang, Y.; Wang, X.; Cai, B. Identification and differentiation of major components in three different “Sheng-ma” crude drug species by UPLC/Q-TOF-MS. Acta Pharm. Sin. B 2017, 7, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Song, X.; Nagai, T.; Yamamoto, M.; Dai, Y.; He, L.; Kiyohara, H.; Yao, X.; Yao, Z. Chemical profile of Cimicifuga heracleifolia Kom. And immunomodulatory effect of its representative bioavailable component, cimigenoside on Poly(I:C)-induced airway inflammation. J. Ethnopharmacol. 2021, 267, 113615. [Google Scholar] [CrossRef]
- Bain, B.J. Structure and function of red and white blood cells and platelets. Medicine 2021, 49, 183–188. [Google Scholar] [CrossRef]
- Parente, J. Diagnostics for White Blood Cell Abnormalities: Leukocytosis and Leukopenia. Physician Assist. Clin. 2019, 4, 625–635. [Google Scholar] [CrossRef]
- Yun, J.-W.; You, J.-R.; Kim, Y.-S.; Cho, E.-Y.; Kim, S.-H.; Yoon, J.-H.; Kwon, E.; Chung, D.H.; Kim, Y.T.; Jang, J.-J.; et al. Pre-clinical in vitro and in vivo safety evaluation of Cimicifuga heracleifolia. Regul. Toxicol. Pharm. 2015, 73, 303–310. [Google Scholar] [CrossRef]
- Xie, K.; Chen, C.H.; Tsai, S.P.; Lu, P.J.; Wu, H.; Zeng, Y.; Ye, Y.; Tu, H.; Wen, C.; Huang, M.; et al. Loss of Life Expectancy by 10 Years or More From Elevated Aspartate Aminotransferase: Finding Aspartate Aminotransferase a Better Mortality Predictor for All-Cause and Liver-Related than Alanine Aminotransferase. Am. J. Gastroenterol. 2019, 114, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xia, Y.; Li, S.; Cheng, X.; Hu, S.; Gao, Y.; Zhou, X.; Wang, G.; Zheng, Q. A retrospective pilot study to examine the potential of aspartate aminotransferase to alanine aminotransferase ratio as a predictor of postoperative acute kidney injury in patients with hepatocellular carcinoma. Ann. Clin. Biochem. 2019, 56, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kim, W.R.; Poterucha, J.J. Evaluation of Elevated Liver Enzymes. Clin. Liver Dis. 2012, 16, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.; Kowdley, K.V. Approach to a Patient with Elevated Serum Alkaline Phosphatase. Clin. Liver Dis. 2012, 16, 199–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDaniel, M.J. Hepatic Function Testing: The ABCs of the Liver Function Tests. Physician Assist. Clin. 2019, 4, 541–550. [Google Scholar] [CrossRef]
- Domínguez-Pérez, M.; Simoni-Nieves, A.; Rosales, P.; Nuño-Lámbarri, N.; Rosas-Lemus, M.; Souza, V.; Miranda, R.U.; Bucio, L.; Uribe Carvajal, S.; Marquardt, J.U.; et al. Cholesterol burden in the liver induces mitochondrial dynamic changes and resistance to apoptosis. J. Cell. Physiol. 2019, 234, 7213–7223. [Google Scholar] [CrossRef] [PubMed]
- Manoeuvrier, G.; Bach-Ngohou, K.; Batard, E.; Masson, D.; Trewick, D. Diagnostic performance of serum blood urea nitrogen to creatinine ratio for distinguishing prerenal from intrinsic acute kidney injury in the emergency department. BMC Nephrol. 2017, 18, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mckay, L.I.; Cidlowski, J.A. Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: Mechanisms of mutual antagonism. Mol. Endocrinol. 1998, 12, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).