Research Progress on Typical Quaternary Ammonium Salt Polymers
Abstract
:1. Introduction
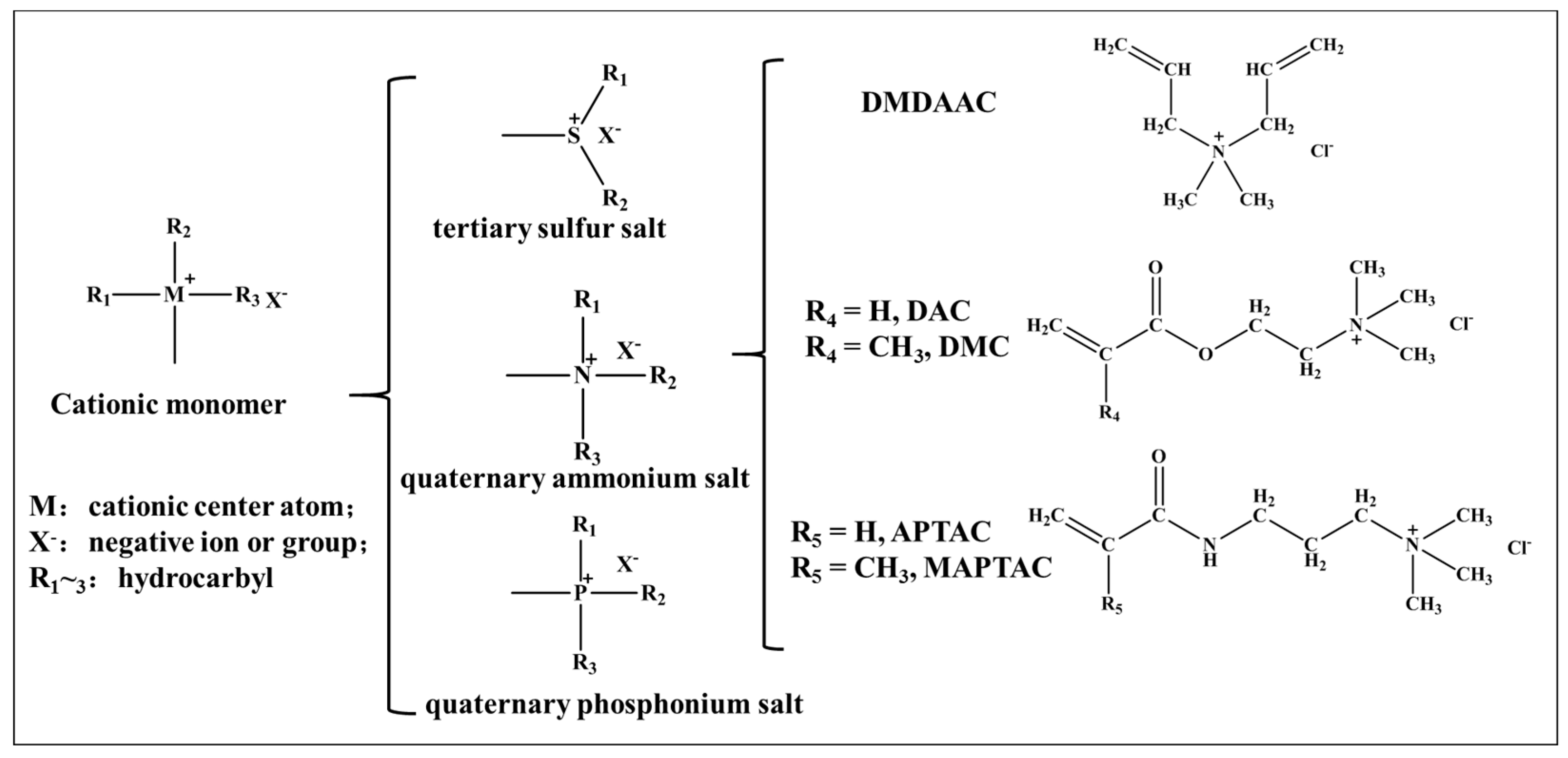
2. Typical Quaternary Ammonium Salt Monomers
2.1. Properties and Synthetic Methods of the Monomer DMDAAC
2.2. Properties and Synthetic Methods of the Monomer DAC (or DMC)
2.3. Properties and Synthetic Methods of the Monomer APTAC (or MAPTAC)
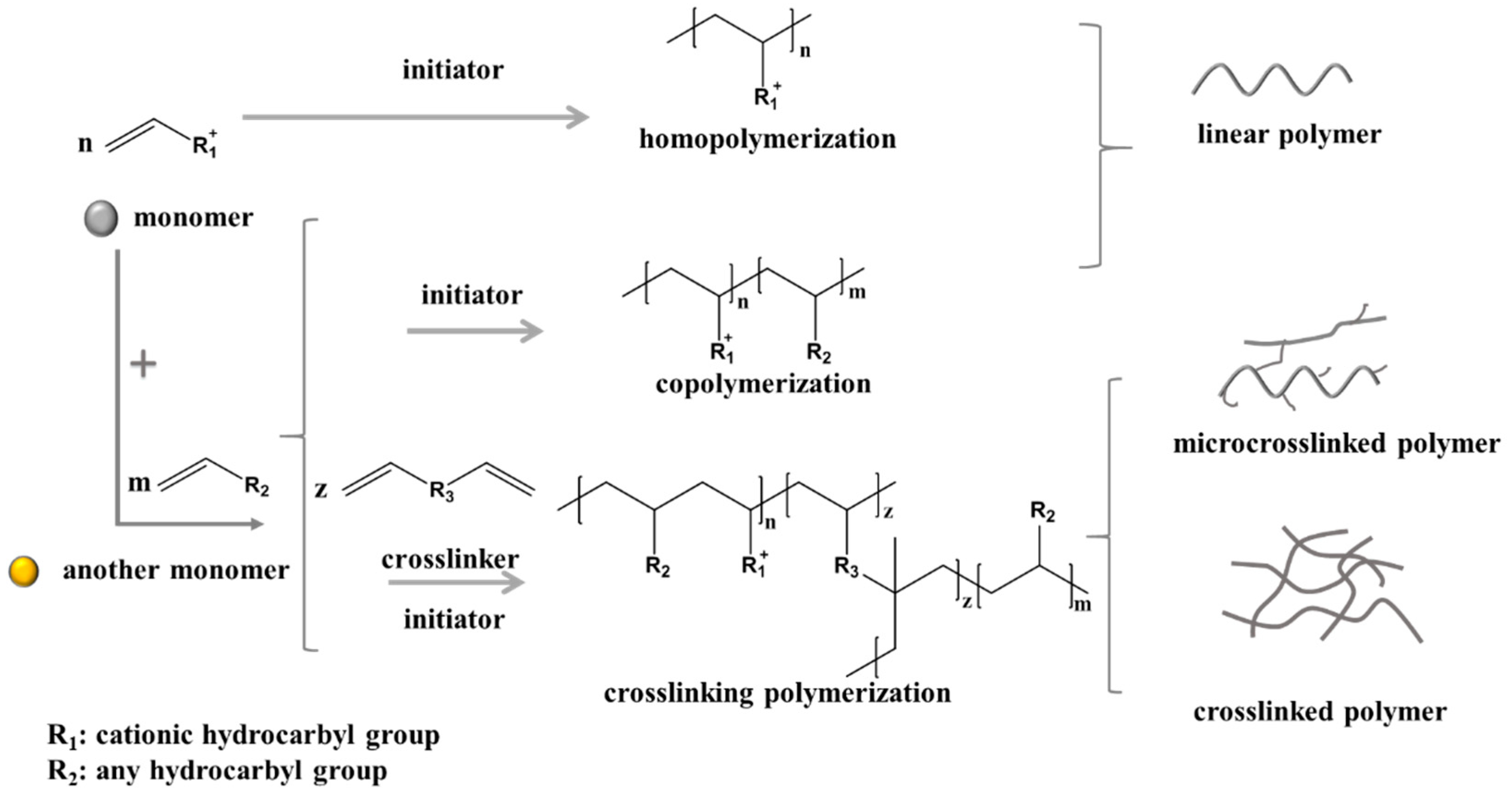
3. Research Progress on the Synthesis of Quaternary Ammonium Salt Polymers
3.1. Synthesis of Polymers with High and Serial Intrinsic Viscosity
3.1.1. Progress in the Synthesis of DMDAAC Polymers
3.1.2. Progress in the Synthesis of DAC (or DMC) Polymers
3.1.3. Progress in the Synthesis of APTAC (or MAPTAC) Polymers
3.1.4. Synthetic Processes and Products of Quaternary Ammonium Salt Polymers
3.2. Studies on Polymers with Narrow Molecular Weight Distribution and High Monomer Conversion
3.2.1. Synthesis of Polymers with Narrow Molecular Weight Distributions
3.2.2. Synthesis of Polymers with High Monomer Conversion
3.3. Synthesis of Polymers with Special Functions
4. Studies on the Relationships between Structures and Properties of Polymers
4.1. Molecular Weights and Application Performance
4.2. Relationships between Charge Densities and Properties
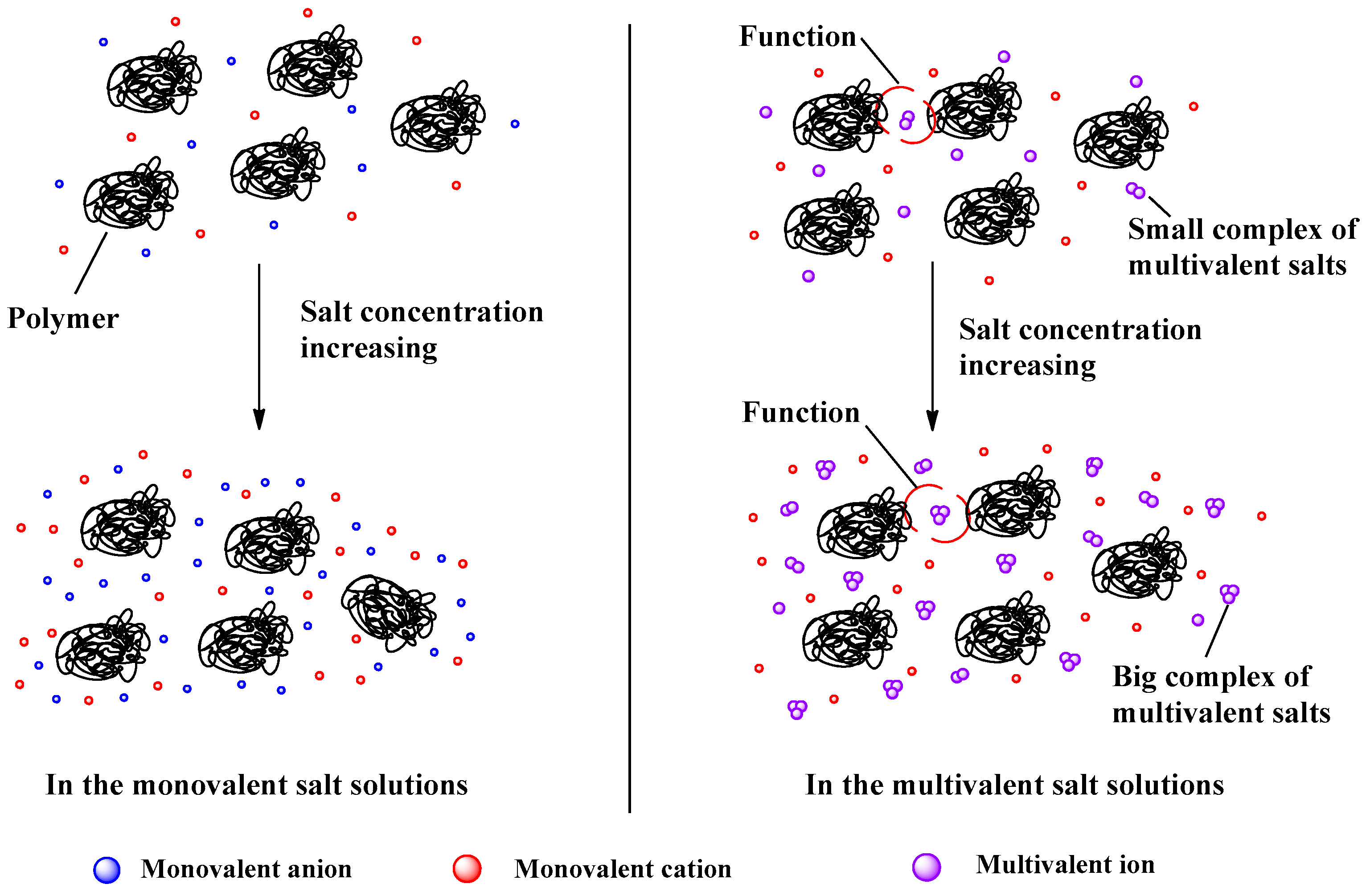
4.3. Solution Properties of Polymers
4.4. Stability of Polymers
4.4.1. Thermal Stability
4.4.2. Hydrolytic Stability
4.5. Differences in the Microstructures of Polymers
4.5.1. Microstructural Differences of Units
4.5.2. Differences of Linear and Branched Structures
4.5.3. Molecular Structure Modification Based on Natural Polymers
5. Research Progress on Applications of Quaternary Ammonium Salt Polymers
5.1. Water Treatment
5.1.1. Raw Water Treatment
5.1.2. Sludge Treatment
5.2. Daily Chemicals
5.2.1. Polymers Containing a DMDAAC Unit
5.2.2. Polymers Containing an APTAC (or MAPTAC) Unit
5.3. Oil Exploitation
5.3.1. Common Polymers
5.3.2. Characteristic Polymers
5.4. Papermaking and Textiles
5.4.1. Papermaking Additive
5.4.2. Textile Auxiliary
5.5. Other Fields
5.5.1. Medicine
5.5.2. Electronic Devices
5.5.3. Fuel Cells
6. Future Perspectives
6.1. Improvement of the Molecular Weight
6.2. The Relationship between the Structure and Properties
6.3. The Fields of Application of Polymers
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| diallyl dimethyl ammonium chloride | DMDAAC |
| acryloyl oxygen ethyl trimethyl ammonium chloride | DAC |
| methyl acryloyl oxygen ethyl trimethyl ammonium chloride | DMC |
| acrylamide propyl trimethyl ammonium chloride | APTAC |
| methyl acrylamide propyl trimethyl ammonium chloride | MAPTAC |
| dimethyl amine ethyl acrylate | DMAEA |
| methyl dimethyl amine ethyl acrylate | DMAEMA |
| dimethyl amine propyl acrylamide | DMPA |
| dimethyl amine propyl methyl acrylamide | DMPMA |
| acrylamide | AM |
| poly-diallyl dimethyl ammonium chloride | PDMDAAC |
| copolymer of DMDAAC with AM | PDA |
| poly-acryloyl oxygen ethyl trimethyl ammonium chloride | PDAC |
| poly-methyl acryloyl oxygen ethyl trimethyl ammonium chloride | PDMC |
| copolymer of DAC with AM | P(DAC-AM) |
| copolymer of DMC with AM | P(DMC-AM) |
| poly-methyl acrylamide propyl trimethyl ammonium chloride | PMAPTAC |
| poly-acrylamide propyl trimethyl ammonium chloride | PAPTAC |
| copolymer of MAPTAC with AM | P(MAPTAC-AM) |
| copolymer of APTAC with AM | P(APTAC-AM) |
| reversible addition fragmentation chain transfer | RAFT |
| 3-chloro-2-hydroxypropyl methyl diallyl ammonium chloride | CMDA |
| critical micelle concentration | CMC |
| humic acid | HA |
| bovine serum albumin | BSA |
| sodium alginate | SA |
| acrylic acid | AA |
| 2-acrylamide-2-methyl propane sulfonic acid | AMPS |
| styrene sulfonate | SSS |
References
- Ribeiro, R.T.; Galvao, C.N.; Betancourt, Y.P.; Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Microbicidal dispersions and coatings from hybrid nanoparticles of poly (methyl Methacrylate), poly (diallyl dimethyl ammonium) chloride, lipids, and surfactants. Int. J. Mol. Sci. 2019, 20, 6150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niesyto, K.; Neugebauer, D. Linear copolymers based on choline ionic liquid carrying anti-tuberculosis drugs: Influence of anion type on physicochemical properties and drug release. Int. J. Mol. Sci. 2021, 22, 284. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jordan, D.; Lowe, R.M.; Cramm, J.; Cheng, W.; Wei, M. Low-Molecular-Weight Dry Powder Polymer for Use as Paper-Making Dry Strength Agent. WIPO Patent 2017214616A1, 14 December 2017. [Google Scholar]
- Gamez-Garcia, M.; Petty, D. Shampoo Compositions Having Reduced Squeakiness Effect, Process for Preparing the Same and Method of Use. WIPO Patent 2017087656A1, 26 May 2017. [Google Scholar]
- Chen, Y.; Wang, H. Fabrication of conductive microparticles as anodal electrode in microfluidic microbial fuel cell. Energy Procedia 2014, 61, 1565–1568. [Google Scholar] [CrossRef] [Green Version]
- Pisárčik, M.; Pupák, M.; Lukáč, M.; Devínsky, F.; Hubčík, L.; Bukovský, M.; Horváth, B. The synthesis, self-assembled structures, and microbicidal activity of cationic gemini surfactants with branched tridecyl chains. Molecules 2019, 24, 4380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Yang, Q.; Zhang, Y. Thermal decomposition behavior and mechanism study of cationic polyacrylamide. J. Therm. Anal. Calorim. 2021, 146, 1371–1381. [Google Scholar] [CrossRef]
- Zhang, M.; Cai, Z.; Xie, L.; Zhang, Y.; Tang, L.; Zhou, Q.; Qiang, Z.; Zhang, H.; Zhang, D.; Pan, X. Comparison of coagulative colloidal microbubbles with monomeric and polymeric inorganic coagulants for tertiary treatment of distillery wastewater. Sci. Total Environ. 2019, 694, 133649–133660. [Google Scholar] [CrossRef]
- Jin, X.; Li, S. Phosphine-Functionalized Polyether Quaternary Phosphonium Salt Ionic Liquid, and Method Based on the Same for Double-Phase Hydroformylation of Olefin. Chinese Patent 105017319A, 4 November 2015. [Google Scholar]
- Zarejousheghani, M.; Jaafar, A.; Wollmerstaedt, H.; Rahimi, P.; Borsdorf, H.; Zimmermann, S.; Joseph, Y. Rational design of molecularly imprinted polymers using quaternary ammonium cations for glyphosate detection. Sensors 2021, 21, 296. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, T.; Gong, Z. Process improvement of the tWIPO Patent -step method for the synthesis of dimethyldiallylammonium chloride. Proc. China Assoc. Sci. Technol. 2008, 4, 268–273. [Google Scholar]
- Hunter, W.E.; Sieder, T.P. Diallyl Dimethyl Ammonium Chloride and Polydiallyl Dimethyl Ammonium Chloride. U.S. Patent 4151202A, 24 April 1979. [Google Scholar]
- Wandrey, C.; Hernandez-Barajas, J.; Hunkeler, D. Diallyldimethylammonium chloride and its polymers. Adv. Polym. Sci. 1999, 145, 123–182. [Google Scholar]
- Butler, G.B.; Ingley, F.L. Preparation and polymerization of unsaturated quaternary ammonium compounds. II. Halogenated allyl derivatives. J. Am. Chem. Soc. 1951, 73, 895–896. [Google Scholar] [CrossRef]
- Harada, S.; Arai, K. Cyclocopolymerization of diallyl compounds and sulfur dioxide. II. Diallyldimethylammonium chloride and sulfur dioxide. Makromol. Chem. 1967, 107, 64–77. [Google Scholar] [CrossRef]
- Boothe, J.E. Dimethyl Diallyl Ammonium Chloride. U.S. Patent 3461163A, 12 August 1969. [Google Scholar]
- Fang, Z.; Yang, Y.; Gu, J.; Yang, Z.; Dai, F.; Zheng, H.; He, W.; Liu, C.; Zhu, N.; Guo, K. Synthesis and scale-up of water-soluble quaternary cationic monomers in a continuous flow system. React. Chem. Eng. 2019, 4, 919–926. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Zhang, Y.; Liu, D. A Purification Method for the Cationic Monomer of Dimethyldiallylammonium Chloride with High Purity. Chinese Patent 106518689B, 18 January 2019. [Google Scholar]
- Xu, Q.; Liu, H.; Zheng, H.; Liang, J. UV-H2O2 preparation of cationic polyacrylamide (CPAM) and its application in sludge dewatering. Sci. Adv. Mater. 2019, 11, 842–853. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Y.; Sun, W.; Shah, K.J.; Xu, Y.; Zheng, H. Efficient cationic flocculant MHCS-g-P(AM-DAC) synthesized by UV-induced polymerization for algae removal. Sep. Purif. Technol. 2019, 210, 10–19. [Google Scholar] [CrossRef]
- Beg, M.; Sharma, S.; Ojha, U. Effect of cationic copolyelectrolyte additives on drilling fluids for shales. J. Pet. Sci. Eng. 2018, 161, 506–514. [Google Scholar] [CrossRef]
- Paul, J.-M.; Graire, C.; Riechl, A. Method for Purifying the Azeotropic Fraction Generated During the Synthesis of N,N-dimethyl Aminoethyl Acrylate. U.S. Patent 20120035389A1, 19 February 2012. [Google Scholar]
- Schmitt, B.; Knebel, J.; Caspari, M. Synthesis of Alkylaminoalkyl (meth)Acrylate by Transesterification. U.S. Patent 200402491.91A1, 9 December 2004. [Google Scholar]
- Nair, M.; Calbick, C.J. Process for Preparation of Unsaturated Quaternary Ammonium Salts. WIPO Patent 2011011352A1, 27 January 2011. [Google Scholar]
- Guliashvili, T.; Vasconcellos, S.R. Methods of Preparing Novel Halide Anion Free Quaternary Ammonium Salt Monomers, Polymerization Methods Therefor, and Methods of Use of the Resulting Polymers. U.S. Patent 20120125863A1, 24 May 2012. [Google Scholar]
- Urbanova, A.; Ezenwajiaku, I.H.; Nikitin, A.N.; Sedlak, M.; Vale, H.M.; Hutchinson, R.A.; Lacik, I. PLP-SEC investigation of the influence of electrostatic interactions on the radical propagation rate coefficients of cationic monomers TMAEMC and MAPTAC. Macromolecules 2021, 54, 3204–3222. [Google Scholar] [CrossRef]
- Humbert, H.; Laping, K. Crystalline Acrylamido- and Methacrylamidoalkyltrialkylammonium Chlorides. German Patent 3330326A1, 7 March 1985. [Google Scholar]
- Yu, L.; Xu, R.; Dong, X.; Liu, Y.; Sun, Y. Protein adsorption to (3-acrylamido propyl) trimethyl ammonium chloride-grafted Sepharose gel: Charge density reduction via copolymerizing with electroneutral monomer drastically increases uptake rate. J. Chromatogr. A 2020, 1629, 461483–461496. [Google Scholar] [CrossRef]
- Mallon, J.J.; Farinato, R.S.; Rosati, L.; Freeman, J.J., Jr. Cationic Water-Soluble Polymer Precipitation in Salt Solutions. WIPO Patent 9814490A1, 9 April 1998. [Google Scholar]
- Rivas, B.L.; del Carmen Aguirre, M.; Pereira, E. Cationic water-soluble polymers with the ability to remove arsenate through an ultrafiltration technique. J. Appl. Polym. Sci. 2007, 106, 89–94. [Google Scholar] [CrossRef]
- Muneharu, M.; Keizo, M.; Hisanari, M. Preparation of N-Substituted Acrylamide. Japanese Patent S5976044A, 28 April 1984. [Google Scholar]
- Takao, Y.; Oogami, H. Method of Manufacturing N-Monosubstituted (meth) Acrylamides. U.S. Patent 5587515A, 24 December 1996. [Google Scholar]
- Wenzel, F.; Arndt, P.J.; Schlosser, F.; Besecke, S.; Hohage, H.J.; Schroeder, G. Method for Making N-Substituted Acrylamides and Methacrylamides. U.S. Patent 4206143A, 3 June 1980. [Google Scholar]
- Jerry, B. Some homo-and copolymerlzation studies of diallyldimethyl ammonium chloride. Macromol. Sci. Chem. 1970, 4, 1419–1430. [Google Scholar]
- Abdollahi, M.; Ziaee, F.; Alamdari, P.; Koolivand, H. A comprehensive study on the kinetics of aqueous free-radical homo- and copolymerization of acrylamide and diallyldimethylammonium chloride by online 1H-NMR spectroscopy. J. Polym. Res. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Williams, J.D.; Robles, F.E.; Ransohoff, J.A. High Molecular Weight Poly(dialkyldiallylammonium halide). WIPO Patent /1999/045037, 10 September 1999. [Google Scholar]
- Liu, B.; Zheng, H.; Deng, X.; Xu, B.; Sun, Y.; Liu, Y.; Liang, J. Formation of cationic hydrophobic micro-blocks in P(AM-DMC) by template assembly: Characterization and application in sludge dewatering. RSC Adv. 2017, 7, 6114–6122. [Google Scholar] [CrossRef] [Green Version]
- Schuller, W.H.; Thomas, W.M. Composition Comprising a Linear Copolymer of a Quaternary Ammonium Compound and an Ethylenically Unsaturated Copolymerizable Compound. U.S. Patent 2923701A, 2 February 1960. [Google Scholar]
- Liao, Y.; Zheng, X.; Zhang, Z.; Xu, B.; Sun, Y.; Liu, Y.; Zheng, H. Ultrasound-assisted polymerization of P(AM-DMDAAC): Synthesis, characterization and sludge dewatering performance. J. Environ. Chem. Eng. 2017, 5, 5439–5447. [Google Scholar] [CrossRef]
- Demarteau, J.; Fernandez de Anastro, A.; Shaplov, A.S.; Mecerreyes, D. Poly(diallyldimethylammonium) based poly(ionic liquid) di- and triblock copolymers by PISA as matrices for ionogel membranes. Polym. Chem. 2020, 11, 1481–1488. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, K. Method for Preparing Copolymer of Dimethyl Diallyl Ammonium Chloride and Acrylamide Through Initiation of Composite Initiator. Chinese Patent 102206303A, 5 October 2011. [Google Scholar]
- Nishikaji, T.; Watanabe, K.; Sawayama, S.; Matsumoto, S. Trimethylammonioethyl Methacrylate. German Patent 2608868A1, 16 September 1976. [Google Scholar]
- Anon, Copolymer of a carboxamide and (meth)Acryloyloxyethyltrimethylammonium chloride. Res. Discl. 1980, 198, 411.
- Arndt, P.J.; Lowitz, J.; Wenzel, F. Liquid, Directly Polymerizable Mixture of Acrylamide and Quaternary Products of Tertiary Aminoalkyl Esters or Tertiary Aminoalkyl Amides of Acrylic or Methacrylic Acid. German Patent 2848627B1, 7 February 1980. [Google Scholar]
- Takeda, H.; Aoyama, K. Water-Soluble Polymer-Containing Emulsion Compositions. Japanese Patent 06025540A, 26 May 1994. [Google Scholar]
- Ringsdorf, H.; Thunig, D. On the kinetics of the polymerization of some ammonium methacrylates with different alkyl chain lengths in aqueous solution. Makromol. Chem. 1977, 178, 2205–2210. [Google Scholar] [CrossRef]
- Zhang, G.; Li, M. Synthsis and characterization of the cationic polyelectrolyte (PDMC). J. Hubei Univ. Sci. Technol. 1999, 3, 71–75. [Google Scholar]
- Zhang, Y.; Tang, L.; Wang, T.; Liu, Z. Synthesis Method of PDMC with High Molecular Weight. Chinese Patent 105017452A, 4 November 2015. [Google Scholar]
- Huang, C.; Yan, M.; Jiang, Y.; Yang, Q.; Chen, Y.; Wang, S. Study on the synthesis of CPAM paper reinforcing agent in aqueous tWIPO Patent -phase system. Asian J. Chem. 2013, 25, 1301–1306. [Google Scholar]
- Djibrine, B.Z.; Zheng, H.; Wang, M.; Liu, S.; Tang, X.; Khan, S.; Jimenez, A.N.; Feng, L. An effective flocculation method to the kaolin wastewater treatment by a cationic polyacrylamide (CPAM): Preparation, characterization, and flocculation performance. Int. J. Polym. Sci. 2018, 2018, 5294251. [Google Scholar] [CrossRef]
- Cheng, Z.; Dong, Z.; Su, M.; Zhang, Y.; Wang, Z.; He, P. Synthesis of cationic polyacrylamide via inverse emulsion polymerization method for the application in water treatment. J. Macromol. Sci. Part A Pure Appl. Chem. 2019, 56, 76–85. [Google Scholar] [CrossRef]
- Goel, N.K.; Joshi, R.; Bhardwaj, Y.K.; Varshney, L. Role of radiolytically generated species in polymerization of [2-(acryloyloxy)ethyl]trimethylammonium chloride (AETC) in aqueous medium: Steady state and pulse radiolysis study. Radiat. Phys. Chem. 2013, 92, 66–70. [Google Scholar] [CrossRef]
- Millard, P.-E.; Herth, G.; Friedrich, S. Preparation and Use of New Water Soluble Cationic Polymers. WIPO Patent 2013153004A1, 17 October 2013. [Google Scholar]
- Zhang, Y.; Wang, M.; Chen, T. Preparation Method of High-Relative-Molecular-Mass Poly Acryloyloxyethyl Trimethyl Ammonium Chloride. Chinese Patent 109880004A, 14 June 2019. [Google Scholar]
- Jean, C.; Claude, T. Strong Cationic Polyelectrolytes in a Powder from Acrylamide and Quaternized or Salt-Combined Dimethylaminoethyl Acrylate and Their Use for Flocculation of Solid-Material Suspensions and the Coalescence of Emulsions. French Patent 2471391A1, 19 June 1981. [Google Scholar]
- Zhang, Y.; Chen, T. Preparation Method of P(DAC-AM) with Serial Cationicities and High Intrinsic Viscosity. Chinese Patent 109880005B, 8 June 2021. [Google Scholar]
- Rivas, B.L.; Pereira, E.D.; Horta, A.; Renamayor, C.S. Macromolecular size of polyelectrolytes containing ammonium and sulfonic acid groups, as determined by light scattering. Eur. Polym. J. 2003, 40, 203–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y. Preparation Method of Poly(methacrylamidopropyltrimethylammonium) Chloride with Serialized Characteristic Viscosity. Chinese Patent 109912739A, 21 June 2019. [Google Scholar]
- Phillips, K.G.; Ballweber, E.G.; Edwards, K.R. Water-in-Oil Emulsions Containing Copolymers of MAPTAC and Acrylamide. Canada Patent 1133788A1, 19 October 1982. [Google Scholar]
- Tanaka, H. Copolymerization of cationic monomers with acrylamide in an aqueous solution. J. Polym. Sci. Polym. Chem. Ed. 1986, 24, 29–36. [Google Scholar] [CrossRef]
- Yuan, Z.; Cheng, Y. Study on preparation and flocculation perpormance of cationic macromolecule flocculant. J. Chem. Ind. Eng. 2011, 32, 5–10. [Google Scholar]
- Li, X.; Ning, J.; Chen, L.; Wang, H.; Tian, X. Aqueous Solution Polymerization Preparation Method for (meth)Acrylamido Propyl Trimethyl Ammonium Chloride and Acrylamide Copolymer. Chinese Patent 103570868A, 12 February 2014. [Google Scholar]
- Bi, K.; Jiang, J.; Tang, X.; Lu, W. Aqueous Solution Polymerization Preparation Method of Methacrylamidopropyltrimethylammonium Chloride and Acrylamide copolymer. Chinese Patent 105111366A, 2 December 2015. [Google Scholar]
- Li, X.; Ning, J.; Chen, L.; Wang, H.; Tian, X. A Kind of Preparation Method of High Relative Molecular Mass Acrylamido Propyl Trimethyl Ammonium Chloride Cationoid Monomer Homopolymers. Chinese Patent 104497184B, 33 March 2019. [Google Scholar]
- Fu, X.; Chen, T.; Wang, C.; Zhang, Y. Rheological Behavior of a Quaternary Ammonium Copolymer in the Presence of Inorganic Salts. J. Macromol. Sci. Part B Phys. 2021, 60, 18–29. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Jia, X. Synthesis of ultra high molecular weight poly(dimethyldiallyl ammonium chloride). Russ. J. Appl. Chem. 2016, 89, 315–323. [Google Scholar] [CrossRef]
- Xiong, Q. Primary Research on Preparation Process of Low Residuce Monomers and Narrow Distribution PDA with Cationicity of 70% and 50%; Nanjing University of Science and Technology: Nanjing, China, 2013. [Google Scholar]
- Chiefari, J.; Mayadunne, R.T.A.; Moad, C.L.; Moad, G.; Rizzardo, E.; Postma, A.; Thang, S.H. Thiocarbonylthio Compounds (SC(Z)S−R) in Free Radical Polymerization with Reversible Addition-Fragmentation Chain Transfer (RAFT Polymerization). Effect of the Activating Group Z. Macromolecules 2003, 36, 2273–2283. [Google Scholar] [CrossRef]
- Plaud, B.; Meretoja, O.; Hofmockel, R.; Raft, J.; Stoddart, P.A.; van Kuijk, J.H.; Hermens, Y.; Mirakhur, R.K. Reversal of Rocuronium-induced Meeting Abstracts with Sugammadex in Pediatric and Adult Surgical Patients. J. Am. Soc. Anesthesiol. 2009, 110, 284–294. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Shi, X.; Zhang, Z.; Kuchel, R.P.; Namivandi-Zangeneh, R.; Corrigan, N.; Jung, K.; Liang, K.; Boyer, C. Porphyrinic Zirconium Metal–Organic FrameWIPO Patent rks (MOFs) as Heterogeneous Photocatalysts for PET-RAFT Polymerization and Stereolithography. Angew. Chem. 2021, 133, 5549–5556. [Google Scholar] [CrossRef]
- Ohno, S.; Ishihara, K.; Yusa, S.-I. Formation of Polyion Complex (PIC) Micelles and Vesicles with Anionic pH-Responsive Unimer Micelles and Cationic Diblock Copolymers in Water. Langmuir 2016, 32, 3945–3953. [Google Scholar] [CrossRef]
- Huang, B.; Jiang, J.; Kang, M.; Liu, P.; Sun, H.; Li, B.-G.; Wang, W.-J. Synthesis of block cationic polyacrylamide precursors using an aqueous RAFT dispersion polymerization. RSC Adv. 2019, 9, 12370–12383. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Lv, X.; An, Z. Modular Monomers with Tunable Solubility: Synthesis of Highly Incompatible Block Copolymer Nano-Objects via RAFT Aqueous Dispersion Polymerization. ACS Macro Lett. 2017, 6, 224–228. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. RAFT polymerization and some of its applications. Chem. Asian J. 2013, 8, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Sponchioni, M.; Capasso Palmiero, U.; Manfredini, N.; Moscatelli, D. RAFT copolymerization of oppositely charged monomers and its use to tailor the composition of nonfouling polyampholytes with an UCST behaviour. React. Chem. Eng. 2019, 4, 436–446. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, T.; Wang, M. Preparation Method of PDAC with Serialized Relative Molecular Mass and High Monomer Conversion Rate. Chinese Patent 109824807B, 12 February 2021. [Google Scholar]
- Luo, J.; Feng, Y. Synthesis of an amphoteric polymer auxiliary agent and its application to chrome-free leather. J. Soc. Leather Technol. Chem. 2018, 102, 298–303. [Google Scholar]
- Kou, R.; Zhang, J.; Chen, Z.; Liu, G. Counterion Specificity of Polyelectrolyte Brushes: Role of Specific Ion-Pairing Interactions. Chem. Phys. Chem. 2018, 19, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Loehmann, O.; Micciulla, S.; Soltwedel, O.; Schneck, E.; von Klitzing, R. Swelling Behavior of Composite Systems: Mutual Effects between Polyelectrolyte Brushes and Multilayers. Macromolecules 2018, 51, 2996–3005. [Google Scholar] [CrossRef]
- Mishra, R.K.; Ramasamy, K.; Ban, N.N.; Majeed, A.B.A. Synthesis of poly[3-(methacryloylamino) propyl trimethylammonium chloride-co-methacrylic acid] copolymer hydrogels for controlled indomethacin delivery. J. Appl. Polym. Sci. 2013, 128, 3365–3374. [Google Scholar] [CrossRef]
- Mei, L.; Ren, Y.; Loontjens, T.J.A.; van der Mei, H.C.; Busscher, H.J. Contact-killing of adhering streptococci by a quaternary ammonium compound incorporated in an acrylic resin. Int. J. Artif. Organs 2012, 35, 854–863. [Google Scholar] [CrossRef]
- Wei, W.; Qi, X.; Li, J.; Zhong, Y.; Zuo, G.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Synthesis and characterization of a novel cationic hydrogel base on salecan-g-PMAPTAC. Int. J. Biol. Macromol. 2017, 101, 474–480. [Google Scholar] [CrossRef]
- Pal, P.; Pandey, J.P.; Sen, G. Synthesis, characterization and flocculation studies of a novel graft copolymer towards destabilization of carbon nano-tubes from effluent. Polymer 2017, 112, 159–168. [Google Scholar] [CrossRef]
- Ying, C.; Tian, G.; Liang, Y.; Qi, Z.; Ji, L. Ammonium persulfate-initiated polymerization of cationic starch-grafted-cationic polyacrylamide flocculant for the enhanced flocculation of oil sludge suspension. J. Dispers. Sci. Technol. 2019, 40, 1246–1255. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhang, F.; Chen, W.; Wei, X.; Zhang, Y.; Lu, Y.; Luo, H.; Fan, L. Study on nano-chitosan grafting quaternary ammonium salt modified polyacrylamide for flocculation and sterilization. Desalin. Water Treat. 2020, 178, 123–135. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.-M.; Sun, D.; Yuan, T.-Q.; Song, G.-Y.; Shi, Q.; Zheng, L.; Wang, S.-F.; Sun, R.-C. Chemosynthesis, characterization and application of lignin-based flocculants with tunable performance prepared by short-wavelength ultraviolet initiation. Ind. Crops Prod. 2020, 157, 112897–112908. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y. Molecular-weight-controlled synthesis and dye-fixing properties of poly(dimethyldiallylammonium chloride). J. Vinyl Addit. Technol. 2010, 16, 277–283. [Google Scholar] [CrossRef]
- Huang, H.; Huang, C.S.; Cheng, W. Process for Fast Dissolution of Powder Comprising Low Molecular Weight Acrylamide-Based Polymer and Papermaking. WIPO Patent 2019028001A1, 7 February 2019. [Google Scholar]
- Hietaniemi, M.; Karppi, A.; Rautiainen, J. Polymeric Structure and Its Use in Paper and Board Making Applications. WIPO Patent 2020260760A1, 30 December 2020. [Google Scholar]
- Zhao, X.; Zhang, Y. Bacteria-removing and bactericidal efficiencies of PDADMAC composite coagulants in enhanced coagulation treatment. CLEAN-Soil Air Water 2013, 41, 37–42. [Google Scholar] [CrossRef]
- Chen, T.; Fu, X.; Zhang, L.; Zhang, Y. Viscosity behavior of P(DAC-AM) with serial cationicity and intrinsic viscosity in inorganic salt solutions. Polymers 2019, 11, 1944. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Chen, T.; Zhang, Y.; Hou, Y.; Zhong, X.; Huang, B. Influence of organic flocculants on the flocculation performance of aerobic sludge. Desalin. Water Treat. 2020, 189, 45–52. [Google Scholar] [CrossRef]
- Yang, K.; Chen, J.; Liang, S.; Wang, Y.; Yao, C. Preparation of super high concentration cationic polyacrylamide emulsion and its flocculation with cationic polymers. Polym. Adv. Technol. 2020, 31, 2711–2721. [Google Scholar] [CrossRef]
- Chen, T.; Fu, X.; Zhang, Y.; Liu, C.; Zhong, X. A novel way of dealing with waste sludge from poly-aluminum chloride (PAC) production. Desalin. Water Treat. 2019, 151, 342–349. [Google Scholar] [CrossRef]
- Jia, X. Researches on the Preparation, Polymerization Mechanism and Relationships between Structures, Properties and Performances of Poly(dimethyldiallylammonium chloride); Najing University of Science and Technology: Najing, China, 2010. [Google Scholar]
- Rabiee, A.; Ershad-Langroudi, A.; Zeynali, M.E. A survey on cationic polyelectrolytes and their applications: Acrylamide derivatives. Rev. Chem. Eng. 2015, 31, 239–261. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Y. Dependence of intrinsic viscosity and molecular size on molecular weight of partially hydrolyzed polyacrylamide. J. Appl. Polym. Sci. 2021, 138, 50850–50860. [Google Scholar] [CrossRef]
- Su, G.; Geng, T.; Jiang, Y.-J.; Ju, H.-B.; Wang, Y.-K. Synthesis, characterization, flocculation and antistatic properties of poly(methacryloyloxyethyl trimethyl ammonium chloride). Tenside Surfactants Deterg. 2020, 57, 318–325. [Google Scholar]
- Lin, L.; Li, X.; Shi, C.; Mao, Y. Desorption of hydrolyzed poly (AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures. E-Polym. 2019, 19, 527–534. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Huang, C.-W.; Tsai, P.-S. Preparation of poly [3-(methacryloylamino) propyl] trimethylammonium chloride coated mesh for oil–water separation. Desal. Wat. Treat 2019, 158, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhang, Y. Dye-fixing Behaviors of Novel Reactive Cationic Copolymers of 3-Chloro-2 hydroxypropylmethyldiallylammonium Chloride and Dimethyldiallylammonium Chloride (P(CMDA-DMDAAC) s) on Cotton Fabric. J. Chem. Soc. Pak. 2013, 35, 282–289. [Google Scholar]
- Zhang, X.; Jia, X.; Zhang, Y.; Tao, X. Synthesis and characterization of poly (N,N-diallyl-nmethylpropylammonium chloride). Fine Chem. 2019, 36, 1–8. [Google Scholar]
- Guo, K.; Gao, Y.; Gao, B.; Feng, Q.; Shen, X.; Liu, C.; Yue, Q.; Xu, X. Structure-activity relationships of the papermill sludge-based flocculants in different dye wastewater treatment. J. Clean. Prod. 2020, 266, 121944–121953. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Fakoorpoor, S.M.; Hosseini, S.H. Novel cationic-modified salep as an efficient flocculating agent for settling of cement slurries. Carbohydr. Polym. 2013, 93, 506–516. [Google Scholar] [CrossRef]
- Cantley, M.; Laget, P. Environmental Risks of Biotechnologies: Economic Sector Perspectives. Enviro. Biotech. 2016, 9, 407–446. [Google Scholar]
- Tanaka, T.S.; Pirbazari, M. Effects of cationic polyelectrolytes on the removal of suspended particulates during direct filtration. J.-Am. Water Work. Assoc. 1986, 78, 57–65. [Google Scholar] [CrossRef]
- Haarhoff, J.; Cleasby, J.L. Direct filtration of Chlorella with cationic polymer. J. Environ. Eng. 1989, 115, 348–366. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Zhao, X.; Gao, N.; Fu, T. The characteristics of sludge from enhanced coagulation processes using PAC/PDMDAAC composite coagulants in treatment of micro-polluted raw water. Sep. Purif. Technol. 2015, 147, 125–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Li, X.; Liu, C.; Zhu, L. Algae-removal efficiency of AS/PDM used for the Taihu Lake prechlorination algae-rich water in summer. J. Chem. Eng. Chin. Univ. 2011, 25, 331–336. [Google Scholar]
- Zhao, X.; Zhang, Y.; Li, X.; Liu, C.; Zhu, L. Algae removal efficiencies of AS/PDMDAAC coagulants. J.-Am. Water Work. Assoc. 2010, 102, 119–128. [Google Scholar] [CrossRef]
- Shen, X.; Gao, B.; Guo, K.; Yu, C.; Yue, Q. PAC-PDMDAAC pretreatment of typical natural organic matter mixtures: Ultrafiltration membrane fouling control and mechanisms. Sci. Total Environ. 2019, 694, 133816. [Google Scholar] [CrossRef]
- Wang, T.; Tang, X.; Zhang, S.; Zheng, J.; Zheng, H.; Fang, L. Roles of functional microbial flocculant in dyeing wastewater treatment: Bridging and adsorption. J. Hazard. Mater. 2020, 384, 121506–121513. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, H.; Guan, Q.; Teng, H.; Zhao, C.; Zhao, C. Fabricating a flocculant with controllable cationic microblock structure: Characterization and sludge conditioning behavior evaluation. Ind. Eng. Chem. Res. 2016, 55, 2892–2902. [Google Scholar] [CrossRef]
- Ramesh, M.; Doucette, C.C.; Cooper, A.J. Antifouling, Antimicrobial Composition of Polymer(s), Ammonium Salt(s) and a Chlorine Source. WIPO Patent 2010093847A1, 19 August 2010. [Google Scholar]
- Ueda, M.; Inui, A.; Kozu, Y. Hair Cosmetic Compositions Comprising Surfactants and Cationic Polymers and Oils. U.S. Patent 20170119648A1, 4 May 2017. [Google Scholar]
- Franzke, M.; Moenks, M.; Schiemann, H.; Florig, E.; Baecker, S.; Roettger, C.; Gaenger, K. Clear, Tow-Phase, Foam-Forming Aerosol Hairstyling Product. WIPO Patent 2006050788A1, 18 May 2006. [Google Scholar]
- Homola, A.M.; Dunton, R.K. Methods, Compositions, and Dental Delivery Systems for the Protection of the Surfaces of the Teeth. WIPO Patent 9802136A1, 22 January 1998. [Google Scholar]
- Ihikawa, A.; Fujii, Y.; Miyake, T. Nonionic Surfactant-Based Laundry Detergent Containing Antimicrobial Quanteranry Ammonium Polymers. Japanese Patent 200260787, 26 February 2002. [Google Scholar]
- Yamazaki, R. Skin Cleansing Composition. European Patent 1384470A1, 28 January 2004. [Google Scholar]
- Gao, Y.; Tong, R. Hair Styling Composition. Chinese Patent 106420402A, 22 February 2017. [Google Scholar]
- Sun, J. Hair Moisturizing Caring Agent. Chinese Patent 104622725A, 20 May 2015. [Google Scholar]
- Wang, X.; Yang, W.; Guo, J.; Tan, J. Prepn of Water Soluble Quarternary Copolymer Softener. Chinese Patent 1322876A, 21 November 2001. [Google Scholar]
- Hayakawa, K. Skin or Hair Care Cosmetic Composition. Chinese Patent 1522684A, 25 August 2004. [Google Scholar]
- Gijsbert, K.; Michael, F.; Nathalie, S.; Tuttu, N.; Kulcsar, L. Composition Comprising Conditioning and/or Styling Polymer. European Patent 2914239A1, 9 September 2015. [Google Scholar]
- Zhou, X.; Lin, Y.; Zhang, W.; Zhang, C. Anti-Dandruff and Itching-Relieving Hair-Washing Composition and Preparation Method Thereof. Chinese Patent 107951792A, 24 April 2018. [Google Scholar]
- Zeng, W.; Qin, G.; Chen, H.; Huang, N.; Zeng, J. Shower Gel and Preparation Method Thereof. Chinese Patent 107375063A, 24 November 2017. [Google Scholar]
- Bai, X.; Yang, Y.; Xiao, D.; Pu, X.; Wang, X. Synthesis, characterization, and performance evaluation of the AM/AMPS/DMDAAC/SSS quadripolymer as a fluid loss additive for water-based drilling fluid. J. Appl. Polym. Sci. 2015, 132, 41762–41772. [Google Scholar] [CrossRef]
- Lü, T.; Qi, D.; Zhao, H.; Cheng, Y. Synthesis of hydrophobically modified flocculant by aqueous dispersion polymerization and its application in oily wastewater treatment. Polym. Eng. Sci. 2015, 55, 1–7. [Google Scholar] [CrossRef]
- Tian, G.; Chen, Y.; Liang, Y.; Gao, Y. Synthesis of nanocomposites from cationic polyacrylamide and modified carbon black: Application as flocculants for oily sludge suspension. Appl. Organomet. Chem. 2019, 33, 4620–4630. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, M.; Xu, Y.; Xia, X.; Zhang, C.; Yu, Y.; Feng, Y.; Guo, J. Inhibitory effects of functionalized polycarboxylate retarder on aberrant thickening phenomena of oil well cement at high temperature. Constr. Build. Mater. 2021, 274, 121994. [Google Scholar] [CrossRef]
- Peiffer, D.G.; Lundberg, R.D.; Sedillo, L.; Newlove, J.C. Fluid Loss Control in Oil Field Cements. U.S. Patent 4626285A, 2 December 1986. [Google Scholar]
- Wang, J.J.; Zheng, J.; Farrar, D.; Song, F. Synthesis and Application of High Pressure High Temperature Fluid Loss Additive and Rheology Stabilizer. WIPO Patent 2013138156A1, 19 September 2013. [Google Scholar]
- Butler, G.B.; Angelo, R.J. Preparation and polymerization of unsaturated quaternary ammonium compounds. VIII. A proposed alternating intramolecular-intermolecular chain propagation1. J. Am. Chem. Soc. 1957, 79, 3128–3131. [Google Scholar] [CrossRef]
- Shixichiro, S. Ink-Jet Recording Paper Containing Polydimethyldiallylammonium Chloride. Japanese Patent 10114144A, 6 May 1998. [Google Scholar]
- Nakamura, T.; Chiba, M.; Matsuhisa, S. Method for Improving the Interlayer Adhesion of Combination-Formed Cardboard. Japanese Patent 2002294595A, 9 October 2002. [Google Scholar]
- Borkar, S.; Putnam, M.C. Surface Application of Polymers and Polymer Mixtures to Improve Paper Strength. WIPO Patent 2011057044A2, 5 December 2011. [Google Scholar]
- Shan, Y.Y.; Fu, Y.J.; Qin, M.H. In Synthesis of star cationic polyacrylamide and its application in the retention and drainage system of papermaking. Adv. Mater. Res. 2012, 36, 2256–2259. [Google Scholar]
- Zhao, F.; He, J. Preparations of novel flocculants and its applications in the paper-making wastewater. Tenside Surfactants Deterg. 2011, 48, 318–322. [Google Scholar] [CrossRef]
- Yang, K.; Chen, J.; Yao, C. Cationic polyacrylamide emulsion with ultra-high concentration as a flocculant for paper mill wastewater treatment. BioResources 2020, 15, 3173–3189. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, X.; Ma, J. Synthesis of cationic fluorinated polyacrylate copolymer by RAFT emulsifier-free emulsion polymerization and its application as waterborne textile finishing agent. Dyes Pigment. 2017, 139, 102–109. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.-n.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.-h. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Maiti, C.; Dey, D.; Mandal, S.; Dhara, D. Thermoregulated formation and disintegration of cationic block copolymer vesicles: Fluorescence resonance energy transfer study. J. Phys. Chem. B. 2014, 118, 2274–2283. [Google Scholar] [CrossRef]
- Alasino, R.V.; Bierbrauer, K.L.; Beltramo, D.M.; Correa, S.G.; Bianco, I.D. Cationic Polymers for Biotechnological Applications. Front. Biomater. 2016, 2, 3–27. [Google Scholar]
- Kostova, B.; Georgieva, D.; Dundarova, M.; Ivanova, S.; Ivanova-Mileva, K.; Tzankova, V.; Christova, D. Design and study of the potential of crosslinked cationic polymers as drug delivery systems for dermal application. J. Appl. Polym. Sci. 2018, 135, 46420–46429. [Google Scholar] [CrossRef]
- Lv, X.; Liu, C.; Song, S.; Qiao, Y.; Hu, Y.; Li, P.; Li, Z.; Sun, S. Construction of a quaternary ammonium salt platform with different alkyl groups for antibacterial and biosensor applications. RSC Adv. 2018, 8, 2941–2949. [Google Scholar] [CrossRef] [Green Version]





| Polymer | Researcher | Cationicity/% | Key Conditions | [η]/(dL·g−1) |
|---|---|---|---|---|
| PDMDAAC | Ranohoff | - | Co-60, radiation polymerization, 20 h | 7.7 (injector) |
| PDMDAAC | Jia Xu | - | stepwise temperature, 9 h | 4.70 |
| PDA | Zhang Y J | 5–50 | redox initiator, stepwise temperature | 21.0–7.0 |
| PDAC | Zhang Y J | - | APS, stepwise temperature | 14.6 |
| P(DAC-AM) | Zhang Y J | 10–90 | redox initiator, stepwise temperature | 26.3–15.1 |
| PDMC | Zhang Y J | - | KPS, stepwise temperature | 14.5 |
| P(DMC-AM) | Zhang Y J | 10–90 | compound initiator, stepwise temperature | 18.2–14.1 |
| PMAPTAC | Zhang Y J | - | stepwise temperature | 8.2 |
| P(MAPTAC-AM) | Li X X | 5–50 | stepwise temperature | 17.2–9.7 |
| PAPTAC | Li X X | - | stepwise temperature | unspecified |
| P(APTAC-AM) | Li X X | 13~75 | stepwise temperature | 21.5~9.7 |
| Polymer | K | α | Temperature/°C | Condition |
|---|---|---|---|---|
| PDMDAAC | 4.61 × 10−3 | 0.81 | 25 | 8.9 × 104 < Mw < 4.7 × 105 |
| PDMDAAC | 1.01 × 10−2 | 0.767 | 30 | [η] < 1.57 dL/g |
| PDMDAAC | 0.313 | 0.504 | 30 | 1.57 dL/g < [η] < 4.49 dL/g |
| Other typical cationic polymers | 4.75 × 10−3 | 0.8 | 30 | high molecular weight polymer |
| Partially hydrolyzed polyacrylamide | 6.98 × 10−4 | 0.91 | 0.99 dL/g < [η] < 2.87 dL/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Zhang, Y.; Jia, X.; Wang, Y.; Chen, T. Research Progress on Typical Quaternary Ammonium Salt Polymers. Molecules 2022, 27, 1267. https://doi.org/10.3390/molecules27041267
Fu X, Zhang Y, Jia X, Wang Y, Chen T. Research Progress on Typical Quaternary Ammonium Salt Polymers. Molecules. 2022; 27(4):1267. https://doi.org/10.3390/molecules27041267
Chicago/Turabian StyleFu, Xingqin, Yuejun Zhang, Xu Jia, Yongji Wang, and Tingting Chen. 2022. "Research Progress on Typical Quaternary Ammonium Salt Polymers" Molecules 27, no. 4: 1267. https://doi.org/10.3390/molecules27041267
APA StyleFu, X., Zhang, Y., Jia, X., Wang, Y., & Chen, T. (2022). Research Progress on Typical Quaternary Ammonium Salt Polymers. Molecules, 27(4), 1267. https://doi.org/10.3390/molecules27041267






