Zn(II) and Co(II) 3D Coordination Polymers Based on 2-Iodoterephtalic Acid and 1,2-bis(4-pyridyl)ethane: Structures and Sorption Properties
Abstract
:1. Introduction
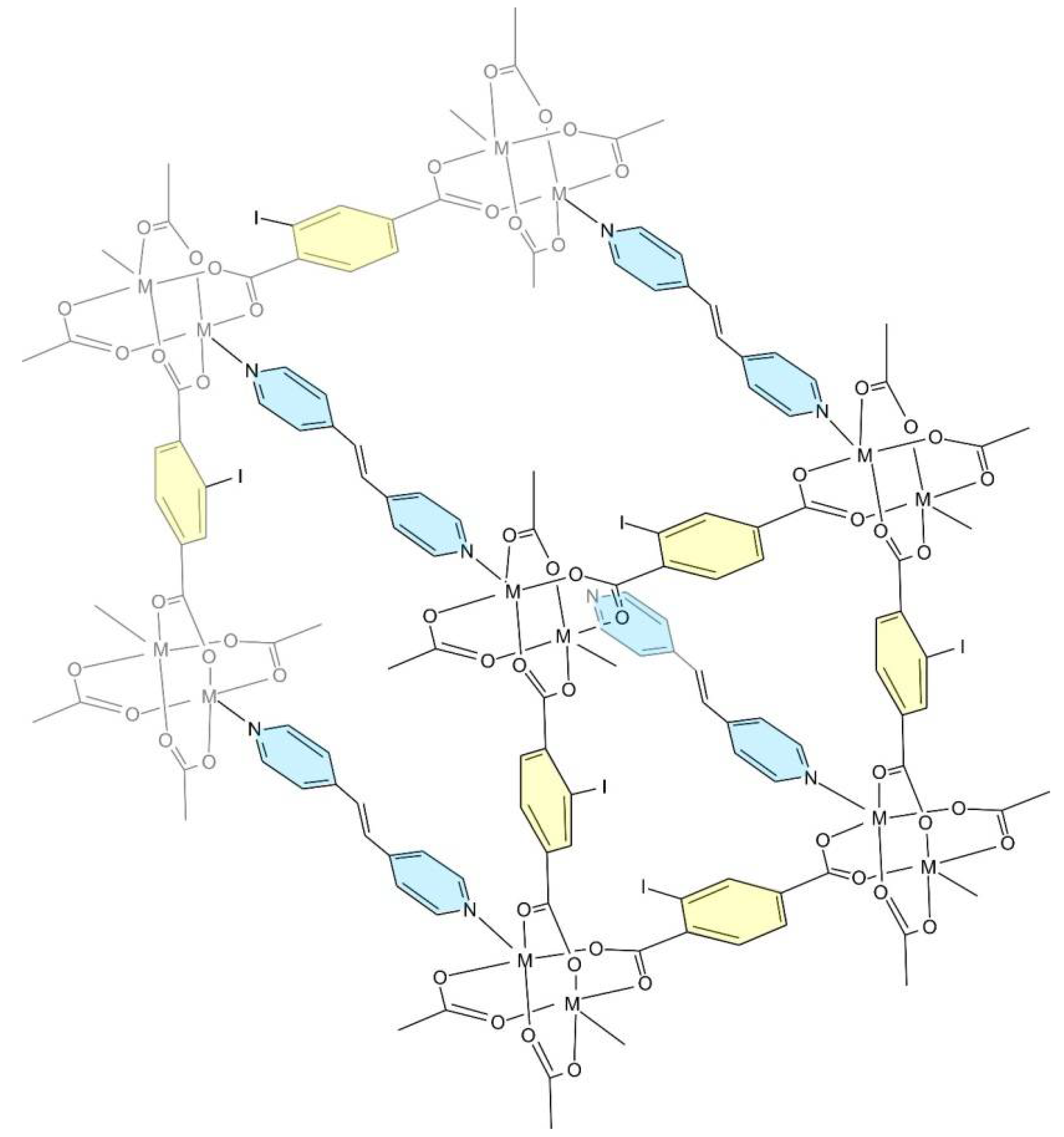
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Sapchenko, S.A.; Demakov, P.A.; Samsonenko, D.G.; Dybtsev, D.N.; Schröder, M.; Fedin, V.P. A Cryptand Metal–Organic Framework as a Platform for the Selective Uptake and Detection of Group I Metal Cations. Chem.-A Eur. J. 2017, 23, 2286–2289. [Google Scholar] [CrossRef] [PubMed]
- Bolotov, V.A.; Kovalenko, K.A.; Samsonenko, D.G.; Han, X.; Zhang, X.; Smith, G.L.; MCormick, L.J.; Teat, S.J.; Yang, S.; Lennox, M.J.; et al. Enhancement of CO2 Uptake and Selectivity in a Metal–Organic Framework by the Incorporation of Thiophene Functionality. Inorg. Chem. 2018, 57, 5074–5082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Da Silva, I.; Fazzi, R.; Sheveleva, A.M.; Han, X.; Spencer, B.F.; Sapchenko, S.A.; Tuna, F.; McInnes, E.J.L.; Li, M.; et al. Iodine Adsorption in a Redox-Active Metal-Organic Framework: Electrical Conductivity Induced by Host-Guest Charge-Transfer. Inorg. Chem. 2019, 58, 14145–14150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapchenko, S.A.; Dybtsev, D.N.; Samsonenko, D.G.; Belosludov, R.V.; Belosludov, V.R.; Kawazoe, Y.; Schröder, M.; Fedin, V.P. Selective gas adsorption in microporous metal-organic frameworks incorporating urotropine basic sites: An experimental and theoretical study. Chem. Commun. 2015, 51, 13918–13921. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal-organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal-organic materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [Green Version]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic methods for the functionalization of metal-organic frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Chen, B.; Qian, G. Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Coord. Chem. Rev. 2014, 273–274, 76–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, X.; Yang, X.; Zhou, H.-C. Luminescent sensors based on metal-organic frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, W.; Yin, Y.; Fang, W.; Xue, H. Metal-organic framework-based sensors for the detection of toxins and foodborne pathogens. Food Control 2022, 133, 108684. [Google Scholar] [CrossRef]
- Mohan, B.; Kumar, S.; Xi, H.; Ma, S.; Tao, Z.; Xing, T.; You, H.; Zhang, Y.; Ren, P. Fabricated Metal-Organic Frameworks (MOFs) as luminescent and electrochemical biosensors for cancer biomarkers detection. Biosens. Bioelectron. 2022, 197, 113738. [Google Scholar] [CrossRef]
- Gheorghe, A.; Lugier, O.; Ye, B.; Tanase, S. Metal-organic framework based systems for CO2 sensing. J. Mater. Chem. C 2021, 9, 16132–16142. [Google Scholar] [CrossRef]
- Dey, G.; Shadab; Aijaz, A. Metal-Organic Framework Derived Nanostructured Bifunctional Electrocatalysts for Water Splitting. ChemElectroChem 2021, 8, 3782–3803. [Google Scholar] [CrossRef]
- Li, S.; Shan, S.; Chen, S.; Li, H.; Li, Z.; Liang, Y.; Fei, J.; Xie, L.; Li, J. Photocatalytic degradation of hazardous organic pollutants in water by Fe-MOFs and their composites: A review. J. Environ. Chem. Eng. 2021, 9, 105967. [Google Scholar] [CrossRef]
- Xia, T.; Lin, Y.; Li, W.; Ju, M. Photocatalytic degradation of organic pollutants by MOFs based materials: A review. Chin. Chem. Lett. 2021, 32, 2975–2984. [Google Scholar] [CrossRef]
- Huang, D.; Wang, G.; Cheng, M.; Zhang, G.; Chen, S.; Liu, Y.; Li, Z.; Xue, W.; Lei, L.; Xiao, R. Optimal preparation of catalytic Metal-organic framework derivatives and their efficient application in advanced oxidation processes. Chem. Eng. J. 2021, 421, 127817. [Google Scholar] [CrossRef]
- Zhai, Z.; Yan, W.; Dong, L.; Deng, S.; Wilkinson, D.P.; Wang, X.; Zhang, L.; Zhang, J. Catalytically active sites of MOF-derived electrocatalysts: Synthesis, characterization, theoretical calculations, and functional mechanisms. J. Mater. Chem. A 2021, 9, 20320–20344. [Google Scholar] [CrossRef]
- Belousov, A.S.; Suleimanov, E.V. Application of metal-organic frameworks as an alternative to metal oxide-based photocatalysts for the production of industrially important organic chemicals. Green Chem. 2021, 23, 6172–6204. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Maxakato, N.W. Porous metal-organic framework (MOF)-based and MOF-derived electrocatalytic materials for energy conversion. Mater. Today Energy 2021, 21, 100816. [Google Scholar] [CrossRef]
- Sahoo, R.; Das, M.C. C2s/C1 hydrocarbon separation: The major step towards natural gas purification by metal-organic frameworks (MOFs). Coord. Chem. Rev. 2021, 442, 213998. [Google Scholar] [CrossRef]
- Wu, Y.; Weckhuysen, B.M. Separation and Purification of Hydrocarbons with Porous Materials. Angew. Chem. Int. Ed. 2021, 60, 18930–18949. [Google Scholar] [CrossRef]
- Demakov, P.A.; Sapchenko, S.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Coordination polymers based on zinc(ii) and manganese(ii) with 1,4-cyclohexanedicarboxylic acid. Russ. Chem. Bull. 2018, 67, 490–496. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Kovalenko, K.A.; Samsonenko, D.G.; Barsukova, M.O.; Dybtsev, D.N.; Fedin, V.P. Exceptionally effective benzene/cyclohexane separation using a nitro-decorated metal-organic framework. Chem. Commun. 2020, 56, 8241–8244. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sensharma, D.; Qazvini, O.T.; Dutta, S.; Macreadie, L.K.; Ghosh, S.K.; Babarao, R. Advances in adsorptive separation of benzene and cyclohexane by metal-organic framework adsorbents. Coord. Chem. Rev. 2021, 437, 213852. [Google Scholar] [CrossRef]
- Hu, J.-M.; Blatov, V.A.; Yu, B.; Van Hecke, K.; Cui, G.-H. An unprecedented “strongly” self-catenated MOF containing inclined catenated honeycomb-like units. Dalt. Trans. 2016, 45, 2426–2429. [Google Scholar] [CrossRef]
- Ye, C.-R.; Wang, W.-J.; Chen, W.; Xiao, Y.; Zhang, H.-F.; Dai, B.-L.; Chen, S.-H.; Wu, X.-D.; Li, M.; Huang, X.-C. Harnessing Shape Complementarity for Upgraded Cyclohexane Purification through Adaptive Bottlenecked Pores in an Imidazole-Containing MOF. Angew. Chem. Int. Ed. 2021, 60, 23590–23595. [Google Scholar] [CrossRef]
- Lysova, A.A.; Kovalenko, K.A.; Dybtsev, D.N.; Klyamkin, S.N.; Berdonosova, E.A.; Fedin, V.P. Hydrocarbon adsorption in a series of mesoporous metal-organic frameworks. Microporous Mesoporous Mater. 2021, 328, 111477. [Google Scholar] [CrossRef]
- Bozbiyik, B.; Duerinck, T.; Lannoeye, J.; De Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Adsorption and separation of n-hexane and cyclohexane on the UiO-66 metal-organic framework. Microporous Mesoporous Mater. 2014, 183, 143–149. [Google Scholar] [CrossRef]
- Zheng, Y.; Yong, J.; Zhu, Z.; Chen, J.; Song, Z.; Gao, J. Spin crossover in metal–organic framework for improved separation of C2H2/CH4 at room temperature. J. Solid State Chem. 2021, 304, 122554. [Google Scholar] [CrossRef]
- Wang, Y.; He, M.; Gao, X.; Li, S.; Xiong, S.; Krishna, R.; He, Y. Exploring the Effect of Ligand-Originated MOF Isomerism and Methoxy Group Functionalization on Selective Acetylene/Methane and Carbon Dioxide/Methane Adsorption Properties in Two NbO-Type MOFs. ACS Appl. Mater. Interfaces 2018, 10, 20559–20568. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liu, K.; Fu, S.; Wang, L.; Liang, C.; Li, G.; Li, C.; Shi, Z. Microporous Cu metal-organic framework constructed from V-shaped tetracarboxylic ligand for selective separation of C2H2/CH4 and C2H2/N2 at room temperature. J. Solid State Chem. 2018, 265, 285–290. [Google Scholar] [CrossRef]
- Mao, H.; Li, S.-H.; Zhang, A.-S.; Xu, L.-H.; Lu, H.-X.; Lv, J.; Zhao, Z.-P. Furfural separation from aqueous solution by pervaporation membrane mixed with metal organic framework MIL-53(Al) synthesized via high efficiency solvent-controlled microwave. Sep. Purif. Technol. 2021, 272. [Google Scholar] [CrossRef]
- Zhou, P.; Cheng, J.; Yan, Y.; Xu, S.; Zhou, C. Ultrafast preparation of hydrophobic ZIF-67/copper mesh via electrodeposition and hydrophobization for oil/water separation and dyes adsorption. Sep. Purif. Technol. 2021, 272, 118871. [Google Scholar] [CrossRef]
- Wei, X.; Wang, C.-C.; Li, Y.; Wang, P.; Wei, Q. The Z-scheme NH2-UiO-66/PTCDA composite for enhanced photocatalytic Cr(VI) reduction under low-power LED visible light. Chemosphere 2021, 280, 130734. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Norouzi, F.; Khavasi, H.R. Iodine decorated-UiO-67 MOF as a fluorescent sensor for the detection of halogenated aromatic hydrocarbons. New J. Chem. 2020, 44, 8937–8943. [Google Scholar] [CrossRef]
- Kalaj, M.; Momeni, M.R.; Bentz, K.C.; Barcus, K.S.; Palomba, J.M.; Paesani, F.; Cohen, S.M. Halogen bonding in UiO-66 frameworks promotes superior chemical warfare agent simulant degradation. Chem. Commun. 2019, 55, 3481–3484. [Google Scholar] [CrossRef] [PubMed]
- Bertani, R.; Sgarbossa, P.; Venzo, A.; Lelj, F.; Amati, M.; Resnati, G.; Pilati, T.; Metrangolo, P.; Terraneo, G. Halogen bonding in metal–organic–supramolecular networks. Coord. Chem. Rev. 2010, 254, 677–695. [Google Scholar] [CrossRef]
- Novikov, A.S.; Ivanov, D.M.; Avdontceva, M.S.; Kukushkin, V.Y. Diiodomethane as a halogen bond donor toward metal-bound halides. CrystEngComm 2017, 19, 2517–2525. [Google Scholar] [CrossRef] [Green Version]
- Kinzhalov, M.A.; Kashina, M.V.; Mikherdov, A.S.; Mozheeva, E.A.; Novikov, A.S.; Smirnov, A.S.; Ivanov, D.M.; Kryukova, M.A.; Ivanov, A.Y.; Smirnov, S.N.; et al. Dramatically Enhanced Solubility of Halide-Containing Organometallic Species in Diiodomethane: The Role of Solvent Complex Halogen Bonding. Angew. Chem. Int. Ed. 2018, 57, 12785–12789. [Google Scholar] [CrossRef]
- Afanasenko, A.M.; Avdontceva, M.S.; Novikov, A.S.; Chulkova, T.G. Halogen and hydrogen bonding in cis-dichlorobis(propionitrile)platinum(II) chloroform monosolvate. Z. Krist.-Cryst. Mater. 2016, 231, 435–440. [Google Scholar] [CrossRef]
- Zelenkov, L.E.; Ivanov, D.M.; Avdontceva, M.S.; Novikov, A.S.; Bokach, N.A. Tetrachloromethane as halogen bond donor toward metal-bound halides. Z. Krist.-Cryst. Mater. 2019, 234, 9–17. [Google Scholar] [CrossRef]
- Torubaev, Y.V.; Skabitsky, I.V.; Saratov, G.A.; Barzilovich, P.Y. Halogen vs. ionic bonding: An unusual isomorphism between the neutral (C5Me5)2Fe/C2I2 cocrystal and ionic [(C5Me5)2Fe]Br3 crystal. Mendeleev Commun. 2021, 31, 58–61. [Google Scholar] [CrossRef]
- Torubaev, Y.V.; Skabitsky, I.V. Halogen bonding in crystals of free 1,2-diiodo-ethene (C2H2I2) and its π-complex [CpMn(CO)2](π-C2H2I2). Z. Krist.-Cryst. Mater. 2020, 235, 599–607. [Google Scholar] [CrossRef]
- Johnson, M.T.; Džolić, Z.; Cetina, M.; Wendt, O.F.; Öhrström, L.; Rissanen, K. Neutral organometallic halogen bond acceptors: Halogen bonding in complexes of PCPPdX (X = Cl, Br, I) with iodine (I2), 1,4- diiodotetrafluorobenzene (F4DIBz), and 1,4-diiodooctafluorobutane (F8DIBu). Cryst. Growth Des. 2012, 12, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.J.; Li, C.; Liu, R.; Jin, W.J. Phosphorescence of several cocrystals assembled by diiodotetrafluorobenzene and three ring angular diazaphenanthrenes via CI··· N halogen bond. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 792–799. [Google Scholar] [CrossRef]
- Eliseeva, A.A.; Ivanov, D.M.; Novikov, A.S.; Kukushkin, V.Y. Recognition of the π-hole donor ability of iodopentafluorobenzene-a conventional σ-hole donor for crystal engineering involving halogen bonding. CrystEngComm 2019, 21, 616–628. [Google Scholar] [CrossRef]
- Eliseeva, A.A.; Ivanov, D.M.; Novikov, A.S.; Rozhkov, A.V.; Kornyakov, I.V.; Dubovtsev, A.Y.; Kukushkin, V.Y. Hexaiododiplatinate(ii) as a useful supramolecular synthon for halogen bond involving crystal engineering. Dalt. Trans. 2020, 49, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Okrut, A.; Feldmann, C. {[P(o-tolyl)3]Br}2[Cu2Br6](Br2) An Ionic Compound Containing Molecular Bromine. Inorg. Chem. 2008, 47, 3084–3087. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, D.; Feldmann, C. Bromine-rich Zinc Bromides: Zn6Br12(18-crown-6)2×(Br2)5, Zn4Br8(18-crown-6)2×(Br2)3, and Zn6Br12(18-crown-6)2×(Br2)2. Inorg. Chem. 2016, 55, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Torubaev, Y.V.; Skabitskiy, I.V.; Pavlova, A.V.; Pasynskii, A.A. First structural evidence of a Se–Br–Br halogen-bonded molecular complex. New J. Chem. 2017, 41, 3606–3611. [Google Scholar] [CrossRef]
- Puttreddy, R.; von Essen, C.; Rissanen, K. Halogen Bonds in Square Planar 2,5-Dihalopyridine-Copper(II) Bromide Complexes. Eur. J. Inorg. Chem. 2018, 2018, 2393–2398. [Google Scholar] [CrossRef] [Green Version]
- Puttreddy, R.; von Essen, C.; Peuronen, A.; Lahtinen, M.; Rissanen, K. Halogen bonds in 2,5-dihalopyridine-copper(ii) chloride complexes. CrystEngComm 2018, 20, 1954–1959. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Haddad, S.F.; Turnbull, M.M.; Landee, C.P.; Willett, R.D. Copper–halide bonds as magnetic tunnels; structural, magnetic and theoretical studies of trans-bis(2,5-dibromopyridine)dihalo copper(ii) and trans-bis(2-bromopyridine)dibromo copper(ii). CrystEngComm 2013, 15, 3111–3118. [Google Scholar] [CrossRef]
- Awwadi, F.; Haddad, S.F.; Willett, R.D.; Twamley, B. The Analogy of C−Br···Br−C, C−Br···Br−Fe, and Fe−Br···Br−Fe Contacts: Crystal Structures of (26DAPH)FeBr 4 and (26DA35DBPH) 2 FeBr 4 ·Br. Cryst. Growth Des. 2010, 10, 158–164. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Turnbull, M.M.; Alwahsh, M.I.; Haddad, S.F. May halogen bonding interactions compete with Cu⋯Cl semi-coordinate bonds? Structural, magnetic and theoretical studies of two polymorphs of trans-bis(5-bromo-2-chloro pyridine)dichlorocopper(ii) and trans -bis(2,5-dichloropyridine)dichlorocopper(ii). New J. Chem. 2018, 42, 10642–10650. [Google Scholar] [CrossRef]
- Christine, T.; Tabey, A.; Cornilleau, T.; Fouquet, E.; Hermange, P. Syntheses of o-iodobenzyl alcohols-BODIPY structures as potential precursors of bimodal tags for positron emission tomography and optical imaging. Tetrahedron 2019, 75, 130765. [Google Scholar] [CrossRef]
- Zaguzin, A.S.; Sukhikh, T.S.; Kolesov, B.A.; Sokolov, M.N.; Fedin, V.P.; Adonin, S.A. Iodinated vs non-iodinated: Comparison of sorption selectivity by [Zn2(bdc)2dabco]n and superstructural 2-iodoterephtalate-based metal–organic framework. Polyhedron 2022, 212, 115587. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.-F.; Guan, Z.-H.; Hou, L.; Cui, B.; Wang, Y.-Y. Cleavage of a C-C σ bond between two phenyl groups under mild conditions during the construction of Zn(II) organic frameworks. Green Chem. 2016, 18, 5418–5422. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. IUCr Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Alexeev, A.V.; Gromilov, S.A. Quantitative phase analysis on a single crystal X-ray diffractometer equipped with a two-dimensional flat detector. J. Struct. Chem. 2010, 51, 156–165. [Google Scholar] [CrossRef]
- Alexeev, A.V.; Gromilov, S.A. X-Ray Diffraction Study of Micro Amounts of Polycrystalline Samples. J. Struct. Chem. 2010, 51, 744–757. [Google Scholar] [CrossRef]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
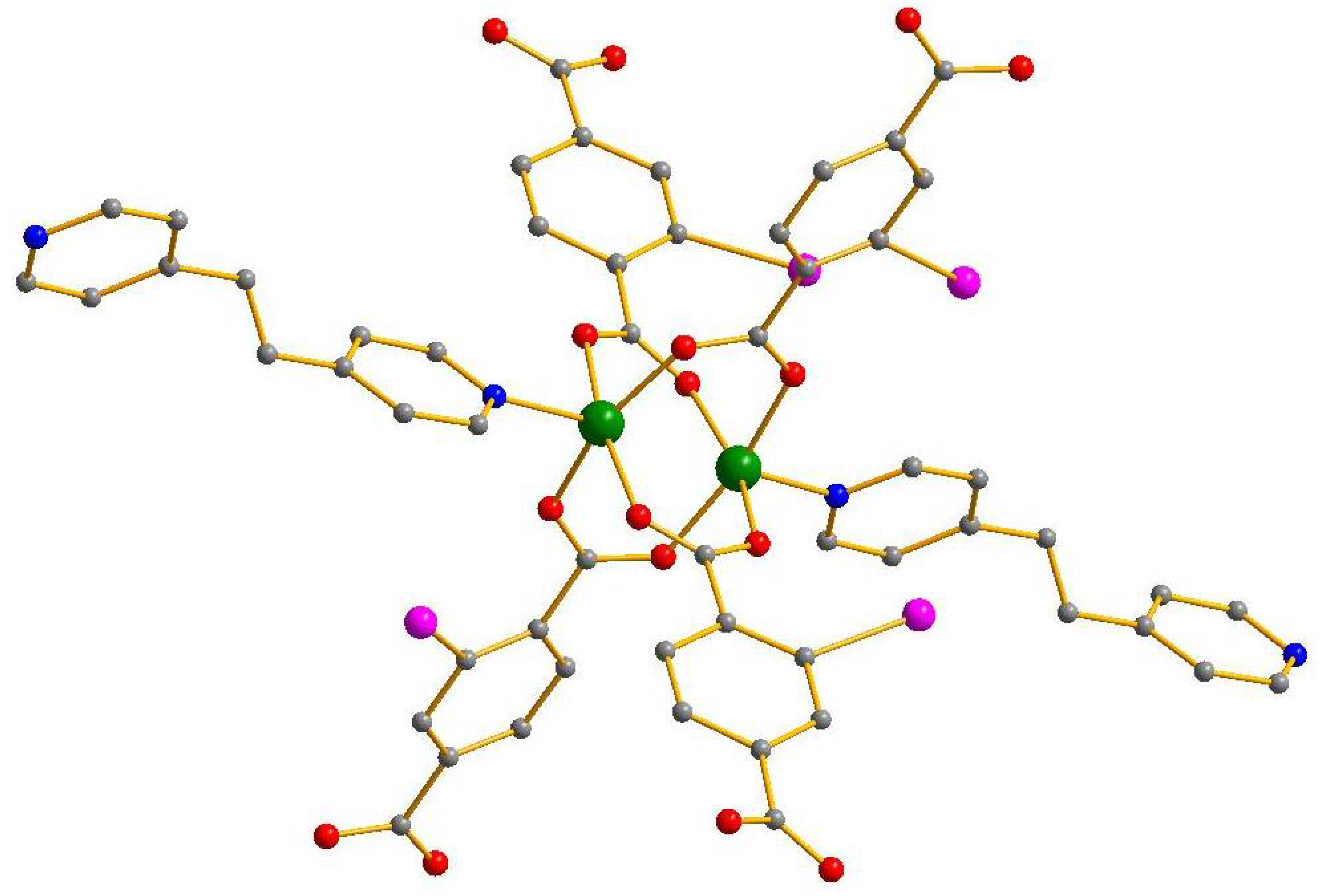
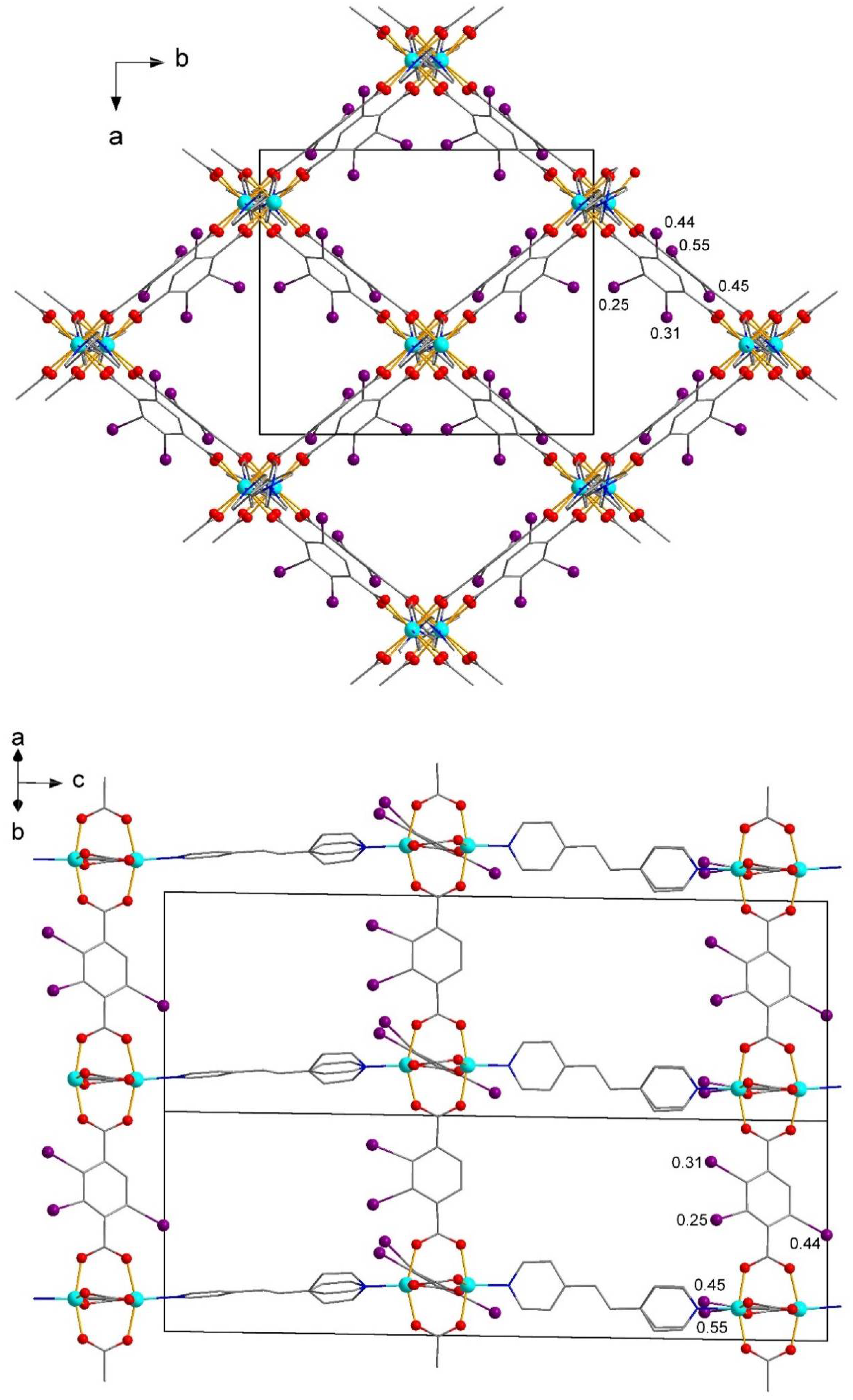
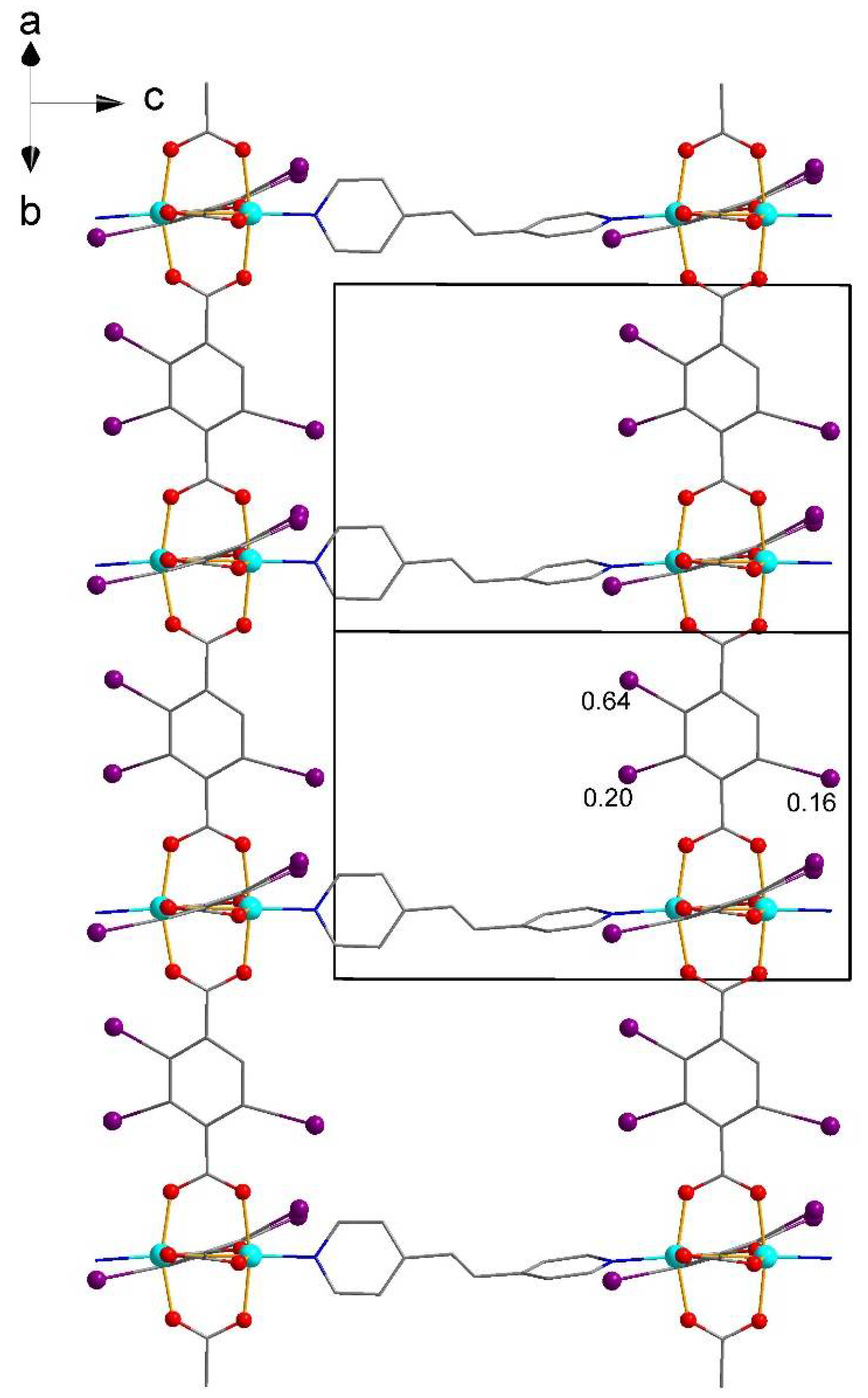
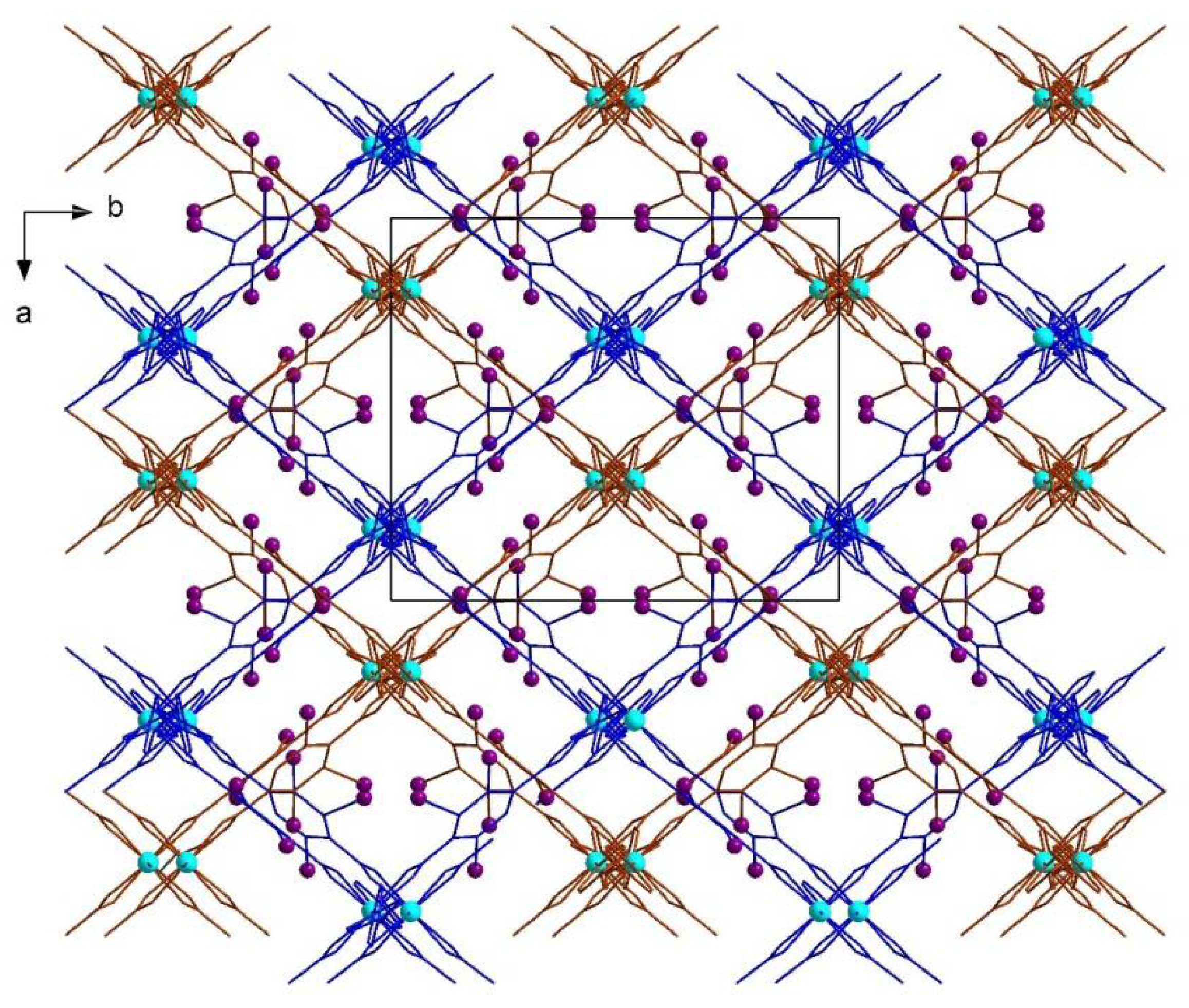
| No. | Substrates | 1 | 2 | [Zn2(2-I-bdc)2dabco] | [Zn2(bdc)2dabco] |
|---|---|---|---|---|---|
| 1 | 1,2-dichloroethane:benzene | 2.54:1 | 2.57:1 | 1.5:1 | 1.2:1 |
| 2 | Chloroform:benzene | 3.3:1 | 1:1 | 1.3:1 | 2.0:1 |
| 3 | Benzene:cyclohexane | 43.8:1 | 10:1 | 15:1 | 25:1 |
| 4 | 1,2-dibromoethane:benzene | 8.3:1 | 3.1:1 | n/a | n/a |
| 5 | 1-butanol:1-bromobutane | 1.3:1 | 1.17:1 | 0.38:1 | 0.5:1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaguzin, A.S.; Sukhikh, T.S.; Sakhapov, I.F.; Fedin, V.P.; Sokolov, M.N.; Adonin, S.A. Zn(II) and Co(II) 3D Coordination Polymers Based on 2-Iodoterephtalic Acid and 1,2-bis(4-pyridyl)ethane: Structures and Sorption Properties. Molecules 2022, 27, 1305. https://doi.org/10.3390/molecules27041305
Zaguzin AS, Sukhikh TS, Sakhapov IF, Fedin VP, Sokolov MN, Adonin SA. Zn(II) and Co(II) 3D Coordination Polymers Based on 2-Iodoterephtalic Acid and 1,2-bis(4-pyridyl)ethane: Structures and Sorption Properties. Molecules. 2022; 27(4):1305. https://doi.org/10.3390/molecules27041305
Chicago/Turabian StyleZaguzin, Alexander S., Taisiya S. Sukhikh, Ilyas F. Sakhapov, Vladimir P. Fedin, Maxim N. Sokolov, and Sergey A. Adonin. 2022. "Zn(II) and Co(II) 3D Coordination Polymers Based on 2-Iodoterephtalic Acid and 1,2-bis(4-pyridyl)ethane: Structures and Sorption Properties" Molecules 27, no. 4: 1305. https://doi.org/10.3390/molecules27041305
APA StyleZaguzin, A. S., Sukhikh, T. S., Sakhapov, I. F., Fedin, V. P., Sokolov, M. N., & Adonin, S. A. (2022). Zn(II) and Co(II) 3D Coordination Polymers Based on 2-Iodoterephtalic Acid and 1,2-bis(4-pyridyl)ethane: Structures and Sorption Properties. Molecules, 27(4), 1305. https://doi.org/10.3390/molecules27041305








