A Mixture of Topical Forms of Polydeoxyribonucleotide, Vitamin C, and Niacinamide Attenuated Skin Pigmentation and Increased Skin Elasticity by Modulating Nuclear Factor Erythroid 2-like 2
Abstract
:1. Introduction
2. Results
2.1. PVN Showed Highest Effect on Decreasing Melanin Amount among Various Combinations of Niacinamide, Vitamin C, and PDRN
2.2. PVN Increased the Expression of pNRF2 and HO-1 and Decreased NADPH Oxidase Levels in Both In Vitro and In Vivo Models of UV-B Radiation
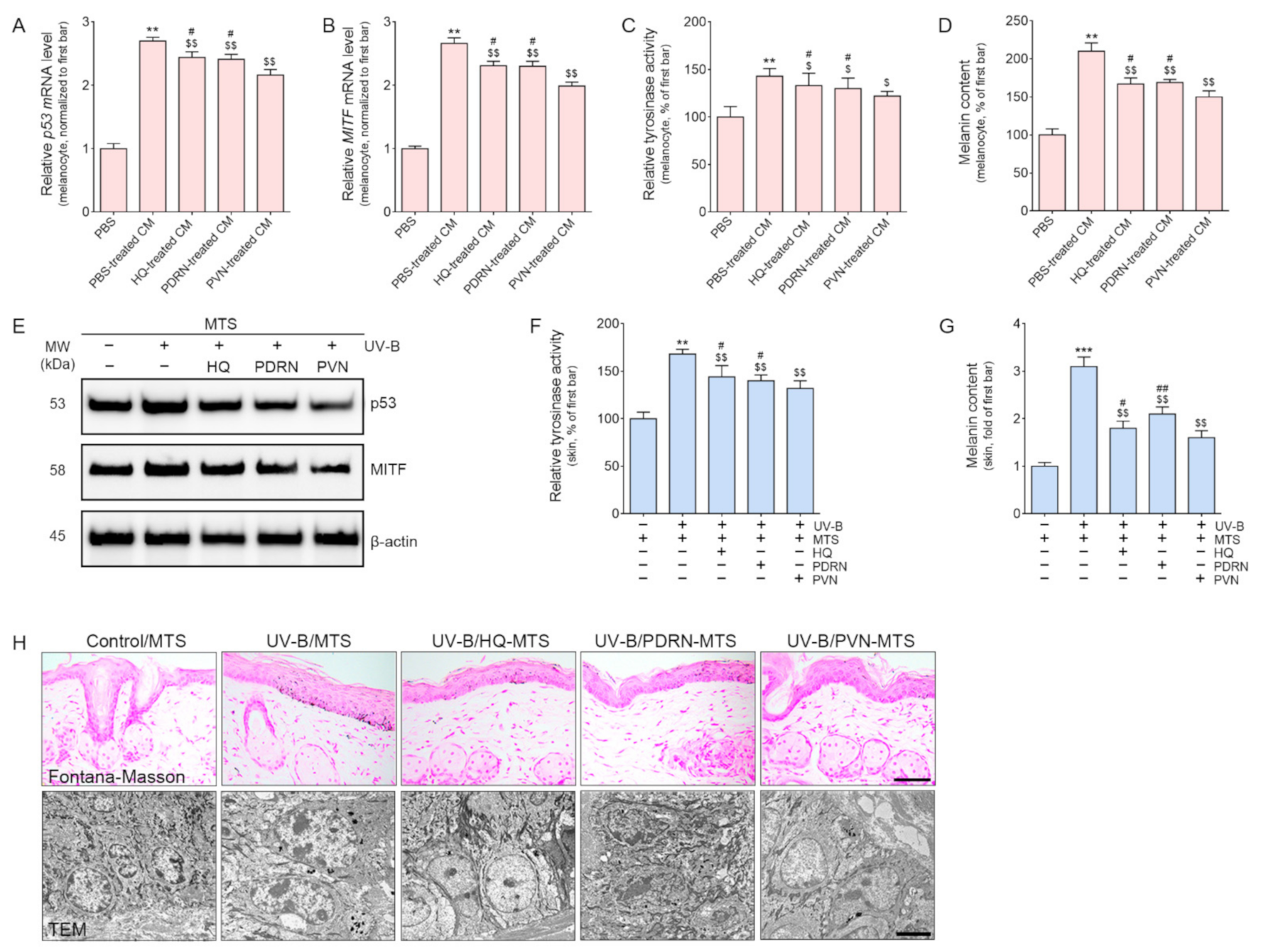
2.3. PVN Decreased Melanogenesis by Decreasing p53/MITF/Tyrosinase Expression in Both In Vitro and In Vivo Models of UV-B Radiation
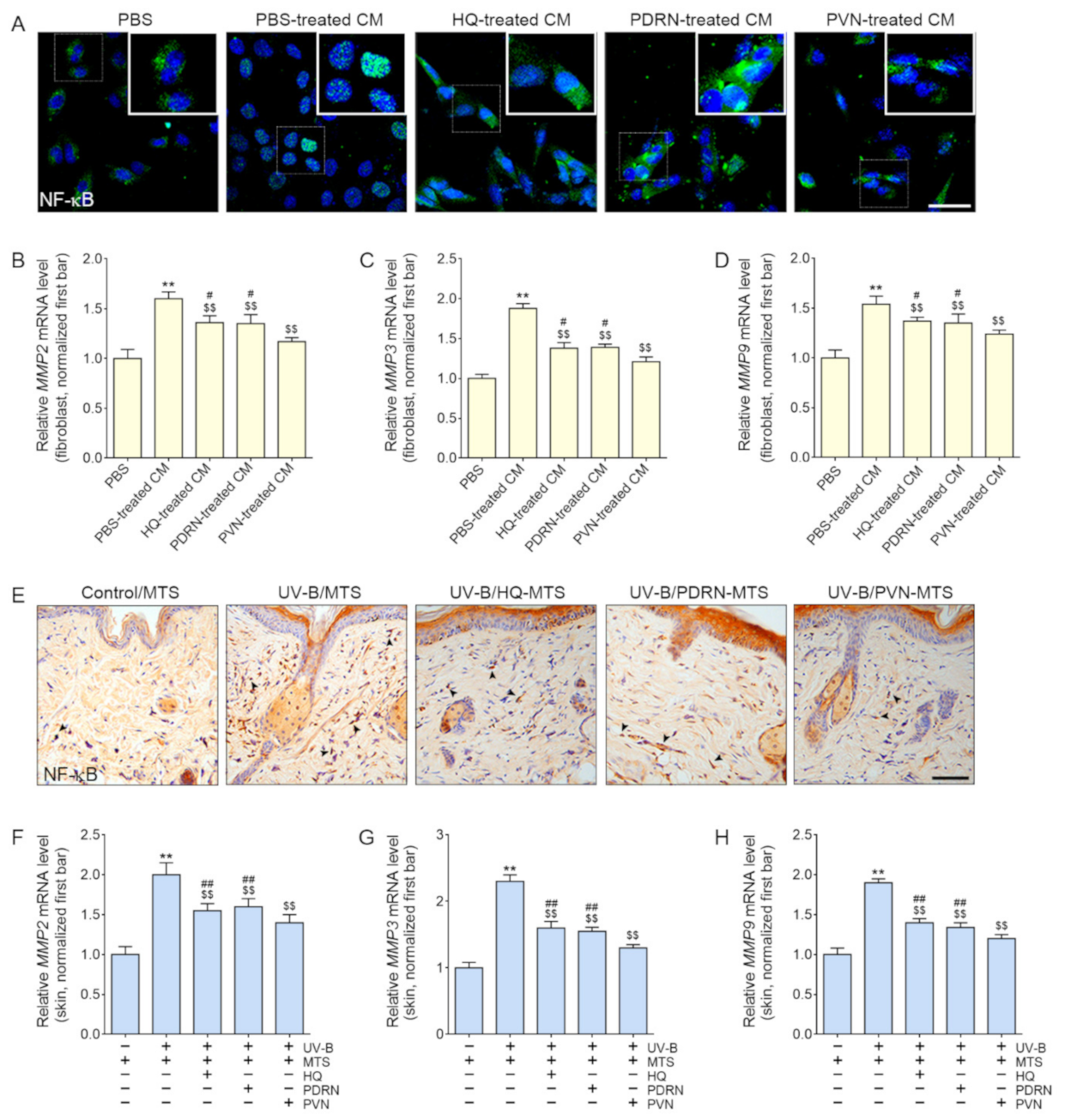
2.4. PVN Decreased the Expression of NF-κB and MMP2/3/9 in Both In Vitro and In Vivo Models of UV-B Radiation
2.5. PVN Increased the Expression of Collagen and Elastin Fibers in the Skin after UV-B Radiation
3. Discussion
4. Materials and Methods
4.1. Experimental Model
4.1.1. Cell Culture
4.1.2. Preparation of PVN
4.1.3. In Vitro Models
4.1.4. UV-B-Radiated Mice Model
4.2. Sample Preparation
4.2.1. RNA Extraction and cDNA Synthesis
4.2.2. Protein Isolation
4.2.3. Paraffin-Embedded Tissue
4.3. Melanin Content Assays
4.4. Immunocytochemistry
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Western Blotting
4.7. Immunohistochemistry
4.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.9. Histological Analysis
4.9.1. Fontana–Masson Staining
4.9.2. Masson Trichrome Staining
4.9.3. Verhoeff Staining
4.10. Transmission Electron Microscopy Analysis (TEM)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Petersen, M.J.; Hansen, C.; Craig, S. Ultraviolet A irradiation stimulates collagenase production in cultured human fibroblasts. J. Invest. Derm. 1992, 99, 440–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannarozzo, G.; Fazia, G.; Bennardo, L.; Tamburi, F.; Amoruso, G.F.; Del Duca, E.; Nisticò, S.P. A New 675 nm Laser Device in the Treatment of Facial Aging: A Prospective Observational Study. Photobiomodul. Photomed. Laser Surg. 2021, 39, 118–122. [Google Scholar] [CrossRef]
- Nistico, S.P.; Silvestri, M.; Zingoni, T.; Tamburi, F.; Bennardo, L.; Cannarozzo, G. Combination of Fractional CO2 Laser and Rhodamine-Intense Pulsed Light in Facial Rejuvenation: A Randomized Controlled Trial. Photobiomodul. Photomed. Laser Surg. 2021, 39, 113–117. [Google Scholar] [CrossRef]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective Tissue and Fibroblast Senescence in Skin Aging. J. Invest. Derm. 2021, 141, 985–992. [Google Scholar] [CrossRef]
- Park, J.E.; Pyun, H.B.; Woo, S.W.; Jeong, J.H.; Hwang, J.K. The protective effect of Kaempferia parviflora extract on UVB-induced skin photoaging in hairless mice. Photodermatol. Photoimmunol. Photomed. 2014, 30, 237–245. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, R.; Kumarasinghe, P.; Bhardwaj, S.; Srivastava, N.; Madaan, A.; Parsad, D. Melanocyte abnormalities and senescence in the pathogenesis of idiopathic guttate hypomelanosis. Int. J. Derm. 2018, 57, 559–565. [Google Scholar] [CrossRef]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Khlgatian, M.K.; Hadshiew, I.M.; Asawanonda, P.; Yaar, M.; Eller, M.S.; Fujita, M.; Norris, D.A.; Gilchrest, B.A. Tyrosinase gene expression is regulated by p53. J. Invest. Derm. 2002, 118, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Nahhas, A.F.; Mohammad, T.F.; Hamzavi, I.H. Vitiligo Surgery: Shuffling Melanocytes. J. Investig. Derm. Symp. Proc. 2017, 18, 34–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huggins, R.H.; Henderson, M.D.; Mulekar, S.V.; Ozog, D.M.; Kerr, H.A.; Jabobsen, G.; Lim, H.W.; Hamzavi, I.H. Melanocyte-keratinocyte transplantation procedure in the treatment of vitiligo: The experience of an academic medical center in the United States. J. Am. Acad. Derm. 2012, 66, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhu, J.; Mi, C.; Xu, B.; Jiao, C.; Li, Y.; Xu, D.; Liu, W.; Xu, Z. Melatonin antagonizes Mn-induced oxidative injury through the activation of keap1-Nrf2-ARE signaling pathway in the striatum of mice. Neurotox Res. 2015, 27, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Jang, H.D. Nrf2-mediated HO-1 induction coupled with the ERK signaling pathway contributes to indirect antioxidant capacity of caffeic acid phenethyl ester in HepG2 cells. Int. J. Mol. Sci 2014, 15, 12149–12165. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Roberts, S.J.; Datla, S.r.; Dusting, G.J. NO modulates NADPH oxidase function via heme oxygenase-1 in human endothelial cells. Hypertension 2006, 48, 950–957. [Google Scholar] [CrossRef] [Green Version]
- Marrot, L.; Jones, C.; Perez, P.; Meunier, J.R. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment. Cell Melanoma Res. 2008, 21, 79–88. [Google Scholar] [CrossRef]
- Kokot, A.; Metze, D.; Mouchet, N.; Galibert, M.D.; Schiller, M.; Luger, T.A.; Böhm, M. Alpha-melanocyte-stimulating hormone counteracts the suppressive effect of UVB on Nrf2 and Nrf-dependent gene expression in human skin. Endocrinology 2009, 150, 3197–3206. [Google Scholar] [CrossRef] [Green Version]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Derm. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef]
- Yanagishita-Nakatsuji, S.; Fukai, K.; Ohyama, A.; Umekoji, A.; Sowa-Osako, J.; Tsuruta, D. Probable allergic contact dermatitis from hydroquinone presenting as leukomelanoderma: Report of two cases. J. Derm. 2017, 44, e330–e331. [Google Scholar] [CrossRef]
- Mishra, S.N.; Dhurat, R.S.; Deshpande, D.J.; Nayak, C.S. Diagnostic utility of dermatoscopy in hydroquinone-induced exogenous ochronosis. Int. J. Derm. 2013, 52, 413–417. [Google Scholar] [CrossRef]
- Findlay, G.H. Ochronosis following skin bleaching with hydroquinone. J. Am. Acad. Derm. 1982, 6, 1092–1093. [Google Scholar] [CrossRef]
- Kari, F.W.; Bucher, J.; Eustis, S.L.; Haseman, J.K.; Huff, J.E. Toxicity and carcinogenicity of hydroquinone in F344/N rats and B6C3F1 mice. Food Chem. Toxicol. 1992, 30, 737–747. [Google Scholar] [CrossRef]
- Tsutsui, T.; Hayashi, N.; Maizumi, H.; Huff, J.; Barrett, J.C. Benzene-, catechol-, hydroquinone- and phenol-induced cell transformation, gene mutations, chromosome aberrations, aneuploidy, sister chromatid exchanges and unscheduled DNA synthesis in Syrian hamster embryo cells. Mutat Res. 1997, 373, 113–123. [Google Scholar] [CrossRef]
- Yu, M.H.; Lee, S.O. Hydroquinone stimulates cell invasion through activator protein-1-dependent induction of MMP-9 in HepG2 human hepatoma cells. Food Chem. Toxicol. 2016, 89, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Saade, D.S.; Maymone, M.B.C.; De La Garza, H.; Secemsky, E.A.; Kennedy, K.F.; Vashi, N.A. Trends in Use of Prescription Skin Lightening Creams. Int. J. Environ. Res. Public Health 2021, 18, 5650. [Google Scholar] [CrossRef]
- Sheth, V.M.; Pandya, A.G. Melasma: A comprehensive update: Part II. J. Am. Acad. Derm. 2011, 65, 699–714. [Google Scholar] [CrossRef]
- Dlova, N.C.; Hamed, S.H.; Tsoka-Gwegweni, J.; Grobler, A. Skin lightening practices: An epidemiological study of South African women of African and Indian ancestries. Br. J. Derm. 2015, 173, 2–9. [Google Scholar] [CrossRef]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calò, M.; Lo Cascio, P.; Stagno d’Alcontres, F.; Squadrito, F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological Activity and Clinical Use of PDRN. Front. Pharm. 2017, 8, 224. [Google Scholar] [CrossRef]
- An, J.; Park, S.H.; Ko, I.G.; Jin, J.J.; Hwang, L.; Ji, E.S.; Kim, S.H.; Kim, C.J.; Park, S.Y.; Hwang, J.J.; et al. Polydeoxyribonucleotide Ameliorates Lipopolysaccharide-Induced Lung Injury by Inhibiting Apoptotic Cell Death in Rats. Int. J. Mol. Sci. 2017, 18, 1847. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.W.; Hyun, H.; Lee, S.; Kim, S.Y.; Kim, G.T.; Um, S.; Hong, S.O.; Chun, H.J.; Yang, D.H. The Effect of Polydeoxyribonucleotide Extracted from Salmon Sperm on the Restoration of Bisphosphonate-Related Osteonecrosis of the Jaw. Mar. Drugs 2019, 17, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.Y.; Park, J.U.; Choi, M.H.; Kim, S.; Kim, H.E.; Jeong, S.H. Polydeoxyribonucleotide-delivering therapeutic hydrogel for diabetic wound healing. Sci. Rep. 2020, 10, 16811. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Yang, C.E.; Roh, T.S.; Kim, J.H.; Lee, J.H.; Lee, W.J. Scar Prevention and Enhanced Wound Healing Induced by Polydeoxyribonucleotide in a Rat Incisional Wound-Healing Model. Int. J. Mol. Sci. 2017, 18, 1698. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.R.; Park, G.Y.; Lee, S.C. Treatment of Full-Thickness Rotator Cuff Tendon Tear Using Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Polydeoxyribonucleotides in a Rabbit Model. Stem Cells Int. 2018, 2018, 7146384. [Google Scholar] [CrossRef]
- Thellung, S.; Florio, T.; Maragliano, A.; Cattarini, G.; Schettini, G. Polydeoxyribonucleotides enhance the proliferation of human skin fibroblasts: Involvement of A2 purinergic receptor subtypes. Life Sci. 1999, 64, 1661–1674. [Google Scholar] [CrossRef]
- Bigliardi, P. Treatment of acute radiodermatitis of first and second degrees with semi-greasy placenta ointment. Int. J. Tissue React. 1982, 4, 153–154. [Google Scholar]
- Belletti, S.; Uggeri, J.; Gatti, R.; Govoni, P.; Guizzardi, S. Polydeoxyribonucleotide promotes cyclobutane pyrimidine dimer repair in UVB-exposed dermal fibroblasts. Photodermatol. Photoimmunol. Photomed. 2007, 23, 242–249. [Google Scholar] [CrossRef]
- Philips, N.; Auler, S.; Hugo, R.; Gonzalez, S. Beneficial regulation of matrix metalloproteinases for skin health. Enzym. Res. 2011, 8, 427285. [Google Scholar] [CrossRef] [Green Version]
- Labat-Robert, J.; Fourtanier, A.; Boyer-Lafargue, B.; Robert, L. Age dependent increase of elastase type protease activity in mouse skin. Effect of UV-irradiation. J. Photochem. Photobiol. B 2000, 57, 113–118. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, M.J.; Kweon, D.K.; Lim, S.T.; Lee, S.J. Polydeoxyribonucleotide Activates Mitochondrial Biogenesis but Reduces MMP-1 Activity and Melanin Biosynthesis in Cultured Skin Cells. Appl. Biochem. Biotechnol. 2020, 191, 540–554. [Google Scholar] [CrossRef]
- Porsch, H.; Bernert, B.; Mehić, M.; Theocharis, A.D.; Heldin, C.H.; Heldin, P. Efficient TGFβ-induced epithelial-mesenchymal transition depends on hyaluronan synthase HAS2. Oncogene 2013, 32, 4355–4365. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I.L.; Scherer, P.E. Dermal adipocytes and hair cycling: Is spatial heterogeneity a characteristic feature of the dermal adipose tissue depot? Exp. Derm. 2016, 25, 258–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telang, P.S. Vitamin C in dermatology. Indian Derm. Online J. 2013, 4, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, G.; Garvey, D.R.; Singh, C.K.; Mintie, C.A.; Ahmad, N. Effects and Mechanism of Nicotinamide Against UVA- and/or UVB-mediated DNA Damages in Normal Melanocytes. Photochem. Photobiol. 2019, 95, 331–337. [Google Scholar] [CrossRef]
- Ratcliffe, D.R.; Iqbal, J.; Hussain, M.M.; Cramer, E.B. Fibrillar collagen type I stimulation of apolipoprotein B secretion in Caco-2 cells is mediated by beta1 integrin. Biochim. Biophys. Acta 2009, 1791, 1144–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philips, N.; Chalensouk-Khaosaat, J.; Gonzalez, S. Simulation of the Elastin and Fibrillin in Non-Irradiated or UVA Radiated Fibroblasts, and Direct Inhibition of Elastase or Matrix Metalloptoteinases Activity by Nicotinamide or Its Derivatives. J. Cosmet. Sci. 2018, 69, 47–56. [Google Scholar] [PubMed]
- Virador, V.M.; Kobayashi, N.; Matsunaga, J.; Hearing, V.J. A standardized protocol for assessing regulators of pigmentation. Anal. Biochem. 1999, 270, 207–219. [Google Scholar] [CrossRef]
- Lei, T.C.; Virador, V.M.; Vieira, W.D.; Hearing, V.J. A melanocyte-keratinocyte coculture model to assess regulators of pigmentation in vitro. Anal. Biochem. 2002, 305, 260–268. [Google Scholar] [CrossRef]
- Hakozaki, T.; Minwalla, L.; Zhuang, J.; Chhoa, M.; Matsubara, A.; Miyamoto, K.; Greatens, A.; Hillebrand, G.G.; Bissett, D.L.; Boissy, R.E. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Derm. 2002, 147, 20–31. [Google Scholar] [CrossRef]
- Greatens, A.; Hakozaki, T.; Koshoffer, A.; Epstein, H.; Schwemberger, S.; Babcock, G.; Bissett, D.; Takiwaki, H.; Arase, S.; Wickett, R.R.; et al. Effective inhibition of melanosome transfer to keratinocytes by lectins and niacinamide is reversible. Exp. Derm. 2005, 14, 498–508. [Google Scholar] [CrossRef]
- Ma, H.P.; Chang, H.L.; Bamodu, O.A.; Yadav, V.K.; Huang, T.Y.; Wu, A.; Yeh, C.T.; Tsai, S.H.; Lee, W.H. Collagen 1A1 (COL1A1) Is a Reliable Biomarker and Putative Therapeutic Target for Hepatocellular Carcinogenesis and Metastasis. Cancers 2019, 11, 786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalasho, B.D.; Minokadeh, A.; Zhang-Nunes, S.; Zoumalan, R.A.; Shemirani, N.L.; Waldman, A.R.; Pletzer, V.; Zoumalan, C.I. Evaluating the Safety and Efficacy of a Topical Formulation Containing Epidermal Growth Factor, Tranexamic Acid, Vitamin C, Arbutin, Niacinamide and Other Ingredients as Hydroquinone 4% Alternatives to Improve Hyperpigmentation: A Prospective, Randomized, Controlled Split Face Study. J. Cosmet. Sci. 2020, 71, 263–290. [Google Scholar] [PubMed]
- Murakami, H.; Shimbo, K.; Inoue, Y.; Takino, Y.; Kobayashi, H. Importance of amino acid composition to improve skin collagen protein synthesis rates in UV-irradiated mice. Amino Acids 2012, 42, 2481–2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Derm. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Lu, J.; Guo, J.H.; Tu, X.L.; Zhang, C.; Zhao, M.; Zhang, Q.W.; Gao, F.H. Tiron Inhibits UVB-Induced AP-1 Binding Sites Transcriptional Activation on MMP-1 and MMP-3 Promoters by MAPK Signaling Pathway in Human Dermal Fibroblasts. PLoS ONE 2016, 11, e0159998. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Derm. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Illman, S.A.; Keski-Oja, J.; Lohi, J. Promoter characterization of the human and mouse epilysin (MMP-28) genes. Gene 2001, 275, 185–194. [Google Scholar] [CrossRef]
- Uitto, J. Biochemistry of the elastic fibers in normal connective tissues and its alterations in diseases. J. Invest. Derm. 1979, 72, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Uehara, E.; Hokazono, H.; Hida, M.; Sasaki, T.; Yoshioka, H.; Matsuo, N. GABA promotes elastin synthesis and elastin fiber formation in normal human dermal fibroblasts (HDFs). Biosci. Biotechnol. Biochem. 2017, 81, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Kielty, C.M.; Sherratt, M.J.; Shuttleworth, C.A. Elastic fibres. J. Cell Sci. 2002, 115, 2817–2828. [Google Scholar] [CrossRef]
- Nakamura, T.; Lozano, P.R.; Ikeda, Y.; Iwanaga, Y.; Hinek, A.; Minamisawa, S.; Cheng, C.F.; Kobuke, K.; Dalton, N.; Takada, Y.; et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 2002, 415, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, J.L.; Murphy, G.; Rock, M.J.; Sherratt, M.J.; Shapiro, S.D.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin degradation by matrix metalloproteinases: Implications for connective tissue remodelling. Biochem. J. 1999, 340, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Ménasche, M.; Jacob, M.P.; Godeau, G.; Robert, A.M.; Robert, L. Pharmacological studies on elastin peptides (kappa-elastin). Blood clearance, percutaneous penetration and tissue distribution. Pathol. Biol. 1981, 29, 548–554. [Google Scholar] [PubMed]
- Iliopoulos, F.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. 3-O-ethyl-l-ascorbic acid: Characterisation and investigation of single solvent systems for delivery to the skin. Int. J. Pharm X 2019, 1, 100025. [Google Scholar] [CrossRef] [PubMed]
- Stamford, N.P. Stability, transdermal penetration, and cutaneous effects of ascorbic acid and its derivatives. J. Cosmet. Derm. 2012, 11, 310–317. [Google Scholar] [CrossRef]
- Pinnell, S.R.; Yang, H.; Omar, M.; Monteiro-Riviere, N.; De Buys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical L-ascorbic acid: Percutaneous absorption studies. Derm. Surg. 2001, 27, 137–142. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [Green Version]
- Sawutdeechaikul, P.; Kanokrungsee, S.; Sahaspot, T.; Thadvibun, K.; Banlunara, W.; Limcharoen, B.; Sansureerungsikul, T.; Rutwaree, T.; Oungeun, M.; Wanichwecharungruang, S. Detachable dissolvable microneedles: Intra-epidermal and intradermal diffusion, effect on skin surface, and application in hyperpigmentation treatment. Sci. Rep. 2021, 11, 24114. [Google Scholar] [CrossRef]
- Chun, H.S.; Song, H.S. Analysis of Trend of Studies on Microneedle Treatment System (MTS). J. Pharmacopunct. 2021, 24, 182–190. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Al-Qallaf, B.; Das, D.B. Optimizing microneedle arrays for transdermal drug delivery: Extension to non-square distribution of microneedles. J. Drug Target. 2009, 17, 108–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D. Microneedles: An approach in transdermal drug delivery: A Review. Pharma Tutor. 2018, 6, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharm. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Mohammed, Y.H.; Yamada, M.; Lin, L.L.; Grice, J.E.; Roberts, M.S.; Raphael, A.P.; Benson, H.A.; Prow, T.W. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS ONE 2014, 9, e101956. [Google Scholar] [CrossRef]
- Bora, P.; Kumar, L.; Bansal, A.K. Microneedle technology for advanced drug delivery: Evolving vistas. Dep. Pharm. Technol. NIPER CRIPS 2008, 9, 4. [Google Scholar]
- Larraneta, E.; Lutton, R.E.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Serrano, G.; Almudéver, P.; Serrano, J.M.; Cortijo, J.; Faus, C.; Reyes, M.; Expósito, I.; Torrens, A.; Millán, F. Microneedling dilates the follicular infundibulum and increases transfollicular absorption of liposomal sepia melanin. Clin. Cosmet. Investig. Derm. 2015, 8, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Chen, Y.; Shi, Y. Microneedles: A potential strategy in transdermal delivery and application in the management of psoriasis. RSC Adv. 2020, 10, 14040–14049. [Google Scholar] [CrossRef]
- Aust, M.C.; Fernandes, D.; Kolokythas, P.; Kaplan, H.M.; Vogt, P.M. Percutaneous collagen induction therapy: An alternative treatment for scars, wrinkles, and skin laxity. Plast. Reconstr. Surg. 2008, 121, 1421–1429. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, D.; Signorini, M. Combating photoaging with percutaneous collagen induction. Clin. Derm. 2008, 26, 192–199. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Barakat, M.; Awad, S.; Medhat, W.; El-Fakahany, H.; Farag, H. Multiple microneedling sessions for minimally invasive facial rejuvenation: An objective assessment. Int. J. Derm. 2015, 54, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Velez, M.W.; Ibrahim, O. Microneedling: A new approach for treating textural abnormalities and scars. Semin. Cutan. Med. Surg. 2017, 36, 155–163. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.; McAlister, E.; Courtenay, A.J.; González-Vázquez, P.; Singh, T.R.; Donnelly, R.F. Microneedle applications in improving skin appearance. Exp. Derm. 2015, 24, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W.; Jeong, H.O.; Jang, E.J.; Choi, Y.J.; Kim, D.H.; Kim, S.R.; Lee, K.J.; Lee, H.J.; Chun, P.; Byun, Y.; et al. Characterization of a small molecule inhibitor of melanogenesis that inhibits tyrosinase activity and scavenges nitric oxide (NO). Biochim. Biophys. Acta 2013, 1830, 4752–4761. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Q.; Xu, C.B. A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. Int. J. Clin. Exp. Med. 2017, 10, 14904–14910. [Google Scholar]
- Faucz, L.L.; Will, S.E.; Rodrigues, C.J.; Hesse, H.; Moraes, A.C.; Maria, D.A. Quantitative evaluation of collagen and elastic fibers after intense pulsed light treatment of mouse skin. Lasers. Surg. Med. 2018, 50, 644–650. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Byun, K.-A.; Oh, S.; Yang, J.Y.; Park, H.J.; Chung, M.S.; Son, K.H.; Byun, K. A Mixture of Topical Forms of Polydeoxyribonucleotide, Vitamin C, and Niacinamide Attenuated Skin Pigmentation and Increased Skin Elasticity by Modulating Nuclear Factor Erythroid 2-like 2. Molecules 2022, 27, 1276. https://doi.org/10.3390/molecules27041276
Kim HM, Byun K-A, Oh S, Yang JY, Park HJ, Chung MS, Son KH, Byun K. A Mixture of Topical Forms of Polydeoxyribonucleotide, Vitamin C, and Niacinamide Attenuated Skin Pigmentation and Increased Skin Elasticity by Modulating Nuclear Factor Erythroid 2-like 2. Molecules. 2022; 27(4):1276. https://doi.org/10.3390/molecules27041276
Chicago/Turabian StyleKim, Hyoung Moon, Kyung-A Byun, Seyeon Oh, Jin Young Yang, Hyun Jun Park, Moon Suk Chung, Kuk Hui Son, and Kyunghee Byun. 2022. "A Mixture of Topical Forms of Polydeoxyribonucleotide, Vitamin C, and Niacinamide Attenuated Skin Pigmentation and Increased Skin Elasticity by Modulating Nuclear Factor Erythroid 2-like 2" Molecules 27, no. 4: 1276. https://doi.org/10.3390/molecules27041276
APA StyleKim, H. M., Byun, K.-A., Oh, S., Yang, J. Y., Park, H. J., Chung, M. S., Son, K. H., & Byun, K. (2022). A Mixture of Topical Forms of Polydeoxyribonucleotide, Vitamin C, and Niacinamide Attenuated Skin Pigmentation and Increased Skin Elasticity by Modulating Nuclear Factor Erythroid 2-like 2. Molecules, 27(4), 1276. https://doi.org/10.3390/molecules27041276






