Mammalian Cell Spheroids on Mixed Organic–Inorganic Superhydrophobic Coating
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of the Surfaces
2.2. Cell Behavior on 2D Culture and Agarose-Induced 3D Culture
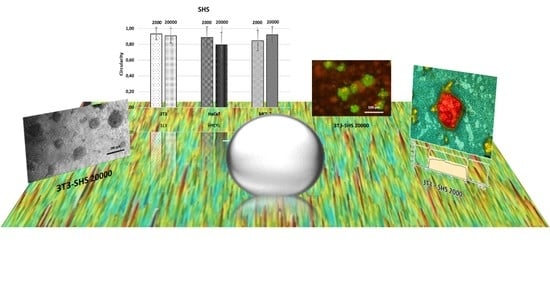
2.3. Cell Behavior on Superhydrophobic Surfaces
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Surface Preparation and Characterization
3.2.2. Cell Cultures
3.2.3. Conventional 2D and Agarose-Induced 3D Culture
3.2.4. Cell Culture in Superhydrophobic Substrates
3.2.5. Profilometry Studies
3.2.6. Fluorescence Microscopy Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Feinberg, A.W.; Wilkerson, W.R.; Seegert, C.A.; Gibson, A.L.; Hoipkemeier-Wilson, L.; Brennan, A.B. Systematic variation of microtopography, surface chemistry and elastic modulus and the state dependent effect on endothelial cell alignment. J. Biomed. Mater. Res. 2008, 86, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wang, Y.; Eberli, D. Cell responses to surface and architecture of tissue engineering scaffolds. In Regenerative Medicine and Tissue Engineering-Cells and Biomaterials; InTechOpen: London, UK, 2011. [Google Scholar]
- Anselme, K.; Ploux, L.; Ponche, A. Cell/Material Interfaces: Influence of surface chemistry and surface topography on cell adhesion. J. Adhes. Sci. Technol. 2010, 24, 831–852. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balikov, D.A.; Crowder, S.W.; Boire, T.C.; Lee, J.B.; Gupta, M.K.; Fenix, A.M.; Lewis, H.N.; Ambrose, C.M.; Short, P.A.; Kim, C.S.; et al. Tunable surface repellency maintains stemness and redox capacity of human mesenchymal stem cells. ACS Appl. Mater. Interfaces 2017, 9, 22994–23006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuji, T.G.; Bin, J.; Yoshimura, A.; Tomura, M.; Tateyama, D.; Minami, I.; Yoshikawa, Y.; Aiba, K.; Heuser, J.E.; Nishino, T.; et al. A 3D sphere culture system containing functional polymers for large-scale human pluripotent stem cell production. Stem Cell Rep. 2014, 24, 734–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caiado Decarli, M.; Amaral, R.; Peres dos Santos, D.; Bueno Tofani, L.; Katayama, E.; Alvarenga Rezende, R.; Lopes da Silva, J.V.; Swiech, K.; Torres Suazo, C.A.; Mota, C.; et al. Cell spheroids as a versatile research platform: Formation mechanisms, high throughput production, characterization and applications. Biofabrication 2021, 13, 032002. [Google Scholar] [CrossRef]
- Nagelkerke, A.; Bussink, J.; Rowan, A.E.; Span, P.N. The mechanical microenvironment in cancer: How physics affects tumours. Semin. Cancer Biol. 2015, 35, 2–70. [Google Scholar] [CrossRef]
- Smyrek, I.; Mathew, B.; Fischer, S.C.; Lissek, S.M.; Becker, S.; Stelzer, S.H.K. E-cadherin, actin, microtubules and FAK dominate different spheroid formation phases and important elements of tissue integrity. Biol. Open 2019, 8, bio037051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luebke-Wheeler, J.L.; Nedredal, G.; Yee, L.; Amiot, B.P.; Nyberg, S.L. E-cadherin protects primary hepatocyte spheroids from cell death by a caspase-independent mechanism. Cell Transplant. 2009, 18, 1281–1287. [Google Scholar] [CrossRef] [Green Version]
- Shao, C.; Chi, J.; Zhang, H.; Fan, Q.; Zhao, Y.; Ye, F. Development of Cell Spheroids by Advanced Technologies. Adv. Mater. Technol. 2020, 5, 2000183. [Google Scholar] [CrossRef]
- Chen, M.; Shah, M.P.; Shelper, T.B.; Nazareth, L.; Barker, M.; Tello Velasquez, J.; Ekberg, J.A.; Vial, M.L.; St John, J.A. Naked liquid marbles: A robust three-dimensional low-volume cell-culturing system. ACS Appl. Mater. Interfaces 2019, 11, 9814–9823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neto, A.I.; Correia, C.R.; Oliveira, M.B.; Rial-Hermida, M.I.; Alvarez-Lorenzo, C.; Reis, R.L.; Mano, J.F. A novel hanging spherical drop system for the generation of cellular spheroids and high throughput combinatorial drug screening. Biomater. Sci. 2015, 3, 581–585. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.B.; Neto, A.I.; Correia, C.R.; Rial-Hermida, M.I.; Alvarez-Lorenzo, C.; Mano, J.F. Superhydrophobic Chips for Cell Spheroids High-Throughput Generation and Drug Screening. ACS Appl. Mater. Interfaces 2014, 6, 9488–9495. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhao, Y.; Wu, W.; Zhao, Q.; Li, G. A superhydrophobic chip integrated with an array of medium reservoirs for long-term hanging drop spheroid culture. Acta Biomater. 2021, 135, 234–242. [Google Scholar] [CrossRef]
- Xu, L.; Chen, S.; Lu, X.; Lu, Q. Durable superamphiphobic silica aerogel surfaces for the culture of 3D cellular spheroids. Natl. Sci. Rev. 2019, 6, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, Y.; Yu, S.J.; Lee, S.Y.; Son, J.G.; Lee, T.G.; Cho, Y.; Shin, J.H.; Lee, E.; Im, S.G. Surface hydrophobicity modulates the key characteristics of cancer spheroids through the interaction with the adsorbed proteins. Adv. Funct. Mater. 2021, 31, 2100775. [Google Scholar] [CrossRef]
- Boban, M.; Mehta, P.; Halvey, A.K.; Repetto, T.; Tuteja, A.; Mehta, G. Novel Omniphobic Platform for Multicellular Spheroid Generation, Drug Screening, and On-Plate Analysis. Anal. Chem. 2021, 93, 8054–8806. [Google Scholar] [CrossRef]
- Moretti, M.; Prina-Mello, A.; Reid, A.J.; Barron, V.; Prendergast, P.J. Endothelial cell alignment on cyclically stretched silicone surfaces. J. Mater. Sci. Mater. Med. 2004, 15, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.A.; Twigg, P.C.; Savage, M.D.; Woon, W.H.; Greig, D. Mechanical investigations on agar gels using atomic force microscopy: Effect of deuteration. Macromol. Mater. Eng. 2012, 297, 214–218. [Google Scholar] [CrossRef]
- Shi, W.; Kwon, J.; Huang, Y.; Tan, J.; Uhl., C.G.; He, R.; Zhou, C.; Liu, Y. Facile tumor spheroids formation in large quantity with controllable size and high uniformity. Sci. Rep. 2018, 8, 6837. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Timmins, N.E.; Brown, C.J.; Fussenegger, M.; Nielsen, L.K. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol. Bioeng. 2003, 83, 173–180. [Google Scholar] [CrossRef]
- Morán, M.C.; Cirisano, F.; Ferrari, M. 3D profilometry and cell viability studies for drug response screening. Mater. Sci. Eng. C 2020, 115, 111142. [Google Scholar] [CrossRef]
- Choudhury, S.; Das, A. Advances in generation of three-dimensional skin equivalents: Pre-clinical studies to clinical therapies. Cytotherapy 2021, 23, 1–9. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian Cell Behavior on Hydrophobic Substrates: Influence of surface properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef] [Green Version]
- Morán, M.C.; Ruano, G.; Cirisano, F.; Ferrari, M. Mammalian cell viability on hydrophobic and superhydrophobic fabrics. Mater. Sci. Eng. C. 2019, 99, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Regenerable superhydrophobic coatings for biomedical fabrics. Coatings 2020, 10, 578. [Google Scholar] [CrossRef]
- Merck KGaA. ECACC Handbook, Fundamental Techniques in Cell Culture Laboratory Handbook, 4th ed.; Merck KGaA: Darmstadt, Germany, 2018. [Google Scholar]
- Gong, X.; Lin, C.; Cheng, J.; Su, J.S.; Zhao, H.; Liu, T.L.; Wen, X.; Zhao, P. Generation of multicellular tumor spheroids with microwell-based agarose scaffolds for drug testing. PLoS ONE 2015, 10, e0130348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, M.; Piccardo, P.; Vernet, J.; Cirisano, F. High Transmittance Superhydrophobic Coatings with Durable Self-Cleaning Properties. Coatings 2021, 11, 493. [Google Scholar] [CrossRef]
- Zeiger, A.S.; Hinton, B.; Van Vliet, K.J. Why the dish makes a difference: Quantitative comparison of polystyrene culture surfaces. Acta Biomater. 2013, 9, 7354–7361. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Bondre, C.; Gladstone, H.B.; Brown, P.O.; Chang, H.Y. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006, 2, e119. [Google Scholar] [CrossRef]
- Carson, S.; Miller, H.B.; Srougi, M.C.; Witherow, D.S. Molecular Biology Techniques: A Classroom Laboratory Manual; Academic Press: London, UK, 2019. [Google Scholar]
- Li, Y.F.; Kumacheva, E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018, 4, eaas8998. [Google Scholar] [CrossRef] [Green Version]
- Karp, J.M.; Yeh, J.; Eng, G.; Fukuda, J.; Blumling, J.; Suh, K.Y.; Cheng, J.; Mahdavi, A.; Borenstein, J.; Langer, R.; et al. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab A Chip 2007, 7, 786–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napolitano, A.P.; Dean, D.M.; Man, A.J.; Youssef, J.; Ho, D.N.; Rago, A.P.; Lech, M.P.; Morgan, J.R. Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels. Biotechniques 2007, 43, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, J.; Khademhosseini, A.; Yeo, Y.; Yang, X.Y.; Yeh, J.; Eng, G.; Blumling, J.; Wang, C.F.; Kohane, D.S.; Langer, R. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials 2006, 27, 5259–5267. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.D.; Liu, J.M.; Chen, Y. Agarose multi-wells for tumour spheroid formation and anti-cancer drug test. Microelectron. Eng. 2016, 158, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Siva Sankar, P.; Che Mat, M.F.; Muniandy, K.; Xiang, B.L.S.; Ling, P.S.; Hoe, S.L.L.; Khoo, A.S.; Mohana-Kumaran, N. Modeling nasopharyngeal carcinoma in three dimensions. Oncol. Lett. 2017, 13, 2034–2044. [Google Scholar] [CrossRef] [Green Version]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef]
- Van Winkle, A.P.; Gates, I.D.; Kallos, M.S. Mass transfer limitations in embryoid bodies during human embryonic stem cell differentiation. Cells Tissues Organs 2012, 196, 34–47. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xu, L.H.; Yu, Q. Cell aggregation induces phosphorylation of PECAM-1 and Pyk2 and promotes tumor cell anchorage-independent growth. Mol. Cancer 2010, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liggieri, L.; Passerone, A. An automatic technique for measuring the surface tension of liquid metals. High Temp. Technol. 1989, 7, 82–86. [Google Scholar] [CrossRef]
- Sharma., A. An ultraviolet-sterilization protocol for microtitre plates. JEMI 2012, 16, 144–147. [Google Scholar]
- McGahon, A.J.; Martin, S.J.; Bissonnette, R.P.; Mahboubi, A.; Shi, Y.; Mogil, R.J.; Nishioka, W.K.; Green, D.R. The end of the (cell) line: Methods for the study of apoptosis in vitro. Methods Cell Biol. 1995, 46, 153–185. [Google Scholar] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian Cell Spheroids on Mixed Organic–Inorganic Superhydrophobic Coating. Molecules 2022, 27, 1247. https://doi.org/10.3390/molecules27041247
Ferrari M, Cirisano F, Morán MC. Mammalian Cell Spheroids on Mixed Organic–Inorganic Superhydrophobic Coating. Molecules. 2022; 27(4):1247. https://doi.org/10.3390/molecules27041247
Chicago/Turabian StyleFerrari, Michele, Francesca Cirisano, and M. Carmen Morán. 2022. "Mammalian Cell Spheroids on Mixed Organic–Inorganic Superhydrophobic Coating" Molecules 27, no. 4: 1247. https://doi.org/10.3390/molecules27041247