Performance of Thyme Oil@Na-Montmorillonite and Thyme Oil@Organo-Modified Montmorillonite Nanostructures on the Development of Melt-Extruded Poly-L-lactic Acid Antioxidant Active Packaging Films
Abstract
:1. Introduction
2. Results
2.1. XRD
2.2. FTIR
2.3. TG–DTA
2.4. Tensile Properties
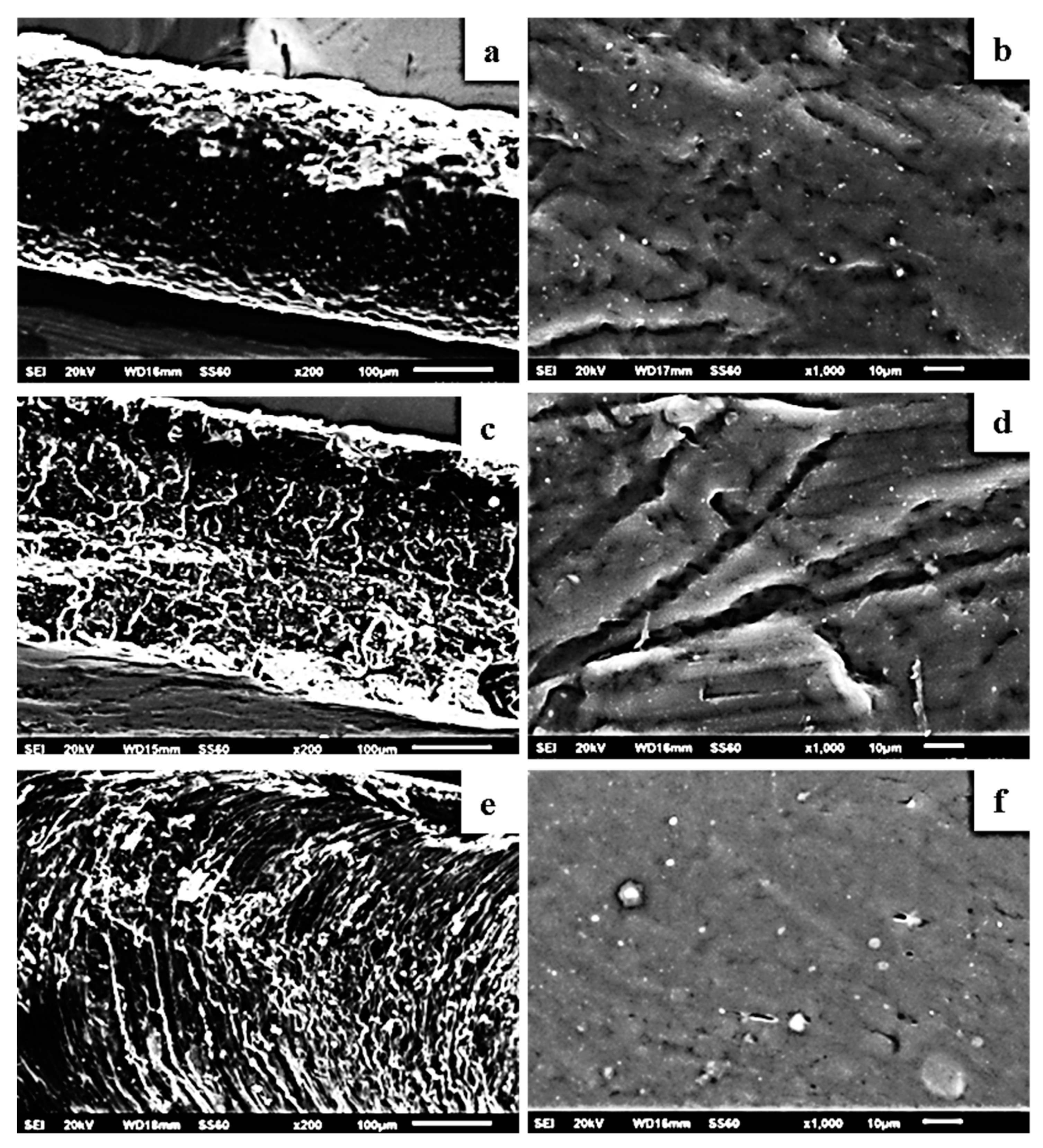
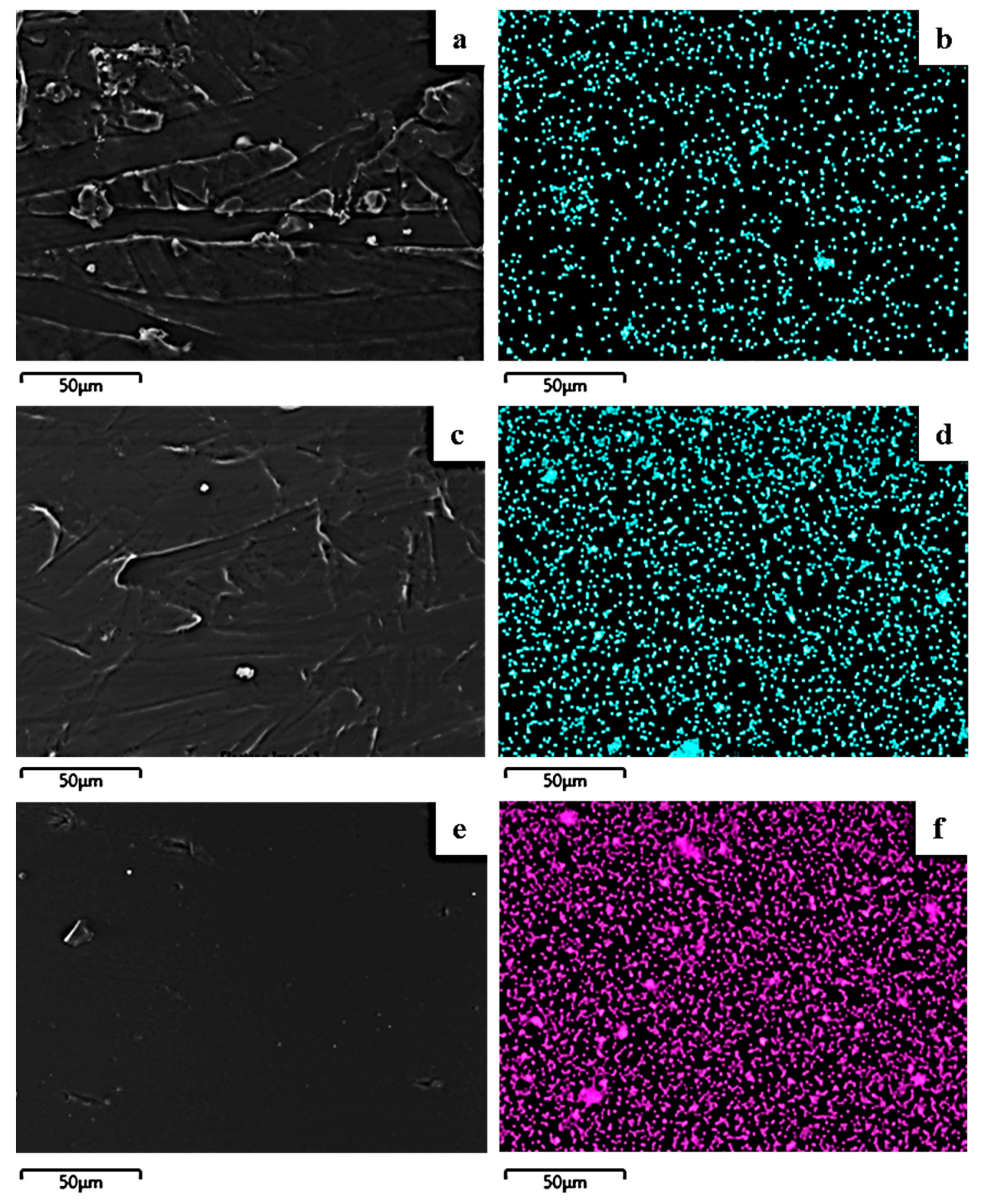
2.5. SEM/EDS Results
2.6. Barrier Properties
2.7. Antioxidant Activity
2.8. Antimicrobial Properties
3. Materials and Methods
3.1. Materials
3.1.1. Used Essential Oil
3.1.2. Used Clay
3.1.3. Used PLLA
3.2. Methods
3.2.1. Preparation of TO@NaMt and TO@OrgMt Nanostructures
3.2.2. Preparation of PLLA/TO@NaMt and PLLA/TO@OrgMt Films
3.3. XRD Analysis
3.4. FTIR Spectrometry
3.5. Thermal Studies TG–DTA
3.6. Tensile Properties
3.7. Scanning Electron Microscopy (SEM)/Energy Dispersive Spectroscopy (EDS)
3.8. Water Vapor Transmission Rate (WVTR)
3.9. Oxygen Permeability (OP)
3.10. Antioxidant Activity
3.11. Antimicrobial Assay
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gross, R.A.; Kalra, B. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional Nanostructured PLA Materials for Packaging and Tissue Engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-Limonene Blends for Food Packaging Applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Gerometta, M.; Rocca-Smith, J.R.; Domenek, S.; Karbowiak, T. Physical and Chemical Stability of PLA in Food Packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-08-100596-5. [Google Scholar]
- Heydari-Majd, M.; Ghanbarzadeh, B.; Shahidi-Noghabi, M.; Najafi, M.A.; Hosseini, M. A New Active Nanocomposite Film Based on PLA/ZnO Nanoparticle/Essential Oils for the Preservation of Refrigerated Otolithes Ruber Fillets. Food Packag. Shelf Life 2019, 19, 94–103. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/Organoclay Bionanocomposites Impregnated with Thymol and Cinnamaldehyde by Supercritical Impregnation for Active and Sustainable Food Packaging. Compos. Part B Eng. 2019, 176, 107336. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.-K. Prospects of Using Nanotechnology for Food Preservation, Safety, and Security. J. Food Drug Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Leontiou, A.A. Montmorillonite Composite Materials and Food Packaging. In Composites Materials for Food Packaging; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–71. ISBN 978-1-119-16024-3. [Google Scholar]
- Idumah, C.I.; Zurina, M.; Ogbu, J.; Ndem, J.U.; Igba, E.C. A Review on Innovations in Polymeric Nanocomposite Packaging Materials and Electrical Sensors for Food and Agriculture. Compos. Interfaces 2020, 27, 1–72. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Garrido-Miranda, K.A.; Pereira, E.D. Polymer/Clay Nanocomposite Films as Active Packaging Material: Modeling of Antimicrobial Release. Eur. Polym. J. 2015, 71, 461–475. [Google Scholar] [CrossRef]
- Busolo, M.A.; Lagaron, J.M. Antioxidant Polyethylene Films Based on a Resveratrol Containing Clay of Interest in Food Packaging Applications. Food Packag. Shelf Life 2015, 6, 30–41. [Google Scholar] [CrossRef]
- Giannakas, A. Na-Montmorillonite Vs. Organically Modified Montmorillonite as Essential Oil Nanocarriers for Melt-Extruded Low-Density Poly-Ethylene Nanocomposite Active Packaging Films with a Controllable and Long-Life Antioxidant Activity. Nanomaterials 2020, 10, 1027. [Google Scholar] [CrossRef]
- Shemesh, R.; Krepker, M.; Natan, M.; Danin-Poleg, Y.; Banin, E.; Kashi, Y.; Nitzan, N.; Vaxman, A.; Segal, E. Novel LDPE/Halloysite Nanotube Films with Sustained Carvacrol Release for Broad-Spectrum Antimicrobial Activity. RSC Adv. 2015, 5, 87108–87117. [Google Scholar] [CrossRef]
- de Oliveira, L.H.; Trigueiro, P.; Souza, J.S.N.; de Carvalho, M.S.; Osajima, J.A.; da Silva-Filho, E.C.; Fonseca, M.G. Montmorillonite with Essential Oils as Antimicrobial Agents, Packaging, Repellents, and Insecticides: An Overview. Colloids Surf. B Biointerfaces 2022, 209, 112186. [Google Scholar] [CrossRef] [PubMed]
- Cheikh, D.; Majdoub, H.; Darder, M. An Overview of Clay-Polymer Nanocomposites Containing Bioactive Compounds for Food Packaging Applications. Appl. Clay Sci. 2022, 216, 106335. [Google Scholar] [CrossRef]
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Zondervan-van den Beuken, E.; Tobback, P. Active and Intelligent Food Packaging: Legal Aspects and Safety Concerns. Trends Food Sci. Technol. 2008, 19, S103–S112. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Ha, C.-S. Tensile, Water Vapor Barrier and Antimicrobial Properties of PLA/Nanoclay Composite Films. LWT-Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Beltrán, A.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled Release, Disintegration, Antioxidant, and Antimicrobial Properties of Poly (Lactic Acid)/Thymol/Nanoclay Composites. Polymers 2020, 12, 1878. [Google Scholar] [CrossRef]
- Oliver-Ortega, H.; Tresserras, J.; Julian, F.; Alcalà, M.; Bala, A.; Espinach, F.X.; Méndez, J.A. Nanocomposites Materials of PLA Reinforced with Nanoclays Using a Masterbatch Technology: A Study of the Mechanical Performance and Its Sustainability. Polymers 2021, 13, 2133. [Google Scholar] [CrossRef]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A Novel Method for the Preparation of Inorganic and Organo-Modified Montmorillonite Essential Oil Hybrids. Appl. Clay Sci. 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Giannakas, A.; Stathopoulou, P.; Tsiamis, G.; Salmas, C. The Effect of Different Preparation Methods on the Development of Chitosan/Thyme Oil/Montmorillonite Nanocomposite Active Packaging Films. J. Food Proc. Preserv. 2019, 44, e14327. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Karydis-Messinis, A.; Moschovas, D.; Kollia, E.; Tsigkou, V.; Proestos, C.; Avgeropoulos, A.; Zafeiropoulos, N.E. Nanoclay and Polystyrene Type Efficiency on the Development of Polystyrene/Montmorillonite/Oregano Oil Antioxidant Active Packaging Nanocomposite Films. Appl. Sci. 2021, 11, 9364. [Google Scholar] [CrossRef]
- Wasanasuk, K.; Tashiro, K.; Hanesaka, M.; Ohhara, T.; Kurihara, K.; Kuroki, R.; Tamada, T.; Ozeki, T.; Kanamoto, T. Crystal Structure Analysis of Poly(l-Lactic Acid) α Form On the Basis of the 2-Dimensional Wide-Angle Synchrotron X-Ray and Neutron Diffraction Measurements. Macromolecules 2011, 44, 6441–6452. [Google Scholar] [CrossRef]
- Hosen, M.S.; Rahaman, M.H.; Gafur, M.A.; Habib, R.; Qadir, M.R. Preparation and Characterization of Poly(L-Lactic Acid)/Chitosan/Microcrystalline Cellulose Blends. Chem. Sci. Int. J. 2017, 21, 1–10. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Mano, J.F.; Reis, R.L. Novel 3D Scaffolds of Chitosan–PLLA Blends for Tissue Engineering Applications: Preparation and Characterization. J. Supercrit. Fluids 2010, 54, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.V.; Pugazhenthi, G. Properties and Thermal Degradation Kinetics of Polystyrene/Organoclay Nanocomposites Synthesized by Solvent Blending Method: Effect of Processing Conditions and Organoclay Loading. J. Appl. Polym. Sci. 2011, 120, 1322–1336. [Google Scholar] [CrossRef]
- Pugazhenthi, G.; Suresh, K.; Vinoth Kumar, R.; Kumar, M.; Rajkumar Surin, R. A Simple Sonication Assisted Solvent Blending Route for Fabrication of Exfoliated Polystyrene (PS)/Clay Nanocomposites: Role of Various Clay Modifiers. Mater. Today Proc. 2018, 5, 13191–13210. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of Active Packaging Film Made from Poly (Lactic Acid) Incorporated Essential Oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Giannakas, A.; Spanos, C.G.; Kourkoumelis, N.; Vaimakis, T.; Ladavos, A. Preparation, Characterization and Water Barrier Properties of PS/Organo-Montmorillonite Nanocomposites. Eur. Polym. J. 2008, 44, 3915–3921. [Google Scholar] [CrossRef]
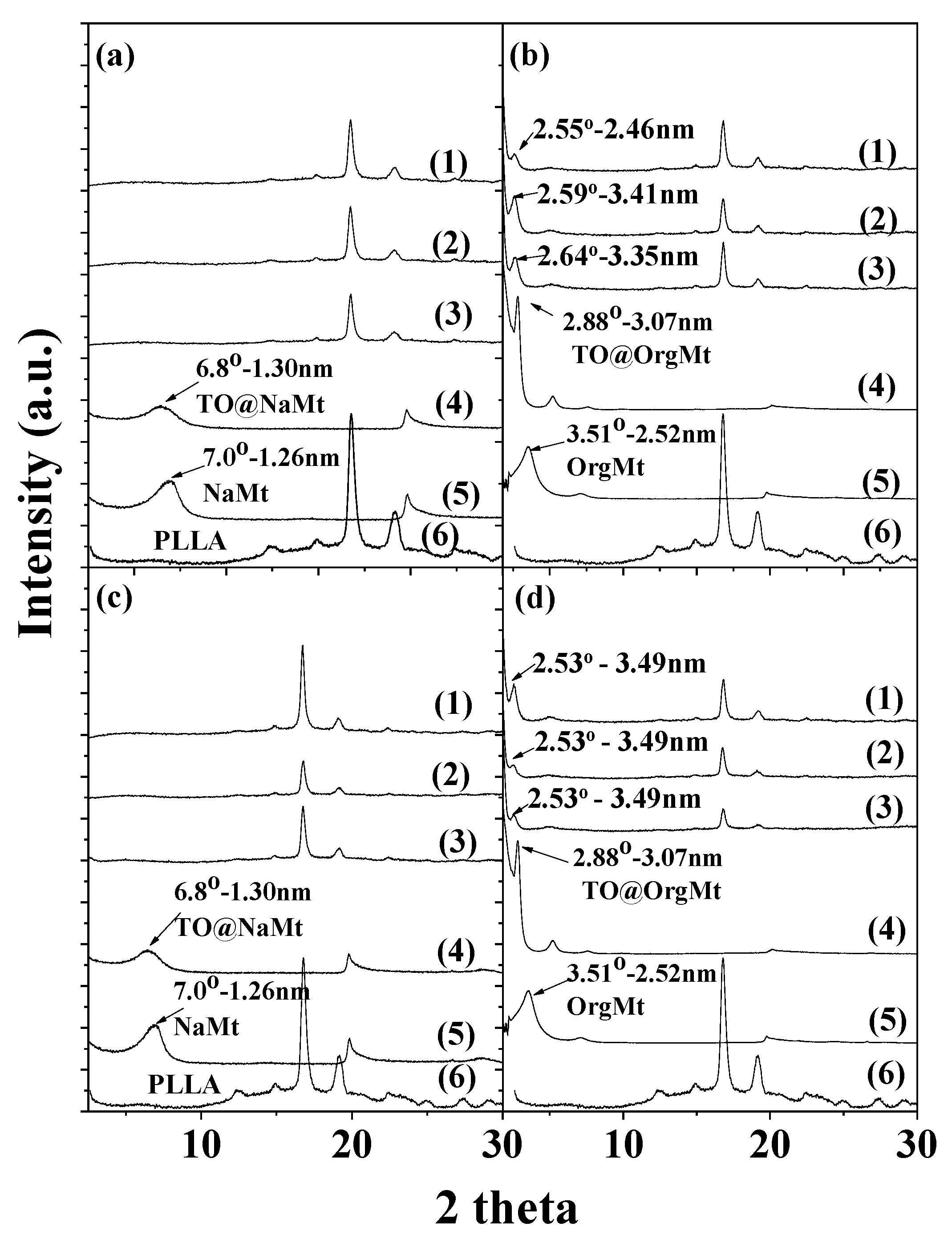
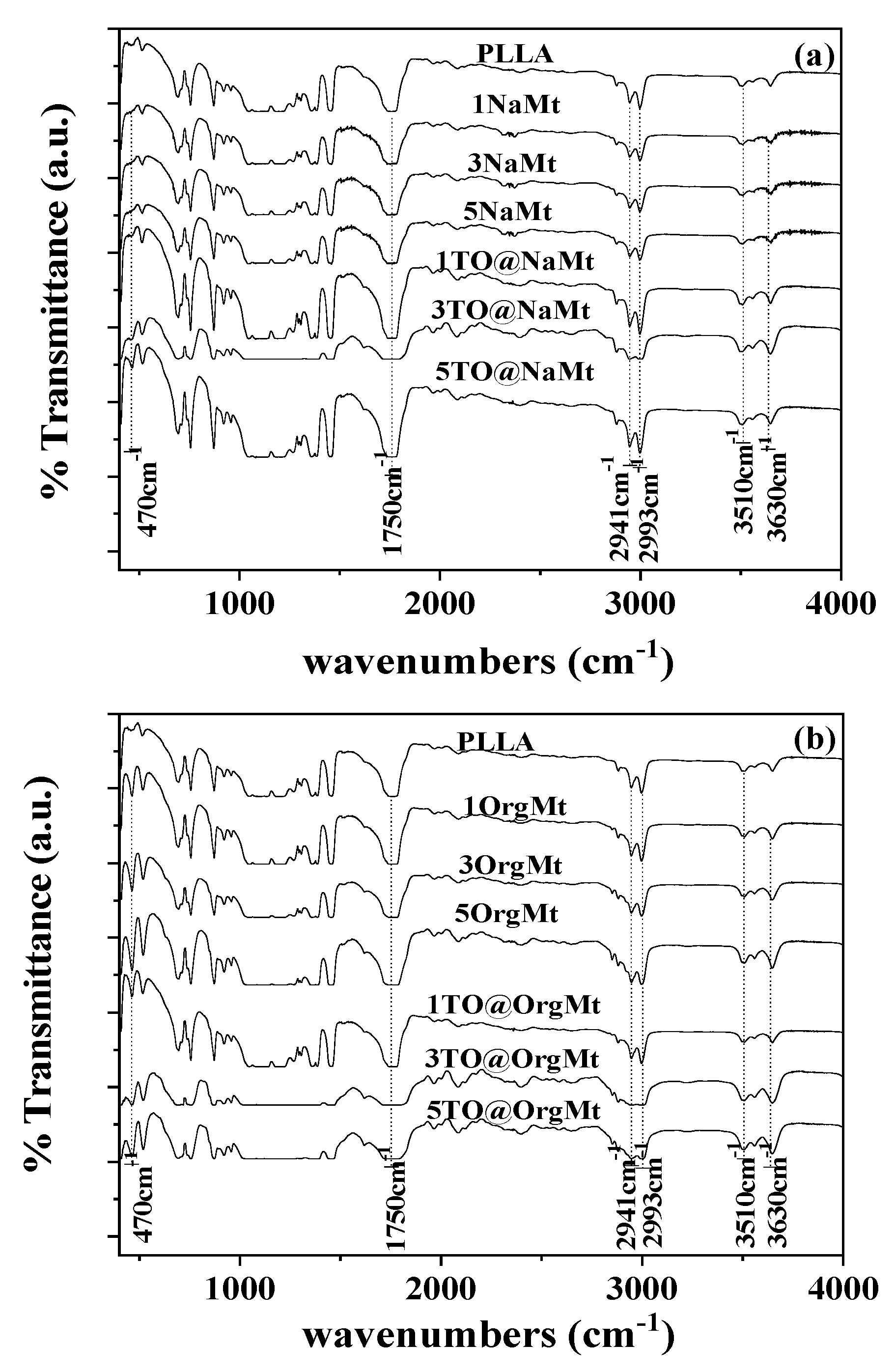
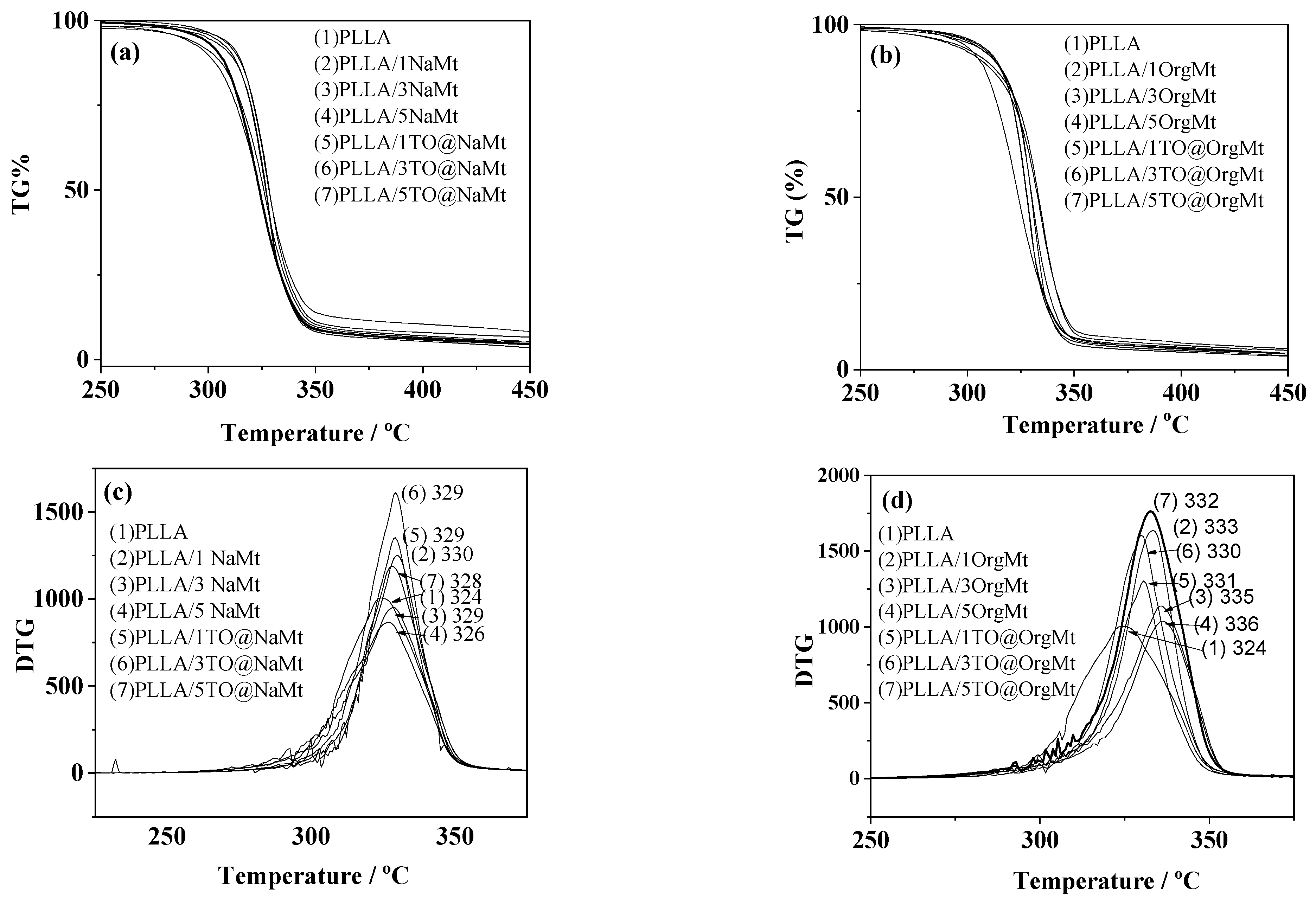
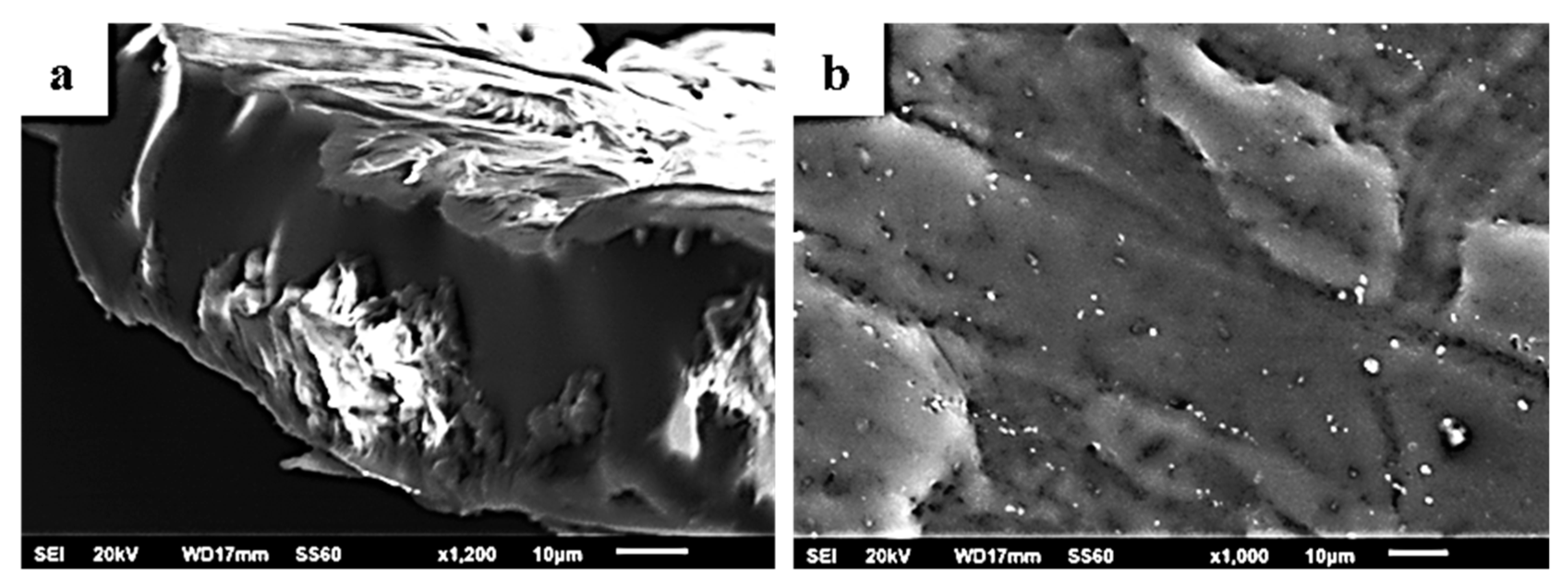



| Code Name | PLLA | NaMt | OrgMt | TO@NaMt | TO@OrgMt |
|---|---|---|---|---|---|
| PLLA | 5 | - | - | - | - |
| PLLA/1NaMt | 4.95 | 0.05 | |||
| PLLA/3NaMt | 4.85 | 0.15 | - | - | - |
| PLLA/5NaMt | 4.75 | 0.25 | |||
| PLLA/1TO@NaMt | 4.95 | 0.05 | |||
| PLLA/3TO@NaMt | 4.85 | - | - | 0.15 | - |
| PLLA/5TO@NaMt | 4.75 | - | - | 0.25 | - |
| PLLA/1OrgMt | 4.95 | 0.05 | |||
| PLLA/3OrgMt | 4.85 | - | 0.15 | - | - |
| PLLA/5OrgMt | 4.75 | 0.25 | |||
| PLLA/1TO@OrgMt | 4.95 | 0.05 | |||
| PLLA/3TO@OrgMt | 4.85 | - | - | - | 0.15 |
| PLLA/5TO@OrgMt | 4.75 | - | - | - | 0.25 |
| Code Name | Tensile E (St. Dev.) (MPa) | σuts (MPa) (St. Dev.) | εb (%) (St. Dev.) |
|---|---|---|---|
| PLLA | 2891.3 (61.9) | 33.9 (4.9) | 1.4 (0.2) |
| PLLA/1NaMt | 2460.2 (232.3) | 31.7 (5.4) | 1.3 (0.2) |
| PLLA/3NaMt | 2660.5 (137.8) | 32.5 (6.0) | 1.3 (0.3) |
| PLLA/5NaMt | 1823.3 (285.4) | 22.0 (6.3) | 1.1 (0.1) |
| PLLA/1TO@NaMt | 2609.5 (137.8) | 32.2 (6.0) | 1.4 (0.1) |
| PLLA/3TO@NaMt | 2910.7 (268.3) | 34.5 (2.1) | 1.4 (0.1) |
| PLLA/5TO@NaMt | 2811.4 (121.7) | 33.5 (3.7) | 1.5 (0.3) |
| PLLA/1OrgMt | 2315.5 (185.2) | 29.9 (3.8) | 1.8 (0.3) |
| PLLA/3OrgMt | 2965 (210.4) | 34.2 (6.2) | 1.5 (0.2) |
| PLLA/5OrgMt | 2483 (235.2) | 22.5 (5.3) | 1.0 (0.2) |
| PLLA/1TO@OrgMt | 2971 (145.8) | 38.7 (4.2) | 1.9 (0.3) |
| PLLA/3TO@OrgMt | 3277 (181.6) | 40.3 (4.8) | 1.9 (0.1) |
| PLLA/5TO@OrgMt | 2758.5 (206.7) | 18.2 (5.3) | 0.9 (0.2) |
| Sample Code Name | WVTR (g·m−2·day−1) | DWV (cm2·s−1) | OTR (cm3·m−2·day−1) | PeO2 (cm2·s−1) | Antioxidant Activity after 24 h |
|---|---|---|---|---|---|
| PLLA | 15.9 ± 1.3 | 9.64 (±0.9) × 10−12 | 12.4 ± 0.9 | 5.39 (±0.6) × 10−10 | n.d. 1 |
| PLLA/1NaMt | 16.1 ± 1.2 | 9.76 (±0.8) × 10−12 | 9.8 ± 0.8 | 4.26 (±0.5) × 10−10 | n.d. |
| PLLA/3NaMt | 16.4 ± 1.4 | 9.96 (±1.1) × 10−12 | 9.4 ± 0.8 | 4.06 (±0.5) × 10−10 | n.d. |
| PLLA/5NaMt | 16.7 ± 1.0 | 10.14 (±0.7) × 10−12 | 9.2 ± 0.7 | 4.00 (±0.4) × 10−10 | n.d. |
| PLLA/1TO@NaMt | 15.0 ± 1.1 | 9.11 (±0.8) × 10−12 | 9.0 ± 0.6 | 3.91 (±0.3) × 10−10 | 11.9 ± 1.2 |
| PLLA/3TO@NaMt | 15.1 ± 1.2 | 9.17 (±0.8) × 10−12 | 8.9 ± 0.6 | 3.88 (±0.3) × 10−10 | 19.7 ± 1.3 |
| PLLA/5TO@NaMt | 15.5 ± 1.3 | 9.41 (±0.9) × 10−12 | 8.8 ± 0.7 | 3.84 (±0.4) × 10−10 | 24.1 ± 1.5 |
| PLLA/1OrgMt | 14.9 ± 0.9 | 9.03 (±0.6) × 10−12 | 9.3 ± 0.8 | 4.03 (±0.5) × 10−10 | n.d. |
| PLLA/3OrgMt | 14.1 ± 0.9 | 8.54 (±0.6) × 10−12 | 8.4 ± 0.7 | 3.65 (±0.4) × 10−10 | n.d. |
| PLLA/5OrgMt | 13.6 ± 0.8 | 8.26 (±0.5) × 10−12 | 6.3 ± 0.5 | 2.72 (±0.2) × 10−10 | n.d. |
| PLLA/1TO@OrgMt | 14.0 ± 1.0 | 8.50 (±0.7) × 10−12 | 9.1 ± 0.6 | 3.95 (±0.3) × 10−10 | 17.2 ± 1.8 |
| PLLA/3TO@OrgMt | 13.6 ± 0.8 | 8.26 (±0.5) × 10−12 | 8.0 ± 0.6 | 3.48 (±0.3) × 10−10 | 38.0 ± 2.1 |
| PLLA/5TO@OrgMt | 13.0 ± 0.7 | 7.92 (±0.4) × 10−12 | 5.3 ± 0.5 | 2.30 (±0.2) × 10−10 | 50.3 ± 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Avgeropoulos, A.; Proestos, C. Performance of Thyme Oil@Na-Montmorillonite and Thyme Oil@Organo-Modified Montmorillonite Nanostructures on the Development of Melt-Extruded Poly-L-lactic Acid Antioxidant Active Packaging Films. Molecules 2022, 27, 1231. https://doi.org/10.3390/molecules27041231
Giannakas AE, Salmas CE, Leontiou A, Moschovas D, Baikousi M, Kollia E, Tsigkou V, Karakassides A, Avgeropoulos A, Proestos C. Performance of Thyme Oil@Na-Montmorillonite and Thyme Oil@Organo-Modified Montmorillonite Nanostructures on the Development of Melt-Extruded Poly-L-lactic Acid Antioxidant Active Packaging Films. Molecules. 2022; 27(4):1231. https://doi.org/10.3390/molecules27041231
Chicago/Turabian StyleGiannakas, Aris E., Constantinos E. Salmas, Areti Leontiou, Dimitrios Moschovas, Maria Baikousi, Eleni Kollia, Vasiliki Tsigkou, Anastasios Karakassides, Apostolos Avgeropoulos, and Charalampos Proestos. 2022. "Performance of Thyme Oil@Na-Montmorillonite and Thyme Oil@Organo-Modified Montmorillonite Nanostructures on the Development of Melt-Extruded Poly-L-lactic Acid Antioxidant Active Packaging Films" Molecules 27, no. 4: 1231. https://doi.org/10.3390/molecules27041231
APA StyleGiannakas, A. E., Salmas, C. E., Leontiou, A., Moschovas, D., Baikousi, M., Kollia, E., Tsigkou, V., Karakassides, A., Avgeropoulos, A., & Proestos, C. (2022). Performance of Thyme Oil@Na-Montmorillonite and Thyme Oil@Organo-Modified Montmorillonite Nanostructures on the Development of Melt-Extruded Poly-L-lactic Acid Antioxidant Active Packaging Films. Molecules, 27(4), 1231. https://doi.org/10.3390/molecules27041231












