Stem Extract from Momordica cochinchinensis Induces Apoptosis in Chemoresistant Human Prostate Cancer Cells (PC-3)
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Stem Extract Demonstrated Antiproliferative Effects against Cancer Cells but Not against Normal Cells
2.2. Stem-Extract-Induced Apoptosis in PC-3 Cells
2.3. The Stem Extract Affected the Expression of Apoptosis-Related Proteins in PC-3 Cells
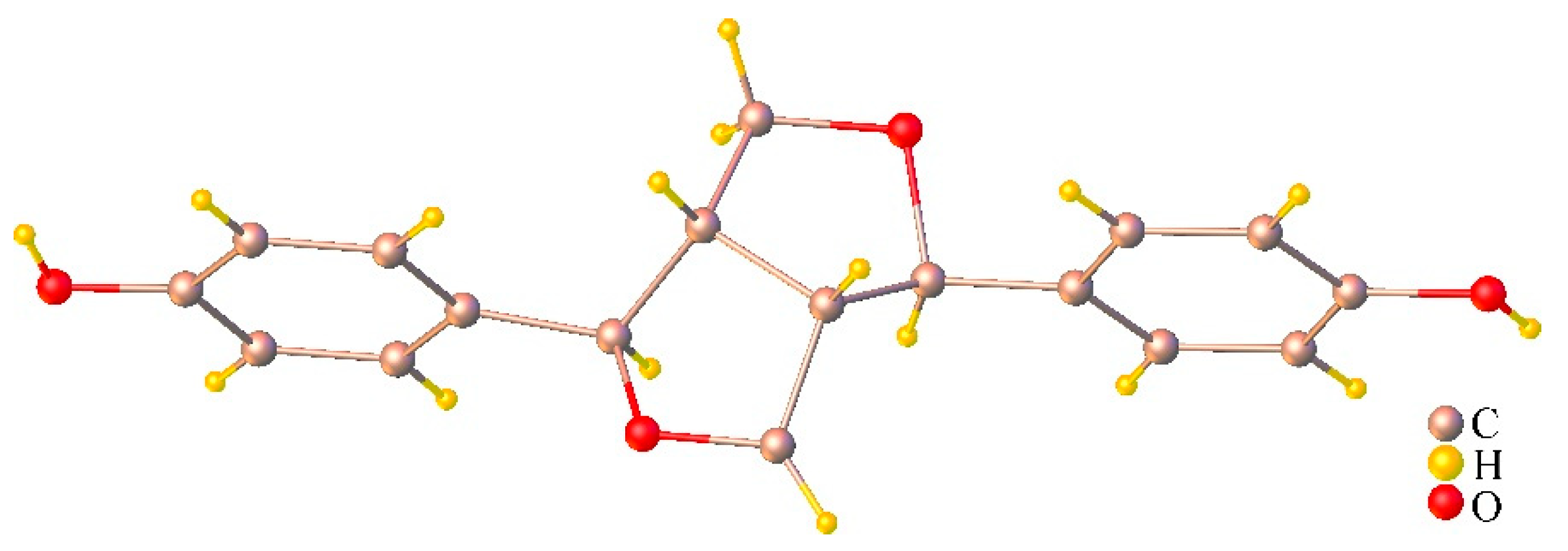
2.4. Chemical Compositions of the Stem Extract and Its Active Compounds
2.5. The Effect of Isolated Compounds on PC-3 Cell Viability
3. Materials and Methods
3.1. General Procedures
3.2. Plant Materials
3.2.1. Preparation of MC Extracts
3.2.2. Phytochemical Isolation of the Stem Extract
3.2.3. Analysis of Fatty Acid Composition
Preparation of Fatty Acid Methyl Esters
3.3. Cell Lines and Cell Culture
3.4. Cell Viability Assay
3.5. Nuclei Staining
3.6. Sub-G1 Apoptosis Assay
3.7. Measurement of Mitochondrial Membrane Potential (MMP)
3.8. Western Blot Analysis
3.9. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular Mechanisms of Apoptosis and Roles in Cancer Development and Treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chipuk, J.E.; Moldoveanu, T.; Llambi, F.; Parsons, M.J.; Green, D.R. The BCL-2 Family Reunion. Mol. Cell 2010, 37, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Wimalasiri, D.; Brkljača, R.; Piva, T.; Urban, S.; Huynh, T. Comparative analysis of carotenoid content in Momordica cochinchinensis (Cucurbitaceae) collected from Australia, Thailand and Vietnam. J. Food Sci. Technol. 2017, 54, 2814–2824. [Google Scholar] [CrossRef]
- Burke, D.S.; Smidt, C.R.; Vuong, L.T. Momordica cochinchinensis, Rosa roxburghii, wolfberry, and sea buckthorn-highly nutritional fruits supported by tradition and science. Curr. Top. Nutraceutical Res. 2005, 3, 259–266. [Google Scholar]
- Ishida, B.K.; Turner, C.; Chapman, M.H.; McKeon, T.A. Fatty Acid and Carotenoid Composition of Gac (Momordica cochinchinensis Spreng) Fruit. J. Agric. Food Chem. 2004, 52, 274–279. [Google Scholar] [CrossRef]
- Xu, J.; Hu, H.; Chen, B.; Yue, R.; Zhou, Z.; Liu, Y.; Zhang, S.; Xu, L.; Wang, H.; Yu, Z. Lycopene Protects against Hypoxia/Reoxygenation Injury by Alleviating ER Stress Induced Apoptosis in Neonatal Mouse Cardiomyocytes. PLoS ONE 2015, 10, e0136443. [Google Scholar] [CrossRef]
- Sitthichai, I.; Wannisa, S.; Apichakarn, S.; Bungorn, S. Protective Effect of Momordica cochinchinensis (L.) Spreng Aril Extract on Essential Testicular Markers in Rats Induced with Valproic Acid. Int. J. Morphol. 2017, 35, 992–999. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Wu, Q.; Zhang, M.; Huang, J. Lycopene inhibits the cell proliferation and invasion of human head and neck squamous cell carcinoma. Mol. Med. Rep. 2016, 14, 2953–2958. [Google Scholar] [CrossRef] [Green Version]
- Wimalasiri, D.; Dekiwadia, C.; Fong, S.Y.; Piva, T.J.; Huynh, T. Anticancer activity of Momordica cochinchinensis (red gac) aril and the impact of varietal diversity. BMC Complement. Med. Ther. 2020, 20, 365. [Google Scholar] [CrossRef]
- Haddad, N.F.; Teodoro, A.J.; Leite de Oliveira, F.; Soares, N.; de Mattos, R.M.; Hecht, F.; Dezonne, R.S.; Vairo, L.; Goldenberg, R.C.; Gomes, F.C.; et al. Lycopene and beta-carotene induce growth inhibition and proapoptotic effects on ACTH-secreting pituitary adenoma cells. PLoS ONE. 2013, 8, e62773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petchsak, P.; Sripanidkulchai, B. Momordica cochinchinensis Aril Extract Induced Apoptosis in Human MCF-7 Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2015, 16, 5507–5513. [Google Scholar] [CrossRef]
- Li, B.-H.; Jiang, L.-N.; Liu, Y.-B. Lycopene exerts anti-inflammatory effect to inhibit prostate cancer progression. Asian J. Androl. 2019, 21, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-R.; Meng, L.-Y.; Lin, Z.-Y.; Shen, Y.; Yu, Y.-Q.; Zhu, Y.-Z. Cochinchina Momordica Seed Extract Induces Apoptosis and Cell Cycle Arrest in Human Gastric Cancer Cells Via PARP and p53 Signal Pathways. Nutr. Cancer 2012, 64, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, Y.; Liu, Y.; Yang, X.O.; Zhan, Y. Momordica cochinchinensis Spreng. seed extract suppresses breast cancer growth by inducing cell cycle arrest and apoptosis. Mol. Med. Rep. 2015, 12, 6300–6310. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Meng, L.; Sun, H.; Zhu, Y.; Liu, H. Cochinchina MomordicaSeed Suppresses Proliferation and Metastasis in Human Lung Cancer Cells by Regulating Multiple Molecular Targets. Am. J. Chin. Med. 2015, 43, 149–166. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Y.-M.; Zhan, Y.-Z.; Liu, C.-X. Momordica cochinchinensis seed extracts suppress migration and invasion of human breast cancer ZR-75-30 cells via down-regulating MMP-2 and MMP-9. Asian Pac. J. Cancer Prev. 2014, 15, 1105–1110. [Google Scholar] [CrossRef] [Green Version]
- Le, A.V.; Huynh, T.T.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Bioactive Composition, Antioxidant Activity, and Anticancer Potential of Freeze-Dried Extracts from Defatted Gac (Momordica cochinchinensis Spreng) Seeds. Medicines 2018, 5, 104. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.; Chin, Y.W.; Yoon, K.d.; Chae, H.S.; Kim, C.Y.; Yoo, H.; Kim, J. Anti-inflammatory properties of a triterpenoidal glycoside from Momordica cochinchinensis in LPS-stimulated macrophages. Immunopharmacol. Immunotoxicol. 2013, 35, 8–14. [Google Scholar] [CrossRef]
- Yu, J.S.; Kim, J.H.; Lee, S.; Jung, K.; Kim, K.H.; Cho, J.Y. Src/Syk-Targeted Anti-Inflammatory Actions of Triterpenoidal Saponins from Gac (Momordica cochinchinensis) Seeds. Am. J. Chin. Med. 2017, 45, 459–473. [Google Scholar] [CrossRef]
- Hardy, S.; El-Assaad, W.; Przybytkowski, E.; Joly, E.; Prentki, M.; Langelier, Y. Saturated Fatty Acid-induced Apoptosis in MDA-MB-231 Breast Cancer Cells: A role for cardiolipin. J. Biol. Chem. 2003, 278, 31861–31870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Chazotte, B. Labeling Mitochondria with JC-1; CSHL Press: Cold Spring Harbor, NY, USA, 2011. [Google Scholar] [CrossRef]
- Reers, M.; Smiley, S.T.; Mottola-Hartshorn, C.; Chen, A.; Lin, M.; Chen, L.B. [29] Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995, 260, 406–417. [Google Scholar] [CrossRef]
- Maji, S.; Panda, S.; Samal, S.K.; Shriwas, O.; Rath, R.; Pellecchia, M.; Emdad, L.; Das, S.K.; Fisher, P.B.; Dash, R. Bcl-2 Antiapoptotic Family Proteins and Chemoresistance in Cancer. Adv. Cancer Res. 2018, 137, 37–75. [Google Scholar] [CrossRef]
- Reiner, T.; de Las Pozas, A.; Parrondo, R.; Palenzuela, D.; Cayuso, W.; Rai, P.; Perez-Stable, C. Mcl-1 protects prostate cancer cells from cell death mediated by chemotherapy-induced DNA damage. Oncoscience 2015, 2, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Willis, S.N.; Wei, A.; Smith, B.; Fletcher, J.; Hinds, M.; Colman, P.M.; Day, C.; Adams, J.; Huang, D. Differential Targeting of Prosurvival Bcl-2 Proteins by Their BH3-Only Ligands Allows Complementary Apoptotic Function. Mol. Cell 2005, 17, 393–403. [Google Scholar] [CrossRef]
- Adams, J.M. Ways of dying: Multiple pathways to apoptosis. Genes Dev. 2003, 17, 2481–2495. [Google Scholar] [CrossRef] [Green Version]
- Willis, S.N.; Chen, L.; Dewson, G.; Wei, A.; Naik, E.; Fletcher, J.I.; Adams, J.M.; Huang, D.C. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005, 19, 1294–1305. [Google Scholar] [CrossRef] [Green Version]
- Kan, L.-D.; Hu, Q.; Chao, Z.-M.; Song, X.; Cao, X.-L. Chemical constituents of unsaponifiable matter from seed oil of Momordica cochinchinensis. China J. Chin. Mater. Med. 2006, 31, 1441–1444. [Google Scholar]
- Zhao, L.M.; Sun, G.G.; Han, L.N.; Liu, L.H.; Ren, F.Z.; Li, L.; Ma, M.; Shan, B.E. P-Hydroxycinnamaldehyde Induces B16-F1 Melanoma Cell Differentiation via the RhoA-MAPK Signaling Pathway. Cell Physiol. Biochem. 2016, 38, 2247–2260. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.; Athukoralage, P.S.; Jamie, J.F. A New Oleanane Triterpenoid from Gordonia ceylanica. Nat. Prod. Lett. 2001, 15, 339–344. [Google Scholar] [CrossRef]
- Rao, M.; Lavie, D. The constituents of Ecballium elaterium L.—XXII, phenolics as minor components. Tetrahedron 1974, 30, 3309–3313. [Google Scholar] [CrossRef]
- Van Minh, C.; Nhiem, N.X.; Yen, H.T.; Van Kiem, P.; Tai, B.H.; Anh, H.L.T.; Hien, T.T.T.; Park, S.; Kim, N.; Kim, S.H. Chemical constituents of Trichosanthes kirilowii and their cytotoxic activities. Arch. Pharmacal Res. 2015, 38, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Bruker. APEX3, SAINT, SADABS and XP; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sedky, N.K.; El-Gammal, Z.; Wahba, A.; Mosad, E.; Waly, Z.Y.; El-Fallal, A.A.; Arafa, R.; El-Badri, N. The molecular basis of cytotoxicity of α-spinasterol from Ganoderma resinaceum: Induction of apoptosis and overexpression of p53 in breast and ovarian cancer cell lines. J. Cell. Biochem. 2018, 119, 3892–3902. [Google Scholar] [CrossRef]
- Ravikumar, Y.; Mahadevan, K.; Manjunatha, H.; Satyanarayana, N. Antiproliferative, apoptotic and antimutagenic activity of isolated compounds from Polyalthia cerasoides seeds. Phytomedicine 2010, 17, 513–518. [Google Scholar] [CrossRef]
- Meneses-Sagrero, S.E.; Navarro-Navarro, M.; Ruiz-Bustos, E.; Del-Toro-Sánchez, C.L.; Jiménez-Estrada, M.; Robles-Zepeda, R.E. Antiproliferative activity of spinasterol isolated of Stegnosperma halimifolium (Benth, 1844). Saudi Pharm. J. 2017, 25, 1137–1143. [Google Scholar] [CrossRef]








| Cell Lines | IC50 Values (mg/mL) | |||
|---|---|---|---|---|
| Leaves | Seeds | Stems | Roots | |
| A549 | 1.98 ± 0.74 | 1.30 ± 0.96 | 0.44 ± 0.87 | 0.74 ± 3.14 |
| HeLa | 0.53 ± 0.37 | 2.09 ± 1.41 | 0.42 ± 1.07 | 0.48 ± 0.38 |
| MDA-MB-231 | 2.10 ± 0.79 | 2.26 ± 1.18 | 0.62 ± 0.74 | 0.77 ± 3.72 |
| PC-3 | 4.25 ± 0.66 | 4.65 ± 0.36 | 0.62 ± 0.74 | 0.73 ± 2.27 |
| Peak | RT | Fatty Acid Names | Mean ± SEM |
|---|---|---|---|
| 1 | 2.292 | Lauric acid (C12:0) | 0.18 ± 0.01 |
| 3 | 2.897 | Myristic acid (C14:0) | 1.54 ± 0.02 |
| 8 | 3.761 | Palmitic acid (C16:0) | 35.07 ± 0.19 |
| 10 | 3.994 | Palmitoleic acid (C16:1) | 1.34 ± 0.02 |
| 15 | 4.827 | Stearic acid (C18:0) | 14.09 ± 0.05 |
| 16 | 5.072 | Oleic acid (C18:1) | 7.30 ± 0.02 |
| 18 | 5.525 | Linoleic acid (C18:2) | 11.85 ± 0.07 |
| 20 | 6.125 | α-Linolenic acid (C18:3) | 27.0 ± 0.09 |
| 21 | 6.245 | Arachidic acid (C20:0) | 1.63 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chainumnim, S.; Saenkham, A.; Dolsophon, K.; Chainok, K.; Suksamrarn, S.; Tanechpongtamb, W. Stem Extract from Momordica cochinchinensis Induces Apoptosis in Chemoresistant Human Prostate Cancer Cells (PC-3). Molecules 2022, 27, 1313. https://doi.org/10.3390/molecules27041313
Chainumnim S, Saenkham A, Dolsophon K, Chainok K, Suksamrarn S, Tanechpongtamb W. Stem Extract from Momordica cochinchinensis Induces Apoptosis in Chemoresistant Human Prostate Cancer Cells (PC-3). Molecules. 2022; 27(4):1313. https://doi.org/10.3390/molecules27041313
Chicago/Turabian StyleChainumnim, Seksom, Audchara Saenkham, Kulvadee Dolsophon, Kittipong Chainok, Sunit Suksamrarn, and Wanlaya Tanechpongtamb. 2022. "Stem Extract from Momordica cochinchinensis Induces Apoptosis in Chemoresistant Human Prostate Cancer Cells (PC-3)" Molecules 27, no. 4: 1313. https://doi.org/10.3390/molecules27041313






_CHAINOK.jpg)


