Component Analysis and Anti-Colorectal Cancer Mechanism via AKT/mTOR Signalling Pathway of Sanghuangporus vaninii Extracts
Abstract
:1. Introduction
2. Results
2.1. Total Polysaccharide and Flavonoids Contents
2.2. UPLC/Q-TOF-MS Analysis
2.3. The Result of CCK8 Cell Proliferation
2.4. Induction of Apoptosis by the Ethanol Extracts of S. vaninii
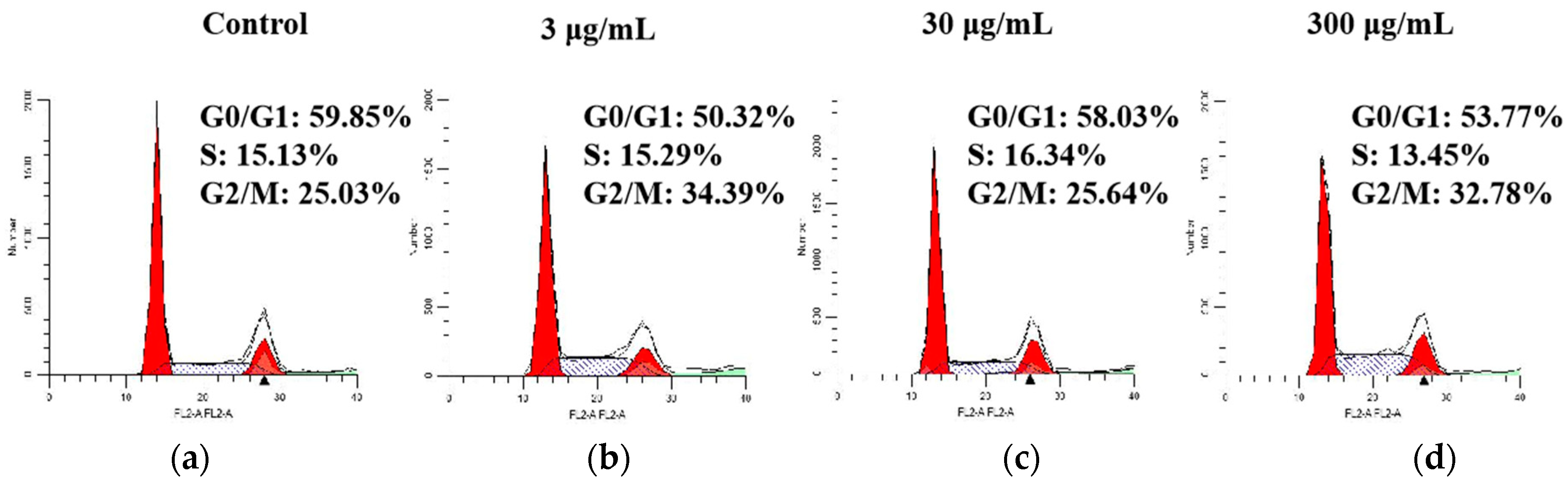
2.5. Effect of SVE60 on Cell Cycle Progression
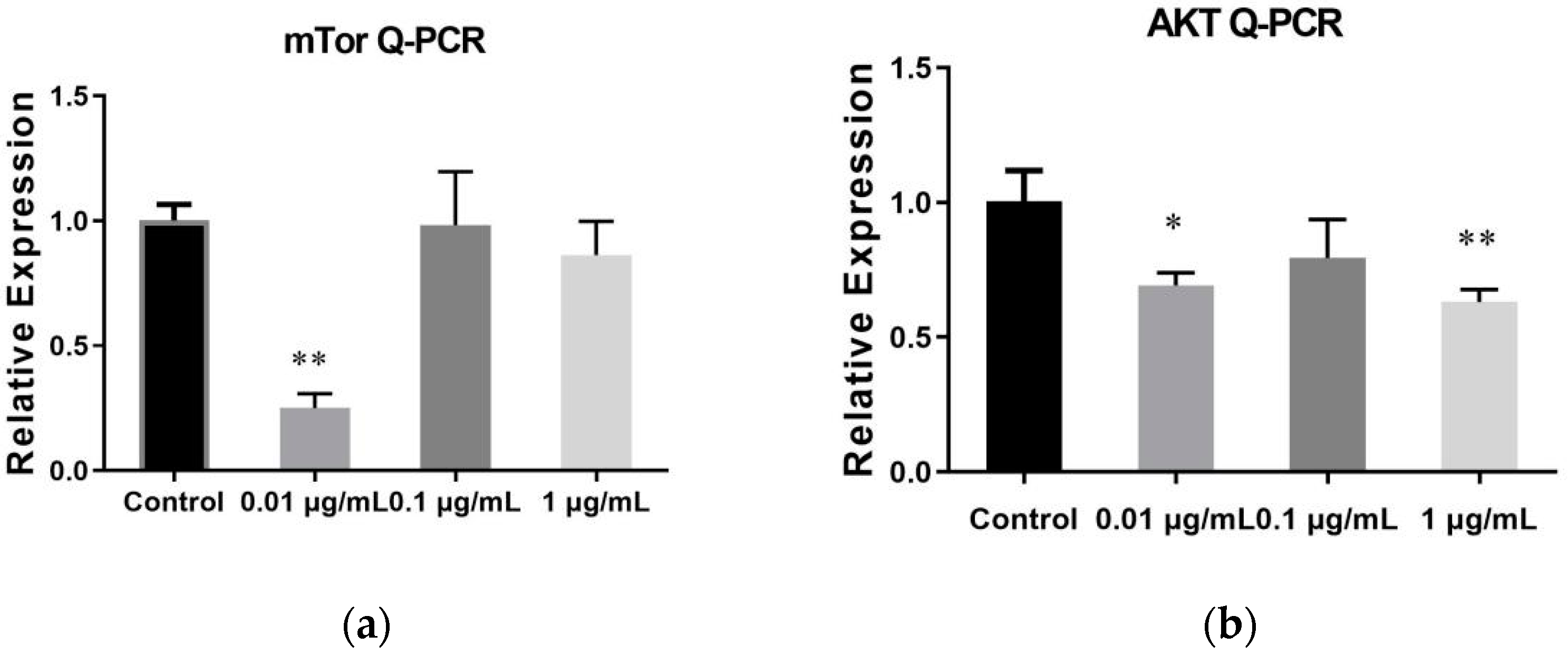
2.6. The Result of qRT-PCR
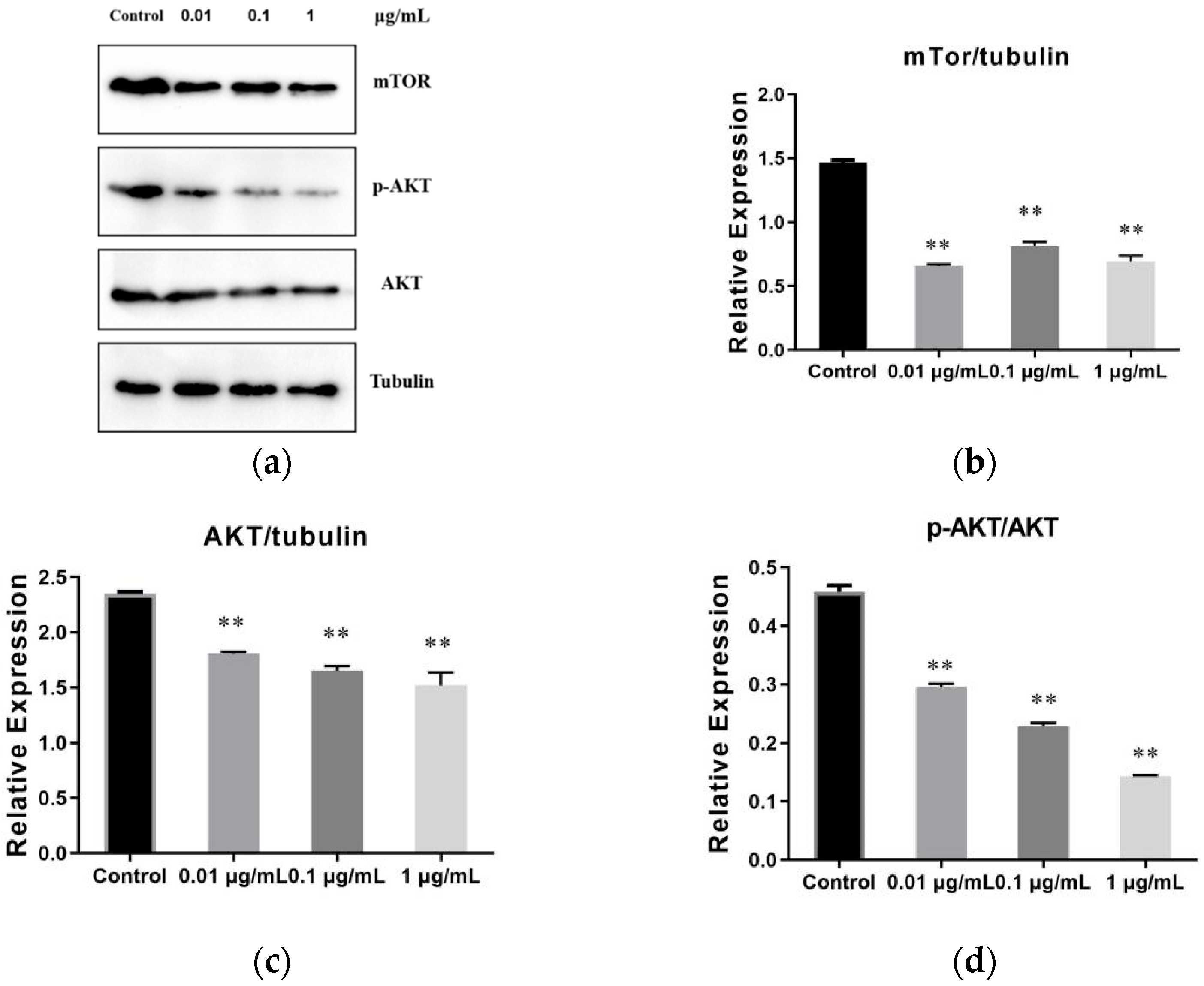
2.7. The Result of SVE60 on the Protein Expression via mTOR Signalling Pathway
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Phellinus Linteus Extracts
3.3. Total Polysaccharide Content
3.4. Total Flavonoids Content
3.5. UPLC-Q-TOF-MS Analytical Conditions
3.6. Cell Culture
3.7. CCK-8 Assay
3.8. Flow Cytometry Cell Apoptosis Analysis
3.9. Cell Cycle Analysis
3.10. Quantitative RT-PCR
3.11. Western Blot
3.12. Statistical Analysis
4. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- De Rosa, M.; Pace, U.; Rega, D.; Costabile, V.; Duraturo, F.; Izzo, P.; Delrio, P. Genetics, diagnosis and management of colorectal cancer (Review). Oncol. Rep. 2015, 34, 1087–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.S.; Lee, J.H.; Lee, S.H. Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin pathways. Mol. Med. Rep. 2015, 12, 3446–3452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- P´opulo, H.; Lopes, J.M.; Soares, P. The mTOR signalling pathway in human cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef]
- Moench, R.; Grimmig, T.; Kannen, V.; Tripathi, S.; Faber, M.N.; Moll, E.-M.; Chandraker, A.; Lissner, R.; Germer, C.-T.; Waaga-Gasser, A.M.; et al. Exclusive inhibition of PI3K/Akt/mTOR signaling is not sufficient to prevent PDGF-mediated effects on glycolysis and proliferation in colorectal cancer. Oncotarget 2016, 7, 68749–68767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demir, S.; Turan, I.; Aliyazicioglu, Y.; Kilinc, K.; Yaman, S.O.; Demir, E.A.; Arslan, A.; Mentese, A.; Deger, O. Morus RubraExtract Induces Cell Cycle Arrest and Apoptosis in Human Colon Cancer Cells through Endoplasmic Reticulum Stress and Telomerase. Nutr. Cancer 2017, 69, 74–83. [Google Scholar] [CrossRef]
- Chen, W.; Tan, H.; Liu, Q.; Zheng, X.; Zhang, H.; Liu, Y.; Xu, L. A Review: The Bioactivities and Pharmacological Applications of Phellinus linteus. Molecules 2019, 24, 1888. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Tian, T.; Miao, H.; Zhao, Y.-Y. Traditional uses, fermentation, phytochemistry and pharmacology of Phellinus linteus: A review. Fitoterapia 2016, 113, 6–26. [Google Scholar] [CrossRef]
- Ikekawa, T.; Nakanishi, M.; Uehara, N.; Chihara, G.; Fukuoka, F. Antitum or action of some basidiomycetes, especially Phellinus linteus. GANN Jpn. J. Cancer Res. 1968, 59, 155–157. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, G.; Yu, M.; Wang, X. Progress in the study of composition and mechanism of antitumor effect of Phellinus igniarius. China Med. Her. 2016, 13, 39–42. [Google Scholar]
- Yoshioka, Y.; Emori, M.; Ikekawa, T.; Fukuoka, F. Isolation, purification, and structure of components from acidic polysaccharides of pleurotus ostreatus (Fr.) Quél. Carbohydr. Res. 1975, 43, 305–320. [Google Scholar] [CrossRef]
- Nakamura, T.; Fukuda, M.; Matsugo, S.; Uzuka, Y. Analysis of mitochondrial DNA restriction fragment length polymorphisms for examining genetic variability among isolates of Phellinus linteus. Mycoscience 2002, 43, 443–445. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef]
- Li, G.; Kim, D.-H.; Kim, T.-D.; Park, B.-J.; Park, H.-D.; Park, J.-I.; Na, M.-K.; Kim, H.-C.; Hong, N.-D.; Lim, K.; et al. Protein-bound polysaccharide from Phellinus linteus induces G2/M phase arrest and apoptosis in SW480 human colon cancer cells. Cancer Lett. 2004, 216, 175–181. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, N.; Yu, S. Research progress on antitumor effects and mechanisms of Phellinus. China J. Chin. Mater. Medica 2014, 39, 4165–4168. [Google Scholar]
- Zhang, C. Hypothesis proved…citric acid (citrate) does improve cancer: A case of a patient suffering from medullary thyroid cancer. Med. Hypotheses 2009, 73, 268–272. [Google Scholar]
- Tanaka, T.; Tanaka, T.; Tanaka, M. Potential Cancer Chemopreventive Activity of Protocatechuic Acid. J. Exp. Clin. Med. 2011, 3, 27–33. [Google Scholar] [CrossRef]
- Guo, D.; Dou, D.; Ge, L.; Huang, Z.; Wang, L.; Gu, N. A caffeic acid mediated facile synthesis of silver nanoparticles with powerful anti-cancer activity. Colloids Surf. B Biointerfaces 2015, 134, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhao, L.; Zhu, Y.; Wu, J.; Hao, C.; Song, S.; Shi, W. Optimization of culture medium for Sanghuangporus vaninii and a study on its therapeutic effects on gout. Biomed. Pharmacother. 2021, 135, 111194. [Google Scholar] [CrossRef]
- Liu, H.-K.; Tsai, T.-H.; Chang, T.-T.; Chou, C.-J.; Lin, L.-C. Lanostane-triterpenoids from the fungus Phellinus gilvus. Phytochemistry 2009, 70, 558–563. [Google Scholar] [CrossRef]
- Wang, G.J.; Tsai, T.-H.; Chang, T.-T.; Chou, C.-J.; Lin, L.-C. Lanostanes from Phellinus igniarius and Their iNOS Inhibitory Activities. Planta Med. 2009, 75, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, A.; Itoh, S.; Tanaka, R.; Kato, S.; Haruna, M.; Kishimoto, K.; Hirayama, H.; Goda, Y.; Mizukami, H.; Ogihara, Y. Identification of novel substituted fused aromatic compounds, meshimakobnol A and B, from natural Phellinus linteus fruit body. Tetrahedron Lett. 2004, 30, 5931–5933. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.-J.; Mo, S.-Y.; Li, S.; Yang, Y.C.; Shi, J.-G. Phelligridimer A, a Highly Oxygenated and Unsaturated 26-Membered Macrocyclic Metabolite with Antioxidant Activity from the Fungus Phellinus igniarius. Org. Lett. 2005, 21, 4733–4736. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-C.; Kuo, P.-C.; Hwang, T.-L.; Chan, Y.-Y.; Chen, C.-H.; Wu, T.-S. Three novel sesquiterpenes from the mycelium of Phellinus linteus. Tetrahedron Lett. 2013, 54, 3332–3335. [Google Scholar] [CrossRef]
- Min, B.-S.; Yun, B.-S.; Lee, H.-K.; Jung, H.-J.; Jung, H.-A.; Choi, J.-S. Two novel furan derivatives from Phellinus linteus with anti-complement activity. Bioorg. Med. Chem. Lett. 2006, 16, 3255–3257. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-F.; Ding, R.; Tan, R.; Liu, J.; Hu, E.-M.; Li, C.-Y.; Liang, G.-Y.; Yi, P. Sesquiterpenes from cultures of the fungus Phellinus igniarius and their Cytotoxicities. Fitoterapia 2020, 140, 104415. [Google Scholar] [CrossRef] [PubMed]
- Norkowska, M.; Myszka, H.; Cyman, M.; Grzywacz, D.; Trzybiński, D.; Sikorski, A.; Liberek, B. 2,3,4,6-tetra-O-Acetyl-D-Gluconic Acid: Crystal Structure and Application in the Synthesis of N-(D-gluconyl) Derivatives of D-Glucosamine. J. Carbohydr. Chem. 2014, 33, 33–47. [Google Scholar] [CrossRef]
- Lv, G.; Song, T.; Cai, W.; Zhang, Z. Comparative study of chemical components and antioxidant activities of wild Sanghuangporus sanghuang and Sanghuangporus vaninii. Mycosystema 2021, 40, 1–11. [Google Scholar]
- Zhao, T.; Wang, Z.; Zhang, N.; Su, X.; Liu, J.; Li, P.; Wang, C. Rapid Identification on Chemical Constituents of Phellinus igniarius by UPLC-Q-TOF-MSE Combined with UNIFI Platform. Spec. Wild Econ. Anim. Plant Res. 2018, 1, 20–25. [Google Scholar]
- Zhu, L.; Cui, B. Progress on the Studies of Medicinal Mushrooms“Sanghuang”Group. J. Fungal Res. 2016, 14, 201–209. [Google Scholar]
- Zan, L.; Bao, H.; Li, D. Review on Polyphenol Components from Medicinal Fungi Sanghuang and Their Biological Activity. Nat. Prod. Res. Dev. 2016, 28, 147–155. [Google Scholar]
- Kitagawa, I.; Baek, N.I.; Kawashima, K.; Yokokawa, Y.; Yoshikawa, M.; Ohashi, K.; Shibuya, H. Indonesian Medicinal Plants, XV.1) Chemical Structures of Five New Resin-Glycosides, Merremosides a, b, c, d and e, from the Tuber of Merrremia mammosa (Convolvulaceae). Chem. Pharm. Bull. 1996, 44, 1680–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Tu, F.-J.; Yu, H.-Y.; Wang, N.-L.; Wang, Z.; Yao, X.-S. Copacamphane, Picrotoxane and Cyclocopacamphane Sesquiterpenes from Dendrobium nobile. Chem. Pharm. Bull. 2008, 56, 854–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohyama, N.; Cai, W.-H.; Kawakami, S.; Sugimoto, S.; Matsunami, K.; Otsuka, H. Symplocosins C-P: Fourteen Triterpene Saponins from the Leaves of Symplocos cochinchinensis var. Philippinensis. Chem. Pharm. Bull. 2020, 68, 1090–1099. [Google Scholar] [CrossRef]
- Vassallo, A.; Pesca, M.; Ambrosio, L.; Malafronte, N.; Melle, N.D.; Piaz, F.D.; Severino, L. Antiproliferative Oleanane Saponins from Dizygotheca Elegantissima. Nat. Prod. Commun. 2012, 7, 1427–1430. [Google Scholar] [CrossRef] [Green Version]
- Matsui, Y.; Kobayashi, K.; Masuda, H.; Kigoshi, H.; Akao, M.; Sakurai, H.; Kumagai, H. Quantitative Analysis of Saponins in a Tea-Leaf Extract and Their Antihypercholesterolemic Activity. Biosci. Biotechnol. Biochem. 2009, 73, 1513–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avula, B.; Bae, J.; Wang, Y.; Wang, M.; Osman, A.G.; Smith, K.; Yuk, J.; Ali, Z.; Plumb, R.; Isaac, G.; et al. Chemical profiling and characterization of phenolic acids, flavonoids, terpene glycosides from Vangueria agrestis using ultra-high-performance liquid chromatography/ion mobility quadrupole time-of-flight mass spectrometry and metabolomics approach. Biomed. Chromatogr. 2020, 34, e4840. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Uddin, G.; Siddiqui, B.; Khan, A.; Farooq, U.; Khan, F.A.; Bukhari, S.M.; Khan, S.B. Bioassay-guided isolation of novel and selective urease inhibitors from Diospyros lotus. Chin. J. Nat. Med. 2017, 15, 865–870. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, X.-T.; Wang, L.; Zhang, X.-Q.; Wang, Y.; Chen, S.-B.; Xiao, P.-G.; Ye, W.-C. Four New Cycloartane (=9,19-Cyclolanostane) Saponins from the Aerial Parts ofThalictrum fortunei. Helv. Chim. Acta 2008, 91, 1961–1966. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourhia, M.; Laasri, F.E.; Aourik, H.; Boukhris, A.; Ullah, R.; Bari, A.; Ali, S.S.; El Mzibri, M.; Benbacer, L.; Gmouh, S. Antioxidant and Antiproliferative Activities of Bioactive Compounds Contained in Rosmarinus officinalis Used in the Mediterranean Diet. Evid.-Based Complement. Altern. Med. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhry, F.; Kawai, H.; Johnson, K.W.; Narula, N.; Shekhar, A.; Chaudhry, F.; Nakahara, T.; Tanimoto, T.; Kim, D.; Adapoe, M.K.; et al. Molecular Imaging of Apoptosis in Atherosclerosis by Targeting Cell Membrane Phospholipid Asymmetry. J. Am. Coll. Cardiol. 2020, 76, 1862–1874. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, M.R.; Khan, A.; Khan, R. In vitro anticancer activity of extracted oil from Parrotiopsis jacquemontiana (Decne) Rehder. Phytomedicine 2021, 91, 153697. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Wei, W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2021, 32, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Leyva, A.; Quintana, A.; Sánchez, M.; Rodríguez, E.N.; Cremata, J.; Sánchez, J.C. Rapid and sensitive anthroneesulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: Method development and validation. Biologicals 2008, 36, 134–141. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.-C.; Zhou, L.-W.; Cui, B.-K.; Chen, Y.-Q.; Decock, C. Current advances in Phellinus sensu lato: Medicinal species, functions, metabolites and mechanisms. Appl. Microbiol. Biotechnol. 2010, 87, 1587–1593. [Google Scholar] [CrossRef]
- Yang, N.-C.; Wu, C.-C.; Liu, R.H.; Chai, Y.-C.; Tseng, C.Y. Comparing the functional components, SOD-like activities, antimutagenicity, and nutrient compositions of Phellinus igniarius and Phellinus linteus mushrooms. J. Food Drug Anal. 2016, 24, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Fotsis, T.; Pepper, M.S.; Aktas, E.; Breit, S.; Rasku, S.; Adlercreutz, H.; Wähälä, K.; Montesano, R.; Schweigerer, L. Flavonoids, dietary inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997, 57, 2916–2921. [Google Scholar]
- Song, T.-Y.; Lin, H.-C.; Yang, N.-C.; Hu, M.-L. Antiproliferative and antimetastatic effects of the ethanolic extract of Phellinus igniarius (Linnearus: Fries) Quelet. J. Ethnopharmacol. 2008, 115, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Holme, J.A.; Gorria, M.; Arlt, V.M.; Øvrebø, S.; Solhaug, A.; Tekpli, X.; Landvik, N.E.; Huc, L.; Fardel, O.; Lagadic-Gossmann, D. Different mechanisms involved in apoptosis following exposure to benzo[a]pyrene in F258 and Hepa1c1c7 cells. Chem. Interact. 2007, 167, 41–55. [Google Scholar] [CrossRef] [PubMed]
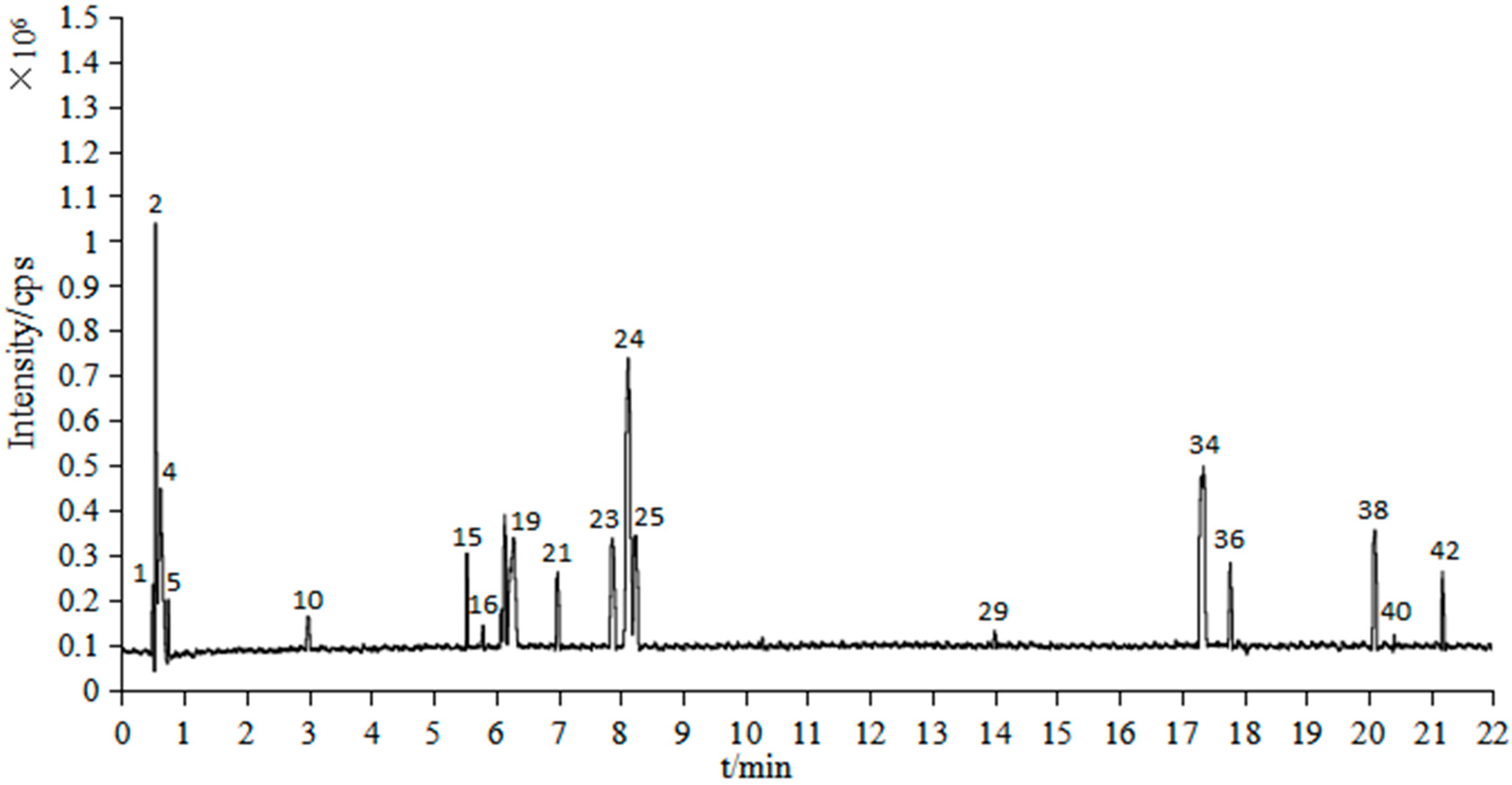



| Peak No. | tR (min) | Identification | Formula | Mass (m/z) | Cacl. Mass (m/z) | mDa | Fragments |
|---|---|---|---|---|---|---|---|
| 1 | 0.50 | 2-Carboxylbenzaldehyde | C8H6O3 | 151.0358 | 151.0395 | −3.7 | 151,128,110 |
| 2 | 0.55 | 8-hydroxyl-5-O-β-D-Glucopyranosylpsoralen | C17H16O10 | 381.0803 | 381.0822 | −1.9 | 381,365,353,258,104 |
| 3 | 0.55 | 7-(α-D-Glucopyranosyloxy)-2,3,4,5,6-pentahydroxyheptanoic acid | C13H24O13 | 387.1141 | 387.1139 | 0.2 | 387,341,245,181,129 |
| 4 | 0.62 | 2,3,4,5-Tetra-O-acetylhexonic acid | C14H20O11 | 365.1058 | 365.1084 | −2.6 | 365,229,205,175,124 |
| 5 | 0.75 | Adenosine | C10H13O4N5 | 268.1047 | 268.1046 | 0.1 | 268,245,229,136,124 |
| 6 | 0.75 | Citric acid | C6H8O7 | 191.0190 | 191.0192 | −0.2 | 191,173,128,111 |
| 7 | 1.34 | Protocatechuic acid | C7H6O4 | 153.0183 | 153.0188 | −0.5 | 153,109 |
| 8 | 2.01 | Protocatechuic aldehyde | C7H6O3 | 137.0235 | 137.0239 | −0.4 | 137,136 |
| 9 | 2.57 | Caffeic acid | C9H8O4 | 179.0342 | 179.0344 | −0.2 | 179,151,135,113 |
| 10 | 2.99 | Ethyl 6-hydroxy-1-cyclohexene-1-carboxylate | C9H14O3 | 171.0999 | 171.1021 | −2.2 | 229,171,158,138 |
| 11 | 3.78 | 2-(2-{2-[2-(2-Methoxyphenoxy) ethoxy]ethoxy} Ethoxy)ethanol | C15H24O6 | 299.1491 | 299.1495 | −0.4 | 299,249,207,147,113 |
| 12 | 4.18 | Osmundacetone | C10H10O3 | 177.0551 | 177.0552 | −0.1 | 177,161,133 |
| 13 | 4.89 | Hispidin | C13H10O5 | 245.0449 | 245.045 | −0.1 | 245,159,113 |
| 14 | 5.33 | Sternbin | C16H14O6 | 301.0706 | 301.0712 | −0.6 | 301,257,249,179,113 |
| 15 | 5.55 | 2,6-bis[3-(3-tert-butyl-2-hydroxy-5-methylphenyl)-3-tricyclo[5.2.1.02,6]decanyl]-4-methylphenol | C49H64O3 | 701.4937 | 701.4934 | 0.3 | 701,680,340,229,138 |
| 16 | 5.80 | Phelligridimer A or isomer | C52H32O20 | 977.1552 | 977.1565 | −1.3 | 977,301,245,229,142 |
| 17 | 5.86 | 4,4′-[2,7-Naphthalenediylbis(oxy)] diphthalic acid | C26H16O10 | 487.0648 | 487.0665 | −1.7 | 487,463,259,181,113 |
| 18 | 6.22 | Davallialactone | C25H20O9 | 463.1021 | 463.1029 | −0.8 | 463,379,259,159,113 |
| 19 | 6.29 | Phelligridimer A or isomer | C52H32O20 | 977.1552 | 977.1565 | −1.3 | 977,301,245,229,142 |
| 20 | 6.88 | 4-dimethyl methoxyphenylmethylene malonate | C13H14O5 | 249.0758 | 249.0763 | −0.5 | 249,219,159,113 |
| 21 | 7.00 | Unknown | C49H78O18 | 955.5246 | 955.5266 | −2.0 | 956,423,301,229,149 |
| 22 | 7.12 | Hosenkoside C | C48H82O20 | 977.5359 | 977.5321 | 3.8 | 978,932,113 |
| 23 | 7.89 | Hypholomine B | C26H18O10 | 491.0981 | 491.0978 | 0.3 | 491.301,183,142 |
| 8.01 | Hypholomine B | C26H18O10 | 489.0830 | 489.0822 | 0.8 | 489,445,199,147,113 | |
| 24 | 8.13 | Acetyl-SSa | C44H70O14 | 823.4819 | 823.4844 | −2.5 | 823,423,203,147,138 |
| 25 | 8.24 | 12-O-Acetylpergularin3-O-[β-D-oleandropyranosyl-(1→4)-β-D-canaropyranosyl-(1→4)-β-D-cymaropyranosyl-(1→4)-β-D-cymaropyranoside] | C50H80O18 | 969.5384 | 969.5423 | −3.9 | 970,423,301,229,149 |
| 26 | 8.30 | Unknown | C36H78O21 | 845.4923 | 845.4957 | −3.4 | 845,445,249,130,113 |
| 27 | 8.43 | Muricatin II | C49H84O20 | 991.5508 | 991.5478 | 3.0 | 992,946,113 |
| 28 | 10.43 | Inoseavin A | C25H18O9 | 461.0863 | 461.0873 | −1.0 | 461,377,159,135,113 |
| 29 | 14.01 | Acetyl-SSa | C44H70O14 | 823.4802 | 823.4844 | −4.2 | 823,801,301,229,142 |
| 30 | 14.15 | Unknown | C36H78O21 | 845.4937 | 845.4957 | −2.0 | 845,799,113 |
| 31 | 14.99 | (3β,16β,24S)-cycloartane-3,16,24,25,30-pentol 3,25-di-β-D-glucopyranoside | C42H72O15 | 815.4830 | 815.4793 | 3.7 | 815,363,249,175,113 |
| 32 | 16.37 | Unknown | C61H66O2 | 829.4944 | 829.4985 | −4.1 | 829,786,385,147,113 |
| 33 | 17.26 | 5′,8′-dihydroxy-5,8-dimethoxy-6,6′-dimethyl-7,3′-binaphthyl- 1,4,1′,4′-tetraone | C24H18O8 | 433.0908 | 433.0923 | −1.5 | 433,385,179,147,113 |
| 34 | 17.36 | Unknown | C56H90O23 | 1131.5933 | 1131.5951 | −1.8 | 1,131,407,229,138 |
| 35 | 17.48 | Unknown | C48H98O30 | 1153.6039 | 1153.6065 | −2.6 | 1,154,599,489,113 |
| 36 | 17.79 | Chakasaponin VI | C59H92O26 | 1217.5917 | 1217.5955 | −3.8 | 1,218,301,229,138 |
| 37 | 18.14 | Unknown | C41H80O24 | 955.4695 | 955.4961 | 0.4 | 955,500,334,207,113 |
| 38 | 20.11 | 12-O-Acetyllineolon3-O-[β-D-oleandropyranosyl-(1→4)-β-D-digitoxopyranosyl-(1→4)-β-D-cymaropyranosyl-(1→4)-β-D-cymaropyranoside] | C50H80O18 | 969.5378 | 969.5423 | −4.1 | 970,767,425,229,149 |
| 39 | 20.20 | Muricatin IV | C49H84O20 | 991.5518 | 991.5478 | 4.0 | 992,946,113 |
| 40 | 20.42 | Cladoloside A4 | C53H82O21 | 1055.5393 | 1055.5427 | −3.4 | 1,056,875,301,229 |
| 41 | 20.50 | Unknown | C44H88O26 | 1031.5485 | 1031.5486 | −0.1 | 1,032,992,207,113 |
| 42 | 21.20 | 3β-O-[β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl]-olean-12-en-28-O-[(3-O-acetyl)-α-L-rhamnopyranosyl] ester | C50H80O18 | 969.5368 | 969.5364 | 0.4 | 970,407,301,229,138 |
| 43 | 21.29 | Merremoside c | C49H84O20 | 991.5511 | 991.5478 | 3.3 | 992,946,179,113 |
| Apoptosis Rate | SVE60 | SVE95 | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | 3 μg/mL | 30 μg/mL | 300 μg/mL | Control | 3 μg/mL | 30 μg/mL | 300 μg/mL | |
| Total | 0.8% | 2.8% | 3.0% | 8.3% | 0.9% | 1.9% | 2.6% | 8.2% |
| Early | 0.7% | 2.3% | 2.7% | 7.7% | 0.8% | 2.3% | 2.7% | 7.7% |
| Late | 0.1% | 0.5% | 0.3% | 0.6% | 0.1% | 0.3% | 0.4% | 0.6% |
| Name | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| AKT | ATGAACGACGTAGCCATTGTG | TTGTAGCCAATAAAGGTGCCAT |
| mTOR | ACCGGCACACATTTGAAGAAG | CTCGTTGAGGATCAGCAAGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Duan, W.; Wang, Y.; Chen, L.; Yang, C.; Gu, X.; Xue, Q.; Li, R.; Zhang, Z. Component Analysis and Anti-Colorectal Cancer Mechanism via AKT/mTOR Signalling Pathway of Sanghuangporus vaninii Extracts. Molecules 2022, 27, 1153. https://doi.org/10.3390/molecules27041153
Guo S, Duan W, Wang Y, Chen L, Yang C, Gu X, Xue Q, Li R, Zhang Z. Component Analysis and Anti-Colorectal Cancer Mechanism via AKT/mTOR Signalling Pathway of Sanghuangporus vaninii Extracts. Molecules. 2022; 27(4):1153. https://doi.org/10.3390/molecules27041153
Chicago/Turabian StyleGuo, Shanshan, Wenwen Duan, Yaxin Wang, Liangmian Chen, Chenchen Yang, Xuezhu Gu, Qinghai Xue, Raorao Li, and Zhijie Zhang. 2022. "Component Analysis and Anti-Colorectal Cancer Mechanism via AKT/mTOR Signalling Pathway of Sanghuangporus vaninii Extracts" Molecules 27, no. 4: 1153. https://doi.org/10.3390/molecules27041153
APA StyleGuo, S., Duan, W., Wang, Y., Chen, L., Yang, C., Gu, X., Xue, Q., Li, R., & Zhang, Z. (2022). Component Analysis and Anti-Colorectal Cancer Mechanism via AKT/mTOR Signalling Pathway of Sanghuangporus vaninii Extracts. Molecules, 27(4), 1153. https://doi.org/10.3390/molecules27041153







