l-Lysine-Based Gelators for the Formation of Oleogels in Four Vegetable Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Nα, Nε-diacyl-l-lysines
2.3. Oleogel Preparation
2.4. Characterization of Vegetable Oils
2.5. Rheological Behavior Measurements
2.6. TEM Measurements
2.7. Oil-Binding Capacity (OBC) Determination
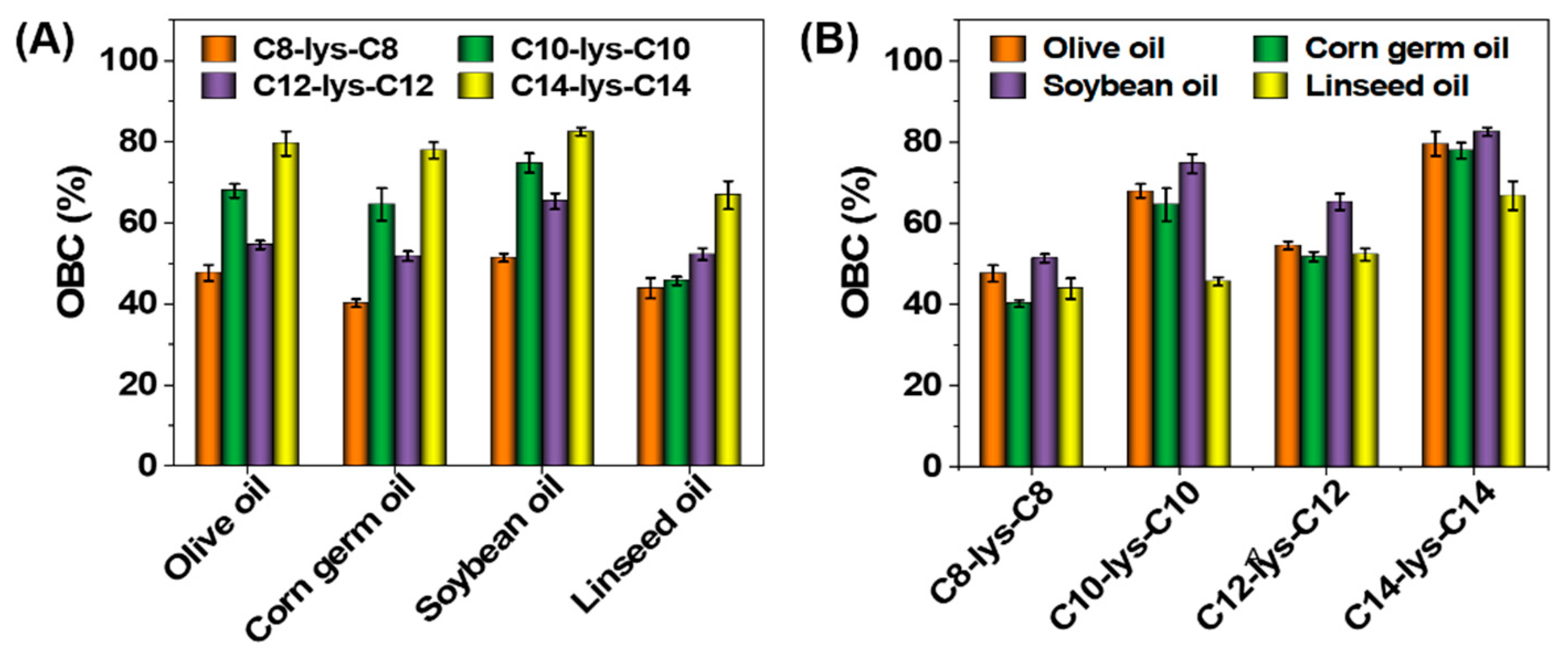
3. Results and Discussion
3.1. Preparation and Characterization of l-Lysine-Based Gelators
3.2. Preparation of Oleogels
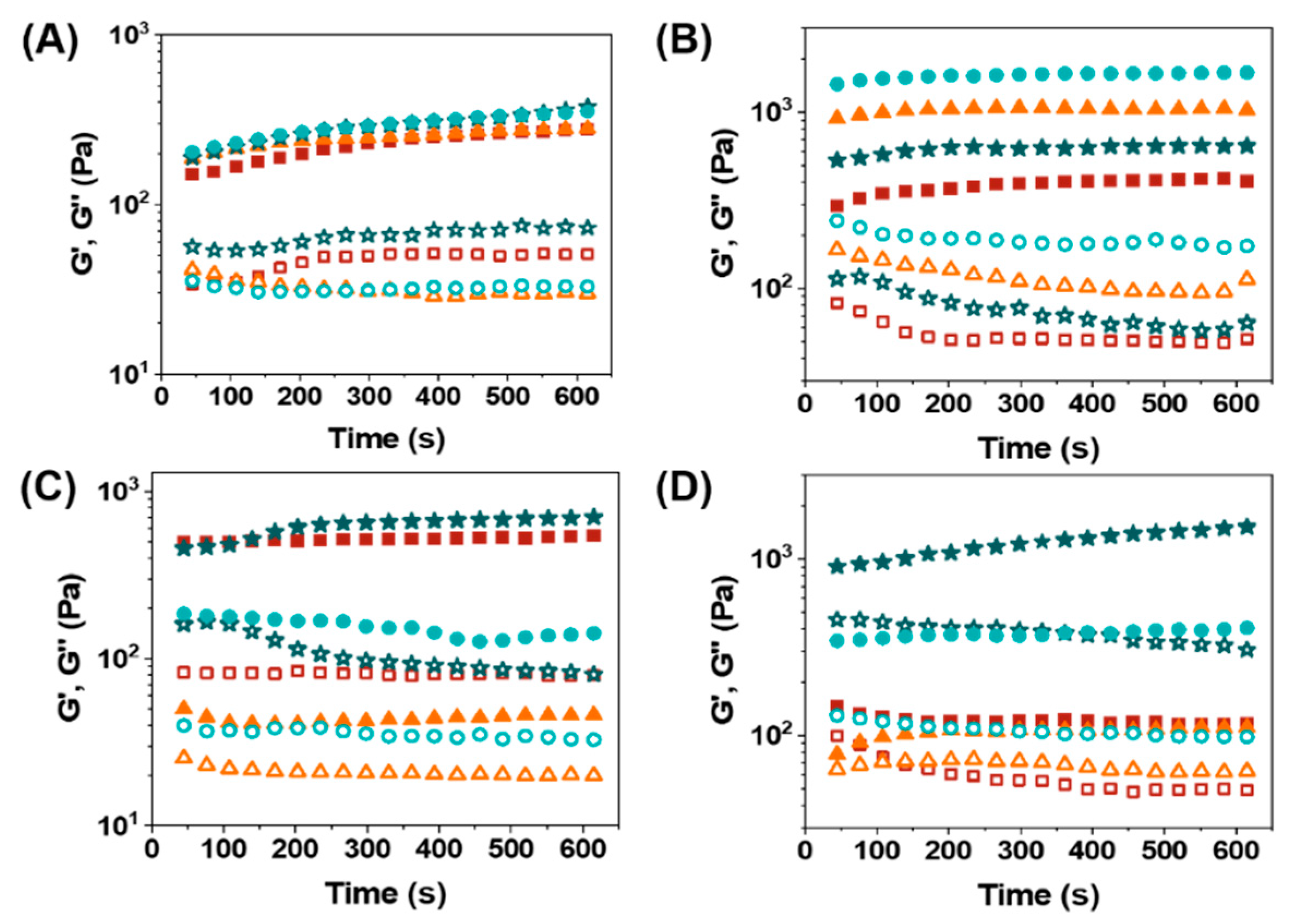
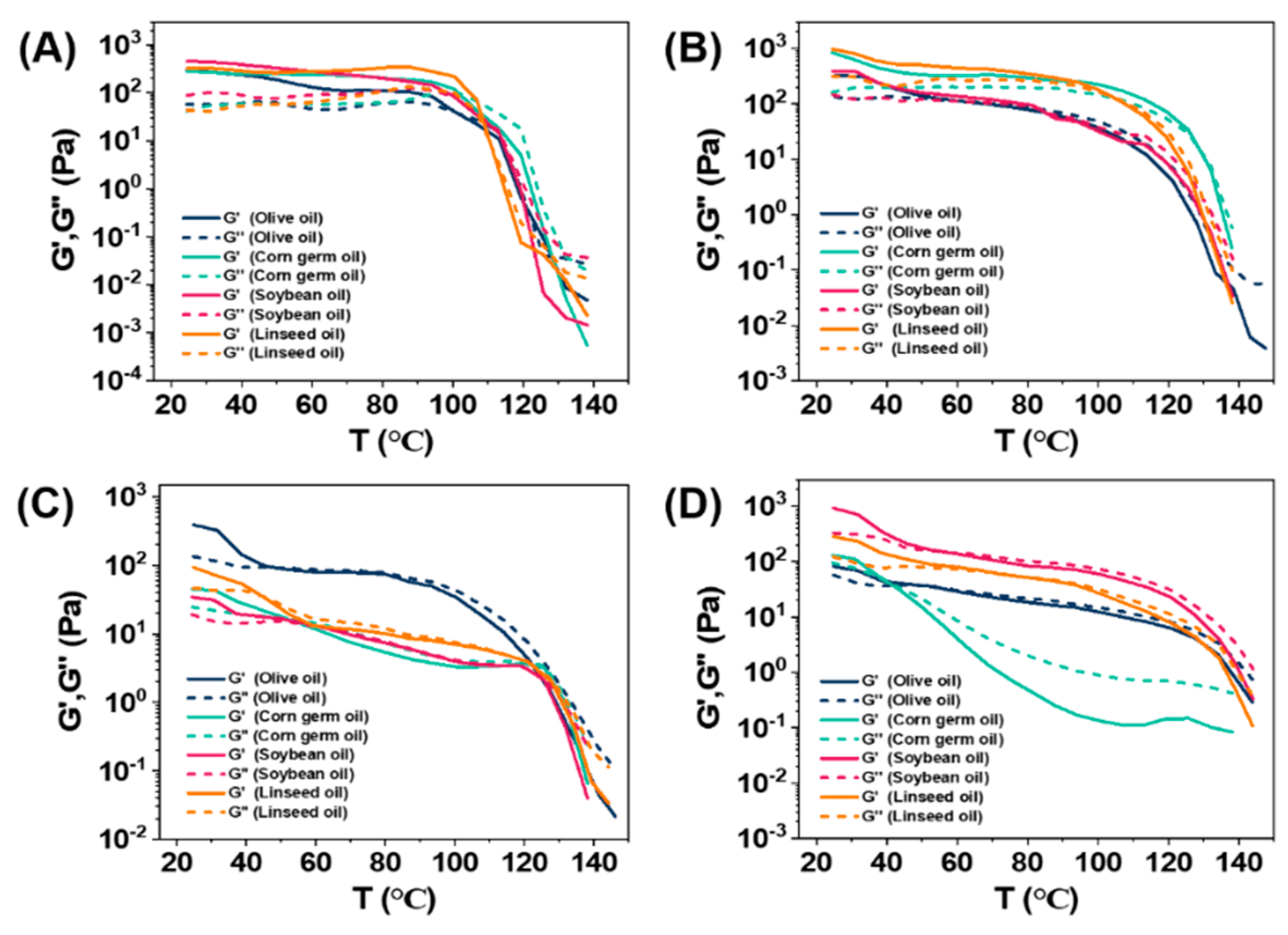
3.3. Rheological Properties
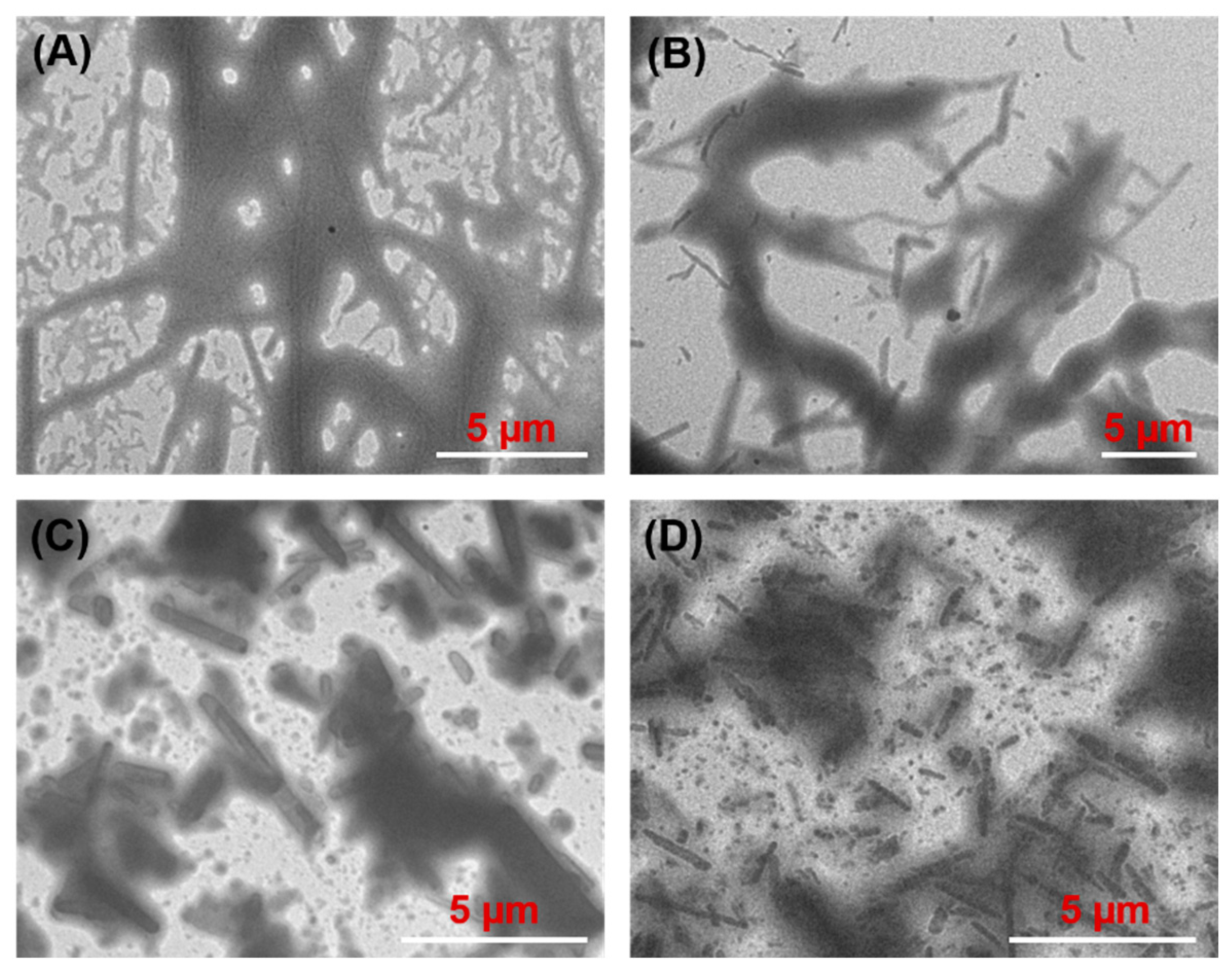
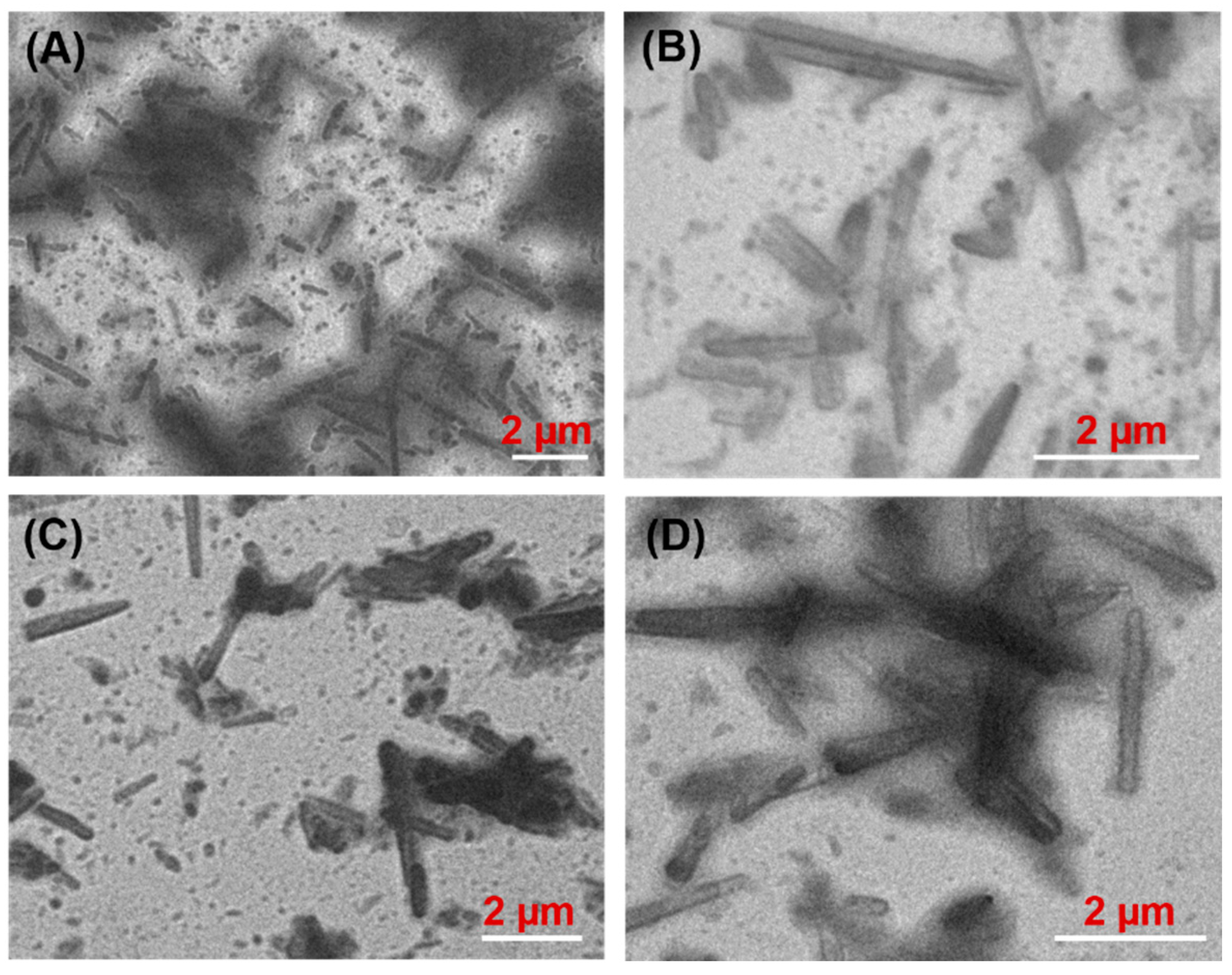
3.4. Morphology of Oleogels
3.5. OBC of Oleogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight gels: The state of the art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef] [Green Version]
- Raeburn, J.; Zamith Cardoso, A.; Adams, D.J. The importance of the self-assembly process to control mechanical properties of low molecular weight hydrogels. Chem. Soc. Rev. 2013, 42, 5143–5156. [Google Scholar] [CrossRef]
- Heeres, A.; van der Pol, C.; Stuart, M.; Friggeri, A.; Feringa, B.L.; van Esch, J. Orthogonal Self-Assembly of low molecular weight hydrogelators and surfactants. J. Am. Chem. Soc. 2003, 125, 14252–14253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifazi, E.L.; Edelsztein, V.C.; Menéndez, G.O.; Samaniego López, C.; Spagnuolo, C.C.; Di Chenna, P.H. Versatile Supramolecular Organogel with Outstanding Stability toward Aqueous Interfaces. ACS Appl. Mater. Interfaces 2014, 6, 8933–8936. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [Green Version]
- Mayr, J.; Saldías, C.; Díaz Díaz, D. Release of small bioactive molecules from physical gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef]
- Rizzo, C.; Andrews, J.L.; Steed, J.W.; D’Anna, F. Carbohydrate-supramolecular gels: Adsorbents for chromium(VI) removal from wastewater. J. Colloid Interfaces Sci. 2019, 548, 184–196. [Google Scholar] [CrossRef] [Green Version]
- Marr, P.C.; Marr, A.C. Ionic liquid gel materials: Applications in green and sustainable chemistry. Green Chem. 2016, 18, 105–128. [Google Scholar] [CrossRef] [Green Version]
- D’Anna, F.; Rizzo, C.; Vitale, P.; Lazzara, G.; Noto, R. Dicationic organic salts: Gelators for ionic liquids. Soft Matter 2014, 10, 9281–9292. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Ma, Z.; Yan, L. Deep eutectic solvents eutectogels: Progress and challenges. Green Chem. Eng. 2021, 2, 359–367. [Google Scholar] [CrossRef]
- Marullo, S.; Meli, A.; Giannici, F.; Anna, F.D. Supramolecular eutecto gels: Fully natural soft materials. ACS Sustain. Chem. Eng. 2018, 6, 12598–12602. [Google Scholar] [CrossRef]
- Terech, P.; Weiss, R.G. Low molecular mass gelators of organic liquids and the properties of their gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, W.; Wang, Y.; Yang, Y.; Xu, L.; Li, S. Degradation of glutamate-based organogels for biodegradable implants: In vitro study and in vivo observation. Mater. Sci. Eng. C 2018, 82, 80–90. [Google Scholar] [CrossRef]
- Hu, B.; Yan, H.; Sun, Y.; Chen, X.; Sun, Y.; Li, S.; Jing, Y.; Li, H. Organogels based on amino acid derivatives and their optimization for drug release using response surface methodology. Artif. Cells Nanomed. Biotechnol. 2020, 48, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S. Formation of oleogels based on edible lipid materials. Curr. Opin. Colloid Interface Sci. 2011, 16, 432–439. [Google Scholar] [CrossRef]
- Patel, A.R. Edible “oleocolloids”: The final frontier in food innovation? J. Agric. Food Chem. 2017, 65, 3432–3433. [Google Scholar] [CrossRef]
- Weiss, R.G. The past, present, and future of molecular gels. What is the status of the field, and where is it going? J. Am. Chem. Soc. 2014, 136, 7519–7530. [Google Scholar] [CrossRef]
- Callau, M.; Sow-Kébé, K.; Nicolas-Morgantini, L.; Fameau, A. Effect of the ratio between behenyl alcohol and behenic acid on the oleogel properties. J. Colloid Interfaces Sci. 2020, 560, 874–884. [Google Scholar] [CrossRef]
- Feng, G.; Chen, H.; Cai, J.; Wen, J.; Liu, X. L-Phenylalanine based Low-Molecular-Weight efficient organogelators and their selective gelation of oil from Oil/Water mixtures. Soft Mater. 2014, 12, 403–410. [Google Scholar] [CrossRef]
- Luo, C.; Yang, B.; Zhou, Y.; Yang, J.; Han, F.; Baocai, X. Gelation properties and application based on amino acids gelators with four kinds of edible oils. Colloid. Surf. A 2020, 585, 124184. [Google Scholar] [CrossRef]
- Pal, A.; Dey, J. L-Cysteine-Derived ambidextrous gelators of aromatic solvents and Ethanol/Water mixtures. Langmuir 2013, 29, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dey, J. Binding of fatty acid amide amphiphiles to bovine serum albumin: Role of amide hydrogen bonding. J. Phys. Chem. B 2015, 119, 7804–7815. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Dey, J. Effect of hydrogen bonding on the physicochemical properties and bilayer Self-Assembly formation of N-(2-Hydroxydodecyl)-l-alanine in aqueous solution. Langmuir 2008, 24, 6018–6026. [Google Scholar] [CrossRef]
- Hai-Kuan, Y.; Hong, Z.; Pei-Ran, Y.; Chao-Hai, H. How do molecular structures affect gelation properties of supramolecular gels? Insights from low-molecular-weight gelators with different aromatic cores and alkyl chain lengths. Colloid. Surf. A 2017, 535, 242–250. [Google Scholar]
- Suzuki, M.; Nakajima, Y.; Yumoto, M.; Kimura, M.; Shirai, H.; Hanabusa, K. Effects of Hydrogen Bonding and van der Waals Interactions on Organogelation Using Designed Low-Molecular-Weight Gelators and Gel Formation at Room Temperature. Langmuir 2003, 19, 8622–8624. [Google Scholar] [CrossRef]
- Suzuki, M.; Hanabusa, K. l-Lysine-based low-molecular-weight gelators. Chem. Soc. Rev. 2009, 38, 967–975. [Google Scholar] [CrossRef]
- Masahiro, S.; Tomoko, A.; Kenji, H. Low-molecular-weight gelators based on Nα-acetyl-Nε-dodecyl-l-lysine and their amphiphilic gelation properties. J. Colloid Interfaces Sci. 2009, 341, 69–74. [Google Scholar]
- Suzuki, M.; Yumoto, M.; Shirai, H.; Hanabusa, K. Supramolecular gels formed by amphiphilic low-molecular-weight gelators of N alpha, N epsilon-diacyl-l-lysine derivatives. Chemistry 2008, 14, 2133–2144. [Google Scholar] [CrossRef]
- Ni, C.; Qi, K.; Jianhong, X.; Na, D.; Li, Y. Supramolecular gels: Using an amide-functionalized imidazolium-based surfactant. J. Colloid Interfaces Sci. 2018, 511, 215–221. [Google Scholar]
- Fayaz, G.; Goli, S.A.H.; Kadivar, M. A novel propolis Wax-Based organogel: Effect of oil type on its formation, crystal structure and thermal properties. J. Am. Oil Chem. Soc. 2017, 94, 47–55. [Google Scholar] [CrossRef]
- Sreenu, M.; Nayak, R.R.; Prasad, R.B.N.; Sreedhar, B. Synthesis, surface and micellar properties of sodium N-oleoyl amino acids. Colloid. Surf. A 2014, 449, 74–81. [Google Scholar] [CrossRef]
- Deepnath, B.; Debmalya, G.; Joykrishna, D. A comparison of the self-assembly behaviour of sodium N-lauroyl sarcosinate and sodium N-lauroyl glycinate surfactants in aqueous and aqueo-organic media. J. Colloid Interfaces Sci. 2018, 529, 314–324. [Google Scholar]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Crystallization kinetics of organogels prepared by rice bran wax and vegetable oils. J. Oleo Sci. 2012, 61, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Doan, C.D.; Van de Walle, D.; Dewettinck, K.; Patel, A.R. Evaluating the Oil-Gelling properties of natural waxes in rice bran oil: Rheological, thermal, and microstructural study. J. Am. Oil Chem. Soc. 2015, 92, 801–811. [Google Scholar] [CrossRef]
- Blake, A.I.; Marangoni, A.G. The use of cooling rate to engineer the microstructure and oil binding capacity of wax crystal networks. Food Biophys. 2015, 10, 456–465. [Google Scholar] [CrossRef]

| Gelator | Structure | Abbreviation | Yield (%) |
|---|---|---|---|
| Nα, Nε-dioctanoyl-l-lysine | 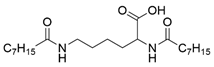 | C8-lys-C8 | 69 |
| Nα, Nε-didecanoyl-l-lysine | 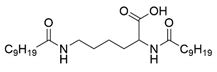 | C10-lys-C10 | 71 |
| Nα, Nε-dilauroyl-l-lysine | 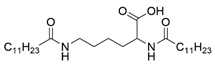 | C12-lys-C12 | 65 |
| Nα, Nε-dimyristoyl-l-lysine |  | C14-lys-C14 | 42 |
| Vegetable Oils | MGC (wt%) | |||
|---|---|---|---|---|
| C8-lys-C8 | C10-lys-C10 | C12-lys-C12 | C14-lys-C14 | |
| Olive oil | 1.08 | 1.28 | 4.28 | 7.79 |
| Corn germ oil | 1.18 | 1.93 | 4.37 | 8.63 |
| Soybean oil | 1.36 | 2.14 | 4.42 | 8.84 |
| Linseed oil | 1.67 | 2.27 | 4.55 | 10.57 |
| Vegetable Oils | SFA 1 | Oleic Acid | Linoleic Acid | Linolenic Acid |
|---|---|---|---|---|
| Olive oil | 18.66 ± 0.11 | 74.55 ± 0.06 | 6.07 ± 0.02 | 0.72 ± 0.10 |
| Corn germ oil | 17.01 ± 0.14 | 30.26 ± 0.03 | 51.70 ± 0.07 | 1.03 ± 0.21 |
| Soybean oil | 18.82 ± 0.21 | 26.83 ± 0.18 | 47.40 ± 0.08 | 6.95 ± 0.23 |
| Linseed oil | 14.04 ± 0.08 | 22.15 ± 0.30 | 14.90 ± 0.19 | 48.91 ± 0.03 |
| Vegetable Oils | Phase Transition Temperature (°C) | |||
|---|---|---|---|---|
| C8-lys-C8 | C10-lys-C10 | C12-lys-C12 | C14-lys-C14 | |
| Olive oil | 101.1 | 62.3 | 48.4 | 51.5 |
| Corn germ oil | 102.5 | 130.9 | 51.5 | 41.1 |
| Soybean oil | 102.5 | 84.2 | 60.8 | 57.0 |
| Linseed oil | 112.9 | 104.4 | 44.9 | 71.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhang, J.; Zhang, G.; Xu, B. l-Lysine-Based Gelators for the Formation of Oleogels in Four Vegetable Oils. Molecules 2022, 27, 1369. https://doi.org/10.3390/molecules27041369
Li Q, Zhang J, Zhang G, Xu B. l-Lysine-Based Gelators for the Formation of Oleogels in Four Vegetable Oils. Molecules. 2022; 27(4):1369. https://doi.org/10.3390/molecules27041369
Chicago/Turabian StyleLi, Qiannan, Jieying Zhang, Guiju Zhang, and Baocai Xu. 2022. "l-Lysine-Based Gelators for the Formation of Oleogels in Four Vegetable Oils" Molecules 27, no. 4: 1369. https://doi.org/10.3390/molecules27041369
APA StyleLi, Q., Zhang, J., Zhang, G., & Xu, B. (2022). l-Lysine-Based Gelators for the Formation of Oleogels in Four Vegetable Oils. Molecules, 27(4), 1369. https://doi.org/10.3390/molecules27041369





