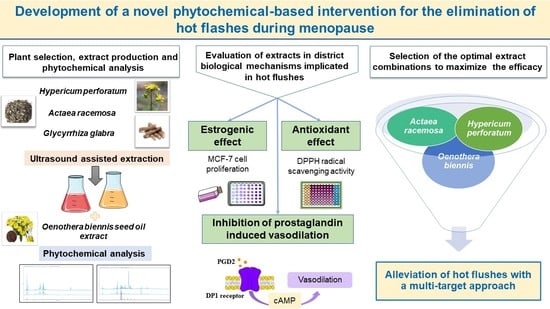
Novel Evidence-Based Combination of Plant Extracts with Multitarget Mechanisms of Action for the Elimination of Hot Flashes during Menopause
Abstract
:1. Introduction
2. Results
2.1. Production of Extracts and Identification of their Phytochemical Footprint
2.2. Estrogenic Effect of the Extracts on the MCF-7 Cells
2.3. Antioxidant Capacity of the Tested Extracts
2.4. Inhibitory Effect of the Extracts on Prostaglandin Induced Vasodilation
2.5. Evaluation of Extract Combinations on the Different Biological Assays
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Plant Materials and Extraction Process
4.3. UPLC-HRMS Analysis of Plant Extracts BC, HP and GG
4.4. High-Performance Liquid Chromatography–Photodiode Array Detection (HPLC–PDA) Analysis of Plant Extracts BC, HP and GG
4.5. Analysis of the Commercial EP Seed Oil
4.6. Evaluation of the Estrogenic Effect of the Extracts on MCF-7 Cells
DPPH Radical Scavenging Assay
4.7. Determination of the Effect of Extracts against Prostaglandin DP1 Receptor Inhibition
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bachmann, G.; Doty, N.J. Menopause. In Principles of Gender-Specific Medicine, 2nd ed.; Academic Press: Salt Lake City, UT, USA, 2010; pp. 449–455. ISBN 9780123742711. [Google Scholar]
- Thurston, R.C.; Joffe, H. Vasomotor Symptoms and Menopause: Findings from the Study of Women’s Health across the Nation. Obstet. Gynecol. Clin. N. Am. 2011, 38, 489–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, R.R. Menopausal hot flashes: Mechanisms, endocrinology, treatment. J. Steroid Biochem. Mol. Biol. 2014, 142, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassarini, J.; Anderson, R.A. New pathways in the treatment for menopausal hot flushes. Lancet 2017, 389, 1775–1777. [Google Scholar] [CrossRef] [Green Version]
- Mehta, J.; Kling, J.M.; Manson, J.A.E. Risks, Benefits, and Treatment Modalities of Menopausal Hormone Therapy: Current Concepts. Front. Endocrinol. (Lausanne) 2021, 12, 564781. [Google Scholar] [CrossRef]
- Kargozar, R.; Azizi, H.; Salari, R. A review of effective herbal medicines in controlling menopausal symptoms. Electron. Physician 2017, 9, 5826–5833. [Google Scholar] [CrossRef] [Green Version]
- Kellogg, D.L.; Zhao, J.L.; Wu, Y.; Johnson, J.M. Nitric oxide and receptors for VIP and PACAP in cutaneous active vasodilation during heat stress in humans. J. Appl. Physiol. 2012, 113, 1512–1518. [Google Scholar] [CrossRef] [Green Version]
- McCord, G.R.; Cracowski, J.L.; Minson, C.T. Prostanoids contribute to cutaneous active vasodilation in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Doshi, S. The role of oxidative stress in menopause. J. Midlife Health 2013, 4, 140. [Google Scholar] [CrossRef]
- Sakavitsi, M.E.; Christodoulou, M.I.; Tchoumtchoua, J.; Fokialakis, N.; Kokkinopoulou, I.K.; Papageorgiou, E.; Argyropoulou, A.; Skaltsounis, L.A.; Halabalaki, M.; Scorilas, A. Comparative HPLC-DAD and UHPLC-ESI(-)-HRMS & MS/MS profiling of Hypericum species and correlation with necrotic cell-death activity in human leukemic cells. Phytochem. Lett. 2017, 20, 481–490. [Google Scholar] [CrossRef]
- Guo, Y.; Yin, T.; Wang, X.; Zhang, F.; Pan, G.; Lv, H.; Wang, X.; Owoicho Orgah, J.; Zhu, Y.; Wu, H. Traditional uses, phytochemistry, pharmacology and toxicology of the genus Cimicifuga: A review. J. Ethnopharmacol. 2017, 209, 264–282. [Google Scholar] [CrossRef]
- Boonmuen, N.; Gong, P.; Ali, Z.; Chittiboyina, A.G.; Khan, I.; Doerge, D.R.; Helferich, W.G.; Carlson, K.E.; Martin, T.; Piyachaturawat, P.; et al. Licorice root components in dietary supplements are selective estrogen receptor modulators with a spectrum of estrogenic and anti-estrogenic activities. Steroids 2016, 105, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galanis, D.; Soultanis, K.; Lelovas, P.; Zervas, A.; Papadopoulos, P.; Galanos, A.; Argyropoulou, K.; Makropoulou, M.; Patsaki, A.; Passali, C.; et al. Protective effect of Glycyrrhiza glabra roots extract on bone mineral density of ovariectomized rats. Biomedicine 2019, 9, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Timoszuk, M.; Bielawska, K.; Skrzydlewska, E. Evening primrose (Oenothera biennis) biological activity dependent on chemical composition. Antioxidants 2018, 7, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Hubing, K.A.; Wingo, J.E.; Ph, D.; Brothers, R.M.; Ph, D.; Coso, D.; Ph, D.; Low, D.A.; Ph, D.; Crandall, C.G.; et al. Nitric oxide synthase inhibition attenuates cutaneous vasodilation during the post-menopausal hot flash. Menopause 2010, 17, 978–982. [Google Scholar] [CrossRef] [Green Version]
- Cagnacci, A.; Venier, M. The controversial history of hormone replacement therapy. Medicina 2019, 55, 602. [Google Scholar] [CrossRef] [Green Version]
- Cvoro, A.; Paruthiyil, S.; Jones, J.O.; Tzagarakis-Foster, C.; Clegg, N.J.; Tatomer, D.; Medina, R.T.; Tagliaferri, M.; Schaufele, F.; Scanlan, T.S.; et al. Selective activation of estrogen receptor-β transcriptional pathways by an herbal extract. Endocrinology 2007, 148, 538–547. [Google Scholar] [CrossRef] [Green Version]
- De Franciscis, P.; Colacurci, N.; Riemma, G.; Conte, A.; Pittana, E.; Guida, M.; Schiattarella, A. A nutraceutical approach to menopausal complaints. Medicina 2019, 55, 544. [Google Scholar] [CrossRef] [Green Version]
- Geller, S.E.; Studee, L. Botanical and Dietary Supplements for Menopausal Symptoms: What Works, What Doesn’t. J. Womens Health 2005, 14, 634–649. [Google Scholar] [CrossRef] [Green Version]
- Kamanna, V.S.; Ganji, S.H.; Kashyap, M.L. The mechanism and mitigation of niacin-induced flushing. Int. J. Clin. Pract. 2009, 63, 1369–1377. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Park, S.J.; Choi, S.; Kim, S.H.; Kang, K.S. In vitro estrogenic and breast cancer inhibitory activities of chemical constituents isolated from Rheum undulatum L. Molecules 2018, 23, 1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oseni, T.; Patel, R.; Pyle, J.; Jordan, V.C. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008, 74, 1656–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodinet, C.; Freudenstein, J. Influence of Cimicifuga racemosa on the proliferation of estrogen receptor-positive human breast cancer cells. Breast Cancer Res. Treat. 2002, 76, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.; Stebbing, J. Black cohosh, hot flushes, and breast cancer. Lancet Oncol. 2015, 16, 137–138. [Google Scholar] [CrossRef]
- Stromeier, S.; Petereit, F.; Nahrstedt, A. Phenolic esters from the rhizomes of Cimicifuga racemosa do not cause proliferation effects in MCF-7 cells. Planta Med. 2005, 71, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.N.; Setzer, W.N. A molecular docking study of phytochemical estrogen mimics from dietary herbal supplements. Silico Pharmacol. 2015, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirmalek, S.A.; Azizi, M.A.; Jangholi, E.; Yadollah-Damavandi, S.; Javidi, M.A.; Parsa, Y.; Parsa, T.; Salimi-Tabatabaee, S.A.; Ghasemzadeh Kolagar, H.; Alizadeh-Navaei, R. Cytotoxic and apoptogenic effect of hypericin, the bioactive component of Hypericum perforatum on the MCF-7 human breast cancer cell line. Cancer Cell Int. 2016, 16, 3. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Rodríguez, M.A.; Zacarías-Flores, M.; Arronte-Rosales, A.; Mendoza-Núnez, V.M. Association between hot flashes severity and oxidative stress among mexican postmenopausal women: A cross-sectional study. PLoS ONE 2019, 14, e0214264. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Nuntanakorn, P.; Jiang, B.; Einbond, L.S.; Yang, H.; Kronenberg, F.; Weinstein, I.B.; Kennelly, E.J. Polyphenolic constituents of Actaea racemosa. J. Nat. Prod. 2006, 69, 314–318. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Rabiee, S.; Larki-Harchegani, A.; Fallahian, A.M.; Moradi, A.; Ataei, S.; Javad, M.T. A comparative study on the effect of “black cohosh” and “evening primrose oil” on menopausal hot flashes. J. Edu. Health Promot. 2018, 1–6. [Google Scholar] [CrossRef]
- Asirvatham-Jeyaraj, N.; Fink, G. Prostaglandin D2-DP1 receptor signaling inhibits sympathetic nerve activity and development of angiotensin II-salt hypertension (874.11). FASEB J. 2014, 28, 874.11. [Google Scholar] [CrossRef]
- Francois, H.; Coffman, T.M. Prostanoids and blood pressure: Which way is up? J. Clin. Investig. 2004, 114, 757–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajirahimkhan, A.; Mbachu, O.; Simmler, C.; Ellis, S.G.; Dong, H.; Nikolic, D.; Lankin, D.C.; Van Breemen, R.B.; Chen, S.N.; Pauli, G.F.; et al. Estrogen Receptor (ER) Subtype Selectivity Identifies 8-Prenylapigenin as an ERβ Agonist from Glycyrrhiza inflata and Highlights the Importance of Chemical and Biological Authentication. J. Nat. Prod. 2018, 81, 966–975. [Google Scholar] [CrossRef] [Green Version]
- Hickl, J.; Argyropoulou, A.; Sakavitsi, M.E.; Halabalaki, M.; Al-Ahmad, A.; Hellwig, E.; Aligiannis, N.; Skaltsounis, A.L.; Wittmer, A.; Vach, K.; et al. Mediterranean herb extracts inhibit microbial growth of representative oral microorganisms and biofilm formation of Streptococcus mutans. PLoS ONE 2018, 13, e0207574. [Google Scholar] [CrossRef]
- Michailidis, D.; Angelis, A.; Nikolaou, P.E.; Mitakou, S.; Skaltsounis, A.L. Exploitation of vitis vinifera, foeniculum vulgare, cannabis sativa and punica granatum by-product seeds as dermo-cosmetic agents. Molecules 2021, 26, 731. [Google Scholar] [CrossRef]
- Panteleon, V.; Marakos, P.; Pouli, N.; Mikros, E.; Andreadou, I. Interactions of a series of novel spiropyranocoumarin derivatives with reactive oxygen species. J. Pharm. Pharmacol. 2003, 55, 1029–1039. [Google Scholar] [CrossRef]
- Panteleon, V.; Kostakis, I.K.; Marakos, P.; Pouli, N.; Andreadou, I. Synthesis and free radical scavenging activity of some new spiropyranocoumarins. Bioorgan. Med. Chem. Lett. 2008, 18, 5781–5784. [Google Scholar] [CrossRef]
- Beaulieu, C.; Guay, D.; Wang, Z.; Leblanc, Y.; Roy, P.; Dufresne, C.; Zamboni, R.; Berthelette, C.; Day, S.; Tsou, N.; et al. Identification of prostaglandin D2 receptor antagonists based on a tetrahydropyridoindole scaffold. Bioorgan. Med. Chem. Lett. 2008, 18, 2696–2700. [Google Scholar] [CrossRef]
- Mitsumori, S.; Tsuri, T.; Honma, T.; Hiramatsu, Y.; Okada, T.; Hashizume, H.; Inagaki, M.; Arimura, A.; Yasui, K.; Asanuma, F.; et al. Synthesis and biological activity of various derivatives of a novel class of potent, selective, and orally active prostaglandin D2 receptor antagonists. 2. 6,6-dimethylbicyclo[3.1.1]heptane derivatives. J. Med. Chem. 2003, 46, 2446–2455. [Google Scholar] [CrossRef]



| Extract Name | DPPH IC50 Value |
|---|---|
| Water extract of HP (HPa) | 148.4 ± 1.0 μg/mL |
| Water/methanol extract of HP (HPb) | 74.7 ± 1.4 μg/mL |
| Water extract of BC (BCa) | 274.0 ± 1.0 μg/mL |
| Water/methanol extract of BC (BCb) | 129.5 ± 1.5 μg/mL |
| Water extract of GG (GGa) | 774.7 ± 1.0 μg/mL |
| Water/methanol extract of GG (GGb) | 1059 ± 1.6 μg/mL |
| Seed oil of EP | No antioxidant effect |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoumani, M.; Nikolaou, P.E.; Argyropoulou, A.; Tseti, I.; Mitakou, S.; Andreadou, I. Novel Evidence-Based Combination of Plant Extracts with Multitarget Mechanisms of Action for the Elimination of Hot Flashes during Menopause. Molecules 2022, 27, 1221. https://doi.org/10.3390/molecules27041221
Tsoumani M, Nikolaou PE, Argyropoulou A, Tseti I, Mitakou S, Andreadou I. Novel Evidence-Based Combination of Plant Extracts with Multitarget Mechanisms of Action for the Elimination of Hot Flashes during Menopause. Molecules. 2022; 27(4):1221. https://doi.org/10.3390/molecules27041221
Chicago/Turabian StyleTsoumani, Maria, Panagiota Efstathia Nikolaou, Aikaterini Argyropoulou, Ioulia Tseti, Sofia Mitakou, and Ioanna Andreadou. 2022. "Novel Evidence-Based Combination of Plant Extracts with Multitarget Mechanisms of Action for the Elimination of Hot Flashes during Menopause" Molecules 27, no. 4: 1221. https://doi.org/10.3390/molecules27041221
APA StyleTsoumani, M., Nikolaou, P. E., Argyropoulou, A., Tseti, I., Mitakou, S., & Andreadou, I. (2022). Novel Evidence-Based Combination of Plant Extracts with Multitarget Mechanisms of Action for the Elimination of Hot Flashes during Menopause. Molecules, 27(4), 1221. https://doi.org/10.3390/molecules27041221