Spherical Sampler Probes Enhance the Robustness of Ambient Ionization Mass Spectrometry for Rapid Drugs Screening
Abstract
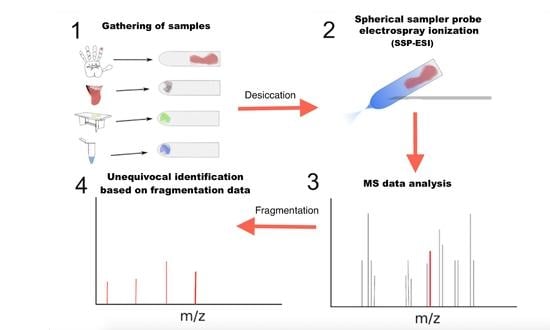
:1. Introduction
2. Results and Discussion
2.1. Characterization of SSP-Emitters for Electrospray Ionization
2.2. The Analysis of Drugs Using Sampler Probes of Spherical Shape
2.3. Method Application
3. Materials and Methods
3.1. Materials
3.2. Determination of the Current–Voltage Curve
3.3. Mass Spectrometry
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feider, C.L.; Krieger, A.; DeHoog, R.J.; Eberlin, L.S. Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Anal. Chem. 2019, 91, 4266–4290. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R.; Yannell, K.E.; Jarmusch, A.K.; Pirro, V.; Ouyang, Z.; Cooks, R.G. Ambient Ionization Mass Spectrometry for Point-of-Care Diagnostics and Other Clinical Measurements. Clin. Chem. 2016, 62, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pekov, S.I.; Bormotov, D.S.; Nikitin, P.V.; Sorokin, A.A.; Shurkhay, V.A.; Eliferov, V.A.; Zavorotnyuk, D.S.; Potapov, A.A.; Nikolaev, E.N.; Popov, I.A. Rapid estimation of tumor cell percentage in brain tissue biopsy samples using inline cartridge extraction mass spectrometry. Anal. Bioanal. Chem. 2021, 413, 2913–2922. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W. Simultaneous Screening of 177 Drugs of Abuse in Urine Using Ultra-performance Liquid Chromatography with Tandem Mass Spectrometry in Drug-intoxicated Patients. Clin. Psychopharmacol. Neurosci. 2013, 11, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Fialkov, A.B.; Lehotay, S.J.; Amirav, A. Less than one minute low-pressure gas chromatography—Mass spectrometry. J. Chromatogr. A 2020, 1612, 460691. [Google Scholar] [CrossRef]
- Ma, X.; Ouyang, Z. Ambient ionization and miniature mass spectrometry system for chemical and biological analysis. Trends Analyt. Chem. 2016, 85, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Lawton, Z.E.; Traub, A.; Fatigante, W.L.; Mancias, J.; O’Leary, A.E.; Hall, S.E.; Wieland, J.R.; Oberacher, H.; Gizzi, M.C.; Mulligan, C.C. Analytical Validation of a Portable Mass Spectrometer Featuring Interchangeable, Ambient Ionization Sources for High Throughput Forensic Evidence Screening. J. Am. Soc. Mass Spectrom. 2017, 28, 1048–1059. [Google Scholar] [CrossRef]
- Lee, C.W.; Su, H.; Cai, Y.D.; Wu, M.T.; Wu, D.C.; Shiea, J. Rapid Identification of Psychoactive Drugs in Drained Gastric Lavage Fluid and Whole Blood Specimens of Drug Overdose Patients Using Ambient Mass Spectrometry. Mass Spectrom. 2017, 6, S0056. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.W.; Chao, Y.Y.; Shiea, J.; Shen, J.H.; Lee, H.H.; Chen, B.H. Ambient mass spectrometry for rapid diagnosis of psychoactive drugs overdose in an unstable patient. Am. J. Emerg. Med. 2018, 36, 530.e1–530.e5. [Google Scholar] [CrossRef]
- Maher, S.; Jjunju, F.; Taylor, S. Colloquium: 100 years of mass spectrometry: Perspectives and future trends. Rev. Mod. Phys. 2015, 87, 113–135. [Google Scholar] [CrossRef] [Green Version]
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberlin, L.S. DESI-MS imaging of lipids and metabolites from biological samples. Methods Mol. Biol. 2014, 1198, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Smoluch, M.; Mielczarek, P.; Silberring, J. Plasma-based ambient ionization mass spectrometry in bioanalytical sciences. Mass Spectrom. Rev. 2016, 35, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.D.; Charipar, N.A.; Mulligan, C.C.; Zhang, X.; Cooks, R.G.; Ouyang, Z. Low-temperature plasma probe for ambient desorption ionization. Anal. Chem. 2008, 80, 9097–9104. [Google Scholar] [CrossRef]
- Smith, B.L.; Hughes, D.M.; Badu-Tawiah, A.K.; Eccles, R.; Goodall, I.; Maher, S. Rapid Scotch Whisky Analysis and Authentication using Desorption Atmospheric Pressure Chemical Ionisation Mass Spectrometry. Sci. Rep. 2019, 9, 7994. [Google Scholar] [CrossRef]
- Morato, N.M.; Pirro, V.; Fedick, P.W.; Cooks, R.G. Quantitative Swab Touch Spray Mass Spectrometry for Oral Fluid Drug Testing. Anal. Chem. 2019, 91, 7450–7457. [Google Scholar] [CrossRef]
- Patrick, W.F.; Ryan, M.B. Swab touch spray mass spectrometry for rapid analysis of organic gunshot residue from human hand and various surfaces using commercial and fieldable mass spectrometry systems. Forensic Chem. 2017, 5, 53–57. [Google Scholar] [CrossRef]
- Lin, C.H.; Liao, W.C.; Chen, H.K.; Kuo, T.Y. Paper spray-MS for bioanalysis. Bioanalysis 2014, 6, 199–208. [Google Scholar] [CrossRef]
- Manicke, N.E.; Bills, B.J.; Zhang, C. Analysis of biofluids by paper spray MS: Advances and challenges. Bioanalysis 2016, 8, 589–606. [Google Scholar] [CrossRef] [Green Version]
- Vandergrift, G.W.; Hessels, A.J.; Palaty, J.; Krogh, E.T.; Gill, C.G. Paper spray mass spectrometry for the direct, semi-quantitative measurement of fentanyl and norfentanyl in complex matrices. Clin. Biochem. 2018, 54, 106–111. [Google Scholar] [CrossRef]
- Rossini, E.L.; Kulyk, D.S.; Ansu-Gyeabourh, E.; Sahraeian, T.; Pezza, H.R.; Badu-Tawiah, A.K. Direct Analysis of Doping Agents in Raw Urine Using Hydrophobic Paper Spray Mass Spectrometry. Mass Spectrom. 2020, 31, 1212–1222. [Google Scholar] [CrossRef]
- Teunissen, S.F.; Fedick, P.W.; Berendsen, B.J.A.; Nielen, M.W.F.; Eberlin, M.N.; Graham Cooks, R.; van Asten, A.C. Novel Selectivity-Based Forensic Toxicological Validation of a Paper Spray Mass Spectrometry Method for the Quantitative Determination of Eight Amphetamines in Whole Blood. J. Am. Soc. Mass Spectrom. 2017, 28, 2665–2676. [Google Scholar] [CrossRef]
- McKenna, J.; Jett, R.; Shanks, K.; Manicke, N.E. Toxicological Drug Screening using Paper Spray High-Resolution Tandem Mass Spectrometry (HR-MS/MS). J. Anal. Toxicol. 2018, 42, 300–310. [Google Scholar] [CrossRef] [Green Version]
- Frey, B.S.; Damon, D.E.; Badu-Tawiah, A.K. Emerging trends in paper spray mass spectrometry: Microsampling, storage, direct analysis, and applications. Mass Spectrom. Rev. 2020, 39, 336–370. [Google Scholar] [CrossRef]
- Jjunju, F.P.M.; Damon, D.E.; Romero-Perez, D.; Young, I.S.; Ward, R.J.; Marshall, A.; Maher, S.; Badu-Tawiah, A.K. Analysis of non-conjugated steroids in water using paper spray mass spectrometry. Sci. Rep. 2020, 10, 10698. [Google Scholar] [CrossRef]
- Espy, R.D.; Muliadi, A.R.; Ouyang, Z.; Cooks, R.G. Spray mechanism in paper spray ionization. Int. J. Mass Spectrom. 2012, 325, 167–171. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, H.; Maas, J.D.; Chappell, W.J.; Manicke, N.E.; Cooks, R.G.; Ouyang, Z. Paper spray ionization devices for direct, biomedical analysis using mass spectrometry. Int. J. Mass Spectrom. 2012, 312, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Kerian, K.S.; Jarmusch, A.K.; Cooks, R.G. Touch spray mass spectrometry for in situ analysis of complex samples. Analyst 2014, 139, 2714–2720. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.M.; Man, S.H.; Che, C.M.; Lau, K.C.; Ng, K.C. Negative electrospray ionization on porous supporting tips for mass spectrometric analysis: Electrostatic charging effect on detection sensitivity and its application to explosive detection. Analyst 2014, 139, 1482–1491. [Google Scholar] [CrossRef]
- Aquino, A.; Mayrink Alves Pereira, G.; Dossi, N.; Piccin, E.; Augusti, R. Reagent-Pencil and Paper Spray Mass Spectrometry: A Convenient Combination for Selective Analyses in Complex Matrixes. J. Am. Soc. Mass Spectrom. 2021, 32, 281–288. [Google Scholar] [CrossRef]
- Li, X.; Tao, W.; Xun, H.; Yao, X.; Wang, J.; Sun, J.; Yue, Y.; Tang, F. Simultaneous Determination of Flavonoids from Bamboo Leaf Extracts Using Liquid Chromatography-Tandem Mass Spectrometry. Rev. Bras. Farmacogn. 2021, 31, 347–352. [Google Scholar] [CrossRef]
- Cochran, K.H.; Barry, J.A.; Muddiman, D.C.; Hinks, D. Direct analysis of textile fabrics and dyes using infrared matrix-assisted laser desorption electrospray ionization mass spectrometry. Anal. Chem. 2013, 85, 831–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filho, J.F.A.; Dos Santos, N.A.; Borges, K.B.; Lacerda, V., Jr.; Pelição, F.S.; Romão, W. Fiber spray ionization mass spectrometry in forensic chemistry: A screening of drugs of abuse and direct determination of cocaine in urine. Rapid Commun. Mass Spectrom. 2020, 34, e8747. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.E.; Shamraeva, M.A.; Larina, I.M.; Bormotov, D.S.; Pekov, S.I.; Shivalin, A.S.; Silkin, S.V.; Eliferov, V.A.; Bocharov, K.V.; Nikolaev, E.N.; et al. The Development of a method for direct mass spectrometry analysis of biological samples using porous samplers. Aerospace Environ. Med. 2021, 55, 99–103. [Google Scholar] [CrossRef]
- Tepper, G.; Kessick, R. Nanoelectrospray aerosols from microporous polymer wick sources. Appl. Phys. Lett. 2009, 94, 084106. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, H.; Manicke, N.E.; Lin, J.M.; Cooks, R.G.; Ouyang, Z. Development, characterization, and application of paper spray ionization. Anal. Chem. 2010, 82, 2463–2471. [Google Scholar] [CrossRef]
- Cheng, S.C.; Jhang, S.S.; Huang, M.Z.; Shiea, J. Simultaneous detection of polar and nonpolar compounds by ambient mass spectrometry with a dual electrospray and atmospheric pressure chemical ionization source. Anal. Chem. 2015, 87, 1743–1748. [Google Scholar] [CrossRef]
- Manicke, N.E.; Abu-Rabie, P.; Spooner, N.; Ouyang, Z.; Cooks, R.G. Quantitative analysis of therapeutic drugs in dried blood spot samples by paper spray mass spectrometry: An avenue to therapeutic drug monitoring. J. Am. Soc. Mass Spectrom. 2011, 22, 1501–1507. [Google Scholar] [CrossRef]
- Manicke, N.E.; Yang, Q.A.; Wang, H.; Oradu, S.; Zheng, O.Y.; Cooks, R.G. Assessment of paper spray ionization for quantitation of pharmaceuticals in blood spots. Int. J. Mass Spectrom. 2011, 300, 123–129. [Google Scholar] [CrossRef]
- Shiea, J.; Bhat, S.M.; Su, H.; Kumar, V.; Lee, C.W.; Wang, C.H. Rapid quantification of acetaminophen in plasma using solid-phase microextraction coupled with thermal desorption electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2019, 34, e8564. [Google Scholar] [CrossRef] [Green Version]
- Schulz, M.; Iwersen-Bergmann, S.; Andresen, H.; Schmoldt, A. Therapeutic and toxic blood concentrations of nearly 1000 drugs and other xenobiotics. Crit. Care 2012, 16, R136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.J.; Liu, X.W.; Kong, X.J.; Qin, Z.; Li, S.H.; Jiao, Z.H.; Li, J.Y. An LC-MS/MS method for the quantification of diclofenac sodium in dairy cow plasma and its application in pharmacokinetics studies. Biomed. Chromatogr. 2019, 33, e4520. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. High-Throughput Ultra-Performance LC–MS-MS Method for Analysis of Diclofenac Sodium in Rabbit Plasma. J. Chromatogr. Sci. 2015, 53, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismaiel, O.; Halquist, M.S.; El-Mammli, M.Y.; Shalaby, A.; Karnes, H.T. Development of a Liquid Chromatography-Negative ESI-Tandem Mass Spectrometry Method for Ibuprofen with Minimization of Matrix Effects Associated with Phospholipids. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 3194–3208. [Google Scholar] [CrossRef]
- Pirro, V.; Jarmusch, A.K.; Vincenti, M.; Cooks, R.G. Direct drug analysis from oral fluid using medical swab touch spray mass spectrometry. Anal. Chim. Acta 2015, 861, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, N.M.; Anderson, K.E. Clinical Pharmacokinetics of Diclofenac. Clin. Pharmacokinet. 1997, 33, 184–213. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamraeva, M.A.; Bormotov, D.S.; Shamarina, E.V.; Bocharov, K.V.; Peregudova, O.V.; Pekov, S.I.; Nikolaev, E.N.; Popov, I.A. Spherical Sampler Probes Enhance the Robustness of Ambient Ionization Mass Spectrometry for Rapid Drugs Screening. Molecules 2022, 27, 945. https://doi.org/10.3390/molecules27030945
Shamraeva MA, Bormotov DS, Shamarina EV, Bocharov KV, Peregudova OV, Pekov SI, Nikolaev EN, Popov IA. Spherical Sampler Probes Enhance the Robustness of Ambient Ionization Mass Spectrometry for Rapid Drugs Screening. Molecules. 2022; 27(3):945. https://doi.org/10.3390/molecules27030945
Chicago/Turabian StyleShamraeva, Mariya A., Denis S. Bormotov, Ekaterina V. Shamarina, Konstantin V. Bocharov, Olga V. Peregudova, Stanislav I. Pekov, Eugene N. Nikolaev, and Igor A. Popov. 2022. "Spherical Sampler Probes Enhance the Robustness of Ambient Ionization Mass Spectrometry for Rapid Drugs Screening" Molecules 27, no. 3: 945. https://doi.org/10.3390/molecules27030945
APA StyleShamraeva, M. A., Bormotov, D. S., Shamarina, E. V., Bocharov, K. V., Peregudova, O. V., Pekov, S. I., Nikolaev, E. N., & Popov, I. A. (2022). Spherical Sampler Probes Enhance the Robustness of Ambient Ionization Mass Spectrometry for Rapid Drugs Screening. Molecules, 27(3), 945. https://doi.org/10.3390/molecules27030945