Bioguided Phytochemical Study of Ipomoea cairica Extracts with Larvicidal Activity against Aedes aegypti
Abstract
:1. Introduction
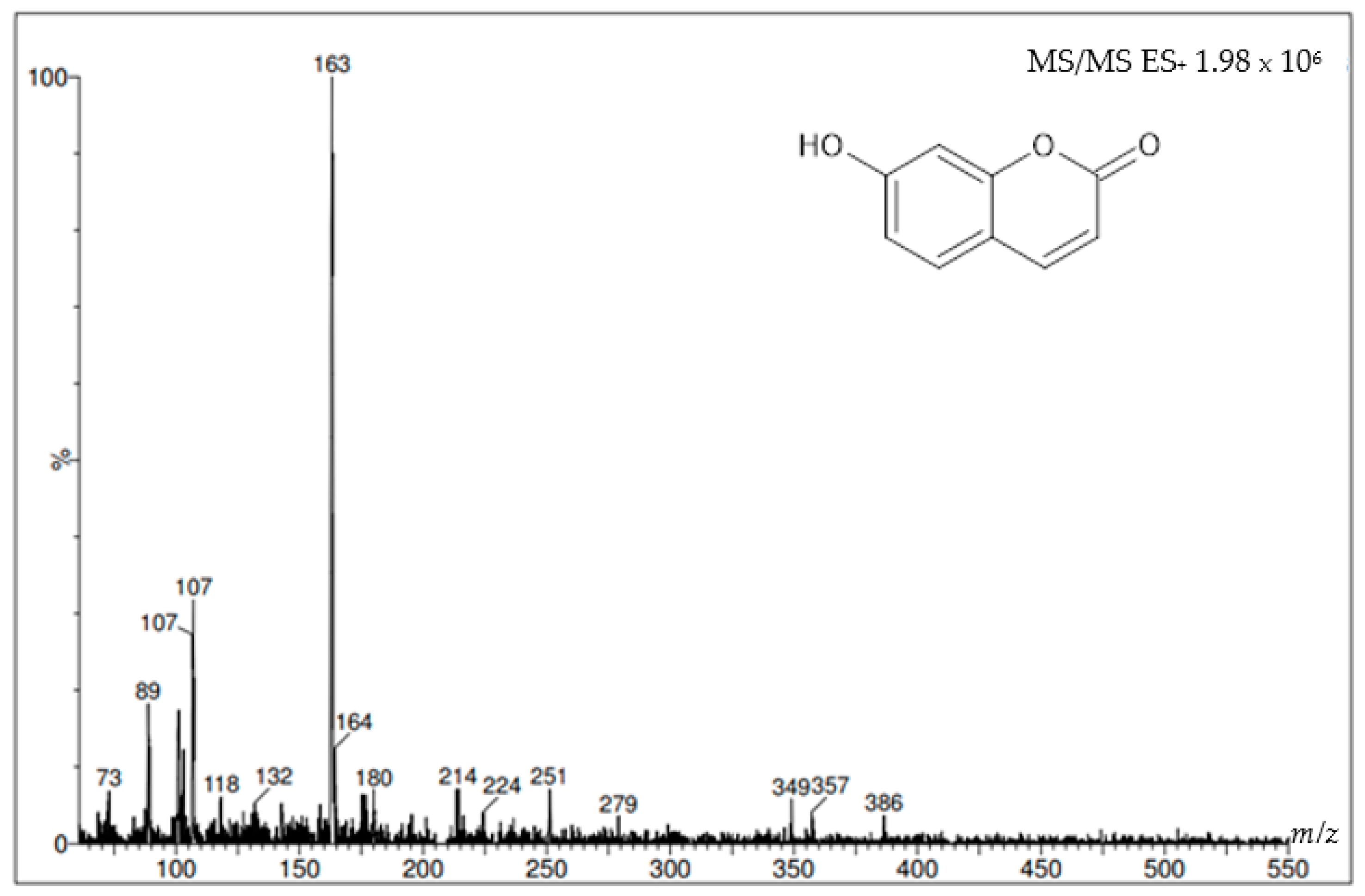
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Sample Processing
4.3. Monitoring Using TLC and Isolation Using Preparative TLC
4.4. High-Efficiency Liquid Chromatography with a Diode Array Detector and Mass Spectrometry (HPLC-DAD-MS/MS)
4.5. Larval Culture
4.6. Bioassay of Larvicidal Activity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kim, S.I.; Ahn, Y.J. Larvicidal activity of lignans and alkaloid identified in Zanthoxylum piperitum bark toward insecticide-susceptible and wild Culex pipiens pallens and Aedes aegypti. Parasites Vectors 2017, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.; Lounibos, P. Ecología de Aedes aegypti y Aedes albopictus en América y la transmisión de enfermedades. Biomédica 2015, 35, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Patterson, J.; Sammon, M.; Garg, M. Dengue, zika and chikungunya: Emerging arboviruses in the new world. West. J. Emerg. Med. 2016, 17, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of Costa Rica Dengue Cases in 2020 Already Exceed All Cases in 2019. Available online: https://www.ministeriodesalud.go.cr/index.php/vigilancia-de-la-salud/analisis-de-situacion-de-salud (accessed on 7 April 2021).
- Yu, B.; Luo, J.; Wang, J.; Zhang, D.; Yu, S.; Kong, L. Pentasaccharide resin glycosides from Ipomoea cairica and their cytotoxic activities. Phytochemistry 2013, 95, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.A.; Amaral, F.A.; Duarte, I.D.G.; Oliveira, P.M.; Alves, R.B.; Silveira, D.; Azevedo, A.O.; Raslan, D.S.; Castro, M.S.A. Antinociceptive effect from Ipomoea cairica extract. J. Ethnopharmacol. 2006, 105, 148–153. [Google Scholar] [CrossRef]
- Arora, S.; Kumar, D. Shiba Phytochemical, antimicrobial and antioxidant activities of methanol extract of leaves and flowers of Ipomoea Cairica. Int. J. Pharm. Pharm. Sci. 2013, 5, 198–202. [Google Scholar]
- Ahbirami, R.; Zuharah, W.F.; Thiagaletchumi, M.; Subramaniam, S.; Sundarasekar, J. Larvicidal efficacy of different plant parts of railway creeper, Ipomoea cairica extract against dengue vector mosquitoes, Aedes albopictus (Diptera: Culicidae) and Aedes aegypti (Diptera: Culicidae). J. Insect Sci. 2014, 14, 180. [Google Scholar] [CrossRef] [Green Version]
- Lima, O.; Braz-Filho, R. Dibenzylbutyrolactone lignans and coumarins from Ipomoea cairica. J. Braz. Chem. Soc. 1997, 8, 235–238. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. A Review of Resistance Mechanisms of Synthetic Insecticides and Botanicals, Phytochemicals, and Essential Oils as Alternative Larvicidal Agents Against Mosquitoes. Front. Physiol. 2020, 10, 1–21. [Google Scholar] [CrossRef]
- Srivastava, D.; Shukla, K. Ipomoea cairica: A medicinal weed with promising health benefits. Int. J. Inf. Res. Rev. 2015, 2, 687–694. [Google Scholar]
- Giatropoulos, A.; Kimbaris, A.; Michaelakis, A.; Papachristos, D.P.; Polissiou, M.G.; Emmanouel, N. Chemical composition and assessment of larvicidal and repellent capacity of 14 Lamiaceae essential oils against Aedes albopictus. Parasitol. Res. 2018, 117, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Hari, I.; Mathew, N. Larvicidal activity of selected plant extracts and their combination against the mosquito vectors Culex quinquefasciatus and Aedes aegypti. Environ. Sci. Pollut. Res. 2018, 25, 9176–9185. [Google Scholar] [CrossRef] [PubMed]
- Chellappandian, M.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Karthi, S.; Thanigaivel, A.; Ponsankar, A.; Kalaivani, K.; Hunter, W.B. Botanical essential oils and uses as mosquitocides and repellents against dengue. Environ. Int. 2018, 113, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, V.; Rodríguez, G.; Argüello, S. Insecticidal activity of ethanolic plant extracts on Aedes aegypti larvae. 2022; submitted. [Google Scholar]
- Chariandy, C.; Seaforth, C.; Phelps, R.; Pollard, G.; Khambay, B. Screening of medicinal plants from Trinidad and Tobago for antimicrobial and insecticidal properties. J. Ethnopharmacol. 1999, 64, 265–270. [Google Scholar] [CrossRef]
- Najafabadi, N.S.; Sahari, M.A.; Barzegar, M.; Hamidi Esfahani, Z. Role of Extraction Conditions in the Recovery of Some Phytochemical Compounds of the Jujube Fruit. J. Agric. Sci. Technol. 2020, 22, 439–451. [Google Scholar]
- Meira, M.; da Silva, E.P.; David, J.M.; David, J.P. Review of the genus Ipomoea: Traditional uses, chemistry and biological activities. Braz. J. Pharmacogn. 2012, 22, 682–713. [Google Scholar] [CrossRef] [Green Version]
- Hildebert, W.; Sabine, B. Plant Drug Analysis: A Thin Layer Chromatography Atlas; Segunda, Ed.; Springer: New York, NY, USA, 1996; pp. 125–147. [Google Scholar]
- Mercolini, L.; Mandrioli, R.; Ferranti, A.; Sorella, V.; Protti, M.; Epifano, F.; Curini, M.; Raggi, M.A. Quantitative evaluation of auraptene and umbelliferone, chemopreventive coumarins in citrus fruits, by HPLC-UV-FL-MS. J. Agric. Food Chem. 2013, 61, 1694–1701. [Google Scholar] [CrossRef]
- Carpinella, M.C.; Ferrayoli, C.G.; Palacios, S.M. Antifungal synergistic effect of scopoletin, a hydroxycoumarin isolated from Melia azedarach L. fruits. J. Agric. Food Chem. 2005, 53, 2922–2927. [Google Scholar] [CrossRef]
- Murugan, K.; Mahesh Kumar, P.; Kovendan, K.; Amerasan, D.; Subrmaniam, J.; Hwang, J. Larvicidal, pupicidal, repellent and adulticidal activity of Citrus sinensis orange peel extract against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 2012, 111, 1757–1769. [Google Scholar] [CrossRef]
- Wang, Z.; Kim, J.-R.R.; Wang, M.; Shu, S.; Ahn, Y.-J.J. Larvicidal activity of Cnidium monnieri fruit coumarins and structurally related compounds against insecticide-susceptible and insecticide-resistant Culex pipiens pallens and Aedes aegypti. Pest. Manag. Sci. 2012, 68, 1041–1047. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Hornick, A.; Langer, T.; Stuppner, H.; Prast, H. Acetylcholinesterase inhibitory activity of scopolin and scopoletin discovered by virtual screening of natural products. J. Med. Chem. 2004, 47, 6248–6254. [Google Scholar] [CrossRef] [PubMed]
- Patar, A.A.; Hassan, W.R.M.; Yusof, F.Z.M. Acute toxicity of malathion, dichlorvos and temephos in climbing perch (Anabas testudineus). Malaysian Appl. Biol. 2015, 44, 37–42. [Google Scholar]
- Costa, M.S.; Santana, A.E.G.; Oliveira, L.L.; Zanuncio, J.C.; Serrão, J.E. Toxicity of squamocin on Aedes aegypti larvae, its predators and human cells. Pest. Manag. Sci. 2017, 73, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Ohshima, M.; Shimada, H.; Akagi, T.; Iwamura, H.; McLaughlin, J.L. Essential structural factors of annonaceous acetogenins as potent inhibitors of mitochondrial complex I. Biochim. Biophys. Acta—Bioenerg. 1998, 1365, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Benelli, G.; Pavela, R.; Canale, A.; Cianfaglione, K.; Ciaschetti, G.; Conti, F.; Nicoletti, M.; Senthil-Nathan, S.; Mehlhorn, H.; Maggi, F. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017, 66, 166–171. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Sánchez-Aldana, D.; Chacón-Vargas, K.F.; Rivera-Chavira, B.E.; Sánchez-Torres, L.E.; Camacho, A.D.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Oviposition deterrent and larvicidal and pupaecidal activity of seven essential oils and their major components against Culex quinquefasciatus say (Diptera: Culicidae): Synergism–antagonism effects. Insects 2018, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Pinto, C.C.C.; De Menezes, J.E.S.A.; Melo, D.S.; Feitosa, C.R.S. Chemical Composition and larvicidal activity against Aedes aegypti of essential oils from Croton jacobinenesis Baill. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2016, 15, 122–127. [Google Scholar]
- Silvério, M.R.S.; Espindola, L.S.; Lopes, N.P.; Vieira, P.C. Plant natural products for the control of Aedes aegypti: The main vector of important arboviruses. Molecules 2020, 25, 3484. [Google Scholar] [CrossRef]
- De Souza Wuillda, A.C.J.; Martins, R.C.C.; Costa, F.D.N. Larvicidal activity of secondary plant metabolites in aedes aegypti control: An overview of the previous 6 years. Nat. Prod. Commun. 2019, 14, 1934578X19862893. [Google Scholar] [CrossRef] [Green Version]
- Mordue(Luntz), A.J.; Nisbet, A.J. Azadirachtin from the neem tree Azadirachta indica: Its action against insects. An. Soc. Entomol. Bras. 2000, 29, 615–632. [Google Scholar] [CrossRef] [Green Version]
- Kishore, N.; Mishra, B.; Tiwari, V.; Tripathi, V. A review on natural products with mosquitosidal potentials. Res. Signpost Chall. Scope Nat. Prod. Med. Chem. 2011, 37661, 335–365. [Google Scholar]


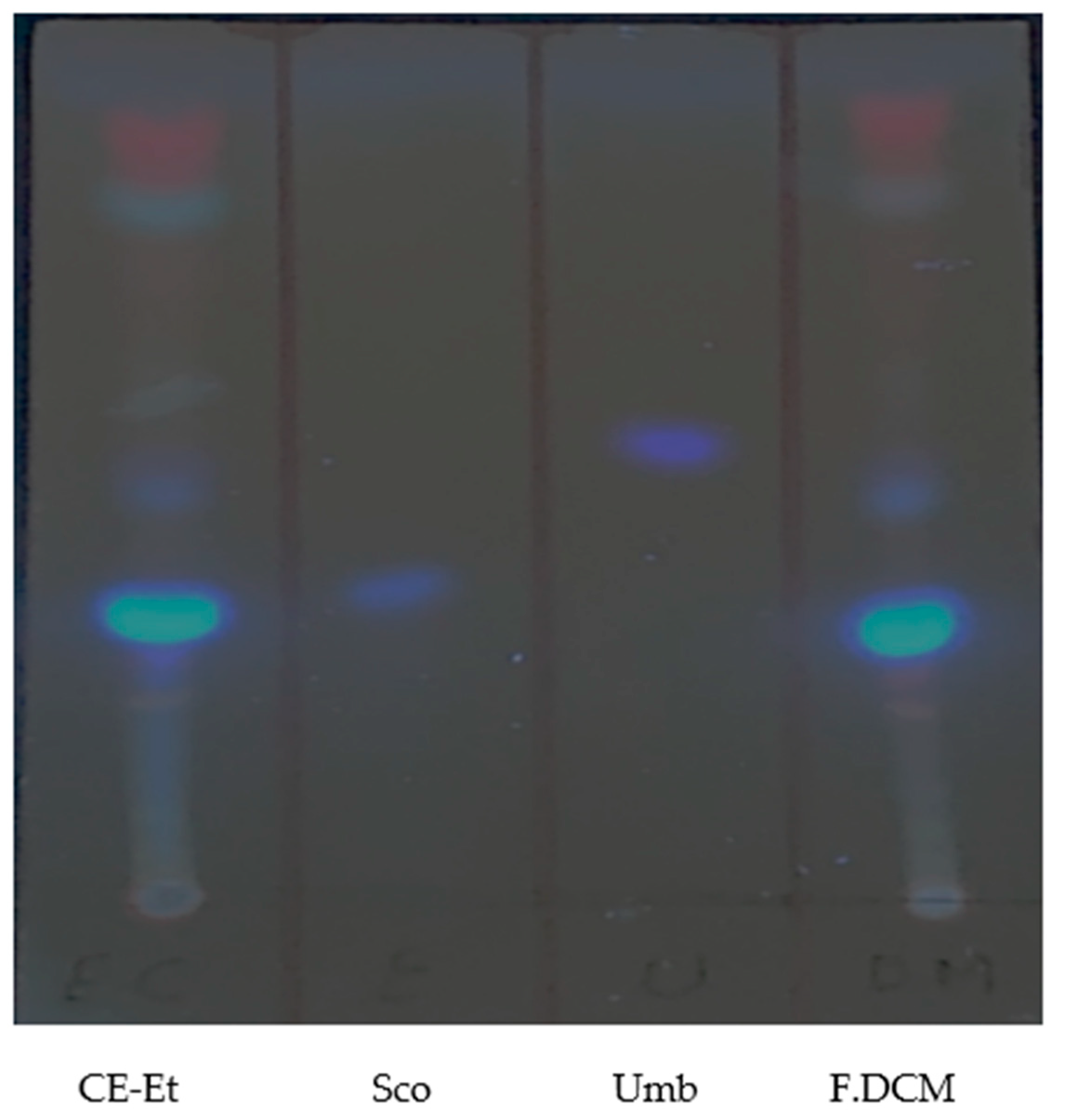
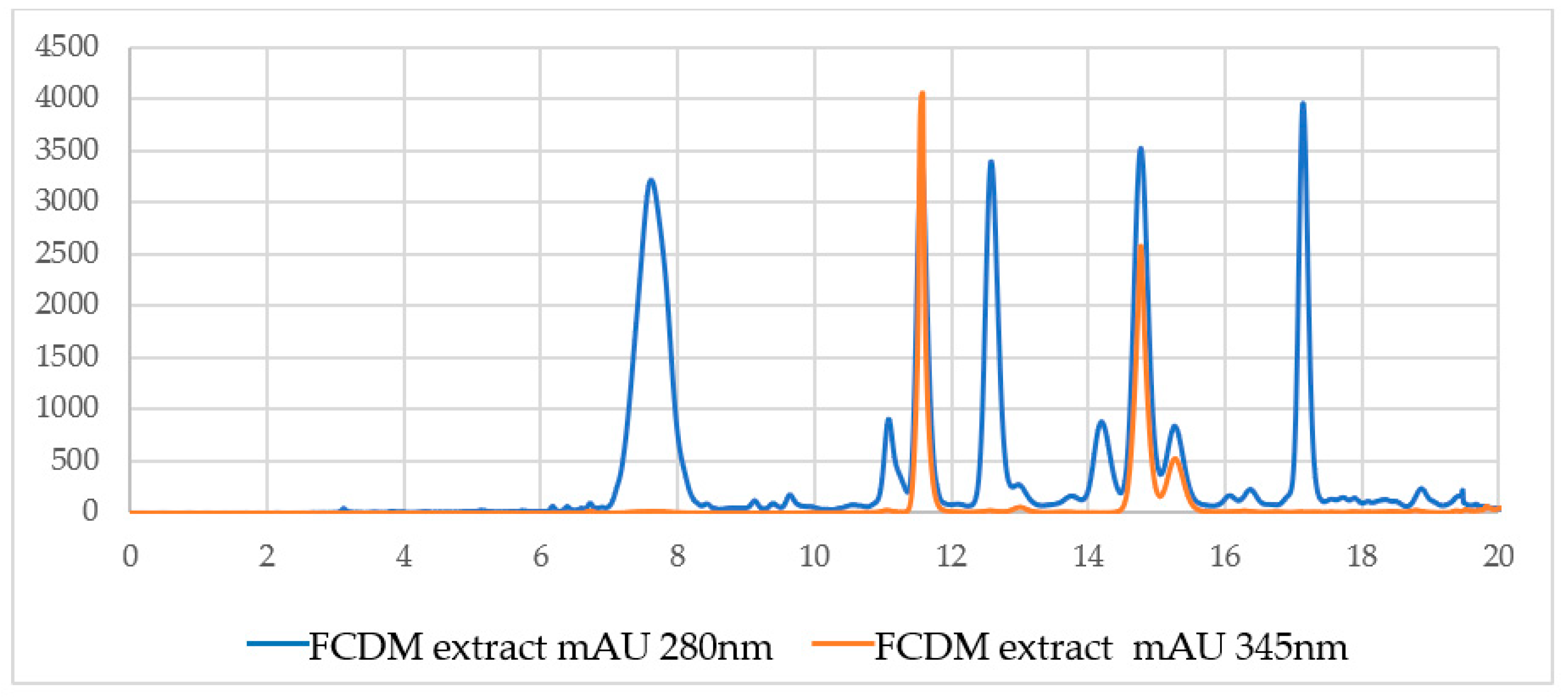
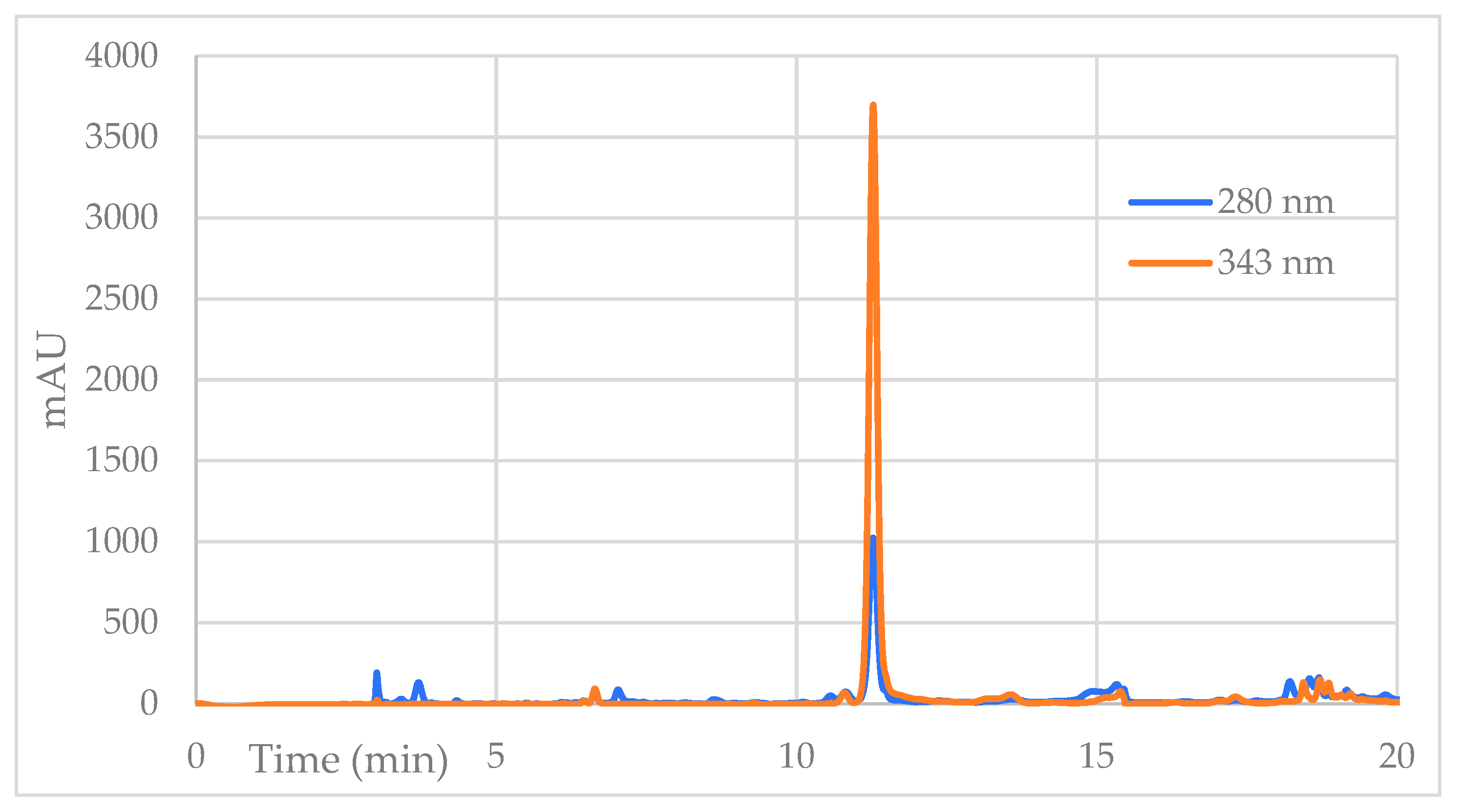
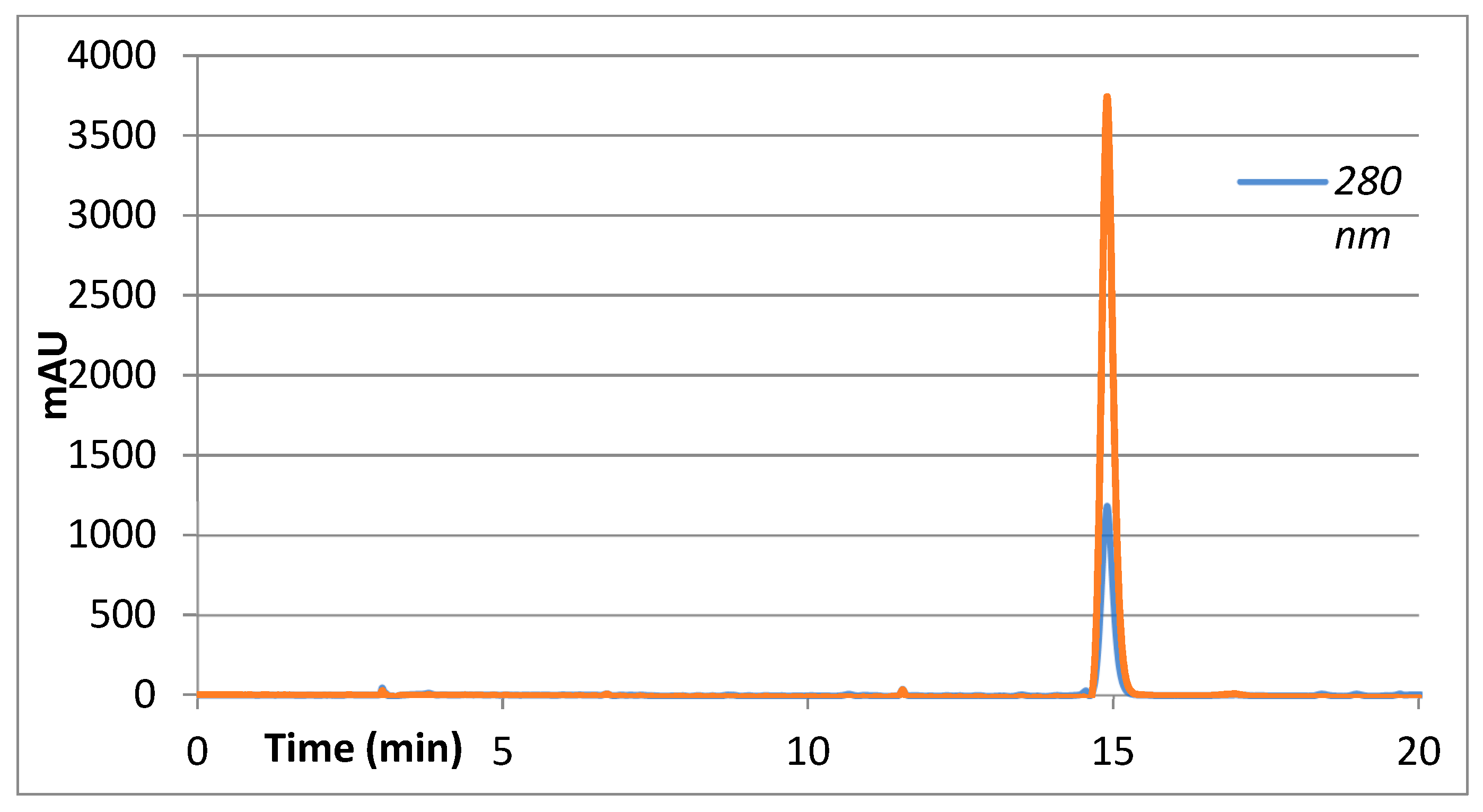


| Sample | Percent Mortality at 24 h |
|---|---|
| Stems | 71.3 ± 4.8% |
| Leaves | 40.0 ± 0.0% |
| Positive control | 100 ± 0% |
| Negative control | 0 ± 0% |
| Sample | Percent Mortality at 24 h |
|---|---|
| Crude ethanolic extract | 71.3 ± 4.8% |
| F.He | 0 ± 0% |
| F.DCM | 100 ± 0% |
| F.Ac | 18.8 ± 2.9% |
| F.Me | 0 ± 0% |
| Positive control | 100 ± 0% |
| Negative control | 0 ± 0% |
| Sample | Percent Mortality at 24 h * |
|---|---|
| F.DCM | 100 ± 0% |
| F.DCM (1) | 0 ± 0% |
| F.DCM (2) | 0 ± 0% |
| F.DCM (3) | 0 ± 0% |
| Positive control | 100 ± 0% |
| Negative control | 0 ± 0% |
| Fraction | LC50 in mg/L (95% CI *) | LC90 in mg/L (95% CI *) | Diagnostic Dose in mg/L (95% CI *) |
|---|---|---|---|
| F.DCM | 30.608 (23.9–39.1) | 79.875 (62.5–98.1) | 349.2 (273.1–446.4) |
| Crude ethanolic extract | 42.1 (32.1–55.0) | 131 (100.0–172.0) | 664 (507–870) |
| Sample | Percent Mortality |
|---|---|
| Scopoletin | 0 |
| Umbelliferone | 0 |
| Scopoletin + Umbelliferone | 0 |
| CONTROL+ | 100.00 |
| CONTROL− | 0.00 |
| Time (min) | Concentration of A | Concentration of B | Flow Rate (mL/min) |
|---|---|---|---|
| 0.01 | 85 | 15 | 1.00 |
| 3.00 | 65 | 35 | 1.00 |
| 7.00 | 60 | 40 | 1.00 |
| 10.00 | 55 | 45 | 1.00 |
| 13.00 | 50 | 50 | 1.00 |
| 15.00 | 50 | 50 | 0.50 |
| 16.00 | 50 | 50 | 0.50 |
| 18.00 | 15 | 85 | 0.50 |
| 18.00 | 15 | 85 | 1.25 |
| 19.00 | 0 | 100 | 1.25 |
| 20.00 | 85 | 15 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez Valverde, V.; Rodríguez Rodríguez, G.; Argüello Vargas, S. Bioguided Phytochemical Study of Ipomoea cairica Extracts with Larvicidal Activity against Aedes aegypti. Molecules 2022, 27, 1348. https://doi.org/10.3390/molecules27041348
Álvarez Valverde V, Rodríguez Rodríguez G, Argüello Vargas S. Bioguided Phytochemical Study of Ipomoea cairica Extracts with Larvicidal Activity against Aedes aegypti. Molecules. 2022; 27(4):1348. https://doi.org/10.3390/molecules27041348
Chicago/Turabian StyleÁlvarez Valverde, Víctor, Gerardo Rodríguez Rodríguez, and Silvia Argüello Vargas. 2022. "Bioguided Phytochemical Study of Ipomoea cairica Extracts with Larvicidal Activity against Aedes aegypti" Molecules 27, no. 4: 1348. https://doi.org/10.3390/molecules27041348
APA StyleÁlvarez Valverde, V., Rodríguez Rodríguez, G., & Argüello Vargas, S. (2022). Bioguided Phytochemical Study of Ipomoea cairica Extracts with Larvicidal Activity against Aedes aegypti. Molecules, 27(4), 1348. https://doi.org/10.3390/molecules27041348






