Elucidation of the Transport Mechanism of Puerarin and Gastrodin and Their Interaction on the Absorption in a Caco-2 Cell Monolayer Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. HPLC Analysis
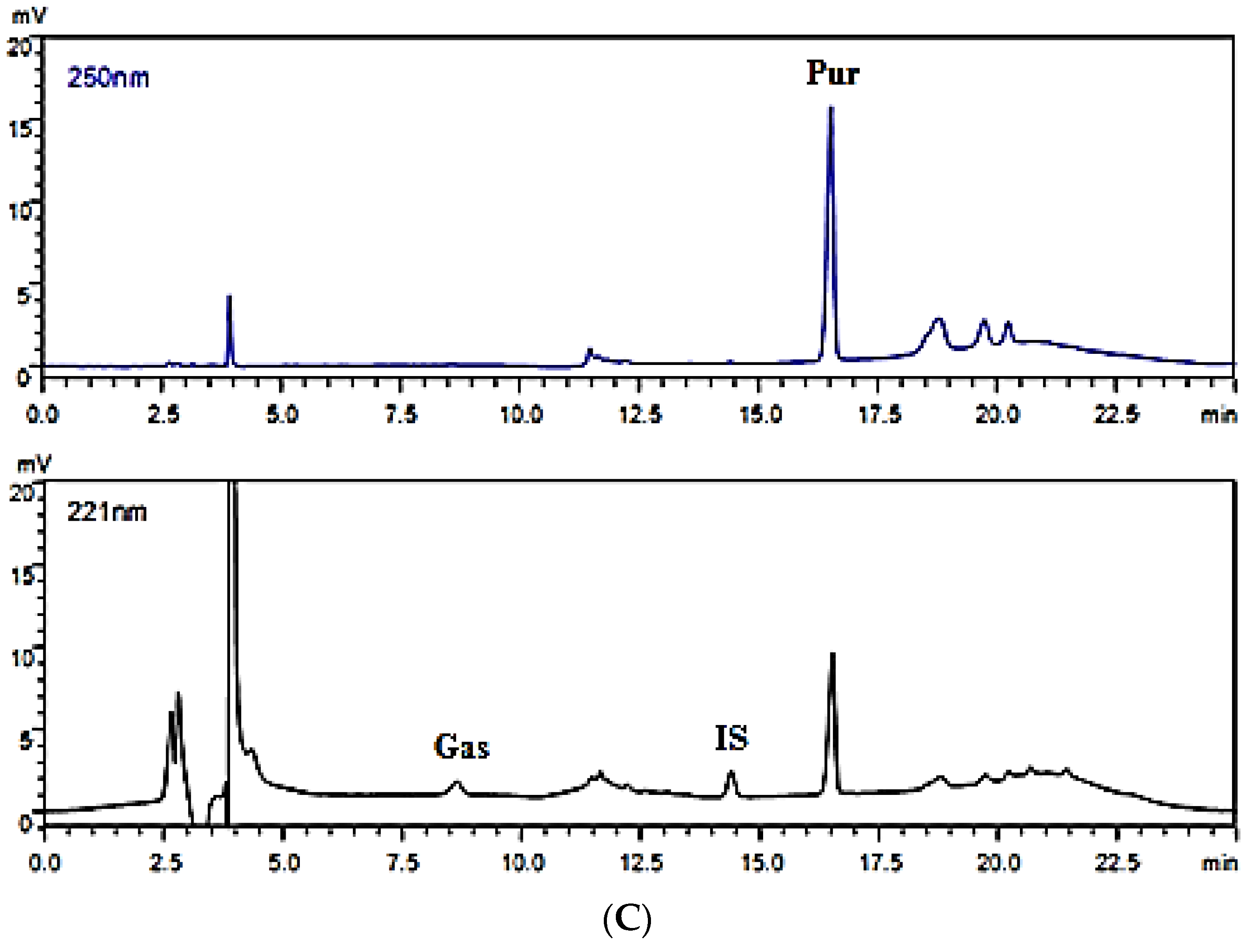
2.3.1. Instruments and Chromatographic Conditions
2.3.2. Preparation of Working Solutions and Internal Standard (IS) Solution
2.3.3. Preparation of Calibration Standard Samples
2.3.4. Preparation of Cell Samples
2.4. Method Validation
2.4.1. Specificity
2.4.2. Linearity
2.4.3. Precision
2.4.4. Accuracy
2.5. Data Analysis
2.5.1. Transmembrane Resistance
2.5.2. Apparent Permeability Coefficient
3. Result
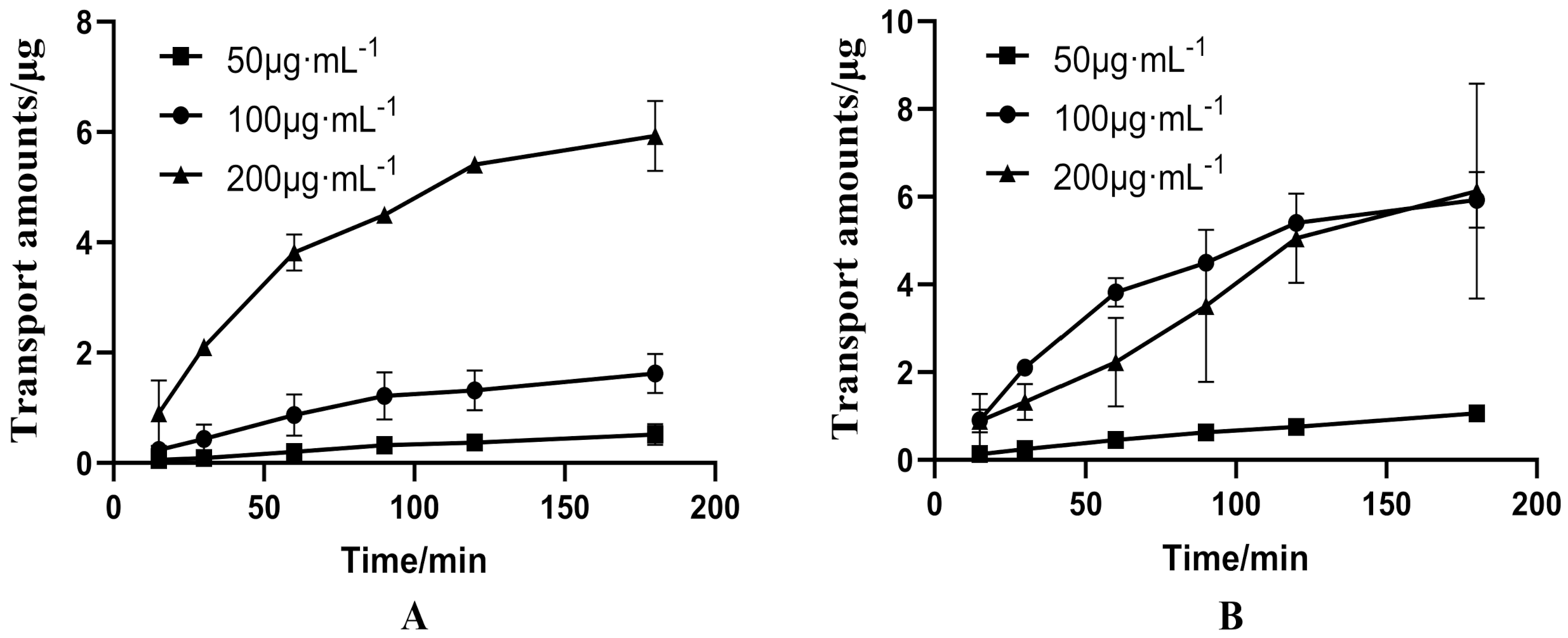
3.1. Bidirectional Transport of PUR and GAS in Caco-2 Cell Model
3.1.1. Bidirectional Transport of Different Concentrations of PUR
3.1.2. Bidirectional Transport of GAS
3.2. Effects of Verapamil and Cyclosporin on Transport of PUR and GAS in Caco-2 Cell Model
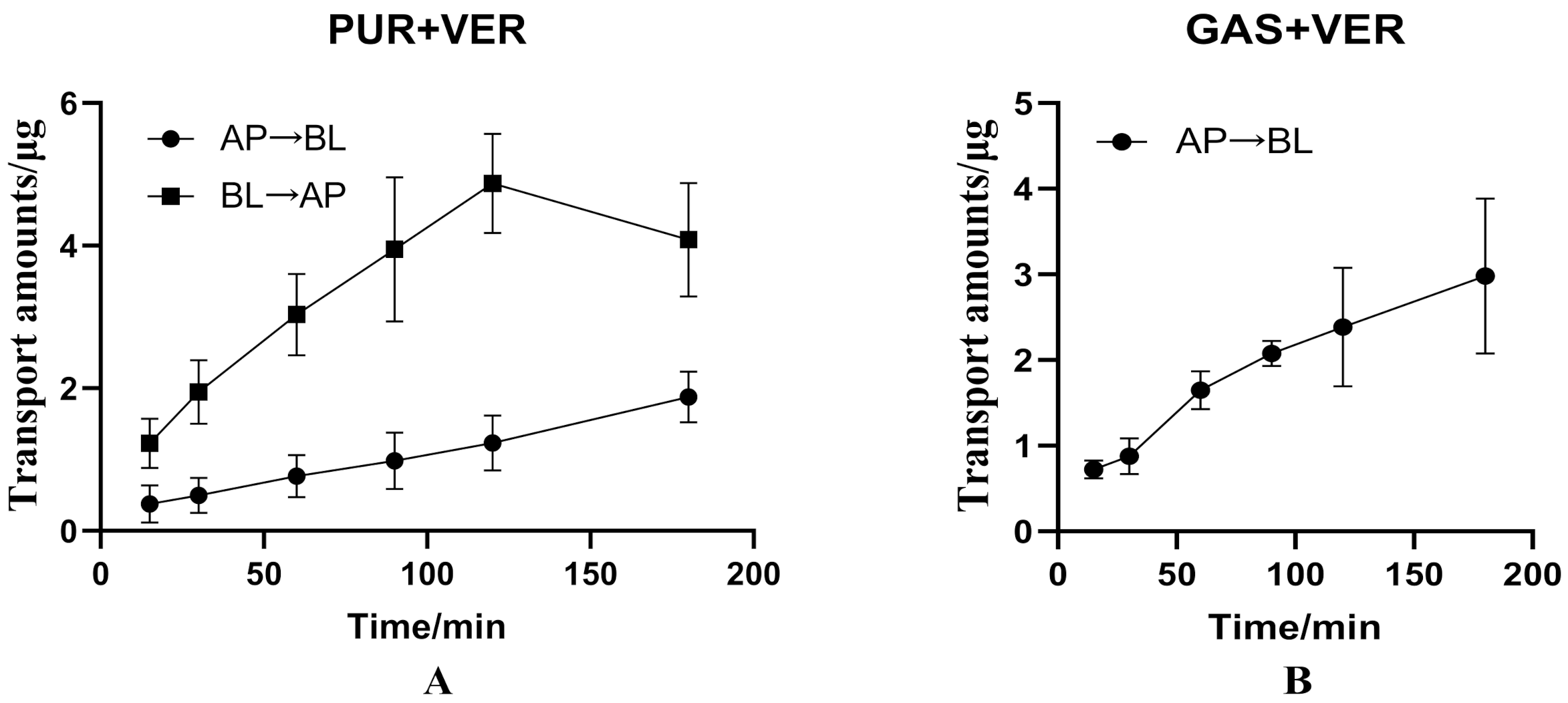
3.2.1. Effects of Verapamil on Bidirectional Transport of PUR
3.2.2. Effects of Verapamil on Transport of GAS from AP to BL Side
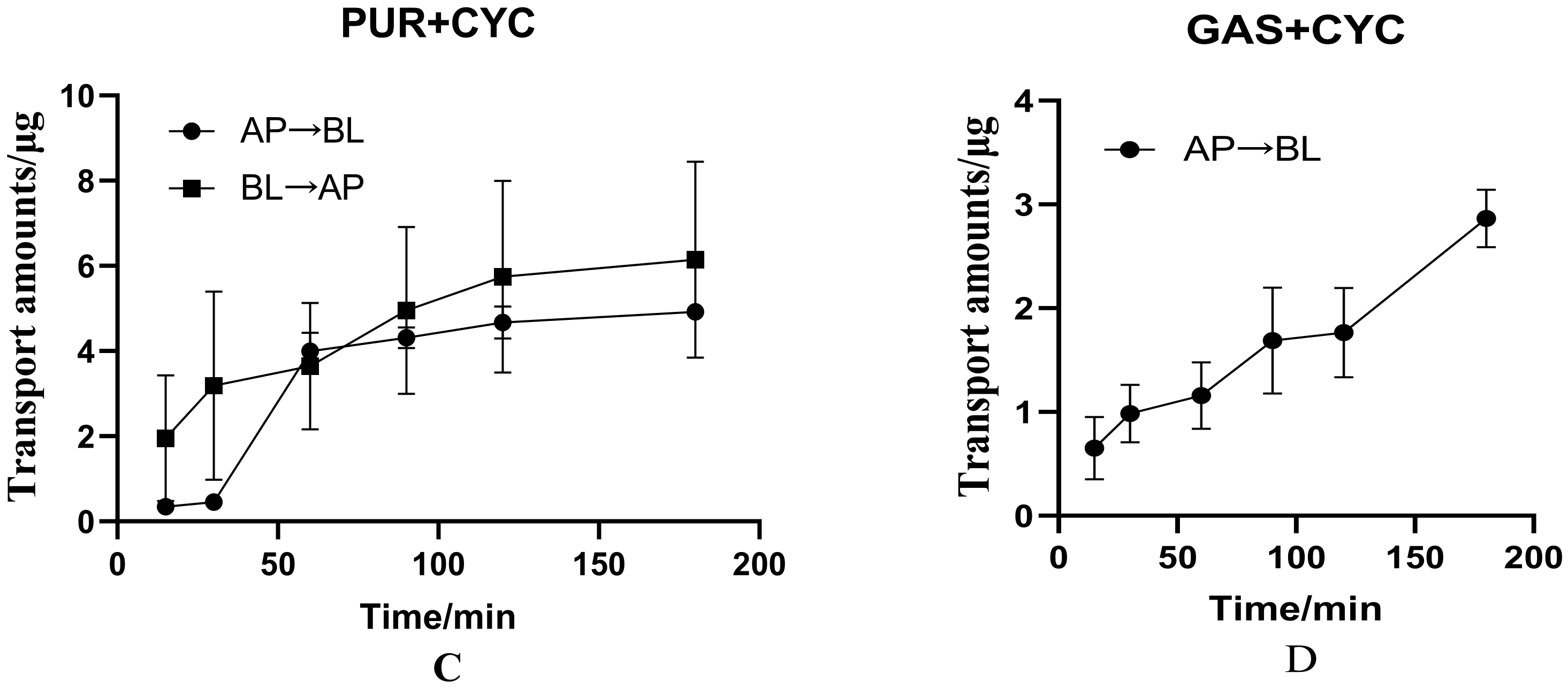
3.2.3. Effects of Cyclosporin on Bidirectional Transport of PUR
3.2.4. Effects of Cyclosporin on Transport of GAS from AP to BL Side
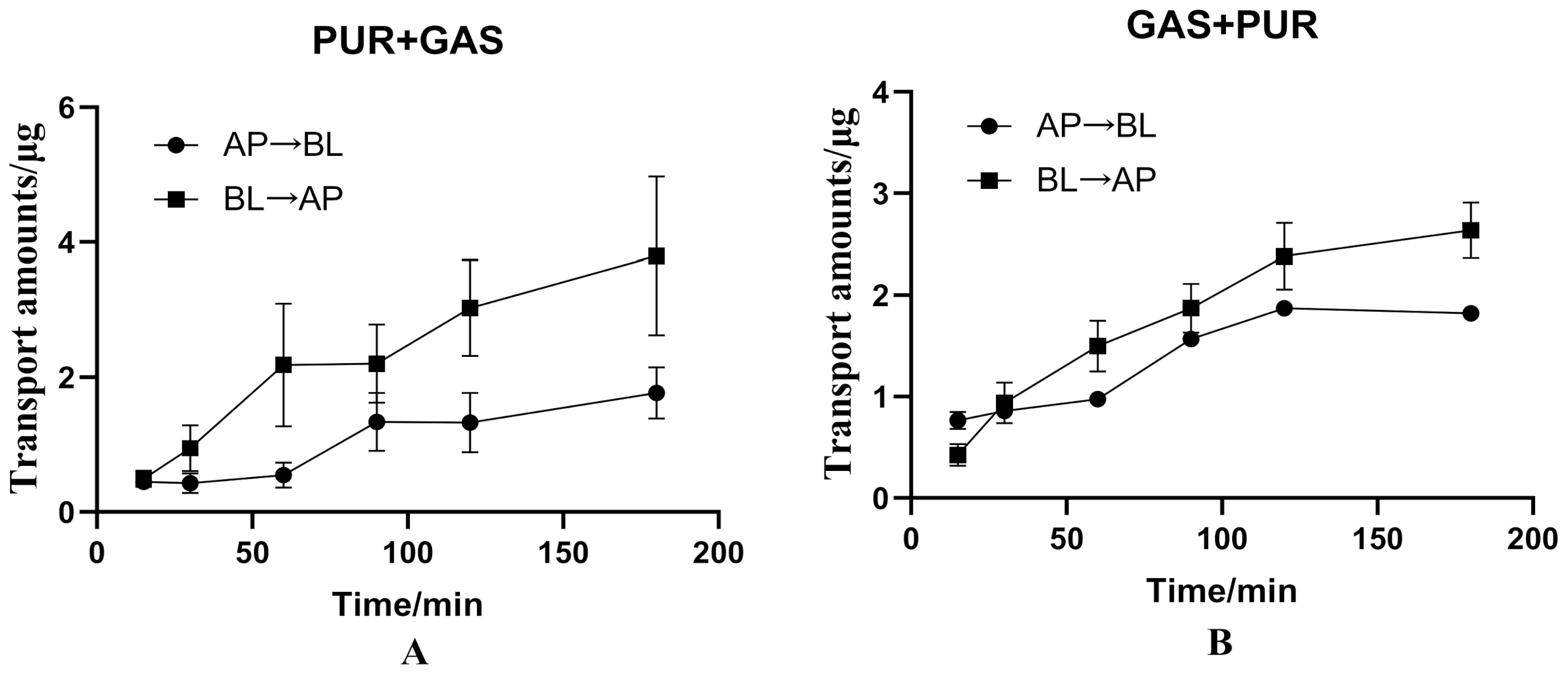
3.3. The Interaction between PUR and GAS on Bidirectional Transport in the Caco-2 Cell Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Maji, A.K.; Pandit, S.; Banerji, P.; Banerjee, D. Pueraria tuberosa: A review on its phytochemical and therapeutic potential. Nat. Prod. Res. 2014, 28, 2111–2127. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Z.-R.; Guo, X.-F.; Song, J.; Zhang, J.-X.; Wang, J.; Dong, W.-G. Synergistic effects of puerarin combined with 5-fluorouracil on esophageal cancer. Mol. Med. Rep. 2014, 10, 2535–2541. [Google Scholar] [CrossRef]
- Wei, S.-Y.; Chen, Y.; Xu, X.-Y. Progress on the pharmacological research of puerarin: A review. Chin. J. Nat. Med. 2014, 12, 407–414. [Google Scholar] [CrossRef]
- Ahmad, B.; Khan, S.; Liu, Y.; Xue, M.; Nabi, G.; Kumar, S.; Alshwmi, M.; Qarluq, A.W. Molecular Mechanisms of Anticancer Activities of Puerarin. Cancer Manag. Res. 2020, 12, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Chen, X.; Li, N.; Jia, W.; Wang, N.; Hou, B.; Yang, H.; Zhang, L.; Qiang, G.; Yang, X.; et al. Puerarin ameliorates skeletal muscle wasting and fiber type transformation in STZ-induced type 1 diabetic rats. Biomed. Pharmacother. 2020, 133, 110977. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yuan, R.; Chen, X.; Xin, Q.; Wang, Y.; Shang, X.; Cong, W.; Chen, K. Puerarin Reduces Blood Pressure in Spontaneously Hypertensive Rats by Targeting eNOS. Am. J. Chin. Med. 2019, 47, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.-G.; Feng, Z.-T.; Wang, J.-F.; Fu, Y.; Yang, S.-B.; Zhang, S.-Z.; Huang, W.-F.; Xiong, L.; Zhou, H.-J.; Tao, W. Puerarin protects rat brain against ischemia/reperfusion injury by suppressing autophagy via the AMPK-mTOR-ULK1 signaling pathway. Neural Regen. Res. 2018, 13, 989–998. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, X.; Dong, G. Effects of verapamil on the pharmacokinetics of puerarin in rats. Xenobiotica 2019, 49, 1178–1182. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Wang, H.; Feng, L. Effects of glycyrrhizin on the pharmacokinetics of puerarin in rats. Xenobiotica 2018, 48, 1157–1163. [Google Scholar] [CrossRef]
- Liu, C.-S.; Liang, X.; Wei, X.-H.; Chen, F.-L.; Tang, Q.-F.; Tan, X.-M. Comparative pharmacokinetics of major bioactive components from Puerariae Radix-Gastrodiae Rhizome extracts and their intestinal absorption in rats. J. Chromatogr. B 2019, 1105, 38–46. [Google Scholar] [CrossRef]
- Jin, M.; He, Q.; Zhang, S.; Cui, Y.; Han, L.; Liu, K. Gastrodin Suppresses Pentylenetetrazole-Induced Seizures Progression by Modulating Oxidative Stress in Zebrafish. Neurochem. Res. 2018, 43, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, L.; Yu, J.; Asare, P.F.; Zhao, Y.-Q. Gastrodin Reduces Blood Pressure by Intervening with RAAS and PPARγin SHRs. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, C.-W.; Liu, Z.-Y.; Zhang, F.-L.; Zhang, L.; Li, F.; Liu, S.-Y.; He, J.-Y.; Xiao, Z.-C. Post-stroke gastrodin treatment ameliorates ischemic injury and increases neurogenesis and restores the Wnt/β-Catenin signaling in focal cerebral ischemia in mice. Brain Res. 2019, 1712, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Guo, S.; Cheng, H.; Liu, M.; Wei, P.; Wang, M.; Mao, Y.; Ke, G. Pharmacokinetic comparative study of tetramethylpyrazine and ferulic acid and their compatibility with different concentration of gastrodin and gastrodigenin on blood–stasis migraine model by blood–brain microdialysis method. J. Pharm. Biomed. Anal. 2019, 177, 112885. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-S.; Chiu, S.-C.; Liu, P.-Y.; Wu, S.-N.; Lai, M.-C.; Huang, C.-W. Gastrodin alleviates seizure severity and neuronal excitotoxicities in the rat lithium-pilocarpine model of temporal lobe epilepsy via enhancing GABAergic transmission. J. Ethnopharmacol. 2021, 269, 113751. [Google Scholar] [CrossRef]
- Mei, Z.-G.; Tan, L.-J.; Wang, J.-F.; Li, X.-L.; Huang, W.-F.; Zhou, H.-J. Fermented Chinese formula Shuan-Tong-Ling attenuates ischemic stroke by inhibiting inflammation and apoptosis. Neural Regen. Res. 2017, 12, 425–432. [Google Scholar] [CrossRef]
- Guo, N.; Jiao, L.M.; Yan, D.X. Clinical study on scalp acupuncture and Tianma Gegen Granules in the treatment of posterior cir-culation ischemia vertigo. Chin. J. Integr. Med. Cardiocerebrovase. Dis. 2016, 14, 2741–2743. [Google Scholar] [CrossRef]
- Jiang, L.; Dai, J.; Huang, Z.; Du, Q.; Lin, J.; Wang, Y. Simultaneous determination of gastrodin and puerarin in rat plasma by HPLC and the application to their interaction on pharmacokinetics. J. Chromatogr. B 2013, 915–916, 8–12. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.R.; Cao, W.Y.; Huang, Z.L.; Du, Q.H. Study on Compatibility Rationality of Gastrodin and Puerarin based on Im-proving Microcirculation. World Sci. Technol. Mod. Tradit. Chin. Med. Mater. Medica. 2013, 15, 244–248. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.R.; Lin, J.H.; Cao, W.Y.; Huang, Z.L. Pilot study on DPPH free radical-scavenging activity and solubility of com-bining gastrodin with puerarin. China J. Tradit. Chin. Med. Pharmacy. 2013, 28, 2397–2400. [Google Scholar]
- Liang, X.-L.; Zhao, L.-J.; Liao, Z.-G.; Zhao, G.-W.; Zhang, J.; Chao, Y.-C.; Yang, M.; Yin, R.-L. Transport properties of puerarin and effect of Radix Angelicae Dahuricae extract on the transport of puerarin in Caco-2 cell model. J. Ethnopharmacol. 2012, 144, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, S.-Y.; Lu, Y.; Liu, C.; Tian, Z.-H.; Yang, C.; Wu, H.-C.; Wang, Z. Puerarin transport across a Calu-3 cell monolayer – an in vitro model of nasal mucosa permeability and the influence of paeoniflorin and menthol. Drug Des. Dev. Ther. 2016, 10, 2227–2237. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.; Yuan, F.; Liu, J.; Liu, W.; Feng, J.; Jin, Y.; Tu, L. Fabrication of Fine Puerarin Nanocrystals by Box–Behnken Design to Enhance Intestinal Absorption. AAPS PharmSciTech 2020, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, E.T.; Nascimento, S.F.; Pires, C.L.; Godinho, L.P.; Churro, C.; Moreno, M.J.; Pardal, M.A. Determination of intestinal absorption of the paralytic shellfish toxin GTX-5 using the Caco-2 human cell model. Environ. Sci. Pollut. Res. 2021, 28, 67256–67266. [Google Scholar] [CrossRef]
- Keemink, J.; Bergström, C.A.S. Caco-2 Cell Conditions Enabling Studies of Drug Absorption from Digestible Lipid-Based Formulations. Pharm. Res. 2018, 35, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Antonescu, I.; Rasmussen, K.F.; Neuhoff, S.; Fretté, X.; Karlgren, M.; Bergström, C.A.S.; Nielsen, C.U.; Steffansen, B. The Permeation of Acamprosate Is Predominantly Caused by Paracellular Diffusion across Caco-2 Cell Monolayers: A Paracellular Modeling Approach. Mol. Pharm. 2019, 16, 4636–4650. [Google Scholar] [CrossRef]
- Lu, C.; Fu, K.; Cao, K.; Wei, J.; Zhou, J.; Zhao, D.; Li, N.; Lu, Y.; Chen, X.; Zhang, Y. Permeability and transport mechanism of trihexyphenidyl hydrochloride in Caco-2 cell monolayer model with a validated UPLC-MS/MS method. J. Pharm. Biomed. Anal. 2019, 178, 112924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Duan, Y.; Feng, Y.; Wang, J. Transepithelial Transport Characteristics of the Cholesterol- Lowing Soybean Peptide, WGAPSL, in Caco-2 Cell Monolayers. Molecules 2019, 24, 2843. [Google Scholar] [CrossRef] [Green Version]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport1PII. Adv. Drug Deliv. Rev. 2001, 46, 27–43. [Google Scholar] [CrossRef]
- Nagayasu, M.; Ozeki, K.; Sakurai, Y.; Tsutsui, H.; Onoue, S. Simplified Method to Determine the Efflux Ratio on P-Glycoprotein Substrates Using Three-Compartment Model Analysis for Caco-2 Cell Assay Data. Pharm. Res. 2019, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, W.; Ou, X.; Luo, G.; Xie, Y.; Sun, R.; Wang, Y.; Qi, X.; Hu, M.; Liu, Z.; et al. Breast Cancer Resistance Protein and Multidrug Resistance Protein 2 Determine the Disposition of Esculetin-7-O-Glucuronide and 4-Methylesculetin-7-O-Glucuronide. Drug Metab. Dispos. 2019, 47, 203–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Sun, Y. Efflux mechanism and pathway of verapamil pumping by human P-glycoprotein. Arch. Biochem. Biophys. 2020, 696, 108675. [Google Scholar] [CrossRef]
- Nielsen, S.; Westerhoff, A.M.; Gé, L.G.; Carlsen, K.L.; Pedersen, M.D.L.; Nielsen, C.U. MRP2-mediated transport of etoposide in MDCKII MRP2 cells is unaffected by commonly used non-ionic surfactants. Int. J. Pharm. 2019, 565, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Yamazaki, M. Role of P-Glycoprotein in Pharmacokinetics: Clinical implications. Clin. Pharmacokinet. 2003, 42, 59–98. [Google Scholar] [CrossRef]
- Li, J. Compatibility Rationality Study of Puerarin and Gastrodin Based on Physico Chemical Pharmacological Function Absorption of Drugs; Beijing University of Chinese Medicine, Beijing University of Chinese Medicine: Beijing, China, 2013. [Google Scholar]
- Jin, X.; Luong, T.-L.; Reese, N.; Gaona, H.; Collazo-Velez, V.; Vuong, C.; Potter, B.; Sousa, J.C.; Olmeda, R.; Li, Q.; et al. Comparison of MDCK-MDR1 and Caco-2 cell based permeability assays for anti-malarial drug screening and drug investigations. J. Pharmacol. Toxicol. Methods 2014, 70, 188–194. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Yee, S. In Vitro Permeability Across Caco-2 Cells (Colonic) Can Predict In Vivo (Small Intestinal) Absorption in Man—Fact or Myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef]



| Analyte | Linear Range (μg·mL−1) | Linear Regression Equations | Correlation Coefficient (r) | LLOQs (μg·mL−1) |
|---|---|---|---|---|
| L-PUR | 0.025–2.5 | y = 1.8823x + 0.07998 | 0.9999 | 0.025 |
| H-PUR | 2.5–100 | y = 1.8758x − 0.3433 | 0.9999 | 2.5 |
| L-GAS | 0.1–5 | y = 0.2159x + 0.0002 | 0.9999 | 0.1 |
| H-GAS | 5–100 | y = 0.2107x − 0.1703 | 0.9998 | 5 |
| Groups | Concentration (μg·mL−1) | Papp(AP→BL) (×10−6 cm·s−1) | Papp(BL→AP) (×10−6 cm·s−1) | ER |
|---|---|---|---|---|
| L-PUR | 50 | 1.225 ± 0.600 | 1.766 ± 0.090 | 1.442 ± 0.073 |
| M-PUR | 100 | 1.285 ± 0.332 | 4.539 ± 0.166 | 3.531 ± 0.129 |
| H-PUR | 200 | 1.137 ± 0.391 | 3.017 ± 0.787 | 2.654 ± 0.693 |
| GAS | 100 | 2.407 ± 0.134 | 2.866 ± 0.809 | 1.191 ± 0.336 |
| Groups | Concentration (μg·mL−1) | Papp(AP→BL) (×10−6 cm·s−1) | Papp(BL→AP) (×10−6 cm·s−1) | ER |
|---|---|---|---|---|
| PUR + Ver | 100 | 1.413 ± 0.381 | 3.004 ± 0.724 | 2.126 ± 0.513 |
| PUR + Cyc | 100 | 4.759 ± 0.405 | 4.014 ± 0.565 | 0.843 ± 0.119 |
| GAS + Ver | 100 | 2.647 ± 1.000 | — | — |
| GAS + Cyc | 100 | 2.402 ± 0.130 | — | — |
| Groups | Concentration (μg·mL−1) | Papp(AP→BL) (×10−6 cm·s−1) | Papp(BL→AP) (×10−6 cm·s−1) | ER |
|---|---|---|---|---|
| PUR + GAS | 100 | 1.425 ± 0.412 | 3.108 ± 0.982 | 2.181 ± 0.689 |
| GAS + PUR | 100 | 2.229 ± 0.086 | 2.566 ± 0.306 | 1.151 ± 0.137 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Xiong, Y.; Tu, Y.; Zhang, W.; Zhang, Q.; Nie, P.; Yan, X.; Liu, H.; Liu, R.; Xu, G. Elucidation of the Transport Mechanism of Puerarin and Gastrodin and Their Interaction on the Absorption in a Caco-2 Cell Monolayer Model. Molecules 2022, 27, 1230. https://doi.org/10.3390/molecules27041230
Jiang L, Xiong Y, Tu Y, Zhang W, Zhang Q, Nie P, Yan X, Liu H, Liu R, Xu G. Elucidation of the Transport Mechanism of Puerarin and Gastrodin and Their Interaction on the Absorption in a Caco-2 Cell Monolayer Model. Molecules. 2022; 27(4):1230. https://doi.org/10.3390/molecules27041230
Chicago/Turabian StyleJiang, Li, Yanling Xiong, Yu Tu, Wentong Zhang, Qiyun Zhang, Peng Nie, Xiaojun Yan, Hongning Liu, Ronghua Liu, and Guoliang Xu. 2022. "Elucidation of the Transport Mechanism of Puerarin and Gastrodin and Their Interaction on the Absorption in a Caco-2 Cell Monolayer Model" Molecules 27, no. 4: 1230. https://doi.org/10.3390/molecules27041230





