Development of Ultrasound-Assisted Extraction to Produce Skin-Whitening and Anti-Wrinkle Substances from Safflower Seed
Abstract
:1. Introduction
2. Results
2.1. Model Fitting
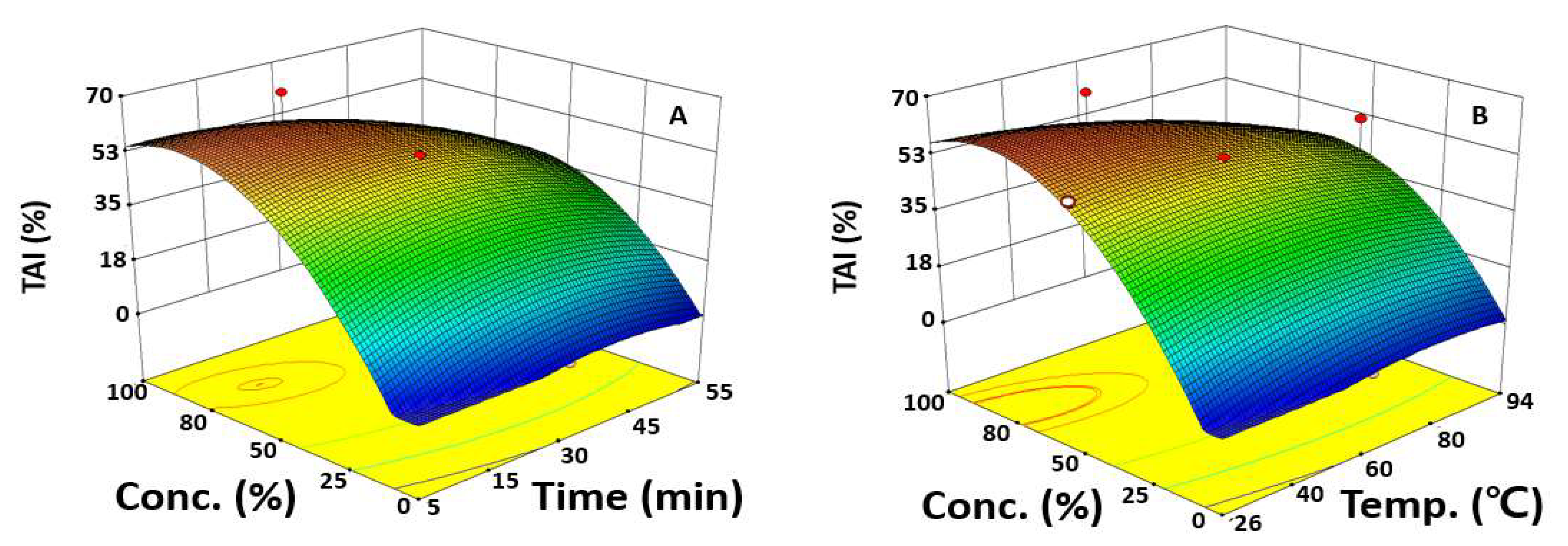
2.2. Optimization of UAE Condition for Maximizing TAI
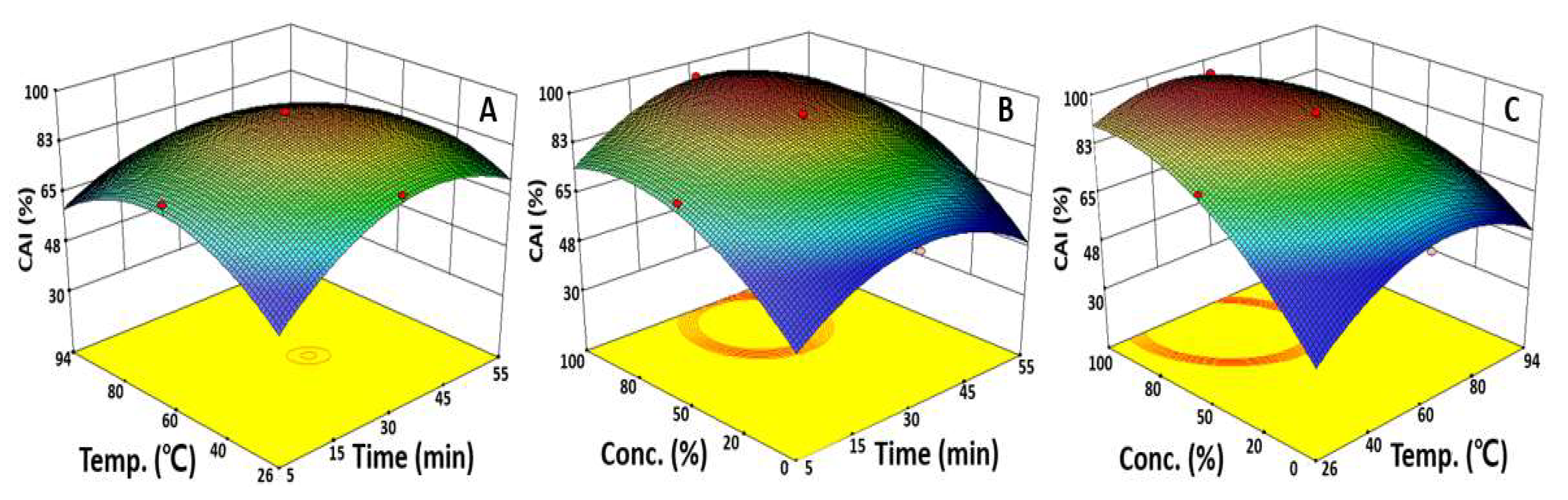
2.3. Optimization of UAE Condition for Maximizing CAI
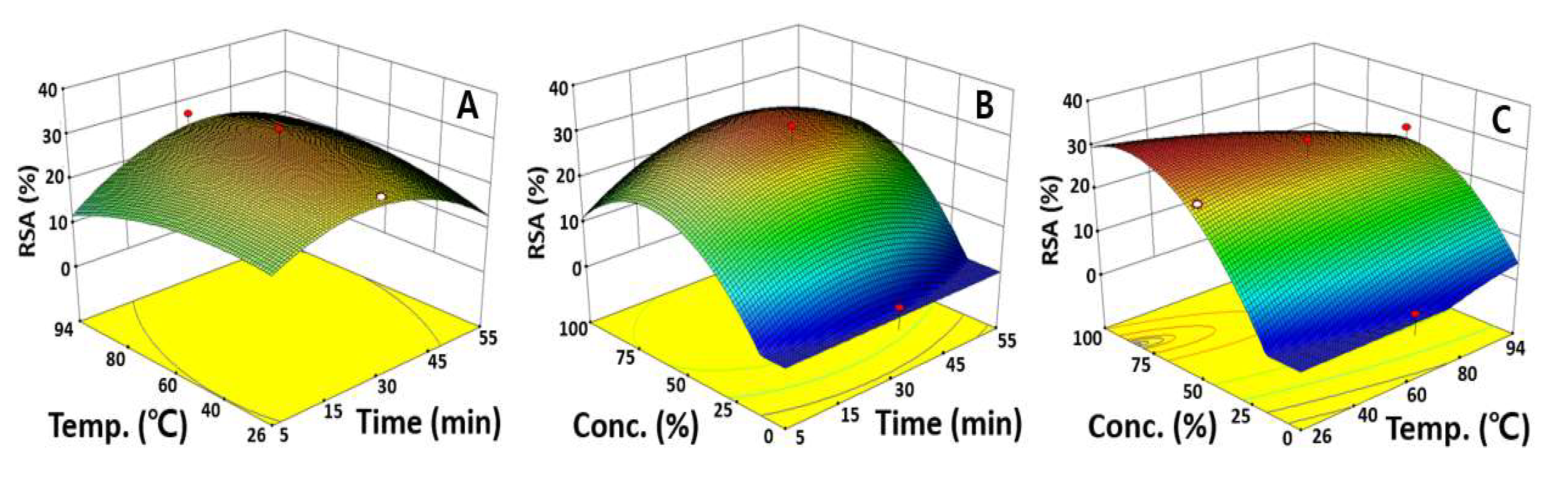
2.4. Optimization of UAE Condition for Maximizing RSA
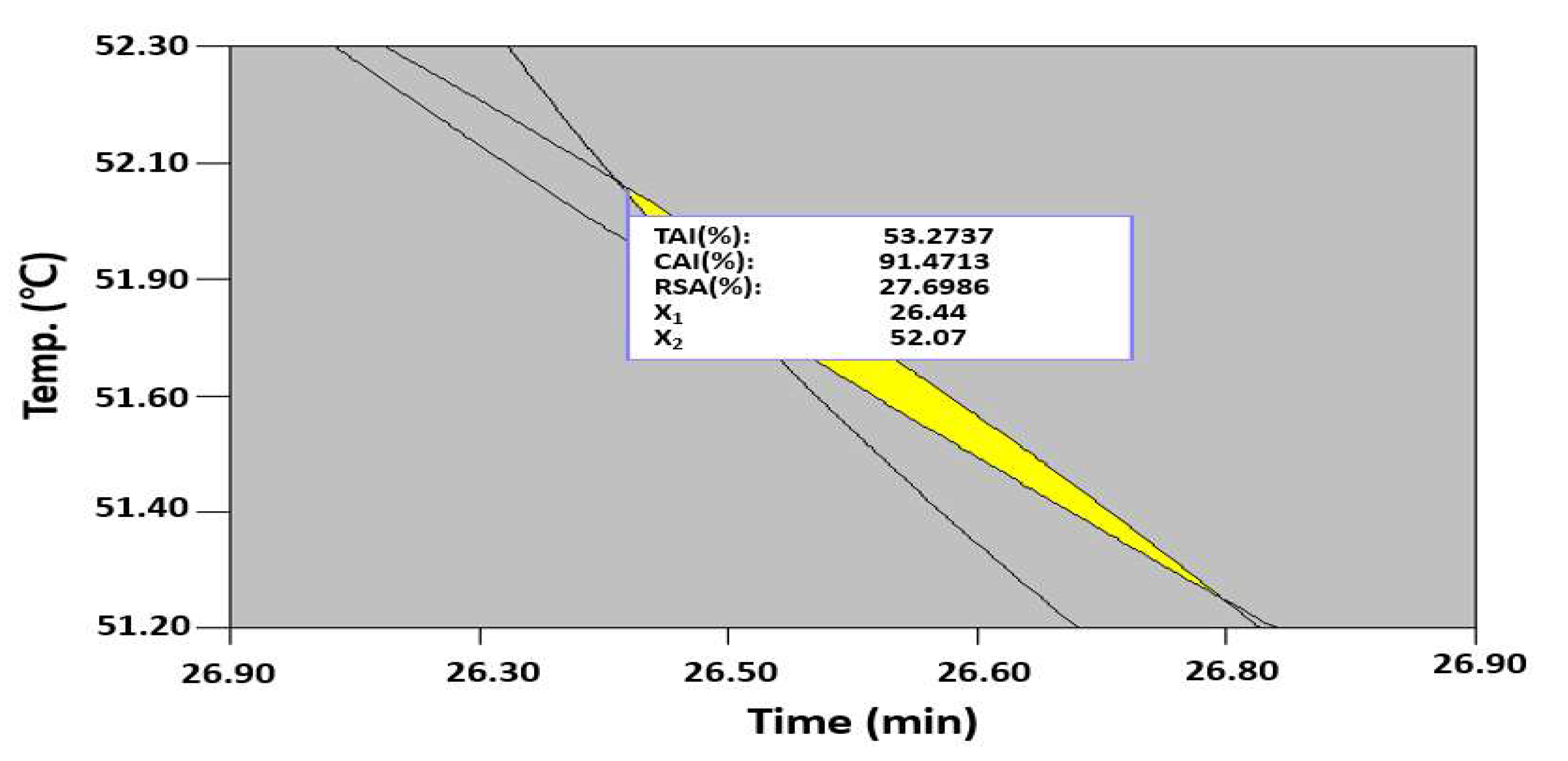
2.5. Model Validation
2.6. LC-MS/MS Analysis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Ultrasound-Assisted Extraction
3.3. Measurement of Tyrosinase Activity Inhibitory (TAI)
3.4. Measurement of Collagenase Activity Inhibitory (CAI)
3.5. Measurement of Radical Scavenging Activity (RSA)
3.6. LC-MS/MS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shin, K.O.; Park, H.S. Antiaging cosmeceuticals in Korea and open innovation in the era of the 4th industrial revolution: From research to business. Sustainability 2019, 11, 898. [Google Scholar] [CrossRef] [Green Version]
- Clatici, V.G.; Racoceanu, D.; Dalle, C.; Voicu, C.; Tomas-Aragones, L.; Marron, S.E.; Wollina, U.; Fica, S. Perceived age and life style. The specific contributions of seven factors involved in health and beauty. Maedica 2017, 12, 191. [Google Scholar]
- Syukkalova, E.A.; Sadetskaya, A.V.; Demidova, N.D.; Bobrysheva, N.P.; Osmolowsky, M.G.; Voznesenskiy, M.A.; Osmolovskaya, O.M. The effect of reaction medium and hydrothermal synthesis conditions on morphological parameters and thermal behavior of calcium phosphate nanoparticles. Ceram. Int. 2021, 47, 2809–2821. [Google Scholar] [CrossRef]
- Kim, M.H.; Hwang, C.S. Influence of shopping orientation of female consumers in their 20s and 30s on motivation for brand-switching in purchasing cosmeceuticals-Focusing on whitening, sunscreen, and anti-aging treatments. J. Korean Soc. Cloth. Text. 2013, 37, 334–347. [Google Scholar]
- Son, K.H.; Heo, M.Y. The evaluation of depigmenting efficacy in the skin for the development of new whitening agents in Korea. Int. J. Cosmet. Sci. 2013, 35, 9–18. [Google Scholar] [CrossRef]
- Arung, E.T.; Shimizu, K.; Kondo, R. Inhibitory effect of artocarpanone from Artocarpus heterophyllus on melanin biosynthesis. Biol. Pharm. Bull. 2006, 29, 1966–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zítka, O.; Kukacka, J.; Krizkov, S.; Húska, D.; Adam, V.; Masarik, M.; Prusa, R.; Kizek, R. Matrix metalloproteinases. Curr. Med. Chem. 2010, 17, 3751–3768. [Google Scholar] [CrossRef]
- Lim, S.B.; Kim, M.U.; Lee, E.H.; Kim, Y.J. Anti-oxidant and inhibitory activities on eastase, collagenase, hyaluronidase, and alpha-Glucosidase of Cedrela sinensis Fruits. J. Korean Soc. Food Sci. Nutr. 2018, 47, 1085–1092. [Google Scholar] [CrossRef]
- Kim, G.E.; Kang, H.K.; Seo, E.S.; Jung, S.H.; Park, J.S.; Kim, D.H.; Kim, D. Glucosylation of the flavonoid, astragalin by Leuconostoc mesenteroides B-512FMCM dextransucrase acceptor reactions and characterization of the products. Enzyme Microb. Technol. 2012, 50, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zheng, Y.W.; Liu, Q.; Liu, L.P.; Luo, F.L.; Zhou, H.C.; Ohkohchi, N.; Li, Y.M. Reactive oxygen species in skin repair, regeneration, aging, and inflammation. In Reactive Oxygen Species (ROS) in Living Cells; Filip, C., Albu, E., Eds.; IntechOpen: London, UK, 2018; pp. 69–87. [Google Scholar]
- Jesumani, V.; Du, H.; Pei, P.; Zheng, C.; Cheong, K.L.; Huang, N. Unravelling property of polysaccharides from Sargassum sp. as an anti-wrinkle and skin whitening property. Int. J. Biol. Macromol. 2019, 140, 216–224. [Google Scholar] [CrossRef]
- Chang, T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; An, C.; Hwang, S.D.; Kim, Y.S. Ceriporia lacerata mycelium culture medium as a novel anti-aging microbial material for cosmeceutical application. Cosmetics 2021, 8, 101. [Google Scholar] [CrossRef]
- Fernando, I.S.; Sanjeewa, K.A.; Samarakoon, K.W.; Kim, H.S.; Gunasekara, U.K.D.S.S.; Park, Y.J.; Abeytunga, D.T.U.; Lee, W.W.; Jeon, Y.J. The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: Antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J. Appl. Phycol. 2018, 30, 3223. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L.; Duan, Z. Five individual polyphenols as tyrosinase inhibitors: Inhibitory activity, synergistic effect, action mechanism, and molecular docking. Food Chem. 2019, 297, 124910. [Google Scholar] [CrossRef]
- Kolbe, L.; Mann, T.; Gerwat, W.; Batzer, J.; Ahlheit, S.; Scherner, C.; Wenck, H.; Stäb, F. 4-n-butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 19–23. [Google Scholar] [CrossRef]
- Kim, H.; Choi, J.; Cho, J.K.; Kim, S.Y.; Lee, Y.S. Solid-phase synthesis of kojic acid-tripeptides and their tyrosinase inhibitory activity, storage stability, and toxicity. Bioorg. Med. Chem. Lett. 2004, 14, 2843–2846. [Google Scholar] [CrossRef]
- Gam, D.H.; Hong, J.W.; Yeom, S.H.; Kim, J.W. Polyphenols in peanut shells and their antioxidant activity: Optimal extraction conditions and the evaluation of anti-obesity effects. J. Nutr. Health 2021, 54, 116–128. [Google Scholar] [CrossRef]
- Schillaci, C.; Nepravishta, R.; Bellomaria, A. Antioxidants in food and pharmaceutical research. J. Pharm. Sci. 2014, 1, 9–15. [Google Scholar]
- Kim, M.J.; Kim, J.Y.; Choi, S.W.; Hong, J.T.; Yoon, K.S. Anti-wrinkle effect of safflower (Carthamus tinctorius L.) seed extract (II). J. Soc. Cosmet. Sci. Korea 2004, 30, 449–456. [Google Scholar]
- Döker, F.; Takadas, O. Extraction method and solvent effect on safflower seed oil production. Chem. Process Eng. 2017, 51, 9–17. [Google Scholar]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemour, K.; Labdelli, A.; Adda, A.; Dellal, A.; Talou, T.; Merah, O. Phenol content and antioxidant and antiaging activity of safflower seed oil (Carthamus tinctorius L.). Cosmetics 2019, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Bae, C.S.; Park, C.H.; Cho, H.J.; Han, H.J.; Kang, S.S.; Choi, S.H.; Uhm, C.S. Therapeutic effects of safflower (Carthamus tinctorius L.) seed powder on osteoporosis. App. Microsc. 2002, 32, 285–290. [Google Scholar]
- Kim, J.H.; He, M.T.; Kim, M.J.; Yang, C.Y.; Shin, Y.S.; Yokozawa, T.; Cho, E.J. Safflower (Carthamus tinctorius L.) seed attenuates memory impairment induced by scopolamine in mice via regulation of cholinergic dysfunction and oxidative stress. Food Funct. 2019, 10, 3650–3659. [Google Scholar] [CrossRef] [PubMed]
- Gam, D.H.; Hong, J.W.; Jeon, S.J.; Baek, D.H.; Kim, J.W. Development of Ultrasound-assisted Extraction for Production of Bioactive Compounds with Whitening and Anti-wrinkle Effects from Sargassum Horneri. Korean Soc. Bioeng. J. 2020, 35, 294–302. [Google Scholar]
- Kim, K.C.; Kim, J.S. Effect of varying ethanol concentrations on the extraction properties and physiological activity of Artemisia annua L. Korean J. Food Sci. Technol. 2020, 52, 130–137. [Google Scholar]
- Han, M.R.; Lee, Y.S.; Kim, N.W. Anti-wrinkle and antioxidative effects of ethanolic extracts of Inula Flos, Chrysanthemi Flos and Carthami Flos. J. Invest. Cosmetol. 2013, 22, 29–37. [Google Scholar]
- Kong, M.R.; Seo, S.J.; Han, M.R.; Lee, Y.S. Anti-oxidation and anti-aging activity of three compositae species at different extraction temperatures. J. Invest. Cosmetol. 2016, 12, 29–37. [Google Scholar]
- Kim, Y.J.; Park, B.S.; Son, S.Y.; Yun, J.Y.; Cho, Y.J. Beauty food activities of Polygala japonica Houtt. J. App. Biol. Chem. 2018, 61, 51–57. [Google Scholar] [CrossRef]
- Choi, W.S. Development of functional beverage using yam (Dioscorea opposita Thunb.). Food Ind. Nutr. 2012, 17, 20–22. [Google Scholar]
- Han, K.Y.; Choi, J.Y. Establishment of optimum extraction conditions for antioxidant activity of chickpea by response surface methodology. Food Serv. Ind. J. 2016, 12, 25–34. [Google Scholar]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.H.; Kim, J.K.; Lee, J.M.; Moon, K.D. Effect of pretreatment conditions on effective components of extracts from safflower (Carthamus tinctorius L.) seed. J. Korean Soc. Food Sci. Nutr. 2002, 31, 367–372. [Google Scholar]
- Meena, P.R.; Khandelwal, R.K. Study of Semiconductor Photocatalysed Oxidation of Malic Acid Used in Cosmetic and Food Industries. J. Pharm. Bio. Sci. 2016, 6, 98–114. [Google Scholar]
- Zhao, L.; Zhao, M.Y.; Phey, C.P.; Yang, H. Efficacy of low concentration acidic electrolysed water and levulinic acid combination on fresh organic lettuce (Lactuca sativa Var. Crispa L.) and its antimicrobial mechanism. Food Control. 2019, 101, 241–250. [Google Scholar]
- Arias, P.L.; Cecilia, J.A.; Gandarias, I.; Iglesias, J.; Granados, M.L.; Mariscal, R.; Morales, G.; Moreno-Tost, R.; Maireles-Torres, P. Oxidation of lignocellulosic platform molecules to value-added chemicals using heterogeneous catalytic technologies. Catal. Sci. Technol. J. 2020, 10, 2721–2757. [Google Scholar] [CrossRef]
- Kim, H.N.; Cho, D.W.; Yoon, U.C.; Jun, H.K. Identification of anti-microbial material originated from Opuntia ficus-indica var. saboten Makino. J. Life Sci. 2007, 17, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Lewińska, A.; Domżał-Kędzia, M.; Kierul, K.; Bochynek, M.; Pannert, D.; Nowaczyk, P.; Łukaszewicz, M. Targeted hybrid nanocarriers as a system enhancing the skin structure. Molecules 2021, 26, 1063. [Google Scholar] [CrossRef]
- Jo, C.; Son, J.H.; Shin, M.G.; Byun, M.W. Irradiation effects on color and functional properties of persimmon (Diospyros kaki L. folium) leaf extract and licorice (Glycyrrhiza Uralensis Fischer) root extract during storage. Radiat. Phys. Chem. 2003, 67, 143–148. [Google Scholar] [CrossRef]
- Gam, D.H.; Park, J.H.; Hong, J.W.; Jeon, S.J.; Kim, J.H.; Kim, J.W. Effects of Sargassum thunbergii Extract on Skin Whitening and Anti-Wrinkling through Inhibition of TRP-1 and MMPs. Molecules 2021, 26, 7381. [Google Scholar] [CrossRef]
- Marxen, K.; Vanselow, K.H.; Lippemeier, S.; Hintze, R.; Ruser, A.; Hansen, U.P. Determination of DPPH radical oxidation caused by methanolic extracts of some microalgal species by linear regression analysis of spectrophotometric measurements. Sensors 2007, 7, 2080–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Run No. | Extraction Conditions | TAI (%) | CAI (%) | RSA (%) | ||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | ||||
| 1 | 15.0 | 40.0 | 20.0 | 21.7 | 61.3 | 5.21 |
| 2 | 45.0 | 40.0 | 20.0 | 22.4 | 68.4 | 3.15 |
| 3 | 15.0 | 80.0 | 20.0 | 19.4 | 67.0 | 8.95 |
| 4 | 45.0 | 80.0 | 20.0 | 21.5 | 73.3 | 10.7 |
| 5 | 15.0 | 40.0 | 80.0 | 63.5 | 80.6 | 28.8 |
| 6 | 45.0 | 40.0 | 80.0 | 39.9 | 96.9 | 29.5 |
| 7 | 15.0 | 80.0 | 80.0 | 38.7 | 82.2 | 17.8 |
| 8 | 45.0 | 80.0 | 80.0 | 33.4 | 79.1 | 23.5 |
| 9 | 5.00 | 60.0 | 50.0 | 52.6 | 76.6 | 21.9 |
| 10 | 55.0 | 60.0 | 50.0 | 43.9 | 73.3 | 19.8 |
| 11 | 30.0 | 26.0 | 50.0 | 52.2 | 80.6 | 25.4 |
| 12 | 30.0 | 94.0 | 50.0 | 51.6 | 68.3 | 27.8 |
| 13 | 30.0 | 60.0 | 0.00 | 1.20 | 61.3 | 2.13 |
| 14 | 30.0 | 60.0 | 100 | 59.6 | 94.5 | 20.9 |
| 15 | 30.0 | 60.0 | 50.0 | 50.1 | 92.9 | 27.6 |
| 16 | 30.0 | 60.0 | 50.0 | 51.9 | 91.7 | 23.5 |
| 17 | 30.0 | 60.0 | 50.0 | 50.7 | 93.7 | 31.9 |
| Response | Quadratic Regression Equation | * R2 | ** p |
|---|---|---|---|
| TAI (%) | YTAI = −31.60456 + 0.61586X1 + 0.50459X2 + 2.17259X3 + 8.20833 × 10−3X1X2 − 5.85417 × 10−3X1X3 − 8.80556 × 10−3X2X3 − 014449X12 − 4.90116 × 10−3X22 − 0.010971X32 | 0.8823 | 0.0149 |
| CAI (%) | YCAI= −45.04668 + 2.20245X1 + 2.27937X2 + 1.20087X3 − 8.41667 ×10−3X1X2 − 5.55556 × 10−3X1X3 − 5.58333 × 10−3X2X3 − 0.026532X12 − 0.015366X22 − 5.59490 × 10−3X32 | 0.9330 | 0.0024 |
| RSA (%) | YRSA = −33.11801 + 0.52341X1 + 0.49596X2 + 1.27826X3 + 3.67083 × 10−3X1X2 + 1.86389 × 10−3X2X3 − 5.89375 × 10−3X1X3 − 0.013739 × 10−3X12 − 2.64607 × 10−3X22 − 7.28493 × 10−3X32 | 0.9233 | 0.0038 |
| Variable | TAI (%) | CAI (%) | RSA (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | F | p | Sum of Squares | F | p | Sum of Squares | F | p | |
| Model | 4157.32 | 5.84 | 0.0149 | 2066.32 | 10.83 | 0.0024 | 1397.69 | 9.37 | 0.0038 |
| X1 | 121.48 | 1.53 | 0.2555 | 32.45 | 1.53 | 0.2559 | 0.48 | 0.029 | 0.8698 |
| X2 | 92.33 | 1.17 | 0.3162 | 50.59 | 2.39 | 0.1663 | 0.21 | 0.012 | 0.9146 |
| X3 | 2571.85 | 32.46 | 0.0007 | 1128.02 | 53.22 | 0.0002 | 771.91 | 46.56 | 0.0002 |
| X1X2 | 48.51 | 0.61 | 0.4596 | 51.01 | 2.41 | 0.1648 | 9.70 | 0.59 | 0.4693 |
| X1X3 | 125.61 | 1.59 | 0.2483 | 5.00 × 10−3 | 2.35 × 10−4 | 0.9882 | 5.63 | 0.34 | 0.5784 |
| X2X3 | 98.70 | 1.25 | 0.3012 | 89.78 | 4.24 | 0.0786 | 100.04 | 6.03 | 0.0437 |
| X12 | 119.51 | 1.51 | 0.2589 | 403.24 | 19.02 | 0.0033 | 108.13 | 6.52 | 0.0379 |
| X22 | 43.49 | 0.55 | 0.4829 | 427.48 | 20.17 | 0.0028 | 12.68 | 0.76 | 0.4109 |
| X32 | 1052.51 | 13.28 | 0.0082 | 273.74 | 12.91 | 0.0088 | 464.09 | 27.99 | 0.0011 |
| Xi | Independent Variables | Coded Levels | ||||
|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | +1 | +1.68 | ||
| X1 | Extraction time (min) | 5.00 | 15.0 | 30.0 | 45.0 | 55.0 |
| X2 | Extraction temperature (°C) | 26.0 | 40.0 | 60.0 | 80.0 | 94.0 |
| X3 | Ethanol concentration (%) | 0.00 | 20.0 | 50.0 | 80.0 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeom, S.-H.; Gam, D.-H.; Kim, J.-H.; Kim, J.-W. Development of Ultrasound-Assisted Extraction to Produce Skin-Whitening and Anti-Wrinkle Substances from Safflower Seed. Molecules 2022, 27, 1296. https://doi.org/10.3390/molecules27041296
Yeom S-H, Gam D-H, Kim J-H, Kim J-W. Development of Ultrasound-Assisted Extraction to Produce Skin-Whitening and Anti-Wrinkle Substances from Safflower Seed. Molecules. 2022; 27(4):1296. https://doi.org/10.3390/molecules27041296
Chicago/Turabian StyleYeom, Suh-Hee, Da-Hye Gam, Jun-Hee Kim, and Jin-Woo Kim. 2022. "Development of Ultrasound-Assisted Extraction to Produce Skin-Whitening and Anti-Wrinkle Substances from Safflower Seed" Molecules 27, no. 4: 1296. https://doi.org/10.3390/molecules27041296
APA StyleYeom, S.-H., Gam, D.-H., Kim, J.-H., & Kim, J.-W. (2022). Development of Ultrasound-Assisted Extraction to Produce Skin-Whitening and Anti-Wrinkle Substances from Safflower Seed. Molecules, 27(4), 1296. https://doi.org/10.3390/molecules27041296








