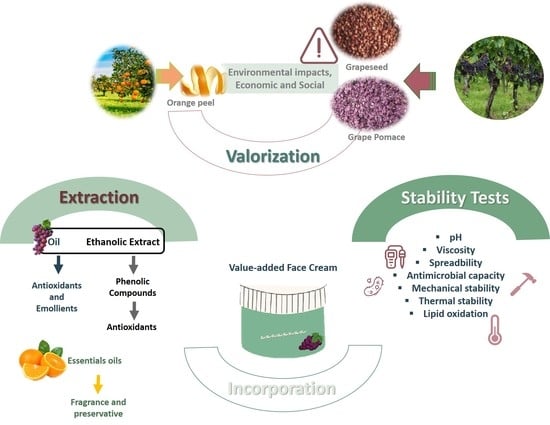
A Potential Valorization Strategy of Wine Industry by-Products and Their Application in Cosmetics—Case Study: Grape Pomace and Grapeseed
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction of Extracts and Oils from Grapeseed and Grape Pomace
2.2. Antioxidant Capacity
2.3. Antimicrobial Capacity
2.4. Stability Tests
2.5. Lipid Oxidation
3. Materials and Methods
3.1. Reagents and Agro-Industrial by-Products
3.2. Methods
3.2.1. Extraction of Phenolic Extract and Oil from Grapeseed and Grape Pomace
3.2.2. Extraction of Essential Oil from Orange Peels
3.2.3. Antioxidant Capacity
3.2.4. Antimicrobial Capacity
3.2.5. Face Cream Moisturizer Production
3.2.6. Stability Tests
3.2.7. Lipid Oxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| BAC | Bioactive Compounds |
| EO | Essential Oil |
| GP | Grape Pomace |
| GS | Grapeseed |
| PC | Phenolic Compounds |
| PV | Peroxide Value |
References
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M.; Cardoso, S.M.; Fazio, A. Management of Fruit Industrial By-Products-A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulo, F.; Santos, L. Deriving valorization of phenolic compounds from olive oil by-products for food applications through microencapsulation approaches: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 61, 920–945. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Sim, Y.Y.; Nyam, K.L. Application of Hibiscus cannabinus L. (kenaf) leaves extract as skin whitening and anti-aging agents in natural cosmetic prototype. Ind. Crops Prod. 2021, 167, 113491. [Google Scholar] [CrossRef]
- Tavares, C.S.; Martins, A.; Miguel, M.G.; Carvalheiro, F.; Duarte, L.C.; Gameiro, J.A.; Figueiredo, A.C.; Roseiro, L.B. Bioproducts from forest biomass II. Bioactive compounds from the steam-distillation by-products of Cupressus lusitanica Mill. and Cistus ladanifer L. wastes. Ind. Crops Prod. 2020, 158, 112991. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel application and industrial exploitation of winery by—Products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef]
- Khdair, A.; Abu-Rumman, G. Sustainable Environmental Management and Valorization Options for Olive Mill Byproducts in the Middle East and North Africa (MENA) Region. Processes 2020, 8, 671. [Google Scholar] [CrossRef]
- Rodrigues, F.; de la Luz Cádiz-Gurrea, M.; Nunes, M.A.; Pinto, D.; Vinha, A.F.; Linares, I.B.; Oliveira, M.B.P.P.; Carretero, A.S. 12—Cosmetics. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 393–427. ISBN 978-0-12-813572-3. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V. Valorization of Winemaking By-Products as a Novel Source of Antibacterial Properties: New Strategies to Fight Antibiotic Resistance. Molecules 2021, 26, 2331. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C. Bioactive Compounds from Vine Shoots, Grape Stalks, and Wine Lees: Their Potential Use in Agro-Food Chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef]
- Soto, M.L.; Falqué, E.; Domínguez, H. Relevance of Natural Phenolics from Grape and Derivative Products in the Formulation of Cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50. ISBN 978-0-12-814774-0. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phyther. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef]
- Chanchal, D.; Swarnlata, S. Novel approaches in herbal cosmetics. J. Cosmet. Dermatol. 2008, 7, 89–95. [Google Scholar] [CrossRef]
- Nunes, M.; Rodrigues, F.; Oliveira, M. 11. Grape Processing By-Products as Active Ingredients for Cosmetic Proposes. In Handbook of Grape Processing By-Products: Sustainable Solutions; Academic Press: London, UK, 2017. [Google Scholar] [CrossRef]
- Domingues, P.M.; Santos, L. Essential oil of pennyroyal (Mentha pulegium): Composition and applications as alternatives to pesticides—New tendencies. Ind. Crops Prod. 2019, 139, 111534. [Google Scholar] [CrossRef]
- Pasini Deolindo, C.T.; Monteiro, P.I.; Santos, J.S.; Cruz, A.G.; Cristina da Silva, M.; Granato, D. Phenolic-rich Petit Suisse cheese manufactured with organic Bordeaux grape juice, skin, and seed extract: Technological, sensory, and functional properties. LWT 2019, 115, 108493. [Google Scholar] [CrossRef]
- Milinči, D.D.; Kosti, A.Ž.; Gaši, U.M.; Levi, S.; Stanojevi, d.P.; Bara, M.B.; Teši, Ž.L.; Nedovi, V.; Peši, M.B. Skimmed Goat’s Milk Powder Enriched with Grape Pomace Seed Extract: Phenolics and Protein Characterization and Antioxidant Properties. Biomolecules 2021, 11, 965. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Zungur Bastıoğlu, A. Production of phenolic enriched mushroom powder as affected by impregnation method and air drying temperature. LWT 2020, 122, 109036. [Google Scholar] [CrossRef]
- Soto, M.L.; Parada, M.; Falqué, E.; Domínguez, H. Personal-Care Products Formulated with Natural Antioxidant Extracts. Cosmetics 2018, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Surini, S.; Mubarak, H.; Ramadon, D. Cosmetic serum containing grape (Vitis vinifera L.) seed extract phytosome: Formulation and in vitro penetration study. J. Young Pharm. 2018, 10, s51–s55. [Google Scholar] [CrossRef] [Green Version]
- Hubner, A.; Sobreira, F.; Neto, A.V.; de Oliveira Pinto, C.A.S.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The Synergistic Behavior of Antioxidant Phenolic Compounds Obtained from Winemaking Waste’s Valorization, Increased the Efficacy of a Sunscreen System. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef] [Green Version]
- Korichi, R.; Tranchant, J.F. Decorative Products; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420069686. [Google Scholar] [CrossRef]
- Romes, N.B.; Wahab, R.A.; Hamid, M.A. The role of bioactive phytoconstituents-loaded nanoemulsions for skin improvement: A review. Biotechnol. Biotechnol. Equip. 2021, 35, 711–729. [Google Scholar] [CrossRef]
- Costa, R.; Santos, L. Delivery systems for cosmetics—From manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Application of microencapsulated essential oils in cosmetic and personal healthcare products—A review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef]
- Han, H.; Yılmaz, H.; Gülçin, İ. Antioxidant Activity of Flaxseed (Linum usitatissimum L.) shell and Analysis of Its Polyphenol Contents by LC-MS/MS. Rec. Nat. Prod. 2018, 12, 397–402. [Google Scholar]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application—A review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Ribeiro, B.S.; de Fatima Ferreira, M.; Moreira, J.L.; Santos, L. Simultaneous Distillation–Extraction of Essential Oils from Rosmarinus officinalis L. Cosmetics 2021, 8, 117. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Baral, E.R.; Koteswararao, R.; Dhyani, A.; Cho, M.H.; Cho, S. Bio-sorbents, industrially important chemicals and novel materials from citrus processing waste as a sustainable and renewable bioresource: A review. J. Adv. Res. 2020, 23, 61–82. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.-H.; Mousavi Khaneghah, A. Recent advances in orange oil extraction: An opportunity for the valorisation of orange peel waste a review. Int. J. Food Sci. Technol. 2019, 54, 925–932. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef] [Green Version]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Saqib, S.; Zaman, W.; Ayaz, A.; Habib, S.; Bahadur, S.; Hussain, S.; Muhammad, S.; Ullah, F. Postharvest disease inhibition in fruit by synthesis and characterization of chitosan iron oxide nanoparticles. Biocatal. Agric. Biotechnol. 2020, 28, 101729. [Google Scholar] [CrossRef]
- Francenia Santos-Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Bjelica, M.; Vujasinović, V.; Rabrenović, B.; Dimić, S. Some Chemical Characteristics and Oxidative Stability of Cold Pressed Grape Seed Oils Obtained from Different Winery Waste. Eur. J. Lipid Sci. Technol. 2019, 121, 1800416. [Google Scholar] [CrossRef]
- Ghafoor, K.; Uslu, N.; Musa Özcan, M.; Al Juhaimi, F.; Babiker, E.E.; Mohamed Ahmed, I.A.; Azmi, I.U. Influence of grape variety on bioactive compounds, antioxidant activity, and phenolic compounds of some grape seeds grown in Turkey. J. Food Process. Preserv. 2020, 44, e14980. [Google Scholar] [CrossRef]
- Luchian, C.E.; Cotea, V.V.; Vlase, L.; Toiu, A.M.; Colibaba, L.C.; Răschip, I.E.; Nadăş, G.; Gheldiu, A.M.; Tuchiluş, C.; Rotaru, L. Antioxidant and antimicrobial effects of grape pomace extracts. BIO Web Conf. 2019, 15, 04006. [Google Scholar] [CrossRef]
- Burčová, Z.; Kreps, F.; Schmidt, Š.; Strižincová, P.; Jablonský, M.; Kyselka, J.; Ház, A.; Šurina, I. Antioxidant activity and the tocopherol and phenol contents of grape residues. BioResources 2019, 14, 4146–4156. [Google Scholar] [CrossRef]
- Phan Thi, T.; Ninh The, S. Antioxidant of Trans-Resveratrol: A Comparison between OH and CH Groups Based on Thermodynamic Views. J. Chem. 2020, 2020, 8869023. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic Compounds; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128132784. [Google Scholar] [CrossRef]
- Hogervorst, J.C.; Miljić, U.; Puškaš, V. Extraction of Bioactive Compounds from Grape Processing By-Products; Academic Press: London, UK, 2017; ISBN 9780128098714. [Google Scholar] [CrossRef]
- Fitrasyah, S.I.; Ariani, A.; Rahman, N.; Nurulfuadi, N.; Aiman, U.; Nadila, D.; Pradana, F.; Rakhman, A.; Hartini, D.A. Analysis of chemical properties and antioxidant activity of sambiloto (Andrographis paniculata nees.) leaf tea formula as a functional drink in preventing coronavirus diseases and degenerative diseases. Open Access Maced. J. Med. Sci. 2021, 9, 196–201. [Google Scholar] [CrossRef]
- Baydar, N.G.; Özkan, G.; Çetin, E.S. Characterization of grape seed and pomace oil extracts. Grasas y Aceites 2007, 58, 29–33. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Asghar, M.; Habib, S.; Zaman, W.; Hussain, S.; Ali, H.; Saqib, S. Synthesis and characterization of microbial mediated cadmium oxide nanoparticles. Microsc. Res. Tech. 2020, 83, 1574–1584. [Google Scholar] [CrossRef]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four virginia-grown grape varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef]
- Dabetic, N.M.; Todorovic, V.M.; Djuricic, I.D.; Antic Stankovic, J.A.; Basic, Z.N.; Vujovic, D.S.; Sobajic, S.S. Grape Seed Oil Characterization: A Novel Approach for Oil Quality Assessment. Eur. J. Lipid Sci. Technol. 2020, 122, 1900447. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Augusta, A.; Smânia, A.; Smânia, E.F.A.; Maraschin, M.; Ferreira, S.R.S. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [Green Version]
- Krasowska, A.; Lukaszewicz, M. Review paper Comparison of antimicrobial activity of three commercially used quaternary ammonium surfactants Porównanie przeciwdrobnoustrojowej aktywności trzech komercyjnie stosowanych. Sepsis 2012, 5, 170–174. [Google Scholar] [CrossRef]
- Kamaruzaman, N.; Yusop, S.M. Determination of stability of cosmetic formulations incorporated with water-soluble elastin isolated from poultry. J. King Saud Univ.Sci. 2021, 33, 101519. [Google Scholar] [CrossRef]
- Fagionato Masiero, J.; Barbosa, E.J.; de Oliveira Macedo, L.; de Souza, A.; Nishitani Yukuyama, M.; Arantes, G.J.; Bou-Chacra, N.A. Vegetable oils in pharmaceutical and cosmetic lipid-based nanocarriers preparations. Ind. Crops Prod. 2021, 170, 113838. [Google Scholar] [CrossRef]
- Dermiş, S.; Can, S.; Doğru, B. Determination of Peroxide Values of Some Fixed Oils by Using the mFOX Method. Spectrosc. Lett. 2012, 45, 359–363. [Google Scholar] [CrossRef]
- Thomsen, B.R.; Taylor, R.; Madsen, R.; Hyldig, G.; Blenkiron, P.; Jacobsen, C. Investigation of Lipid Oxidation in the Raw Materials of a Topical Skin Formulation: A Topical Skin Formulation Containing a High Lipid Content. J. Am. Oil Chem. Soc. 2018, 95, 185–196. [Google Scholar] [CrossRef]
- Andersen, F.A. Final Report on the Safety Assessment of BHT 1. Int. J. Toxicol. 2002, 21, 19–94. [Google Scholar] [CrossRef]
- Seifu, T.; Abera, A. Extraction of essential oil from orange peel using different methods and effect of solvents, time, temperature to maximize yield. Int. J. Eng. Sci. Comput. 2019, 9, 24300–24308. [Google Scholar]
- El-Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic profile and antioxidant activities of olive mill wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I.R.N.A. Potential application of grape (Vitis vinifera L.) stem extracts in the cosmetic and pharmaceutical industries: Valorization of a by-product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- Navarro-Pérez, Y.M.; Cedeño-Linares, E.; Norman-Montenegro, O.; Ruz-Sanjuan, V.; Mondeja-Rivera, Y.; Hernández-Monzón, A.M.; González-Bedia, M.M. Prediction of the physical stability and quality of O/W cosmetic emulsions using full factorial design [Predicción de la estabilidad física y calidad de emulsiones cosméticas O/W mediante diseño factorial completo]. J. Pharm. Pharmacogn. Res. 2021, 9, 98–112. [Google Scholar]
- Chen, M.X.; Alexander, K.S.; Baki, G. Formulation and Evaluation of Antibacterial Creams and Gels Containing Metal Ions for Topical Application. J. Pharm. 2016, 2016, 5754349. [Google Scholar] [CrossRef] [Green Version]
- Kumara, T.P.P.; Sunil, K.; Arunkumar, B.; Patil, S.B. Formulation and evaluation of multipurpose topical cream by using natural Schiff base active ingredients. AIP Conf. Proc. 2020, 2274, 50001. [Google Scholar] [CrossRef]
- Nalur, S.; Decker, E.A. Rapid, Sensitive, Iron-Based Spectrophotometric Methods for Determination of Peroxide Values of Food Lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef]






| Objectives | Results | Reference | |
|---|---|---|---|
| Food Industry | Application of the grape skin and seeds extract, rich in antioxidants in Petit Suisse cheese and evaluation of its technological, sensorial, and functional properties. | No difference was detected, neither in sensorial acceptance nor in total phenolic content in fortified cheese | [22] |
| Protein and phenolic characterization and evaluation of antioxidant properties of goat’s milk powder enriched with grapeseed extract | The addition of grapeseed extract enhanced the antioxidant capacity of the milk. | [23] | |
| Development of a functional food ingredient by incorporation of grape pomace extract. | Higher bioactive content and better physical properties. | [24] | |
| Cosmetic Industry | Evaluation of the potential use of grape pomace extract in personal-care products | The extract presented appropriate characteristics to be incorporated in cosmetics and good acceptability by consumers | [25] |
| Comparison between a cosmetic serum with phytosomes of grapeseed extract and a serum containing grapeseed extract and evaluation of phenolic penetration in skin | The incorporation of phytosomes from grapeseed extract can enhance the penetration of phenolic compounds into the skin | [26] | |
| Incorporation of phenolic extract from grape pomace in sunscreen, and evaluation of their antioxidant, and their photostability, and in vitro sun protection factor | The antioxidant activity was higher in formulations with the extract. The alliance between the extract and UV filters increased the sun protection factor of 81%. | [27] |
| DPPH (% DPPH Inhibition) | DPPH (IC50) (μgsample·mLDPPH−1) | |
|---|---|---|
| Phenolic Extract GP | 90.8 ± 0.8 | 48.9 ± 0.5 |
| Phenolic Extract GS | 87.5 ± 0.5 | 55.9 ± 0.7 |
| GP Oil | 33.7 ± 0.2 | 296.1 ± 0.9 |
| GS Oil | 34.6 ± 0.2 | 283.3 ± 0.9 |
| Microorganism | dhalo (mm) | |||
|---|---|---|---|---|
| Phenolic Extract GP | Phenolic Extract GS | GP Oil | GS Oil | |
| S. aureus | 12.7 ± 0.9 | 12.0 ± 0.0 | ND | ND |
| S. epidermidis | 14.3 ± 0.5 | 12.2 ± 0.2 | ND | ND |
| E. coli | ND | ND | ND | ND |
| Microorganism. | dhalo (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FB | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
| S. aureus | ND | ND | ND | ND | ND | ND | 18.3 ± 0.9 | 20.7 ± 1.7 | ND | ND |
| S. epidermidis | ND | ND | ND | ND | ND | ND | 23.0 ± 0.8 | 23.7 ± 0.9 | ND | ND |
| E. coli | ND | ND | ND | ND | ND | ND | 9.7 ± 0.9 | 12.7 ± 0.9 | ND | ND |
| Ingredients | FB | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | |||||||||||
| Phase A | Ultrapure Water | 74.0 | 73.9 | 73.9 | 73.9 | 73.6 | 73.6 | 73.6 | 73.6 | 73.6 | 73.6 |
| Glycerin | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 | |
| Xanthan Gum | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | |
| Phase B | Coconut Oil | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 |
| Sweet Almond Oil | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | |
| Soy Lecithin | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | - | - | 3.0 | 3.0 | |
| Betaine | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 3.8 | 3.8 | 0.8 | 0.8 | |
| Formulation Stabilizers | BHT | - | 0.1 | - | - | - | - | - | - | - | - |
| Phenolic Extract GS | - | - | 0.1 | - | 0.1 | - | 0.1 | - | 0.1 | - | |
| Phenolic Extract GP | - | - | - | 0.1 | - | 0.1 | - | 0.1 | - | 0.1 | |
| GS Oil | - | - | - | - | 0.1 | 0.1 | 0.1 | 0.1 | - | - | |
| GP Oil | - | - | - | - | - | - | - | - | 0.1 | 0.1 | |
| Orange EO | - | - | - | - | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, S.M.; Santos, L. A Potential Valorization Strategy of Wine Industry by-Products and Their Application in Cosmetics—Case Study: Grape Pomace and Grapeseed. Molecules 2022, 27, 969. https://doi.org/10.3390/molecules27030969
Ferreira SM, Santos L. A Potential Valorization Strategy of Wine Industry by-Products and Their Application in Cosmetics—Case Study: Grape Pomace and Grapeseed. Molecules. 2022; 27(3):969. https://doi.org/10.3390/molecules27030969
Chicago/Turabian StyleFerreira, Sara M., and Lúcia Santos. 2022. "A Potential Valorization Strategy of Wine Industry by-Products and Their Application in Cosmetics—Case Study: Grape Pomace and Grapeseed" Molecules 27, no. 3: 969. https://doi.org/10.3390/molecules27030969
APA StyleFerreira, S. M., & Santos, L. (2022). A Potential Valorization Strategy of Wine Industry by-Products and Their Application in Cosmetics—Case Study: Grape Pomace and Grapeseed. Molecules, 27(3), 969. https://doi.org/10.3390/molecules27030969